Abstract
BACKGROUND
Oxidative stress has a major pathogenic role for liver damage following chronic hepatitis B. Glutathione peroxidase (Gpx) is necessary in oxidative state mechanism that is generally down-regulated by Hepatitis B virus (HBV) infection. On the other hand, disorders of iron homeostasis have been found out in HBV infected patients. Therefore, the objective of this study was to assess the interplay of Gpx and serum iron on clinical and virological features of patients with chronic HBV infection.
METHODS
One hundred and fifty adult, treatment-naïve, patients with chronic hepatitis B were randomly designated from an ongoing cohort of patients with HBV. Plasma Gpx1 concentration and HBV DNA quantity were measured. Liver stiffness was measured by transient elastography.
RESULTS
Serum iron had a positive association with HBV DNA count in the total population. Serum iron was not associated with liver stiffness. However, HBV DNA was significantly associated with liver stiffness only in male patients. Serum Gpx was inversely associated with liver stiffness. Serum iron and Gpx had indirect effects on liver stiffness via HBV DNA count. We observed dissimilar effects of serum iron on HBV DNA and Gpx on liver stiffness in male and female patients.
CONCLUSION
We identified interplay of serum iron and Gpx1 in relation to level of liver fibrosis in patients with chronic hepatitis B. Our results propose that oxidative stress and serum iron are differentially implicated in the progression of chronic hepatitis B in male and female patients.
Keywords: Glutathione peroxidase (Gpx), Hepatitis B, Iron, Liver Stiffness, HBV DNA
INTRODUCTION
Chronic hepatitis B (CHB) caused by hepatitis B virus (HBV) is a serious illness and a major etiological factor of liver cirrhosis and hepatocellular carcinoma (HCC). Upon infection, HBV enters the hepatocyte and undergoes replication within the infected hepatocyte. Higher replication rate of the virus leads to sustained inflammation and increased fibrosis.1,2 Immune response to HBV results in by-production of reactive oxygen species (ROS) including hydrogen peroxide, superoxide anions, and lipid peroxides, which consequently change the oxidative state of the microenvironment.1,3 Furthermore, HBV also dynamically regulates the oxidative stress via its HBx (hepatitis B x protein) protein.4,5 An imbalance between the production and destruction of ROS results in excess ROS, which induce oxidative damage in cellular components.6 Therefore oxidative stress plays a major pathogenic role in liver injury following CHB. It is suggested that the oxidative DNA damage increases the risk of HBV-induced HCC.6
Glutathione peroxidase (Gpx) is a family of enzymes that constitute a main antioxidant defense system in mammals.7 Gpx1 is the most abundant Gpx isoenzyme, which is also expressed in the liver.8 Gpx1 is localized to the cytoplasm where it reduces H2O2 to water by employing glutathione (GSH) as an electron donor.7 Gpx1 is thought to be the most important H2O2 scavenger.9 Previous findings indicate down-regulation of Gpx in HBV transgenic mice.10 HBV transgenic mice have reduced the level of Gpx protein in the liver, which is significantly less than wild type control. The Gpx enzyme activity was also significantly reduced in the liver of HBV transgenic mice compared with controls. Transfection of HBV genome into the human hepatoma cell lines C3A and HuH-7 resulted in decreased protein level of Gpx in C3A cells.11 Adenoviral delivery of HBx into Hep3B cell line also results in reduced level of glutathione protein and cell viability following H2O2 treatment compared with non-transfected cells.4 HBV-induced oxidative damage was reviewed elsewhere.3 However, the association of serum Gpx1 with HBV progression has not been documented.
Serum iron has been widely studied in viral hepatitis specifically in HCV (Hepatitis C virus) infection.12,13 Serum iron, ferritin, and transferrin are significantly different between HBV patients with cirrhosis and healthy controls as well as between patients with and without cirrhosis. Serum iron and ferritin are increased whereas transferrin is decreased in patients with HBV compared with controls. Serum iron and ferritin were positively correlated with serum ALT in cirrhotic patients with HBV. The MELD (Model for End-Stage Liver Disease) score was inversely correlated with serum transferrin in cirrhotic HBV. Serum iron and ferritin was significantly higher in cirrhotic patients with ALT>40 U/L whereas serum transferrin was significantly lower.14 In a survey of 205 patients with CHB, hepatic iron deposits were found in 35.1% patients, which were significantly more prevalent in male patients.15 The interaction of serum iron and Gpx1 in oxidative-induced injury has been studied in mouse astrocyte.16 The interplay of serum iron and Gpx in HBV infection is not known. Therefore, the main objective of the present study was to evaluate the interplay of Gpx and serum iron on clinical and virological characteristics of patients with chronic HBV.
