Abstract
This study examined the effects of high- vs. moderate-intensity interval training on cardiovascular fitness, leptin levels and ratings of perceived exertion (RPE) in obese female adolescents. Forty-seven participants were randomly assigned to one of three groups receiving either a 1:1 ratio of 15 s of effort comprising moderate-intensity interval training (MIIT at 80% maximal aerobic speed: MAS) or high-intensity interval training (HIIT at 100% MAS), with matched 15 s recovery at 50% MAS, thrice weekly, or a no-training control group. The HIIT and MIIT groups showed improved (p < 0.05) body mass (BM), BMI Z-score, and percentage of body fat (%BF). Only the HIIT group showed decreased waist circumference (WC) (p = 0.017). The effect of exercise on maximal oxygen uptake (VO2max) was significant (p = 0.019, ES = 0.48 and p = 0.010, ES = 0.57, HIIT and MIIT, respectively). The decrease of rate-pressure product (RPP) (p < 0.05, ES = 0.53 and ES = 0.46, HIIT and MIIT, respectively) followed the positive changes in resting heart rate and blood pressures. Blood glucose, insulin level and the homeostasis model assessment index for insulin decreased (p < 0.05) in both training groups. Significant decreases occurred in blood leptin (p = 0.021, ES = 0.67 and p = 0.011, ES = 0.73) and in RPE (p = 0.001, ES = 0.76 and p = 0.017, ES = 0.57) in HIIT and MIIT, respectively. In the post-intervention period, blood leptin was strongly associated with %BF (p < 0.001) and VO2max (p < 0.01) in the HIIT and MIIT groups, respectively, while RPE was strongly associated with BM (p < 0.01) in the HIIT group. The results suggest that high-intensity interval training may produce more positive effects on health determinants in comparison with the same training mode at a moderate intensity.
Keywords: Intermittent exercise, Obesity, Effort perception, Children, Moderate exercise intensity
INTRODUCTION
It is well known that childhood obesity is a complex condition that can lead to a plethora of metabolic and cardiovascular risk factors [1]. To combat this scourge, dietary and/or physical exercise may be proposed [2]. However, although aerobic continuous exercise is generally included in intervention programmes, interval training remains the preferred physical exercise modality in adolescents because it reflects their spontaneous activity patterns [3]. Consequently, the American College of Sports Medicine [4] suggested incorporating interval training in intervention programmes in obese adolescents. However, despite this, interval training exercise modality is still underused in obese adolescents. On the other hand, insulin resistance in children and adolescents is a frequent consequence of obesity [5], and is responsible for some metabolic complications such as type 2 diabetes. However, released hormones such as leptin interact with insulin, contributing to better metabolic functioning [6]. Previously, blood leptin concentration has been shown to be positively correlated with the level of exercise in children [7], and even to decrease after sufficient training programme duration [8]. However, the relationship between plasma leptin, exercise training and body composition is poorly understood and the effects of different exercise intensities on these factors have not been studied in obese adolescent females. Consequently, the optimal exercise intensity for decreasing blood leptin concentration, but also improving anthropometric, physiological and biological data, in the young obese population warrants investigation. Moreover, these data could be important for exercise prescription and for evidence-based exercise programming in obese adolescents.
Previously, Coquart et al. [9] have shown in an obese population and from ratings of perceived exertion (RPE) data that moderate-intensity interval training (MIIT) exercise was perceived as being less strenuous than aerobic continuous exercise despite matching of training relative workload and exercise duration. This subsequently prompted the authors to propose that MIIT should be prescribed rather than continuous aerobic exercise to maintain adherence to exercise programmes in obese populations [9]. However, this remains a matter of ongoing debate [10]. In fact, to the best of the authors’ knowledge, no study has yet examined the interrelationship between the anthropometric, physiological and biological modifications with perceptual responses to maximal exercise (i.e., RPE), following MIIT and HIIT in young obese persons. Such interventions may improve the understanding of exercise prescription for obese adolescents.
Therefore, this study aimed to compare the effects of either MIIT or HIIT on the determinants of cardio-metabolic health, body composition and blood leptin levels in obese adolescent females and to compare the effects of these two training modes on RPE following maximal exercise testing.
We hypothesized that the HIIT programme may yield more favourable results for improving cardio-respiratory fitness parameters than the MIIT programme, and is more efficient for improving leptin level and the RPE after maximal exercise testing in obese adolescent females.
MATERIALS AND METHODS
Participants
Forty-seven healthy obese female adolescents (age = 14.2±1.2 years, BMI Z-score = 3.5±0.5, BF = 40.0±1.5%), recruited from three schools in the region, volunteered to take part in this study. At first, participants were classified according to body mass index (BMI), which was calculated in the standard way, using the algorithm provided by the Centers for Disease Control and Prevention (CDC) (body mass [kg])/(height [m])2. The participants’ BMI was > 97th percentile according to the French standards and the %BF > 34% [11]. Using the CDC growth chart, the BMI scores were transformed to produce age- and sex-adjusted BMI percentiles. To constitute the three groups, subjects were randomized and stratified (according to age and BMI) and the BMI was turned into the BMI Z-score.
None of the participants participated in any systematic exercise training at the time of study enrolment or during the six months that preceded the experiment (except one hour of physical education at school). In addition, participants were instructed not to perform any leisure activity based on exercising. None of the girls used drugs or therapies for obesity, or presented chronic diseases. Moreover, all participants were asked not to consume any medications during the week prior to blood sampling which may have impacted the study testing and progress.
The study protocol was approved by the local Research Ethics committee. Written informed consent was obtained from all volunteers and their parents prior to the study commencement in accordance with the international ethical standards and the Declaration of Helsinki.
Procedure
During the first day, height (cm) was measured in the morning using a wall stadiometer with the participant scantily dressed and without shoes. In the same conditions, BM (kg) and the %BF were assessed with a calibrated bioelectrical impedance scale (TBF-300, Tanita, Tokyo, Japan).
