This meta-analysis demonstrates a substantial decrease in the risk of most opportunistic infections with antiretroviral therapy (ART) use in human immunodeficiency virus-infected children in low- and middle-income countries and estimated cost savings with earlier ART initiation.
Keywords: pediatrics, opportunistic infections, HIV, low- and middle-income countries
Abstract
Background. We conducted a systematic review and meta-analysis to evaluate the incidence and prevalence of 14 opportunistic infections (OIs) and other infections as well as the impact of antiretroviral therapy (ART) among human immunodeficiency virus (HIV)–infected children (aged <18 years) in low- and middle-income countries (LMICs), to understand regional burden of disease, and inform delivery of HIV services.
Methods. Eligible studies described the incidence of OIs and other infections in ART-naive and -exposed children from January 1990 to November 2013, using Medline, Global Health, Embase, Cumulative Index to Nursing and Allied Health Literature, Web of Knowledge, and Literatura Latino Americana em Ciências da Saúde databases. Summary incident risk (IR) and prevalent risk for each OI in ART-naive and ART-exposed children were calculated, and unadjusted odds ratios calculated for impact of ART. The number of OI cases and associated costs averted were estimated using the AIDS impact model.
Results. We identified 4542 citations, and 88 studies were included, comprising 55 679 HIV-infected children. Bacterial pneumonia and tuberculosis were the most common incident and prevalent infections in both ART-naive and ART-exposed children. There was a significant reduction in IR with ART for the majority of OIs. There was a smaller impact on bacterial sepsis and pneumonia, and an increase observed for varicella zoster. ART initiation based on 2010 World Health Organization guidelines criteria for ART initiation in children was estimated to potentially avert >161 000 OIs (2013 UNAIDS data) with estimated cost savings of at least US$17 million per year.
Conclusions. There is a decrease in the risk of most OIs with ART use in HIV-infected children in LMICs, and estimated large potential cost savings in OIs averted with ART use, although there are greater uncertainties in pediatric data compared with that of adults.
(See the Major Article by Low et al on pages 1595–1603.)
In 2014, 2.6 million children aged <15 years worldwide were living with human immunodeficiency virus (HIV), of whom 88% lived in sub-Saharan Africa [1]. The same year, there were 220 000 new infections and 150 000 deaths among children, of which the majority can be attributed to opportunistic infections (OIs) [1, 2]. Although there has been a 60% decline in new pediatric HIV infections since 2000, and a 42% decline in HIV-related deaths, scale-up of antiretroviral therapy (ART) has been much less successful in children compared with adults [1, 3, 4]. As of 2014, only one-third of children <15 years of age in need of treatment were receiving it, compared with two-thirds of adults [5].
Large multicenter cohort studies among HIV-infected children in high-income countries (HICs) [6–8] have demonstrated a decrease in incidence and prevalence of most OIs following ART introduction [9–11], but the effect of ART in low- and middle-income countries (LMICs) has generally been less well documented.
Reliable data on the relative burden of different OIs in children are important for planning delivery of HIV services, which includes drug procurement, use of prophylaxis, and provision of appropriate diagnostic capacity. Our objective was to undertake a comprehensive systematic review and meta-analysis to estimate the incidence and prevalence of key OIs and other infections in HIV-infected children, both before and after the initiation of ART in LMICs across 3 geographic regions (Asia, sub-Saharan Africa, and Latin America), and to evaluate the magnitude of effect of ART on these infections and potential costs averted.
METHODS
Search Strategy and Selection Criteria
A systematic review of the literature was performed using the Medline, Embase, Global Health, Cumulative Index to Nursing and Allied Health Literature, Literatura Latino Americana em Ciências da Saúde, and Web of Science databases, and the Cochrane Library of Systematic Reviews from January 1990 to November 2013, published in English, French, Spanish, and Portuguese (see Supplementary Appendix 1 for the search strategy used). Eligible studies reported the incidence and/or prevalence in children aged <18 years in LMICs (as defined by the 2010 World Bank classification) [12] of 14 OIs and other infections, including cryptococcal meningitis; Pneumocystis pneumonia; candidiasis (oral and esophageal combined); cytomegalovirus retinitis; varicella zoster virus (VZV); herpes simplex virus stomatitis; Kaposi sarcoma; cerebral toxoplasmosis; cryptosporidial diarrhea; Mycobacterium tuberculosis (hereafter “tuberculosis”), which was further subdivided into pulmonary tuberculosis (PTB), extrapulmonary tuberculosis, (EPTB), or all types of tuberculosis combined (cTB); and bacterial infections (bacterial pneumonia, bacterial meningitis, isolated bacteremia, and sepsis). LMICs were divided into 3 regions for the purpose of the analysis: sub-Saharan Africa, Latin America and the Caribbean, and Asia. Eligible study designs were cross-sectional, prospective and retrospective cohort studies, and randomized controlled trials (RCTs). This review used Meta-analysis of Observational Studies in Epidemiology and Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines for systematic reviews [13, 14].
Two independent reviewers (M.-R. B.-L. and O. D.) screened abstracts and titles to identify potentially relevant articles. Eligible studies included >50 HIV-infected children, and provided data from which a cumulative incident risk (IR) of 1 or more of 14 different OIs or infections could be calculated (ie, number of episodes or events as well as a denominator of number of children in the study). Twenty-four authors were contacted to provide additional missing data, and 7 responded. The following data were extracted for each study: study design, duration of follow-up, mean/median age or age groups included, ART status (naive or ART exposed including time on ART), use of cotrimoxazole (CTX) prophylaxis, baseline CD4 cell counts, study setting (urban, rural, mix), and diagnostic methods. See Supplementary Appendix 2 for further details on methodology.
Study Definitions
The ART status of the study population was categorized by study as either “ART naive” (studies conducted prior to the availability of ART, or where <10% of the population were ART exposed) or “ART exposed” (studies where >80% of the population were on ART). Studies where the proportion on ART was >10% but <80%, or which did not provide data on ART use, were excluded. No distinction was made according to ART regimens, as there was heterogeneity over time and by geographic region. Few studies reported duration of time on ART, but this was extracted where available (see Supplementary Appendix 2 for further definitions). For each study, 2 investigators independently rated quality of diagnostic approaches for each OI.