MATERIALS AND METHODS
Patients
One hundred and fifty adult, treatment-naïve, patients with CHB defined as being HBsAg positive and HBeAg negative according to current guidelines were randomly selected from 3505 participants of HBV cohort.17,18 The patients did not have co-infection with human immune-deficiency virus (HIV), hepatitis C virus, hepatitis D virus, and hepatitis G virus. They were not pregnant, and did not have autoimmune hepatitis, history of alcohol consumption, recent blood transfusion, and hemochromatosis. The patients had not received oral or intravenous iron-containing drugs, steroid, pegylated interferon, or nucleoside analog therapy at the time of the sample collection. Written informed consent was obtained from all the individuals. The study was conducted according to the Declaration of Helsinki. The Institutional Review Board and the Ethics Committee of the Digestive Disease Research institute approved the study protocol.
Study Design
At the first visit, demographic data of the participants were recorded. Liver stiffness was assessed by transient elastography, and 10 ml peripheral venous blood was collected. Sera and plasma samples were stored at -70°C until further processing.
Glutathione Peroxidase Measurement
Glutathione Peroxidase concentration was measured in the plasma samples by enzyme-linked immunosorbent assay (ELISA) using the Human Glutathione Peroxidase 1 ELISA Kit (BIOVENDOR-LABORATORNI MEDICINA A.S. Hong Kong, China) according to the manufacturer’s instructions. The lower limit of detection of the kit is 45 pg/mL.
HBV-DNA Quantification
HBV DNA was extracted from 200 µL of plasma using QIAamp DNA Blood Mini Kit (QIAGEN, Valencia, CA, USA) and then quantified in the Light-Cycler (Roche Diagnostics, Mannheim, Germany) by the artus RealArtTM HBV LC PCR (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. The linear range of this assay was 2x101–108 IU/mL.
Liver Stiffness Measurement
Liver stiffness was measured by a trained medical doctor using the FibroScan® 502 machine (EchoSense, Paris, France, 5 MHz) following at least 3 hours fasting. According to the manufacturer’s guidelines the M and XL probes were used for individuals with thoracic perimeter of less than or above 110 cm, respectively. With the patient lying in the dorsal decubitus position with maximal abduction of the right arm, the probe was placed on the skin overlying the right lobe of the liver, through the intercostal spaces. At least 10 measurements were done for each patient and the median value was recorded. Values were considered valid if the interquartile range (IQR) was less than 30% of the median reading and the success rate was at least 60%. The median value was calculated automatically and expressed in kilopascals (kPa). Transient elastography was not performed in cases of any degree of ascites, ferromagnetic tools in the body, pregnancy, or morbid obesity as suggested by the manufacturer. Cut-off for the advanced liver fibrosis was set as equal or greater than 8 KPa.
Statistical Analysis
Statistical analysis was performed using the Stata software version 12 (Stata Statistical Software: Release 12. College Station, TX: StataCorp LP). Data were expressed as mean±SD and number (percent) for continues and categorical data, respectively. Baseline characteristics were tested between male and female patients using independent t test or Chi-square test, as appropriate. Simple linear regression analysis was used to determine the probable risk factors of HBV DNA count and liver stiffness. Also, Pearson correlation was calculated to determine the linear relations between continuous variables. In order to examine the strength of direct and indirect associations among variables, path regression analysis was used, which measures the causal association of the variables in a hypothesized system. P value of less than 0.05 was considered as statistically significant.
RESULTS
Overall, 150 patients with CHB (65 [43.3%] male) with the mean±SD age of 59.2±7.0 years were investigated. Log HBV DNA count and liver stiffness were 2.98±1.05 and 5.16±2.24 kPa, respectively. The mean concentrations of serum iron and Gpx were 69.31±25.96 μg/dL and 2.33±2.87 ng/mL, respectively. The baseline characteristics of patients are summarized in table 1. Overall, 129 (86%) patients had mild fibrosis, 14 (9%) had moderate fibrosis, and 7 (5%) had advanced liver fibrosis on liver stiffness measurement. The levels of Gpx, serum iron, and HBV DNA in different stages of liver fibrosis are summarized in table 2.