The BMI Z-score was identified for each participant. Waist circumference (WC in cm) was measured at the midpoint between the lower part of the ribcage and the iliac crest with participants standing and breathing normally with a non-elastic measuring tape.
During the same day, resting heart rate (HRrest in bpm) was recorded in the morning and after sitting for 10 min, using a heart rate monitor (S-610, Polar, Kempele, Finland). HRrest represented the minimal value of heart rate measured during the last 1 minute of the rest period. Resting systolic (SBP) and diastolic (DBP) blood pressures (mmHg) were measured at rest using the auscultatory method with a stethoscope and sphygmomanometers (Vaquez-Laubry, Spengler, Issoudun, France). The average of three recordings taken one minute apart from the left arm and after being seated for ten minutes was recorded as the criterion value. In order to evaluate ventricular function, the rate-pressure product (RPP), which results from multiplying systolic blood pressure (SBP) by heart rate (HR), was calculated (RPP = SBP × HR/1000) [12].
The next day, blood was sampled using the venipuncture method between 7:00 a.m. and 9:00 a.m. following an overnight fast (∼12 h). Plasma glucose concentrations were determined using the hexokinase method with an automated device (Architect c8000, Abbott, Québec, Canada). The inter-assay coefficient of variation (CV) was 1.7%. Plasma insulin was assayed with radioimmunoassay kits (Immunotech A, Beckman Coulter Company, Marseille, France). The intra- and inter-assay coefficients of variations were 3.3-4.0 and 3.7-4.8%, respectively. Insulin resistance was assessed using the homeostatic model assessment for insulin resistance (HOMA-IR), which has been validated in children and adolescents [13] and was computed as follows: HOMA-IR= [fasting insulin (µU·mL−1) × fasting glucose (mmol·L−1)] ÷ 22.5
Plasma leptin was evaluated using an enzyme-linked immune-sorbent assay (ELISA) kit (Quantikine: human leptin immunoassay) (Linco Research Inc., St. Louis, USA). The intra- and inter-assay coefficients of variation were 3.2-3.2% and 3.5-5.4%, respectively. Each sample was assayed in duplicate and the mean of the two was used in the analyses.
Following the collection of baseline data (the third day), all participants performed graded exercise testing [14] until exhaustion to determine maximal oxygen uptake (VO2max) and maximal aerobic speed (MAS). This maximal test was carried out on a 200 m outdoor track calibrated with cones. The test starts at a running speed of 8.5 km·h−1 and increases by 0.5 km·h−1 every minute until exhaustion. The sustained speed during the last completed stage corresponds to the MAS. During this exercise test, respiratory gas exchange was determined breath-by-breath using a calibrated portable telemetry system (K4b2, Cosmed, Rome, Italy). After that, HR was monitored using a HR monitor (S-610, Polar, Kempele, Finland). Exhaustion was verified by the following criteria: 1) a plateau phenomenon in oxygen uptake; 2) respiratory exchange ratio (RER) ≥ 1.1; 3) peak hear rate ± 10 bpm of the predicted maximal heart rate (220 - age) and 4) voluntary exhaustion. At least three of the four criteria were met or the test was repeated.
Thirty minutes following the graded exercise testing, participants were asked to rate RPE from the French translation [15] of the Foster et al. scale [16].
Following baseline measurement, participants were randomly assigned to 3 groups: 1) MIIT group (n = 16), HIIT group (n = 17) and control group (i.e., non-exercising group; n = 14). Both interval training programmes (i.e., MIIT and HIIT) were performed on an outdoor athletics track. A detailed training programme is presented in table 1.
TABLE 1.
Summary of the interval training programmes (high-intensity interval training: HIIT vs. Moderate-intensity interval training: MIIT)
| HIIT group | MIIT group | |
|---|---|---|
| 10 min of jogging at 50% MAS | ||
| Warm-up period | 5 min of dynamic stretching exercises | |
| 5 accelerations on 20-m with 1 min of recovery between | ||
| Weeks 1-4: 3 sessions × 4 min (15 s/15 s) | ||
| Weeks 5-8: 3 sessions × 6 min (15 s/15 s) | ||
| Interval training period | Weeks 9-12: 3 sessions × 8 min (15 s/15 s) | |
| 100%/50% MAS | 80%/50% MAS | |
| 3-min of inter-session passive recovery period | ||
| Cooling down period | 5 min of jogging at low intensity | |
| 5 min of static stretching exercises | ||
Note: MAS: maximal aerobic speed; HIIT: high-intensity interval training (100%MAS) vs. MIIT: moderate-intensity interval training (80%MAS); MAS: maximal aerobic speed.
Only three week days (Monday, Wednesday and Friday) were devoted to the training programmes during the intervention. During this period, all participants were asked not to participate in any other physical activity, as well as to maintain their usual diet.
After the 12-week intervention period, all anthropometric, physiological, biological and perceptual data were collected again, in the same conditions.
In order to estimate energy intake, participants completed a dietary questionnaire on 4 days (3 week days and one weekend day), before and after the intervention period. Only the first and the last week of the intervention were taken into consideration for data analysis. In order to estimate energy intake (kcal·day−1), data were analyzed using Bilnut 2.01 software (Nutrisoft, Cerelles, France).