Meta-analysis
The cumulative IR for individual OIs for each study in both ART-naive and ART-exposed children were estimated based on the cumulative number of children who developed the specific OI divided by the number of children at risk during the follow-up period, and are presented as a percentage. This approach was used because of inconsistency in incidence reporting across studies, with few reporting annual incidence rates per children-years. Prevalent rate or risk (PR) was defined as the number of children with the OI or other infections divided by the total number of children in the cross-sectional sample. Ninety-five percent confidence intervals (CIs) for both IR and PR were extracted from the papers if available or calculated from the raw data where possible.
Summary IR and PR were calculated by stabilizing the variances of raw percentages using a Tukey-Freeman arcsine square root transformation. Summary risks for individual OIs were then obtained across studies using a random-effects meta-analysis to adjust for the wide variability between studies [15, 16]. Heterogeneity between studies was evaluated using I2 and the P value (Cochran Q statistic) for heterogeneity. A high I2 index (75%) indicates high heterogeneity, whereas a low index (<25%) indicates variability in risk estimates due to within-study variability and therefore low heterogeneity. R software version 2.14.0 was used to perform the analysis. Meta-regression analyses could not be completed because both the number of studies and sample size were limited. The unadjusted odds ratio (OR) was used as a crude estimate for the effect of ART, as there were insufficient data to adjust for confounders. The OR was calculated by applying the calculated IR and PR obtained by random-effects analysis to the absolute number of children affected in the included studies.
OI Cases Averted by the Use of ART
The estimated numbers of HIV-infected children in need of ART were based on the 2010 World Health Organization (WHO) guidelines criteria for ART initiation [17] as we had the most complete dataset for these criteria, than for the WHO 2013 or 2015 criteria, (ie, all HIV-positive children <2 years of age, those aged 2–4 years with a CD4 percentage <25%, and those aged 5–14 years with a CD4 count <350 cells/uL), for 156 LMICs using the 2013 Joint United Nations Programme on HIV/AIDS (UNAIDS) global country estimates for the number of HIV-infected children generated using the AIDS impact model (AIM) [18, 19]. For each country, the AIM model has been used to estimate incidence trends by fitting smooth curves to surveillance and survey data. New infections are followed over time as they progress from higher to lower CD4 counts. The number of OI cases averted was estimated by applying risk difference on specific OI incidence rates in ART-exposed, relative to ART-naive children to the estimated number of children at risk of developing OIs in the 3 geographic regions. The annual cost averted per OI was calculated for 6 OIs (Pneumocystis pneumonia, cryptococcal meningitis, Cryptosporidium, herpes simplex virus, candidiasis, and tuberculosis (PTB and EPTB) where there was both evidence of a decrease in incidence with ART, and where the cost of treating the OI case was known [20], by multiplying the number of OI cases averted by the cost of OI treatment per case. There were insufficient data on treatment costs to include several important infections such as bacterial pneumonia. The 95% CI uncertainty ranges are based on 1000 Monte Carlo simulations assuming normal distributions of incidence risk.
RESULTS
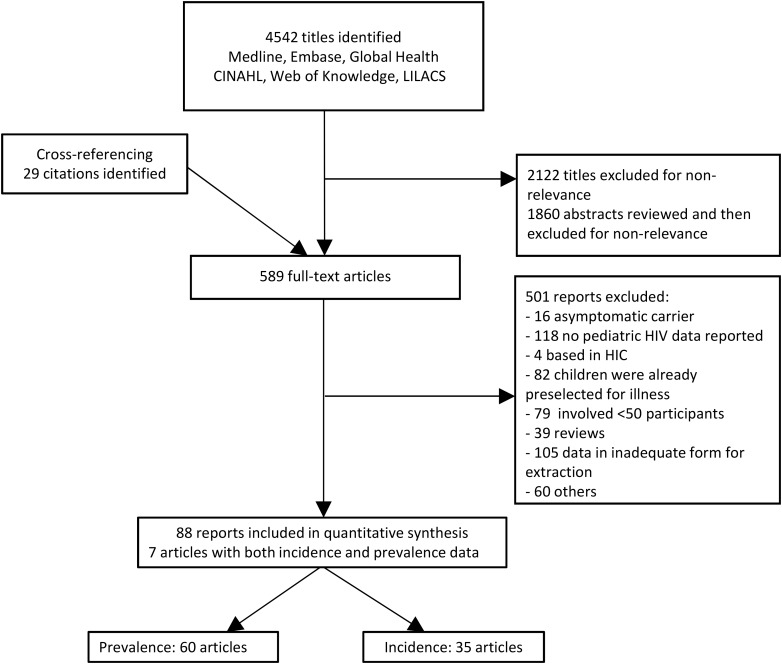
The search strategy identified 4542 citations with an additional 29 identified through cross-referencing. Of these 4571 citations, 589 potentially relevant full-text articles were screened and 88 studies met inclusion criteria for eligibility in quantitative analysis: 7 had both prevalence and incidence data, 53 had prevalence data, and 28 had incidence data (Figure 1). Table 1 and Supplementary Appendix 3 summarize the characteristics of included incidence and prevalence studies, respectively. Supplementary Appendices 6 and 7 include all references to included studies, as well as geographic location of included studies.
Figure 1.
Flowchart of study selection for incidence and prevalence of opportunistic infections in resource-limited settings. Abbreviations: CINAHL, Cumulative Index to Nursing and Allied Health Literature; HIC, high-income country; HIV, human immunodeficiency virus; LILACS, Literatura Latino Americana em Ciências da Saúde.
Table 1.