Table 1 . Baseline characteristics of the patients .
| Feature | Male | Female | Total |
| N (%) | 65 (43.33) | 85 (56.67) | 150 |
| Age, mean±SD | 58.88±7.78 | 59.52±6.39 | 59.24±7.01 |
| Smoking, n (%) | |||
| No | 12 (18.5) | 18 (21.18) | 120 (80.00) |
| Yes | 53 (81.54) | 67 (78.82) | 30 (20.00) |
| Body mass index*, mean±SD | 27.04±4.65 | 29.66±5.09 | 28.55±5.06 |
| Hb g/dL*, mean±SD | 14.02±1.49 | 12.31±1.35 | 13.05±1.64 |
| ALT U/L, mean±SD | 32.54±48.43 | 24.88±33.08 | 28.20±40.48 |
| AST U/L, mean±SD | 27.82±28.94 | 24.11±19.29 | 25.71±23.94 |
| ALP U/L, mean±SD | 267.31±67.37 | 272.06±99.30 | 270.00±86.68 |
| Creatinine mg/dL*, mean±SD | 1.27±0.42 | 1.02±0.21 | 1.13±0.34 |
| Serum Iron μg/dL*, mean±SD | 76.69±26.78 | 63.66±23.97 | 69.31±25.96 |
| Glutathione peroxidase ng/mL, mean±SD | 2.14±2.46 | 2.47±3.15 | 2.33±2.87 |
| HBV DNA log (IU/mL), median (IQR) | 3.05±1.13 | 2.92±0.99 | 2.98±1.05 |
| Fibroscan score (kPa), median (IQR) | 5.51±2.67 | 4.89±1.82 | 5.16±2.24 |
HB, hemoglobin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; HBV, hepatitis B virus; IQR, interquartile range
* These variables were significantly different between male and female patients (p<0.05)
Table 2 . Association of serum iron, serum Gpx, and HBV DNA count with liver stiffness .
| Liver stiffness | Fibrosis | N (%) | HBV DNA | Serum iron | Gpx |
| 0< liver stiffness<7 | Mild | 129 (86%) | 2.84±0.88 | 68.65±25.26 | 2.46±3.00 |
| 7≤ liver stiffness<8 | Moderate | 14 (9%) | 3.48±1.47 | 66.36±27.29 | 1.97±1.87 |
| 8≤ liver stiffness | Sever | 7 (5%) | 4.54±1.53 | 87.28±33.28 | 0.61±0.39 |
Association of Serum Iron with HBV DNA Count
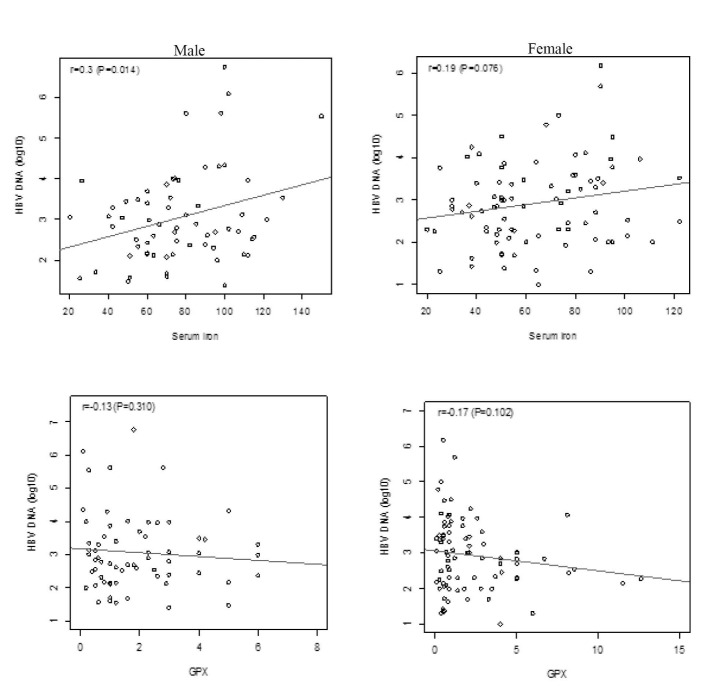
We measured the association between the patients’ features and HBV DNA count. Serum iron had a positive association with HBV DNA count in the total population. Increasing each μg/dL of serum Iron added 0.01 to the HBV DNA log count [p=0.002, 95% CI: (0.004 to 0.017)]. We also observed a negative association between serum Gpx and HBV DNA log count, albeit it was not statistically significant [p=0.05, -0.06 (-0.12 to 0.001)]. We did not detect an interaction between sex and either serum iron or Gpx on the HBV DNA. Therefore, we did not observe a significant difference between the association of serum iron or Gpx on HBV DNA in men and women (figure 1).
Fig. 1 .
Association of serum iron and Gpx with HBV DNA count in patients with chronic HBV.