Statistical Analysis
Data are expressed as the mean ± standard deviation. The normal Gaussian distribution was verified by the Shapiro-Wilk test, whereas homogeneity of variance was assessed using Levene's test. Inter- and intra-group comparisons of the variables were computed by two-way ANOVA (group vs. time) with repeated measurements to determine the main and interaction effects between groups over time. Percentage change between pre-test and post-test was calculated for each parameter. One-way ANOVA was used to determine the difference of the percentage change between groups (MIIT, HIIT and control group). Whenever significant differences in values occurred, a pair-wise multiple comparisons test was performed using a Bonferroni post-hoc test. In addition, post-hoc effect size statistics (ES) for all the statistically significant t ratios were established. These calculations were based on Cohen's classification, and knowledge of the ES enabled the magnitude of the difference to be estimated (i.e., trivial: ES < 0.2, small: 0.2 ≤ ES < 0.5, moderate: 0.5 ≤ ES < 0.8, or large: ES ≥ 0.8).
In order to identify the most relevant associations, stepwise multiple regression was performed, while the correlation between leptin and other parameters was examined using Pearson's test.
Statistical significance was set at p<0.05, and all analyses were performed with SPSS (release 18.0, Chicago, IL, USA).
RESULTS
During the intervention period, all participants completed all the tests. The training groups responded to the exercise training programme conditions as allocated. The participants of HIIT and MIIT attended all training sessions and no one complained or reported any training or test-related injury.
Daily energy intake did not change significantly from pre- to post-intervention and did not differ between groups (p = 0.187). Both training groups’ measurements elicited significant decreases in BM, BMI Z-score and %BF in the post-intervention period. Waist circumference decreased significantly only in the HIIT group (p = 0.017; Table 2). In the between groups comparison, BM, BMI Z-score and WC in the HIIT group were significantly different (p < 0.05) from the two other groups (MIIT and control group).
TABLE 2.
Anthropometric data before and after the intervention period
| HIIT group (n = 17) | MIIT group (n = 16) | Control group(n = 14) | ||||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Height (cm) | 164 ± 5 | 165 ± 5 | 163 ± 4 | 164 ± 4 | 165 ± 5 | 165 ± 5 |
| Body mass (Kg) | 87.3 ± 4.5 | 84.1 ± 4.7a | 88.3 ± 6.0 | 86.6 ± 6.5a | 85.6 ± 5.2 | 85.9 ± 4.2 |
| Body mass index –Z-score | 3.4 ± 0.4 | 3.1 ± 0.3a | 3.9 ± 0.3 | 3.6 ± 0.4a | 3.3 ± 0.5 | 3.3 ± 0.4 |
| Body fat (%) | 40.3 ± 1.5 | 36.4 ± 1.5a | 40.6 ± 1.6 | 37.2 ± 1.2a | 39.8 ± 1.2 | 39.3 ± 0.7 |
| Waist circumference (cm) | 94 ± 6 | 91 ± 6a | 93 ± 4 | 91 ± 4 | 94 ± 4 | 94 ± 4 |
| Energy intake (kcal·day−1) | 2995 ± 101 | 2923 ± 76 | 3010 ± 137 | 2962 ± 111 | 2953 ± 92 | 2927 ± 87 |
Note: Values are mean ± SD
Significantly different with pre-test at p < 0.05.
After the intervention period, no group showed positive changes in HRmax or RER, while SBP, DBP and HRrest decreased significantly (Table 3), leading to a significant decrease in RPP (p = 0.013, ES = 0.53 and p = 0.036, ES = 0.46, HIIT and MIIT, respectively).
TABLE 3.
Physiological, biological and perceptual data before and after the intervention period
| HIIT group (n = 17) | MIIT group (n = 16) | Control group(n = 14) | ||||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Maximal heart rate (bpm) | 203.8 ± 2.4 | 202.8 ± 2.1 | 201.1 ± 2.3 | 200.6 ± 1.9 | 202.5 ± 2.7 | 202.3 ± 2.2 |
| Resting heart rate (bpm) | 70 ± 3 | 67 ± 2aa | 71 ± 3 | 68 ± 2aa | 70 ± 2 | 70 ± 2 |
| Systolic blood pressure (mmHg) | 121 ± 8 | 115 ± 5a | 119 ± 5 | 115 ± 5a | 118 ± 6 | 118 ± 5 |
| Diastolic blood pressure (mmHg) | 80 ± 4 | 74 ± 2a | 79 ± 2 | 75 ± 4a | 75 ± 3 | 74 ± 2 |
| Rate of pressure product | 8.48 ± 0.75 | 7.72 ± 0.46a | 8.53 ± 0.55 | 7.86 ± 0.43a | 8.28 ± 0.46 | 8.31 ± 0.28 |
| Blood glucose (mmol.L-1) | 4.8 ± 0.5 | 4.6 ± 0.4a | 4.9 ± 0.2 | 4.7 ± 0.2a | 4.6 ± 0.4 | 4.6 ± 0.4 |
| Blood insulin (µU.mL-1) | 22.0 ± 1.9 | 16.3 ± 0.84a | 21.6 ± 2.1 | 17.3 ± 1.5a | 20.0 ± 2.0 | 19.2 ± 2.0 |
| HOMA-IR | 4.7 ± 0.7 | 3.3 ± 0.4a | 4.7 ± 0.5 | 3.6 ± 0.4a | 4.1 ± 0.6 | 3.9 ± 0.6 |
| Blood leptin (ηg.mL-1) | 24.1 ± 3.5 | 18.6 ± 2.1a | 25.0 ± 3.1 | 19.1 ± 3.6a | 21.0 ± 2.1 | 21.4 ± 2.4 |
| Maximal oxygen uptake (L.min−1) | 2.98 ± 0.27 | 3.05 ± 0.29a | 3.01 ± 0.30 | 3.12 ± 0.34a | 3.05 ± 0.25 | 3.11 ± 0.27 |
| Respiratory exchange ratio | 1.13 ± 0.02 | 1.11 ± 0.02 | 1.12 ± 0.02 | 1.12 ± 0.03 | 1.13 ± 0.03 | 1.13 ± 0.02 |
| RPE index | 8.6 ± 0.5 | 6.1 ± 0.4aa | 8.4 ± 0.5 | 7.2 ± 0.9a | 8.3 ± 0.5 | 8.2 ± 0.6 |
Note: Values are mean ± SD. RPE: rating of perceived exertion 30 min after the graded exercise test; HOMA-IR: homoeostasis model assessment index for insulin resistance.