Characteristics of Included Studies—Incidence of Opportunistic Infections and Other Infections, by Region
| Infection and Region | Study Design | No. of Patients (Study Range) |
Median Age, mo (No.) |
IQR, mo (No.) | Diagnosis Confirmationa, No. (%) |
|---|---|---|---|---|---|
| Cryptococcal meningitis | |||||
| Asia | 1 cohort | 329 | 54 (1) | 0.60–180 (1) | 1 (100) |
| LAC | 3 cohorts | 2414 (110–1859) | NA | NA | 0 (0) |
| Pneumocystis pneumonia | |||||
| SSA | 1 RCT, 1 cohort | 632 (98–534) | 53 (1) | NA | 0 (0) |
| Asia | 4 cohorts | 1080 (124–317) | 62 (2) | 43–96 (2) | 1 (25) |
| LAC | 4 cohorts | 2700 (110–1859) | 66 (2) | 23–159 (2) | 0 (0) |
| Oral candidiasis | |||||
| SSA | 2 cohorts | 244 (54–190) | NA | NA | 0 (0) |
| Asia | 4 cohorts | 1033 (102–329) | 54 (1) | 0.60–180 (2) | 3 (75) |
| LAC | 2 cohorts | 841 (110–731) | 66 (2) | 23–159 (2) | 0 (0) |
| Esophageal candidiasis | |||||
| SSA | 1 cohort | 1206 | NA | NA | 0 (0) |
| Asia | 3 cohorts | 671 (149–317) | 64 (2) | 22–138 (2) | 2 (67) |
| LAC | 4 cohorts | 3145 (110–1859) | 66 (2) | 23–159 (2) | 0 (0) |
| CMV retinitis | |||||
| Asia | 1 cohort | 329 | 54 (1) | 0.60–180 (1) | 0 (0) |
| LAC | 2 cohorts | 953 (222–731) | 7, 5 (1) | NA | 1 (50) |
| Varicella zoster virus | |||||
| Asia | 4 cohorts | 815 (67–329) | 52 (2) | 0.65–180 (2) | 0 (0) |
| LAC | 2 cohorts | 1176 (158–731) | 60 (2) | 36–108 (1) | 0 (0) |
| Herpes simplex virus | |||||
| SSA | 1 cohort | 190 | NA | NA | 0 (0) |
| Asia | 2 cohorts | 997 (329–668) | 54 (1) | 0.60–180 (1) | 0 (0) |
| LAC | 4 cohorts | 3145 (110–1859) | 66 (2) | 23–159 (2) | 0 (0) |
| Kaposi sarcoma | |||||
| LAC | 1 cohort | 1859 | NA | NA | 0 (0) |
| Cerebral toxoplasmosis | |||||
| Asia | 1 cohort | 329 | 132 (1) | 132–180 (1) | 1 (100) |
| LAC | 4 cohorts | 3145 (110–1859) | 64 (3) | 24–138 (1) | 0 (0) |
| Cryptosporidium diarrhea | |||||
| SSA | 1 cohort | 54 | 13 (1) | NA | 1 (100) |
| Asia | 1 cohort | 329 | 54 (1) | 0.6–180 (1) | 2 (100) |
| LAC | 3 cohorts | 3010 (420–1859) | 60 (1) | 36–108 (1) | 0 (0) |
| Mycobacterium tuberculosis | |||||
| SSA | 5 RCT, 11 cohorts | 13 459 (38–6301) | 43 (12) | 26–75 (9) | 11 (69) |
| Asia | 6 cohorts | 1358 (102–329) | 60 (3) | 15–152 (3) | 3 (50) |
| LAC | 3 cohorts | 1212 (110–731) | 66 (2) | 23–159 (2) | 0 (0) |
| Pulmonary tuberculosis | |||||
| SSA | 4 RCT, 5 cohorts | 4234 (131–1206) | 52 (6) | 30–80 (4) | 7 (78) |
| Asia | 3 cohorts | 931 (285–329) | 54 (1) | 0.60–180 (1) | 3 (100) |
| LAC | 2 cohorts | 481 (110–371) | 72 (1) | 11–210 (1) | 0 (0) |
| Extrapulmonary tuberculosis | |||||
| SSA | 1 RCT, 2 cohorts | 1119 (210–635) | 40 (2) | 26–59 (2) | 2 (68) |
| Asia | 4 cohorts | 1080 (149–329) | 65 (2) | 32–138 (2) | 3 (75) |
| LAC | 1 cohort | 110 | 72 (1) | 11–210 (1) | 0 (0) |
| Bacterial pneumonia | |||||
| SSA | 1 RCT, 7 cohorts | 7421 (54–5752) | 63 (3) | 16–136 (2) | 1 (13) |
| Asia | 3 cohorts | 294 (102–317) | NA | NA | 0 (0) |
| LAC | 2 cohorts | 445–731 | 60 (1) | 36–108 (1) | 0 (0) |
| Bacterial meningitis | |||||
| SSA | 1 RCT, 1 cohort | 939 (405–534) | 53 (2) | 23–90 (1) | 0 (0) |
| Asia | 1 cohort | 192 | NA | NA | 0 (0) |
| LAC | 2 cohorts | 1176 (445–731) | 60 (1) | 36–108 (1) | 0 (0) |
| Isolated bacteremia | |||||
| LAC | 1 cohort | 731 | 60 (1) | 36–108 (1) | 0 (0) |
| Sepsis | |||||
| SSA | 1 RCT, 1 cohort | 6286 | 53 (1) | NA | 1 (50) |
| Asia | 2 cohorts | 397 | 77 (1) | 43–96 (1) | 0 (0) |
| LAC | 1 cohort | 445 | NA | NA | 0 (0) |
Abbreviations: CMV, cytomegalovirus; IQR, interquartile range; LAC, Latin America and the Caribbean; NA, data not available; RCT, randomized controlled trial; SSA, sub-Saharan Africa.
a Diagnosis confirmation is as follows: cryptococcal meningitis (India ink stain of cerebrospinal fluid [CSF]), cerebral toxoplasmosis (immunoglobulin M–positive serology), Pneumocystis pneumonia (bronchoalveolar lavage and stain), bacterial pneumonia (suggestive chest radiograph), bacterial meningitis (CSF culture), isolated bacteremia or sepsis (blood culture), herpes simplex virus stomatitis, varicella zoster virus (viral culture), cytomegalovirus retinitis (viral culture), oral and esophageal candidiasis (microscopy or fungal culture), Kaposi sarcoma (biopsy and histology), cryptosporidial diarrhea (modified acid-fast stool stain), and pulmonary tuberculosis (mycobacterial sputum culture) and extrapulmonary tuberculosis (culture).