Determinant Factors of Liver Stiffness
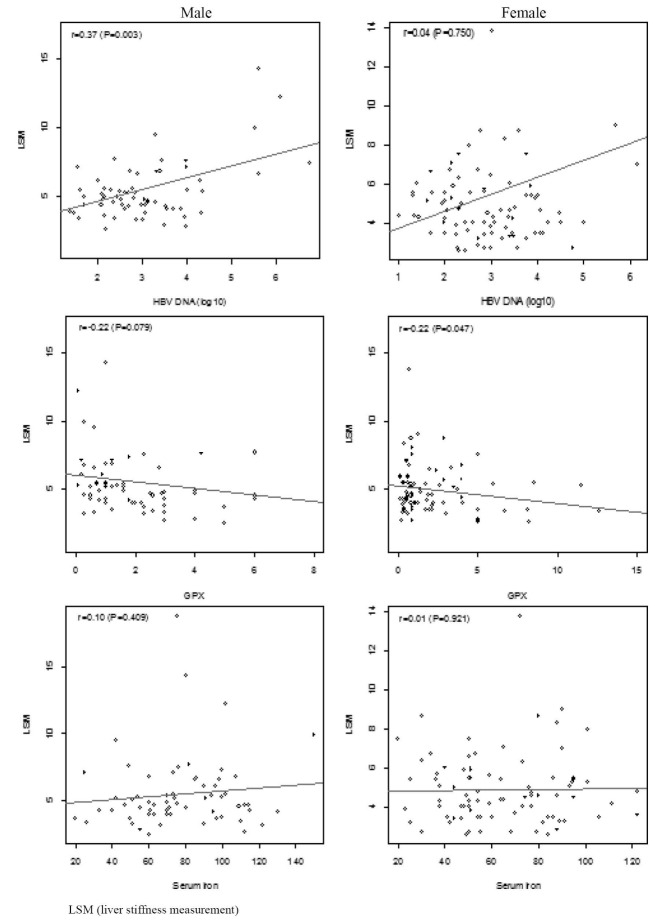
We also measured the association between the patients’ features and liver stiffness. Serum iron was not associated with liver stiffness, however, HBV DNA count was positively associated with it [p=0.006, 0.48 (0.14 to 0.82)]. The effect of HBV DNA on liver stiffness was significantly different between male and female patients (p for interaction=0.018). HBV DNA was significantly associated with liver stiffness only in men [p=0.003, 0.87 (0.31 to 1.42; p=0.003)] and not women [p=0.750, 0.06 (0.33 to 0.46)]. Serum Gpx was inversely associated with liver stiffness [p=0.009, -0.17 (-0.29 to -0.04)] with no significant interaction between sex and Gpx (p for interaction=0.398). However, in the subgroup analysis, the association between Gpx and liver stiffness was not statistically significant in men possibly because of the small sample size. We did not distinguish a significant association between serum iron and liver stiffness (figure 2). More over we did not detect significant associations between age, sex, and smoking and HBV DNA level and liver stiffness. BMI was positively associated with liver stiffness in women. However, the interaction of BMI and sex was not significant. This might be due to the significantly higher BMI in women in our study (data not shown).
Fig. 2 .
Association of serum iron, HBV, Gpx on liver stiffness measurement in patients with chronic HBV.
Interplay of Serum Iron, HBV DNA, and Serum GPx
The directed and undirected dependencies of serum iron, HBV DNA, and Gpx on liver stiffness were analyzed by path analysis. We identified that serum iron was positively associated with HBV DNA log count [p=0.002; 0.01 (0.003 to 0.016)]. Serum Gpx was inversely associated with HBV DNA, albeit not significantly. We also identified that liver stiffness had positive association with HBV DNA [p=0.013; 0.42 (0.09 to 0.75)] but negative with Gpx [p=0.022; -0.14 (-0.26 to -0.02)]. Serum iron and Gpx had indirect effects on liver stiffness via HBV DNA count [P=0.056; 0.004 (0.000 to 0.008)] and [p=0.175; -0.02 (-0.05 to 0.01)], respectively. Consequently, the combined direct and indirect effect of Gpx on liver stiffness was [-0.16 (-0.28 to -0.04), p=0.009]. We observed distinct effects of serum iron on HBV DNA and Gpx on liver stiffness in men and women. Serum iron was significantly associated with HBV DNA in men [p=0.010; 0.01 (0.003 to 0.02)] while Gpx demonstrated a significant inverse association with liver stiffness in women [p=0.035; -0.13 (-0.25 to -0.01)]. We also observed a positive trend in the indirect effect of serum iron on liver stiffness in men [p=0.051; 0.01 (0.000 to 0.021)] (table 3). The pathway diagram is illustrated in figure 3.