Significantly different with pre-test at p < 0.05
Significantly different with pre-test at p < 0.01.
Following HIIT and MIIT programmes, VO2max when expressed in absolute form increased significantly by 2.22±2.99%, p = 0.019, ES = 0.48 and 3.53±1.39%, p = 0.010, ES = 0.57, respectively. In the between groups comparison these two groups were significantly different (p < 0.05) from the control group. Blood glucose and insulin levels, as well as HOMA-IR, decreased significantly in the training groups (Table 3). Blood leptin concentrations underwent positive changes (-22.31±6.58%, p = 0.021, ES = 0.67 and -23.20 ± 10.18%, p = 0.012, ES = 0.72), in high and moderate training programmes, respectively. HIIT and MIIT groups showed a decrease in RPE after 30 min of the graded exercise testing (-29.1%, p= 0.001, ES = 0.76 and -14.3%, p = 0.017, ES = 0.57, respectively).
One-way ANOVA showed significant differences between the three groups in percentage changes (Table 4).
TABLE 4.
Percentage changes between pre-test and post-test in each group
| HIIT group (%) pre vs.post | MIIT group (%) pre vs.post | Control group (%) pre vs.post | ES | ||
|---|---|---|---|---|---|
| Body mass (Kg) | -3.70 ± 1.0b, c | -1.98 ± 1.12c | 0.47 ± 1.75 | F = 5.57 | 0.45 |
| Body mass index –Z-score | -9.22 ± 2.60c | -8.10 ± 5.49c | -1.88 ± 6.33 | F = 9.7 | 0.57 |
| Body fat (%) | -9.60 ± 2.26b, c | -8.24 ± 1.78c | -1.37 ± 1.38 | F = 12.4 | 0.64 |
| Waist circumference (cm) | -3.23 ± 2.38b, c | -2.98 ± 1.26c | -0.31 ± 0.72 | F = 4.8 | 0.42 |
| Resting heart rate (bpm) | -4.39 ± 3.52c | -4.35 ± 2.21c | 0.62 ± 1.80 | F = 7.6 | 0.47 |
| Systolic blood pressure (mmHg) | -4.46 ± 3.32c | -3.48 ± 1.23c | -0.20 ± 2.08 | F = 10.08 | 0.44 |
| Rate of pressure product | -8.63 ± 5.29c | -7.69 ± 1.80c | 0.44 ± 3.40 | F = 12.5 | 0.49 |
| Diastolic blood pressure (mmHg) | -7.17 ± 3.86c | -5.79 ± 3.35c | -2.03 ± 2.97 | F = 5.5 | 0.21 |
| Blood glucose (mmol·L−1) | -4.15 ± 1.39c | -3.99 ± 1.79c | -0.92 ± 2.51 | F = 7.8 | 0.48 |
| Blood insulin (µU·mL−1) | -25.68 ± 4.24b, c | -19.40 ± 6.23c | -3.84 ± 2.46 | F = 14.1 | 0.48 |
| HOMA-IR | -28.74 ± 4.69b, c | -22.58 ± 6.72c | -4.73 ± 3.20 | F = 12.8 | 0.52 |
| Blood leptin (ηg·mL-1) | -22.31 ± 6.58c | -23.20 ± 10.18c | 2.38 ± 2.50 | F = 14.6 | 0.70 |
| Maximal oxygen uptake (L·min−1) | 2.22 ± 2.99c | 3.57 ± 1.39c | 1.68 ± 1.83 | F = 8.75 | 0.52 |
| RPE 30 min after exercise test | -28.97 ± 6.18b, c | -14.38 ± 8.70c | -0.10 ± 0.88 | F = 9.0 | 0.52 |
Note: b Significantly different from MIIT at p < 0.05
Significantly different from control group at p < 0.05. pre vs post: pre-training vs. post-training; HIIT: high-intensity interval training; MIIT: moderate-intensity interval training; ES: effect size; trivial: ES < 0.2, small: 0.2 ≤ ES < 0.5, moderate: 0.5 ≤ ES < 0.8, and large: ES ≥ 0.8.
After the intervention, blood leptin levels were associated with %BF (r=0.74, p < 0.001) in the HIIT group and with VO2max (r=0.72, p < 0.01) in the MIIT group. RPE was associated with body mass (r=0.68, p < 0.01) in the HIIT group.
DISCUSSION
The aims of this study were twofold: (i) to compare the effects of either MIIT or HIIT on determinants of cardio-metabolic health, body composition and on blood leptin levels in obese adolescent females; and (ii) to compare the effects of these two training models on RPE following a bout of maximal exercise testing.
The current study results demonstrate that HIIT and MIIT positively changed cardio-metabolic determinants (i.e., VO2max, HRrest, blood pressures, blood glucose, blood insulin and RPP; Table 3) and decreased blood leptin concentration, while HIIT (100% of MAS) induced better results in body composition and in RPE at maximal effort in a cohort of obese female adolescents, compared to training at moderate intensity (80% of MAS).
Twelve weeks of exercise training in this study resulted in a significant decrease in BM, BMI Z-score and %BF. In fact, we suggest that decreasing BMI Z-score during adolescence is interesting, since it has been considered an important predictor of body fat in adult age [17]. In addition to this result, a further significant decrease in WC was observed at post-training in the HIIT group (-3.2±2.4%). These positive changes are important during childhood and may prevent obesity complications. However, the advantageous significant difference in percentage change of BM, %BF and WC, in the HIIT group when compared to the other groups (Table 4), supports the efficiency of this exercise training mode based on high-intensity exercise.