Incidence of OIs and Other Infections
The 35 studies of incidence (30 cohorts and 5 RCTs) involved 23 266 children. Twenty studies were based in sub-Saharan Africa (n = 13 547 children), 9 in Asia (n = 2207), and 5 in Latin America and the Caribbean (n = 3293). One study was based in multiple sites in LMICs (n = 3946). All studies were outpatient based. The majority of children had vertically acquired HIV infection; of 19 (54%) for whom age was reported, the median age at study report was 41 months (range, 3–156 months). Eleven studies (31%) used appropriate diagnostic methods (for definitions, see Supplementary Appendices 2 and 3 or Table 1), 16 reported use of CTX prophylaxis (46%), 11 baseline CD4 count (31%), 9 WHO stage (26%), 14 duration of time on ART (40%), and 3 age at ART initiation (9%).
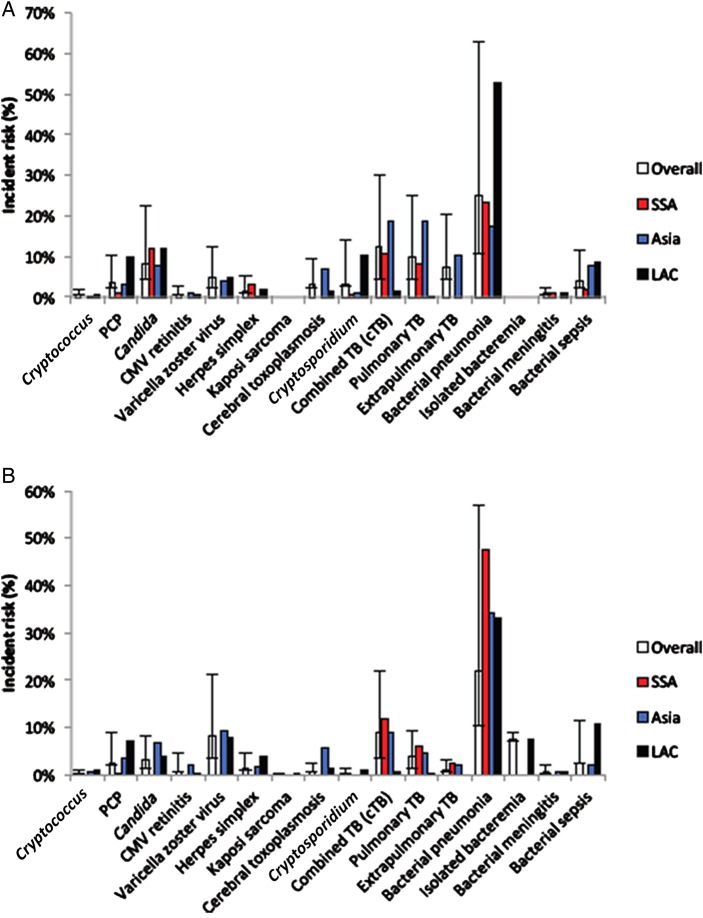
Table 2 and Figure 2 shows the incidence of 14 OIs and related infections in ART-naive and -exposed HIV-infected children. The most common infections in ART-naive children were bacterial pneumonia (25.01% [95% CI, 14.50%–37.91%]; n = 9), cTB (12.36% [95% CI, 7.95%–17.59%]; n = 17), PTB (9.78% [95% CI, 5.37%–15.34%]; n = 10), oral and esophageal candidiasis (8.29% [95% CI, 3.75%–14.39%]; n = 8), and EPTB (7.26% [95% CI, 3.00%–13.18%]; n = 4). A similar profile was observed in ART-exposed children, with bacterial pneumonia (22.11% [95% CI, 11.58%–34.89%]; n = 7), cTB type (8.84% [95% CI, 5.21%–13.31%]; n = 20), and VZV (8.40% [95% CI, 4.78%–12.91%]; n = 5) being the most common infections. A complete summary of all OIs with a regional overview is available in Supplementary Appendices 3 and 4. Tuberculosis was the only OI for which each region had a large enough number of studies reporting incidence to allow for a meaningful comparison by geographic region (Table 3).
Table 2.