Table 3 . Interplay of serum Iron, HBV DNA, and serum GPx in path analysis .
| Factors | Male | Female | ||
| RC (95%CI) | p-value | RC (95%CI) | p-value | |
| Associated with HBV DNA count | ||||
| Serum Iron | 0.01 (0.003 to 0.02) | 0.010 | 0.01 (-0.01 to 0.02) | 0.069 |
| Factors associated with liver stiffness | ||||
| BMI | 0.03 (-0.09 to 0.16) | 0.593 | 0.08 (0.01 to 0.16) | 0.022 |
| HBV DNA | 0.82 (0.29 to 1.35) | 0.003 | 0.002 (-0.37 to 0.38) | 0.990 |
| Gpx | -0.19 (-0.43 to 0.06) | 0.131 | -0.13 (-0.25 to -0.01) | 0.035 |
Fig. 3 .
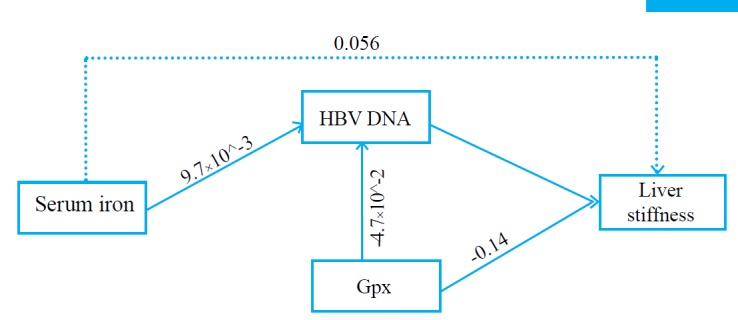
The interaction of serum iron, HBV DNA, and Gpx on liver stiffness.
DISCUSSION
Oxidative stress and serum iron are independently implicated in the pathogenesis of CHB. There is currently no report on the interplay of Gpx and serum iron on HBV DNA and liver fibrosis. We assessed the plasma concentration of Gpx and serum iron in 150 patients with CHB. We did not histologically assess the liver iron deposit, as liver biopsy specimens were not available for the patients. However, we had previously demonstrated the correlation of transient elastography with histological measure of hepatic iron deposition.2,19,20 Therefore, we used the liver stiffness as an indirect measure of both liver fibrosis and liver iron deposit. We demonstrated that plasma level of Gpx was inversely associated with HBV DNA count and liver stiffness whereas serum iron was positively associated with HBV DNA count and was indirectly affecting the liver stiffness. The effect of serum iron on HBV DNA and HBV DNA on liver stiffness was affected by sex. Interestingly, HBV DNA was significantly associated with liver stiffness only in men. In the multivariate path analysis, which relies on the causal relationship of the investigated factors, it was confirmed that serum iron indirectly affected the liver stiffness via its effect on HBV DNA while Gpx1 both directly and indirectly regulated the liver stiffness.
Oxidative stress plays a major pathogenic role in liver injury following CHB.21,22 HBx protein of HBV renders hepatocytes more sensitive to H2O2-induced cell death4 by reducing the liver Gpx content as well as activity.11 We did not compare the serum level of Gpx in patients with CHB with healthy controls. However, we identified an inverse association between serum Gpx concentration and the degree of liver fibrosis. Higher concentration of serum Gpx was detected in patients with lower stages of liver fibrosis. On the other hand, serum iron was identified as an independent predictive factor of HBV DNA count. However, we did not detect a significant association between serum iron and liver stiffness in the path analysis. In other words, although the serum iron is important in regulating the oxidative response in the course of HBV infection, its overall effect is mediated by the regulation of HBV DNA replication and hence, DNA count. The association between HBV DNA and fibrosis had been previously demonstrated.23,24
The results on the association of serum and liver iron on the progression of viral hepatitis are contradictory. Earlier studies have demonstrated that iron homeostasis is disrupted in HBV infected patients leading to increased serum iron as well as hepatic iron deposition.5,25-27, Patients with CHB and cirrhosis have significantly higher level of serum iron compared with those without cirrhosis.14 Similarly, serum ferritin is significantly higher in CHB patients with progressive liver disease (and not the asymptomatic carriers) compared with healthy controls.28 Hepatic iron content was also higher in patients with CHB and higher grades of fibrosis and higher stages, albeit not significantly.29,18 However, in other reports, serum iron and ferritin were not different between cirrhotic and non-cirrhotic patients with CHB30 and were not associated with histological assessment of liver fibrosis.28 In chronic hepatitis C, fibrosis staging is also positively correlated with higher hepatic iron content.29 Serum ferritin and transferrin