Furthermore, the decrease of WC is important in adolescents, since WC has been shown to be significantly associated with high blood pressure [18]. In line with the result of WC noted in this study, two weeks of HIIT induced a significant reduction in WC in obese sedentary men [19]. By contrast, in another study Ciolac et al. [20] reported that even 16 weeks of HIIT did not change WC significantly. In this regard, whether HIIT potentially activates preferential oxidation of abdominal adipose tissue remains to be established.
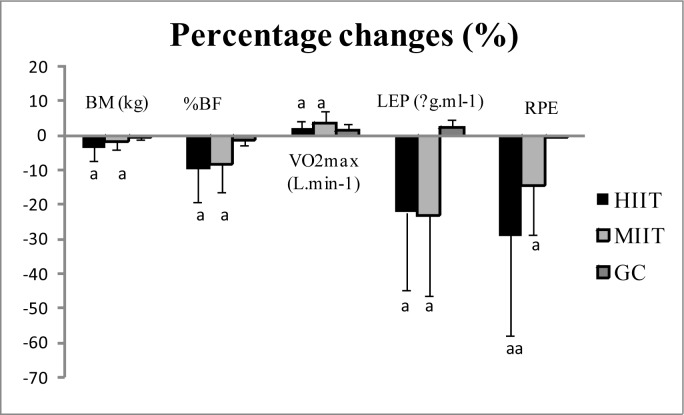
After 12 weeks of training, participants in both groups were able to increase significantly their absolute VO2max (3.53±1.39% vs. 2.22±2.99%, MIIT and HIIT groups, respectively; Figure 1). This may explain the significant effect of moderate- and high-intensity interval training programmes on aerobic capacity. In fact, we presume that a decrease in fat mass as shown in the training groups may help participants’ skeletal muscles in supporting hard effort longer, which allows improvement of VO2max, as has been recently demonstrated after a period of high-intensity interval exercise [20, 21]. In another context and in the case of different training modes, some previous studies [22, 23] showed the effectiveness of exercising with intervals compared to continuous training. In their study, the results revealed greater reductions in cardiovascular risk factors after high-intensity interval exercises when compared with moderate exercises. In fact, obese persons prefer short bursts of intense physical exercises interspersed with short periods of less intensity rather than continuous effort; this could help to improve their cardio respiratory fitness.
FIG. 1.
Exercise-induced change in BM, %BF, VO2max, plasma leptin levels and RPE, in the three groups after the intervention
Note: Values are mean ± SD, a (p < 0.05), aa (p < 0.01).
The present study also revealed that both modes of training decreased HRrest significantly. This resting heart rate adaptation to exercise training could be the result of the high number of calories expended following the training session. Therefore, interval training exercise seems an effective way to condition the heart compared to other forms of exercise, as it burns more calories [24]. This is in line with some previous literature [9, 25] but in contradiction to Ciolac et al. [22] who did not note significant changes in resting heart rate either after HIIT or after continuous moderate exercise training. From a practical point of view, the current study results related to heart rate suggest that interval training may be an efficient way to decrease the resting heart rate in young obese persons.
The reduction in resting arterial blood pressure in participants of both training groups was significant after training. This decrease might be related to an improvement in insulin sensitivity, as reported in the study of Montani et al. [26]. However, this positive decrease of insulin level is itself affected by a decrease in body fat. This insulin level has been shown to be more prominent after high-intensity exercise training than after moderate-intensity exercise training, in a cohort of obese adolescent girls. [21]. Furthermore, the reduction in arterial blood pressure in obese participants of the present study could have possibly occurred consecutively to a decrease in BMI Z-score and WC, as previously indicated [27, 18, 28]. In this regard, it is important to note that other studies showed a great enhancement in the endothelial function [20] and more important reductions in arterial stiffness, following HIIT compared to continuous moderate training [29, 20]. Interestingly, the above factors, related to the heart and having been improved, have led to a decrease in RPP between pre- and post-intervention in the training groups (Table 3). This RPP is the major determinant of myocardial oxygen consumption and of cardiac dysfunction [30]. In this context, the mean values in post-intervention were 7.72±0.46 and 7.86±0.43, in HIIT and MIIT groups, respectively. This is important, since the values were within the tolerated range of values above which pre- hypertension and hypertension can appear [31]. Therefore, both training models were found to be not only efficient but also safe for the participants.
Before the intervention commencement, blood glucose data were all within normal ranges. After the training programme, blood glucose concentration decreased significantly in both the HIIT and MIIT groups to a similar extent. Those latter improvements in blood glucose are probably due to an increase in muscle oxidative capacity and a decrease in blood insulin levels. It is worth mentioning that blood insulin levels decreased in the post-intervention period and this was higher in the high-intensity training group than in the moderate-intensity training group (-25.68 ± 4.24% and -19.40 ± 6.23%, respectively). These decreases in blood insulin concentration are considerable and justify the greater advantage of the intense training. This is not concurrent with some studies [32, 20] which demonstrated a similarity in MIIT vs. HIIT effects on insulin sensitivity. In a different study Tjonna et al. [33] reported the superiority of high-intensity interval exercise in comparison to moderate-intensity continuous exercise to increase insulin sensitivity. However, the literature indicates that the decrease in blood glucose concentration may occur after an increase in GLUT-4 protein content which increases in proportion to increased insulin sensitivity after aerobic training [34]. This decrease of insulin resulted in a larger decrease in HOMA-IR in the HIIT group (-28.74±4.69%) compared with the MIIT group (-22.58±6.72%). In this regard, HIIT seems to be a better approach for the prevention and management of adolescent obesity by regulating hormonal disorders observed in this population to a higher extent than does MIIT.
According to the literature, leptin acts on the central nervous system by reducing appetite and by stimulating energy expenditure [35]. After physical exercise, leptin increases the oxidation of fatty acids in skeletal muscle through the activation of AMP-activated protein kinase (AMPK) by the sympathetic nervous system and α-adrenergic mechanism [36].