Estimated Summary Incident Risk for Opportunistic Infections and Other Infections, by Antiretroviral Status
| Opportunistic Infection | Summary of Incident Risk, % (95% CI) (No. of Studies) |
Unadjusted Odds Ratio (95% CI)a |
|
|---|---|---|---|
| ART Naive | ART Exposed | ||
| Cryptococcus neoformans meningitis | 0.55 (.10–1.36) (3) | 0.25 (.04–.66) (4) | 0.42 (.11–1.83) |
| Pneumocystis pneumonia | 3.48 (1.23–6.82) (7) | 2.49 (.34–6.54) (7) | 0.71 (.50–1.01) |
| Oral and esophageal candidiasis | 8.29 (3.75–14.39) (8) | 3.20 (1.71–5.15) (8) | 0.37 (.29–.46) |
| CMV retinitis | 0.82 (.15–2.04) (2) | 0.85 (.01–3.69) (2) | 0.99 (.24–4.71) |
| Varicella zoster virus | 4.69 (2.44–7.62) (3) | 8.40 (4.78–12.91) (5) | 1.88 (1.23–2.87) |
| Herpes simplex | 1.59 (.43–3.48) (5) | 1.33 (.23–3.32) (5) | 0.86 (.53–1.41) |
| Kaposi sarcoma | NA | 0.06 (.01–.14) (2) | NA |
| Cerebral toxoplasmosis | 3.06 (.85–6.59) (3) | 0.72 (.15–1.71) (5) | 0.23 (.13–.43) |
| Cryptosporidium diarrhea | 2.92 (.00–10.97) (3) | 0.31 (.01–1.06) (7) | 0.10 (.05–.22) |
| Mycobacterium tuberculosis (unspecified types) | 12.36 (7.95–17.59) (15) | 8.84 (5.21–13.31) (18) | 0.69 (.63–.75) |
| Pulmonary tuberculosis | 9.78 (5.37–15.34) (10) | 3.99 (2.66–5.56) (9) | 0.38 (.32–.46) |
| Extrapulmonary tuberculosis | 7.26 (3.00–13.18) (4) | 1.12 (.40–2.18) (6) | 0.15 (.10–.21) |
| Bacterial pneumonia | 25.01 (14.50–37.91) (9) | 22.11 (11.58–34.89) (7) | 0.85 (.78–.92) |
| Isolated bacteremia | NA | 7.50 (.25–1.50) (1) | NA |
| Bacterial meningitis | 0.95 (.48–1.56) (3) | 0.80 (.36–1.42) (3) | 0.85 (.33–2.12) |
| Bacterial sepsis | 3.95 (1.46–7.58) (4) | 2.39 (.00–9.19) (4) | 0.59 (.47–.75) |
Abbreviations: ART, antiretroviral therapy (combines both specified and unspecified); CI, confidence interval; CMV, cytomegalovirus; NA, numbers insufficient to perform analysis.
a Reference is naive population.
Figure 2.
Summary incident risk by region for antiretroviral therapy (ART)–naive (A) and ART-exposed (B) patients. Abbreviations: CMV, cytomegalovirus; LAC, Latin America and the Caribbean; PCP, Pneumocystis pneumonia; SSA, sub-Saharan Africa; TB, tuberculosis.
Table 3.
Estimated Summary Incident Risk for Mycobacterium tuberculosis, by Regiona
| Opportunistic Infection | Summary of Incident Risk, % (95% CI) (No. of Studies) |
|
|---|---|---|
| ART Naive | ART Exposed | |
| Mycobacterium tuberculosis (unspecified types) | 12.36 (7.95–17.59) (15) | 8.84 (5.21–13.31) (18) |
| Sub-Saharan Africa | 10.58 (5.89–16.44) (9) | 9.52 (5.00–15.29) (12) |
| Asia | 23.10 (11.82–36.79) (4) | 9.10 (.00–33.60) (4) |
| Latin America and Caribbean | 1.48 (.50–2.95) (2) | 0.74 (.24–1.51) (2) |
| Pulmonary tuberculosis | 9.78 (5.37–15.34) (10) | 3.99 (2.66–5.56) (9) |
| Sub-Saharan Africa | 8.27 (5.11–12.09) (5) | 6.09 (2.40–11.33) (6) |
| Asia | 18.60 (6.43–35.23) (3) | 4.61 (2.18–7.89) (1) |
| Latin America | 1.48 (.50–2.95) (2) | 0.39 (.01–1.28) (1) |
| Extrapulmonary tuberculosis | 7.26 (3.00–13.18) (4) | 1.12 (.40–2.18) (6) |
| Sub-Saharan Africa | NA | 2.49 (.04–8.53) (3) |
| Asia | 10.27 (6.96–14.12) (3) | 2.101 (.33–11.74) (2) |
| Latin America and Caribbean | 1.31 (.05–4.26) (1) | NA |
Abbreviations: ART, antiretroviral therapy (combines both specified and unspecified); CI, confidence interval; NA, numbers insufficient to perform analysis.
a A study reporting data from multiple international sites in low- and middle-income countries is included in the pooled analysis but not the individual regional analysis, explaining the missing study in the total number of studies compared to the regional analysis.
Prevalence of OIs and Other Infections
The 60 prevalence studies (44 cross-sectional studies, 15 cohorts, and 1 RCT) involved 36 357 children, with data from 47 studies in sub-Saharan Africa, 9 in Asia, and 4 in Latin America and the Caribbean (Supplementary Appendix 3 provides information on included prevalence studies). The median age was 56 months (range, 2–158 months), for the 26 studies where age was reported. Nine studies used appropriate diagnostic approaches. Eight studies reported use of CTX prophylaxis (13%), 22 reported baseline CD4 count (37%), 9 reported WHO stage (15%), 6 reported time on ART (10%), and 8 reported the age at ART onset (13%), and as such adjustment for these variables in subgroup analyses was not possible.
Table 4 shows similar findings to those observed for the IR. The most prevalent infections were bacterial pneumonia (32.51% [95% CI, 21.96%–44.04%]; n = 21), oral and esophageal candidiasis (24.77% [95% CI, 19.31%–30.67%]; n = 26), and bacteremia (23.18% [95% CI, 10.12%–39.62%]; n = 5). A complete summary of all OIs with a regional overview is available in Supplementary Appendices 3 and 4.
Table 4.