saturation are significantly increased in CHC with cirrhosis compared with non-cirrhosis.31 The association of cell-specific hepatic iron deposit was assessed in CHC in relation to adverse clinical events including death, hepatocellular carcinoma, decompensated cirrhosis, and increased liver fibrosis. Iron content of hepatocytes and portal triads were significantly associated with the rate of an adverse clinical event. Serum ferritin and transferrin saturation were also associated with the poor outcome but not significantly. However, serum iron was a significant predictor of an adverse outcome.32 Negative association of iron measures with fibrosis has also been reported in CHC.33
Iron is a hepatotoxic agent, which can affect liver homeostasis and promote non-hemochromatic liver diseases. It is known that Hbx suppresses the Gpx production and reduces the Gpx activity in the liver, as a consequence of which the HBV-infected liver becomes more susceptible to oxidative stress. Concurrent high levels of serum and/or liver iron now can augment the injury and hence, result in hepatitis progression to advanced stages of fibrosis and ultimately cirrhosis. HBV-induced oxidative stress could induce stimulation of hepatic stellate cell, proliferation of myofibroblasts, formation of extracellular matrix, and consequently augmentation of fibrogenesis.34 Moreover, oxidative imbalance could cause DNA damage, which upon failure of the repair mechanisms can become fixated and result in potentially oncogenic mutations.35 Currently, anti-oxidants such as N-acetylcysteine, S-adenosyl-L-methionine, and vitamin E are utilized as supplementary medications in patients who have developed fibrosis.36 We identified interplay of serum iron and Gpx1 in relation to level of liver fibrosis in CHB. It is conceivable that measures, which aim at targeting the ROS imbalance and hence reducing the oxidative stress in HBV-infected liver, could have therapeutic benefits for patients with CHB in terms of preventing the progressive liver damage, fibrosis, and ultimately cirrhosis.
ACKNOWLEDGEMENT
This study was supported in part by a grant from Digestive Disease Research Institute, Tehran University of Medical Sciences.
CONFLICT OF INTEREST
The authors declare no conflict of interest related to this work.
Please cite this paper as:
Moossavi S, Besharat S, Sharafkhah M, Ghanbari R, Sharifi A, Rezanejad P, Pourshams A, Poustchi H, Mohamadkhani A. Inverse Association of Plasma Level of Glutathione Peroxidase with Liver Fibrosis in Chronic Hepatitis B: Potential Role of Iron. Middle East J Dig Dis 2016;8:122-130. DOI: 10.15171/mejdd.2016.17
References
- 1.McMahon BJ. Chronic hepatitis B virus infection. Med Clin North Am. 2014;98:39–54. doi: 10.1016/j.mcna.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Besharat S, Poustchi H, Mohamadkhani A, Katoonizadeh A, Moradi A, Roshandel G. et al. Association of Mutations in the Basal Core Promoter and Pre-core Regions of the Hepatitis B Viral Genome and Longitudinal Changes in HBV Level in HBeAg Negative Individuals: Results From a Cohort Study in Northern Iran. Hepat Mon. 2015;15:e23875. doi: 10.5812/hepatmon.23875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higgs MR, Chouteau P, Lerat H. ‘Liver let die’: oxidative DNA damage and hepatotropic viruses. J Gen Virol. 2014;95:991–1004. doi: 10.1099/vir.0.059485-0. [DOI] [PubMed] [Google Scholar]
- 4.Wu YL, Wang D, Peng XE, Chen YL, Zheng DL, Chen WN. et al. Epigenetic silencing of NAD(P)H:quinone oxidoreductase 1 by hepatitis B virus X protein increases mitochondrial injury and cellular susceptibility to oxidative stress in hepatoma cells. Free Radic Biol Med. 2013;65:632–644. doi: 10.1016/j.freeradbiomed.2013.07.037. [DOI] [PubMed] [Google Scholar]