However, both training groups showed decreased blood leptin concentrations after the intervention period (Table 3). Since blood leptin concentration seems tightly coupled to body fat in humans [37], it has been suggested that the reductions in %BF and BMI Z-score probably allowed a significant decrease of blood leptin concentration in this study. This suggestion is further supported by the finding of Baskin et al. [38] that a beneficial change in serum leptin concentration was associated with a decrease in fat mass.
Interestingly, the current results showed strong associations between blood leptin concentration and %BF after HIIT and between blood leptin concentration and VO2max after MIIT.
According to McMurray et al. [39], the plasma leptin concentration was associated with aerobic fitness (VO2max) after exercise training. Given that both training groups have increased absolute VO2max at post-intervention (figure 1), this could also be a reason for decreasing blood leptin concentration. It may be suggested that leptin sensitivity in obese youth may return to normal functioning when fat mass is reduced and/or the physical activity level is significantly increased (exp: VO2max) as in the HIIT and MIIT groups.
Even though some authors [40] found a 12.29% increase in cardio respiratory fitness level (VO2max) in obese women after 9 weeks of aerobic exercise, the percentage of fat and the leptin concentration were not altered by training. However, the percentage of fat in their study at pre-exercise did not differ much from the present study (43.0% vs. 40.2%) but the relative VO2max of participants at pre-exercise in the present study (data not shown) was higher (34.65± 2.6 ml·kg−1·min−1 vs. 21.95±0.75 ml.kg−1.min−1). Based on these results, it may be presumed that the reduction in leptin levels in this study was the result of a decrease in body fat, more than of aerobic capacity, which is in agreement with the conclusions of some authors [37] and different to others [39]. Consequently the present study data demonstrate that leptin concentration may change with an optimal training intensity. Taking into account the current study results, it is suggested that leptin resistance may exist in obese subjects, who should practice physical training at an optimal intensity in order to positively affect leptin levels.
As previously reported [41], the intra-individual comparison of perceptual values (here measured from RPE) may be used to evaluate the impact of exercise training programmes on physical fitness in obese participants. In the present study, a 12-week training programme was sufficient to significantly decrease the RPE by 2.5 units in the HIIT group and 1.2 units in the MIIT group. Thus, the percentage change was significantly higher in the HIIT group compared to the two other groups (Table 4). In addition, both trained groups perceived the same maximal exercise session as being less hard after the intervention period, regardless of exercise intensity. This result seems to be mainly linked to significant changes in body mass. In their study, Brock et al. [42] reported that overweight and obese individuals perceived physical exercise as more difficult, when they were compared to their lean counterparts. In fact, we did not expect that the practice of high- and moderate-intensity exercises could alter the subject's perceived difficulty after the maximal graded exercise test. Thus we presume that these feelings that appeared after maximal exercise encourage more obese participants to adhere to training programmes, as has been reported [10].
To conclude, both exercise intensities (high and moderate) improved aerobic capacity (as measured by VO2max) and improved blood leptin concentration. In addition, HIIT was better in improving the values of BM, %BF and WC in obese adolescent girls, compared to the MIIT.
This study has limitations. First, the current study results took into consideration only girls, since the difference between boys and girls begins from the age of adolescence and usually boys spend more time in physical education and are more engaged in vigorous- or moderate-intensity physical activity than girls [43]. Thus the present results have to be interpreted only for young obese females.
Secondly, participants’ menstrual cycles were not controlled during the intervention. Therefore, future studies should pay attention to this factor during the exercise protocols.
CONCLUSIONS
The present study demonstrated that, in obese adolescent females, high-intensity and moderate-intensity interval training were efficient in improving aerobic fitness levels (VO2max) and in decreasing blood leptin concentrations following twelve weeks of intervention. However, HIIT was more efficient in improving body composition and RPE after maximal graded exercise. The results suggest that high-intensity interval training may produce more positive effects on health determinants in comparison with the same training mode at a moderate intensity. Therefore, these findings may provide an important first step for incorporating exercise programmes for prevention of obesity.
Acknowledgements
The authors would like to thank the girls for their participation and would like to thank the medical staff for their medical assistance. This study was supported by the Ministry of Higher Education, Scientific Research and Technology of Tunisia.
Conflict of interests
The authors declared no conflict of interests regarding the publication of this manuscript.