Estimated Summary Prevalent Risk for Opportunistic Infections and Other Infections, by Antiretroviral Status
| Opportunistic Infection | Summary of Prevalent Risk, % (95% CI) (No. of Studies) |
Unadjusted Odds Ratio (95% CI)a |
|
|---|---|---|---|
| ART Naive | ART Exposed | ||
| Cryptococcus neoformans meningitis | 3.98 (.89–9.13) (3) | 16.65 (7.33–28.80) (1) | 4.46 (1.45–13.44) |
| Pneumocystis pneumonia | 7.40 (2.28–15.13) (9) | 8.40 (.65–10.52) (1) | 1.43 (.83–1.58) |
| Oral and esophageal candidiasis | 24.77 (19.31–30.67) (26) | 18.23 (5.84–35.49) (8) | 0.68 (.57–0.80) |
| CMV retinitis | 1.02 (.28–2.21) (3) | 0.89 (.34–1.69) (1) | 0.87 (.25–3.18) |
| Varicella zoster virus | 5.33 (2.22–9.67) (7) | 14.73 (4.25–30.05) (1) | 3.12 (2.11–4.62) |
| Herpes simplex | 3.23 (1.02–6.64) (2) | 5.99 (.70–15.95) (3) | 1.82 (.68–5.28) |
| Kaposi sarcoma | 2.98 (1.15–5.61) (4) | 4.18 (.76–10.18) (2) | 1.55 (.39–5.17) |
| Cerebral toxoplasmosis | 1.53 (.06–4.97) (1) | 1.30 (.61–2.24) (1) | 1.29 (.17–27.24) |
| Cryptosporidium diarrhea | 4.98 (2.62–8.05) (3) | 0.20 (.01–.65) (1) | 0.03 (.00–.18) |
| Mycobacterium tuberculosis (unspecified types) | 13.78 (11.26–16.51) (20) | 7.53 (2.80–14.28) (3) | 0.51 (.40–.64) |
| Sub-Saharan Africa | 13.95 (9.86–18.62) (18) | 15.86 (3.18–35.67) (2) | … |
| Asia | 38.19 (27.38–49.63) (1) | NA | … |
| Latin America | 9.57 (4.47–17.40) (1) | 8.89 (6.93–11.19) (1) | … |
| Pulmonary tuberculosis | 13.75 (8.12–20.58) (11) | 8.95 (6.99–11.12) (1) | 0.61 (.46–.82) |
| Extrapulmonary tuberculosis | 5.97 (2.56–10.69) (9) | NA | NA |
| Bacterial pneumonia | 32.51 (21.96–44.04) (21) | 38.16 (12.79–67.66) (2) | 1.28 (1.09–1.52) |
| Bacteremia | 23.18 (10.12–39.62) (5) | 6.57 (4.98–8.35) (3) | 0.23 (.16–.33) |
| Bacterial meningitis | 1.98 (1.08–3.16) (8) | 4.16 (1.41–8.27) (2) | 2.14 (1.24–3.69) |
| Bacterial sepsis | 14.49 (7.66–23.05) (11) | NA | NA |
Abbreviations: ART, antiretroviral therapy (combines both specified and unspecified); CI, confidence interval; CMV, cytomegalovirus; NA, numbers insufficient to perform analysis.
a Reference is naive population.
Impact of ART on Incidence
Tables 2 and 4 show, respectively, the IR and PR, as well as the OR to quantify impact of ART. For IR, the OR ranged from 0.10 to 1.88, and the greatest (>80% reduction in risk) and most statistically significant reductions were seen for Cryptosporidium diarrhea (OR, 0.10 [95% CI, .05–.22]), cerebral toxoplasmosis (OR, 0.23 [95% CI, .13–.43]), and EPTB (OR, 0.15 [95% CI, .10–.21]). For all OIs except EPTB, however, the 95% CIs of the IR in ART-naive and ART-exposed patients overlapped.
Based on the OR of prevalence, Cryptosporidium diarrhea (OR, 0.03[95% CI, .00–.18]) and bacteremia (OR, 0.23 [95% CI, .16–.33]) showed a substantial decrease in burden (≥ 80%) with nonoverlapping CIs between ART-naive children and ART-exposed children. All other OIs showed overlapping CIs in ART-naive compared with ART-exposed children.
There was significant heterogeneity with a very high I2 across studies for all OIs. However, the limited data on potential explanatory variables such as CD4 cell count or use of CTX precluded any further analysis on key sources of heterogeneity.
Global Impact of ART Use on OIs Averted and Cost Savings
Based on difference in incidence for different OIs between ART-naive and -exposed children, and assuming ART initiation in all children meeting WHO 2010 treatment guidelines eligibility criteria [17], ART was estimated to have potentially averted approximately 161 000 (95% CI, 12 200–256 000) cases of OIs annually (based on 2013 UNAIDS country estimates of number of HIV-infected children), mainly in sub-Saharan Africa [3] (Table 5). The majority of OIs averted were tuberculosis, candidiasis, and Cryptosporidium diarrhea. For several OIs, the difference in incidence rate between ART naïve and exposed was small relative to the uncertainty around each estimate, and so CIs for cases and costs averted were very wide and included negative values. When all OIs are combined, this uncertainty is minimised and there is an overall positive effect on cases and costs averted. Total costs averted were $17 700 000 (95% CI, $7 500 000–$25 900 000) annually, but these cost savings excluded bacterial pneumonia, sepsis, cerebral toxoplasmosis, VZV, and cytomegalovirus, for which there were no accurate estimates of treatment costs per case, and as such represent minimum cost savings. More than 90% of the savings came from averted cases of tuberculosis.
Table 5.
Estimated Number of Opportunistic Infection Cases Averted Due to Antiretroviral Therapy, All Regions Combined
| Opportunistic Infection | No. of Cases Averted (95% CI) | Cost |
|
|---|---|---|---|
| Cost per Case, USD | Total Savings, USD (95% CI) | ||
| Mycobacterium tuberculosis | |||
| Pulmonary | 38 100 (3300–65 000) | 182.76 | 7 000 000 (600 000–12 000 000) |
| Extrapulmonary | 41 400 (4800–67 000) | 234.99 | 9 700 000 (1 200 000–16 000 000) |
| Pneumocystis pneumonia | 4850 (−20 000 to 24 000) | 53.97 | 262 000 (−1 100 000 to 1 300 000) |
| Cryptococcal meningitis | 1990 (−1900 to 5100) | 301.00 | 599 000 (−560 000 to 1 500 000) |
| Cryptosporidium | 21 100 (−1160 to 48 000) | 6.65 | 140 000 (−7700 to 320 000) |
| Herpes simplex | 1380 (−12 000 to 11 500) | 3.16 | 4400 (−37 000 to 360 000) |
| Oral and esophageal candidiasis | 34 800 (−3800 to 61 000) | 3.65 | 127 000 (−14 000 to 225 000) |
| Cerebral toxoplasmosis | 15 600 (−2100 to 29 000) | NA | |
| Bacterial pneumonia | 18 800 (−94 000 to 102 000) | NA | |
| Total | 161 300 (12 200–256 000) | 17 700 000 (7 500 000–25 600 000) | |
For several opportunistic infections (OIs), the difference in incidence rate between antiretroviral therapy naïve and exposed was small relative to the uncertainty around each estimate, and so CIs for cases and costs averted were very wide and included negative values. When all OIs are combined, this uncertainty is minimised and there is an overall positive effect on cases and costs averted.