- 5.Gu JM, Lim SO, Oh SJ, Yoon SM, Seong JK, Jung G. HBx modulates iron regulatory protein 1-mediated iron metabolism via reactive oxygen species. Virus Res. 2008;133:167–177. doi: 10.1016/j.virusres.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Nair J, Srivatanakul P, Haas C, Jedpiyawongse A, Khuhaprema T, Seitz HK. et al. High urinary excretion of lipid peroxidation-derived DNA damage in patients with cancer-prone liver diseases. Mutat Res. 2010;683:23–28. doi: 10.1016/j.mrfmmm.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Espinoza SE, Guo H, Fedarko N, DeZern A, Fried LP, Xue QL. et al. Glutathione peroxidase enzyme activity in aging. J Gerontol A Biol Sci Med Sci. 2008;63:505–509. doi: 10.1093/gerona/63.5.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petryszak R, Burdett T, Fiorelli B, Fonseca NA, Gonzalez-Porta M, Hastings E. et al. Expression Atlas update--a database of gene and transcript expression from microarray- and sequencing-based functional genomics experiments. Nucleic Acids Res. 2014;42:D926–932. doi: 10.1093/nar/gkt1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 10.Lee DH, Esworthy RS, Chu C, Pfeifer GP, Chu FF. Mutation accumulation in the intestine and colon of mice deficient in two intracellular glutathione peroxidases. Cancer Res. 2006;66:9845–9851. doi: 10.1158/0008-5472.CAN-06-0732. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q, Na B, Ou JH, Pulliam L, Yen TS. Hepatitis B virus alters the antioxidant system in transgenic mice and sensitizes hepatocytes to Fas signaling. PLoS One. 2012;7:e36818. doi: 10.1371/journal.pone.0036818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vagu C, Sultana C, Ruta S. Serum iron markers in patients with chronic hepatitis C infection. Hepat Mon. 2013;13:e13136. doi: 10.5812/hepatmon.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mifuji-Moroka R, Iwasa M, Miyachi H, Sugimoto R, Tanaka H, Ishihara T. et al. Iron overload and glucose abnormalities in chronic hepatitis C virus infection: phlebotomy lowers risk of new-onset diabetes. Hepatogastroenterology. 2013;60:1736–41. [PubMed] [Google Scholar]
- 14.Mao W, Hu Y, Lou Y, Chen Y, Zhang J. Abnormal serum iron markers in chronic hepatitis B virus infection may be because of liver injury. Eur J Gastroenterol Hepatol. 2015;27:130–136. doi: 10.1097/MEG.0000000000000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sebastiani G, Tempesta D, Alberti A. Hepatic iron overload is common in chronic hepatitis B and is more severe in patients coinfected with hepatitis D virus. J Viral Hepat. 2012;19:e170–176. doi: 10.1111/j.1365-2893.2011.01508.x. [DOI] [PubMed] [Google Scholar]
- 16.Liddell JR, Hoepken HH, Crack PJ, Robinson SR, Dringen R. Glutathione peroxidase 1 and glutathione are required to protect mouse astrocytes from iron-mediated hydrogen peroxide toxicity. J Neurosci Res. 2006;84:578–586. doi: 10.1002/jnr.20957. [DOI] [PubMed] [Google Scholar]
- 17.Poustchi H, Katoonizadeh A, Ostovaneh MR, Moossavi S, Sharafkhah M, Esmaili S. et al. Cohort profile: golestan hepatitis B cohort study- a prospective long term study in northern iran. Middle East J Dig Dis. 2014;6:186–94. [PMC free article] [PubMed] [Google Scholar]
- 18.Pourshams A, Nasiri J, Mohammadkhani A, Nasrollahzadeh D. Hepatitis B in Gonbad-Kavoos: prevalence, risk factors and intrafamilial spreading. Govaresh. 2004;9:222–5. [Google Scholar]
- 19.Hamidieh AA, Shazad B, Ostovaneh MR, Behfar M, Tayebi S, Malekzadeh R. et al. Noninvasive measurement of liver fibrosis using transient elastography in pediatric patients with major thalassemia who are candidates for hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20:1912–7. doi: 10.1016/j.bbmt.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 20.Poustchi H, Eslami M, Ostovaneh MR, Modabbernia A, Saeedian FS, Taslimi S. et al. Transient elastography in hepatitis C virus-infected patients with beta-thalassemia for assessment of fibrosis. Hepatol Res. 2013;43:1276–1283. doi: 10.1111/hepr.12088. [DOI] [PubMed] [Google Scholar]
- 21.Dandri M, Burda MR, Burkle A, Zuckerman DM, Will H, Rogler CE. et al. Increase in de novo HBV DNA integrations in response to oxidative DNA damage or inhibition of poly(ADP-ribosyl)ation. Hepatology. 2002;35:217–223. doi: 10.1053/jhep.2002.30203. [DOI] [PubMed] [Google Scholar]