REFERENCES
- 1.Juonala M, Magnussen CG, Berenson GS, Alison V, Trudy LB, Matthew AS, Srinivasan SR, Daniels SR, Davis PH, Cong Sun WC, Cheung M, Viikari JS, Dwyer T, Raitakari OT. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365(20):1876–1885. doi: 10.1056/NEJMoa1010112. [DOI] [PubMed] [Google Scholar]
- 2.Kelishadi R, Azizi-Soleiman FJ. Controlling childhood obesity:A systematic review on strategies and challenges. Res Med Sci. 2014;19(10):993–1008. [PMC free article] [PubMed] [Google Scholar]
- 3.Crisp NA, Fournier PA, Licari MK, Braham R, Guelfi KJ. Adding sprints to continuous exercise at the intensity that maximizes fat oxidation:implications for acute energy balance and enjoyment. Metabolism. 2012;61(9):1280–1288. doi: 10.1016/j.metabol.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 4.American College of Sports Medicine. ACSM's Guidelines for exercise testing and prescription. 9th edition. Baltimore: Lippincott Williams & Wilkins; 2014. [Google Scholar]
- 5.Caprio S. Insulin resistance in childhood obesity. J Pediatr Endocrinol Metab. 2002;15(1):487–492. [PubMed] [Google Scholar]
- 6.Matsuzawa Y. White adipose tissue and cardiovascular disease. Best Pract Res Clin Endocrinol Metab. 2005;19(4):637–647. doi: 10.1016/j.beem.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Salbe AD, Weyer C, Lindsay RS, Ravussin E, Tataranni PA. Assessing risk factors for obesity between childhood and adolescence:I. Birth weight, childhood adiposity, parental obesity, insulin, and leptin. Pediatrics. 2002;110(2):299–306. doi: 10.1542/peds.110.2.299. [DOI] [PubMed] [Google Scholar]
- 8.Akbarpour M. The effect of aerobic training on serum adiponectin and leptin levels and inflammatory markers of coronary heart disease in obese men. Biol Sport. 2013;30(1):21–7. doi: 10.5604/20831862.1029817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coquart JB, Lemaire C, Dubart AE, Luttembacher DP, Douillard C, Garcin M. Intermittent versus continuous exercise:effects of perceptually lower exercise in obese women. Med Sci Sports Exerc. 2008;40(8):1546–1553. doi: 10.1249/MSS.0b013e31816fc30c. [DOI] [PubMed] [Google Scholar]
- 10.Biddle SJH, Batterham AM. High-intensity interval exercise training for public health:a big HIT or shall we HIT it on the head? Int J Behav Nutr Phys Act. 2015;12:95. doi: 10.1186/s12966-015-0254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jebb S, McCarthy D, Fry T, Prentice AM. New body fat reference curves for children. Obesity Reviews. 2004;(NAASO Suppl):A156. doi: 10.1038/sj.ijo.0803232. [DOI] [PubMed] [Google Scholar]
- 12.Navalta JW, Sedlock DA, Park K. Physiological responses to downhill walking in older and younger individuals. J Exerc Physiol. 2004;7:45–51. [Google Scholar]
- 13.Guzzaloni G, Grugni G, Mazzilli G, Moro D, Morabito F. Comparison between B-cell function and insulin resistance indexes in pre-pubertal and pubertal obese children. Metabolism. 2002;51:1011–6. doi: 10.1053/meta.2002.34029. [DOI] [PubMed] [Google Scholar]
- 14.Chtara M, Chamari K, Chaouachi M, Chaouachi A, Koubaa D, Feki Y, Millet GP, Amri M. Effects of intra-session concurrent endurance and strength training sequence on aerobic performance and capacity. Br J Sports Med. 2005;39:555–560. doi: 10.1136/bjsm.2004.015248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haddad M, Chaouachi A, Castagna C, Hue O, Wong DP, Tebben M, Behm DG, Chamari K. Validity and Psychomotric Evaluation of the Frensh Version of RPE scale in Young Fit Males when monitoring training loads. Sci Sport. 2013;28(2):e29–e35. [Google Scholar]
- 16.Foster C, Florhaug JA, Franklin J, Gottschall L, Hrovatin L, Parker S, Doleshai P, Dodge C. new approach to monitoring exercise testing. J Strength Cond Res. 2001;15(1):109–115. [PubMed] [Google Scholar]
- 17.Guo SS, Huang C, Maynard LM, Demerath E, Towne B, Chumlea WC, Siervogel RM. Body mass index during childhood, adolescence and young adulthood in relation to adult overweight and adiposity:the Fels Longitudinal Study. Int J Obes Relat Metab Disord. 2000;24(12):1628–1635. doi: 10.1038/sj.ijo.0801461. [DOI] [PubMed] [Google Scholar]
- 18.Guimarães ICB, Almeida AM, Santos AS, Barbosa DBV, Guimarães AC. Pressão arterial: efeito do índice de massa corporal e da circunferência abdominal em adolescentes. Arq Bras Cardiol. 2008;90(6):293–9. [Google Scholar]
- 19.Leggate M, Carter WG, Evans MJ, Vennard RA, Sribala-Sundaram S, Nimmo MA. Determination of inflammatory and prominent proteomic changes in plasma and adipose tissue after high-intensity intermittent training in overweight and obese males. J Appl Physiol. 2012;112:1353–1360. doi: 10.1152/japplphysiol.01080.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciolac EG, Bocchi EA, Bortolotto LA, Carvalho VO, Greve JMD, Guimarães GV. Effects of high intensity aerobic interval training vs. moderate exercise on hemodynamic, metabolic and neuro-humoral abnormalities of young normotensive women at high familial risk for hypertension. Hypert Res. 2010;33:836–843. doi: 10.1038/hr.2010.72. [DOI] [PubMed] [Google Scholar]
- 21.Racil G, Ben Ounis O, Hammouda O, Kallel A, Zouhal H, Chamari K, Amri M. Effects of high vs. moderate exercise intensity during interval training on lipids and adiponectin levels in obese young females. Eur J Appl Physiol. 2013;113(10):2531–2540. doi: 10.1007/s00421-013-2689-5. [DOI] [PubMed] [Google Scholar]
- 22.Ciolac EG, Bocchi EA, Greve JM, Guimarães GV. Heart rate response to exercise and cardiorespiratory fitness of young women at high familial risk for hypertension:effects of interval vs continuous training. Eur J Cardiovasc Prev Rehabil. 2011;18(6):824–830. doi: 10.1177/1741826711398426. [DOI] [PubMed] [Google Scholar]