Abbreviations: CI, confidence interval; NA, no cost per case available; USD, US dollars.
DISCUSSION
This systematic review and meta-analysis is the most comprehensive assessment of the incidence and prevalence of the 14 most important OIs and other infections and the effect of ART among HIV-infected children in LMICs. It provides data from a total of 55 679 children in 88 studies, of which 35 had data on incidence, and 60 on prevalence. Our data highlight the high incidence and burden of tuberculosis (approximately 30% for cTB, PTB, and EPTB combined), bacterial pneumonia (25%), and candidiasis (8%)—the most common OIs in both ART-naive and exposed children. This is similar to the pattern of OIs observed in a companion meta-analysis we have conducted among adults [21].
Following ART initiation, the incidence for all OIs, except bacterial pneumonia, tuberculosis, candidiasis, and VZV declined to <2.5%. The greatest effect of ART on incidence was for cerebral toxoplasmosis, Cryptosporidium diarrhea, and EPTB. Statistically significant reduction (nonoverlapping CIs) in incidence was only found for EPTB and reduction in prevalence was only significant for Cryptosporidium and bacteremia. These findings were also similar to those reported in the adult meta-analysis [21].
In general, our results on incidence of OIs and magnitude of effect of ART in LMICs are also consistent with meta-analyses and cohorts from HICs [9–11, 22, 23], with the exception of bacterial pneumonia, which remains very common among ART-exposed children in LMICs. This is probably at least partly accounted for by the high incidence of lower respiratory tract infections in all children (regardless of HIV status) in LMICs [24].
Estimated minimum cost savings of approximately $17 million from use of ART based on the 2010 WHO guidelines criteria for ART initiation in children were considerable as there was considerable imprecision in the estimates of number of cases averted and in cost savings. In addition, this represents an underestimate as several key OIs such as pneumonia were excluded from the cost analysis.
There are several methodological limitations to this analysis. Overall, there was a paucity of well-described or large studies in children relative to a comparable companion review in adults (88 vs 126 eligible studies in adults, [21]), and the overall pooled sample size was approximately a tenth that of the meta-analysis in adults (55 679 children vs 491 000 in adult analysis, [21]), leading to low reliability of individual estimates of incidence of specific OIs and with wide and overlapping CIs between ART naive and exposed. Few studies reported follow-up time, which is crucial in analysis of incidence risks, and differences in duration of follow-up may explain some of the observed differences in incidence of specific OIs. Also, few studies reported data on potentially important confounders of the impact of ART, such as CD4 cell count, age at which ART was initiated, and use of CTX prophylaxis. Overall, this precluded conduct of a meta-regression that would have allowed us to examine contributors to heterogeneity across studies and adjust for important confounders. Interpretation of regional variation in incidence and the effect of ART were limited by the few studies from Asia, and Latin America and the Caribbean, resulting in imprecise estimates in these regions. While our search was comprehensive, we cannot exclude the possibility of publication bias in the studies reported. To limit this possibility, we searched published abstracts and reviewed the gray literature for relevant studies, neither of which yielded additional results.
There is also potential misclassification of some opportunistic and other infections due to limited diagnostic capacity in many LMICs in addition to inherent difficulties in diagnosing such infections in children. A significant proportion of studies failed to meet acceptable standards for the diagnosis of specific OIs: <33% of incidence studies and <25% of prevalence studies used an optimal diagnostic approach.
In summary, despite a more limited evidence base compared to adults, this systematic review based on study data from a >20-year period shows an overall trend of reduced incidence and prevalence for most OIs after ART initiation, and the substantial potential impact and cost savings on OIs averted with earlier ART initiation. The recent publication of the landmark Strategic Timing of Antiretroviral Therapy [25] and TEMPRANO RCT [25, 26] results showed a major reduction in AIDS and non-AIDS-related morbidity and mortality among adults with early ART initiation above a CD4 count of 500 cells/µL. WHO now recommends ART initiation regardless of CD4 count for adults [25]. Although these trials excluded children and adolescents, the 2015 WHO guidelines now also recommend ART initiation in all children and adolescents with a priority for those aged <1 year to further simplify and promote more prompt and ready access to ART among children [3, 27–33]. This is critical as scale-up of ART has been less successful in children [3–5]. Strong mother–child prevention programs, expansion of access to early infant diagnosis, and more robust procurement and supply management systems, including improved pediatric ART formulation, are also critical to reducing the adult–pediatric ART coverage gap, with an ultimate goal of eliminating the pediatric HIV burden.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank John Stover for his support in the cost analysis. We also thank Natasha Larke for her guidance in the statistical analysis and comments on the drafts.
Author contributions. All authors were involved in study design, analysis, and interpretation of the data. M.-R. B.-L., L. M., and P. E. conceived of and designed the project. M.-R. B.-L. and O. D. performed the systematic review and extracted all the data. G. B. and Q. N. performed the statistical analysis. M.-R. B.-L. wrote the first draft. All authors contributed to results interpretation and reviews of the subsequent manuscript.
Disclaimer. The views expressed do not necessarily represent the official views of the World Health Organization (WHO).
Financial support. This research was funded by the WHO.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.United Nations Children's Emergency Fund (UNICEF). Children & AIDS 2015 statistical update. Available at: http://www.childrenandaids.org/situation Accessed 2 January 2016.