- 22.Mohamadkhani A, Katoonizadeh A, Poustchi H. Immune-Regulatory Events in the Clearance of HBsAg in Chronic Hepatitis B: Focuses on HLA-DP. Middle East J Dig Dis. 2015;7:5–13. [PMC free article] [PubMed] [Google Scholar]
- 23.Mohamadkhani A, Sayemiri K, Ghanbari R, Elahi E, Poustchi H, Montazeri G. The inverse association of serum HBV DNA level with HDL and adiponectin in chronic hepatitis B infection. Virol J. 2010;7:228. doi: 10.1186/1743-422X-7-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bai H, Liu H, Chen X, Xu C, Dou X. Influence of age and HBeAg status on the correlation between HBV DNA and hepatic inflammation and fibrosis in chronic hepatitis B patients. Dig Dis Sci. 2013;58:1355–1362. doi: 10.1007/s10620-012-2479-7. [DOI] [PubMed] [Google Scholar]
- 25.Felton C, Lustbader ED, Merten C, Blumberg BS. Serum iron levels and response to hepatitis B virus. Proc Natl Acad Sci USA. 1979;76:2438–2441. doi: 10.1073/pnas.76.5.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patil PS, Mohandas KM, Bhatia SJ, Mehta SA. Serum ferritin and the risk of hepatocellular carcinoma in chronic liver disease of viral etiology: a case-control study. Indian J Gastroenterol. 2014;33:12–18. doi: 10.1007/s12664-013-0367-5. [DOI] [PubMed] [Google Scholar]
- 27.Di Bisceglie AM, Axiotis CA, Hoofnagle JH, Bacon BR. Measurements of iron status in patients with chronic hepatitis. Gastroenterology. 1992;102:2108–2113. doi: 10.1016/0016-5085(92)90339-z. [DOI] [PubMed] [Google Scholar]
- 28.Ghaziani T, Alavian SM, Zali MR, Shahraz S, Agah M, Jensen KP. et al. Serum measures of iron status and HFE gene mutations in patients with hepatitis B virus infection. Hepatol Res. 2007;37:172–178. doi: 10.1111/j.1872-034X.2007.00026.x. [DOI] [PubMed] [Google Scholar]
- 29.Fujita N, Sugimoto R, Urawa N, Araki J, Mifuji R, Yamamoto M. et al. Hepatic iron accumulation is associated with disease progression and resistance to interferon/ribavirin combination therapy in chronic hepatitis C. J Gastroenterol Hepatol. 2007;22:1886–1893. doi: 10.1111/j.1440-1746.2006.04759.x. [DOI] [PubMed] [Google Scholar]
- 30.Chook JB, Ngeow YF, Yap SF, Tan TC, Mohamed R. Combined use of wild-type HBV precore and high serum iron marker as a potential tool for the prediction of cirrhosis in chronic hepatitis B infection. J Med Virol. 2011;83:594–601. doi: 10.1002/jmv.22016. [DOI] [PubMed] [Google Scholar]
- 31.Weiss G, Umlauft F, Urbanek M, Herold M, Loyevsky M, Offner F. et al. Associations between cellular immune effector function, iron metabolism, and disease activity in patients with chronic hepatitis C virus infection. J Infect Dis. 1999;180:1452–8. doi: 10.1086/315052. [DOI] [PubMed] [Google Scholar]
- 32.Lambrecht RW, Sterling RK, Naishadham D, Stoddard AM, Rogers T, Morishima C. et al. Iron levels in hepatocytes and portal tract cells predict progression and outcomes of patients with advanced chronic hepatitis C. Gastroenterology. 2011;140:1490–1500 e1493. doi: 10.1053/j.gastro.2011.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Won JE, Jeong SH, Chung JI, Lee JH, Hwang SH, Kim JW. et al. Hepatic iron, serum ferritin, HFE mutation, and hepatic fibrosis in chronic hepatitis C. Intervirology. 2009;52:239–46. doi: 10.1159/000228547. [DOI] [PubMed] [Google Scholar]
- 34.Bhogal RH, Curbishley SM, Weston CJ, Adams DH, Afford SC. Reactive oxygen species mediate human hepatocyte injury during hypoxia/reoxygenation. Liver Transpl. 2010;16:1303–1313. doi: 10.1002/lt.22157. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka H, Fujita N, Sugimoto R, Urawa N, Horiike S, Kobayashi Y, t al. Hepatic oxidative DNA damage is associated with increased risk for hepatocellular carcinoma in chronic hepatitis C. Br J Cancer. 2008;98:580–6. doi: 10.1038/sj.bjc.6604204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghiassi-Nejad Z, Friedman SL. Advances in antifibrotic therapy. Expert Rev Gastroenterol Hepatol. 2008;2:803–816. doi: 10.1586/17474124.2.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]