- 23.Buchan DS, Ollis S, Thomas NE, Buchanan N, Cooper SM, Malina RM, Baker JS. Physical activity interventions:effects of duration and intensity. Scand J Med Sci Sports. 2011;21(6):e341–e350. doi: 10.1111/j.1600-0838.2011.01303.x. [DOI] [PubMed] [Google Scholar]
- 24.Wisloff U, Stoylen A, Loennchen JP, Bruvold M, Rognmo O, Haram PM, Tjønna AE, Helgerud J, Slørdahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen Ø, Skjærpe T. Superior cardiovascular effect of Aerobic interval training versus moderate continuous training in heart failure patients. A Randomized Study. Circulation. 2007;115:3086–94. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 25.Racil G, Lemaire C, Dubart AE, Garcin M, Coquart JB. Intermittent Exercise is Beneficial to Obese Women Independently of Obesity Class. Jacobs J Physiother Exerc. 2015;1(1):003. [Google Scholar]
- 26.Montani JP, Antic V, Yang Z, Dulloo A. Pathways from obesity to hypertension:from the perspective of a vicious triangle. Int J Obes Relat Metab Disord. 2002;26(2):S28–38. doi: 10.1038/sj.ijo.0802125. [DOI] [PubMed] [Google Scholar]
- 27.Da Silva AC, Rosa AA. Blood pressure and obesity of children and adolescents association with body mass index and waist circumference. Arch Latino am Nutr. 2006;56(3):244–250. [PubMed] [Google Scholar]
- 28.Sarni RS, De Souza FIS, Schoeps DO, Catherino P, Oliveira MCCP, Pessotti CFX, Kochi C, Colugnati FAB. Relação da cintura abdominal com a condição nutricional, perfil lipídico e pressão arterial em pré-escolares de baixo estrato econômico. Arq Bras Cardiol. 2006;87(2):153–8. doi: 10.1590/s0066-782x2006001500013. [DOI] [PubMed] [Google Scholar]
- 29.Guimarães GV, Ciolac EG, Carvalho VO, D’Ávila VM, Bortolotto LA, Bocchi EA. Effects of continuous vs. interval exercise training on blood pressure and arterial stiffness in treated hypertension. Hypert Res. 2010;33:627–632. doi: 10.1038/hr.2010.42. [DOI] [PubMed] [Google Scholar]
- 30.Lin CH. Myocardial Oxygen Consumption Change Predicts Left Ventricular Relaxation Improvement in Obese Humans After Weight Loss. Obesity. 2011;19:1804–1812. doi: 10.1038/oby.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson WR, Gordon NF, Pescatello LS. ACSM's Guide¬lines for Exercise Testing and Prescription. 8th ed. Philadel¬phia: Williams & Wilkins; 2010. [Google Scholar]
- 32.Buchan DS, Ollis S, Thomas NE, Buchanan N, Cooper SM, Malina RM, Baker JS. Physical activity interventions:effects of duration and intensity. Scand J Med Sci Sports. 2011;21(6):e341–e350. doi: 10.1111/j.1600-0838.2011.01303.x. [DOI] [PubMed] [Google Scholar]
- 33.Tjønna AE, Lee SJ, Rognmo Ø, Stølen TO, Bye A, Haram PM, Loennechen JP, Al-Share QY, Skogvoll E, Slørdahl SA, Kemi OJ, Najjar SM, Wisløff U. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome. A pilot study. Circulation. 2008;118:346–354. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Host HH, Hansen PA, Nolte LA, Chen MM, Holloszy JO. Rapid reversal of adaptive increases in muscle GLUT-4 and glucose transport capacity after training cessation. J Appl Physiol. 1998;84:798–802. doi: 10.1152/jappl.1998.84.3.798. [DOI] [PubMed] [Google Scholar]
- 35.Webber J. Energy balance in obesity. Proc Nutr Soc. 2003;62(2):539–543. doi: 10.1079/pns2003256. [DOI] [PubMed] [Google Scholar]
- 36.Minokoshi Y, Toda C, Okamoto S. Regulatory role of leptin in glucose and lipid metabolism in skeletal muscle. Indian J Endocrinol Metab. 2012;16(3):S562–8. doi: 10.4103/2230-8210.105573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baile CA, Della-Fera MA, Martin RJ. Regulation of metabolism and body fat mass by leptin. Annu Rev Nutr. 2000;20:105–127. doi: 10.1146/annurev.nutr.20.1.105. [DOI] [PubMed] [Google Scholar]
- 38.Baskin DG, Blevins JE, Schwatz MW. How the brain regulates food intake and body weight:the role of leptin. J Pediatr Endocrinol Metab. 2001;14:1417–1429. [PubMed] [Google Scholar]
- 39.McMurray RG, Harrell JS, Brown SA. Circulating leptin concentrations are not related to physical activity levels in adolescents. Clin Exerc Physiol. 2000;2:159–164. [Google Scholar]
- 40.Kraemer RR, Kraemer GR, Acevedo EO, Hebert EP, Temple E, Bates M, Etie A, Haltom R, Quinn S, Castracane VD. Effects of aerobic exercise on serum leptin levels in obese women. Eur J Appl Physiol. 1999;80(2):154–158. doi: 10.1007/s004210050572. [DOI] [PubMed] [Google Scholar]
- 41.Coquart JB, Tourny-Chollet C, Lemaître F, Lemaire C, Grosbois JM, Garcin M. Relevance of the measure of perceived exertion for the rehabilitation of obese patients. Ann Phys Rehabil Med. 2012;55(9-10):623–640. doi: 10.1016/j.rehab.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Brock DW, Chandler-Laney PC, Alvarez JA, Gower BA, Gaesser GA, Hunter GR. Perception of exercise difficulty predicts weight regain in formerly overweight women. Obesity. 2010;18(5):982–6. doi: 10.1038/oby.2009.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Belsky J, Booth C, Bradley R, Brownell CA, Campbell SB, Clarke-Stewart A, Friedman SL, Hirsh-Pasek K, Houts RM, Husten A, Knoke B, McCartney K, McKenzie TL, Morrison F, Nader PR, O'Brien M, Payne C, Parke RD, Owen MT, Phillips D, Pianta R, Spieker S, Vandell DL, Robeson WW, Weinraub M. Frequency and intensity of activity of third-grade children in physical education. Arch Pediatr Adolesc Med. 2003;157(2):185–190. doi: 10.1001/archpedi.157.2.185. [DOI] [PubMed] [Google Scholar]