- 2.Joint United Nations Programme on HIV/AIDS (UNAIDS). Global fact sheet. Available at: http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/201207_FactSheet_Global_en.pdf Accessed 11 September 2014. [PubMed]
- 3.Joint United Nations Programme on HIV/AIDS (UNAIDS). UNAIDS global report 2013, 2013. [PubMed]
- 4.World Health Organization (WHO). Global update on the health sector response to HIV. 2014. Geneva, Switzerland: WHO, 2014. [Google Scholar]
- 5.Joint United Nations Programme on HIV/AIDS (UNAIDS). Children and HIV. Available at: http://www.unaids.org/en/resources/documents/2014/name,93710,en.asp Accessed 11 September 2014. [PubMed]
- 6.Buchacz K, Baker R, Palella F et al. AIDS-defining opportunistic illnesses in US patients, 1994–2007: a cohort study. AIDS 2010; 24:1549–59. [DOI] [PubMed] [Google Scholar]
- 7.Smit C, Geskus R, Walker S et al. Effective therapy has altered the spectrum of cause-specific mortality following HIV seroconversion. AIDS 2006; 20:741–9. [DOI] [PubMed] [Google Scholar]
- 8.Palella F, Baker R, Moorman A et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 2006; 43:27–34. [DOI] [PubMed] [Google Scholar]
- 9.Guillén S, García San Miguel L, Resino S et al. Opportunistic infections and organ-specific diseases in HIV-1-infected children: a cohort study (1990–2006). HIV Med 2010; 11:245–52. [DOI] [PubMed] [Google Scholar]
- 10.Nesheim SR, Kapogiannis BG, Soe MM et al. Trends in opportunistic infections in the pre- and post-highly active antiretroviral therapy eras among HIV-infected children in the Perinatal AIDS Collaborative Transmission Study, 1986–2004. Pediatrics 2007; 120:100–9. [DOI] [PubMed] [Google Scholar]
- 11.Gona P, Van Dyke R, Williams P et al. Incidence of opportunistic and other infections in HIV-infected children in the HAART era. JAMA 2006; 296:292–300. [DOI] [PubMed] [Google Scholar]
- 12.World Bank. World Bank classification 2010. Available at: http://data.worldbank.org/about/country-classifications Accessed March 2016.
- 13.Moher D, Liberati A, Tetzlaff J, Altman D. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med 2009; 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stroup D, Berlin J, Morton S et al. Meta-analysis of Observational Studies in Epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283:2008–12. [DOI] [PubMed] [Google Scholar]
- 15.Mills E, Nachega J, Buchan I et al. Adherence to antiretroviral therapy in sub-Saharan Africa and North America: a meta-analysis. JAMA 2006; 296:679–90. [DOI] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–88. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. Antiretroviral therapy for HIV infection in infants and children: towards universal access. Recommendations for a public health approach: 2010 revision. Geneva, Switzerland: WHO, 2010. Available at: http://www.who.int/hiv/pub/paediatric/infants2010/en/. Accessed 11 September 2014. [PubMed] [Google Scholar]
- 18.United Nations Children's Emergency Fund (UNICEF). Children and HIV/AIDS: third stocktaking report. 2008. Available at: http://www.who.int/hiv/pub/ca_stocktaking/en/. Accessed 11 September 2014.
- 19.United Nations Children's Emergency Fund (UNICEF). Children and AIDS. Fifth stocktaking report. 2010. Available at: http://www.unicef.org/publications/index_57005.html. Accessed 11 September 2014.
- 20.Bertozzi S, Gutierrez JP, Opuni M, Walker N, Schwartlander B. Estimating resource needs for HIV/AIDS health care services in low-income and middle-income countries. Health Policy 2004; 69:189–200. [DOI] [PubMed] [Google Scholar]
- 21.Low A, Gavriilidis G, Larke N et al. Incidence of opportunistic infections and the impact of antiretroviral therapy among HIV-infected adults in low and middle income countries: a systematic review and meta-analysis. Clin Infect Dis 2016; 62:1595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiappinia E, Gallia L, Tovob P et al. Changing patterns of clinical events in perinatally HIV-1-infected children during the era of HAART. AIDS 2007; 21:1607–15. [DOI] [PubMed] [Google Scholar]
- 23.Ylitalo N, Brogly S, Hughes MD et al. Risk factors for opportunistic illnesses in children with human immunodeficiency virus in the era of highly active antiretroviral therapy. Arch Pediatr Adolesc Med 2006; 160:778–87. [DOI] [PubMed] [Google Scholar]
- 24.Theodoratou E, McAllister D, Reed C et al. Global, regional, and national estimates of pneumonia burden in HIV-infected children in 2010: a meta-analysis and modelling study. Lancet Infect Dis 2014; 14:1250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.TEMPRANO ANRS 12136 Study Group. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015; 373:808–22. [DOI] [PubMed] [Google Scholar]
- 27.van Dijk JH, Sutcliffe CG, Munsanje B, Hamangaba F, Thuma PE, Moss WJ. Barriers to the care of HIV-infected children in rural Zambia: a cross-sectional analysis. BMC Infect Dis 2009; 16:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimani-Murage EW, Manderson L, Norris SA, Kahn K. “It's my secret”: barriers to paediatric HIV treatment in a poor rural South African setting. AIDS Care 2013; 25:744–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Dijk JH, Moss WJ, Hamangaba F, Munsanje B, CG S. Scaling-up access to antiretroviral therapy for children: a cohort study evaluating care and treatment at mobile and hospital-affiliated HIV clinics in rural Zambia. PLoS One 2014; 9:e104884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penazzato M, Davies MA, Apollo T, Negussie E, Ford N. Task shifting for the delivery of pediatric antiretroviral treatment: a systematic review. J Acquir Immune Defic Syndr 2014; 65:414–22. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization (WHO). Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendation for a public health approach. Geneva, Switzerland: WHO, 2013. [PubMed] [Google Scholar]
- 32.International AIDS Society. IAS-ILF/CIPHER thematic roundtable on paediatric HIV. 2014 19 July 2014.
- 33.World Health Organization (WHO). Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Available at: http://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/. Accessed 11 September 2014. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.