Flucytosine, an essential medicine for AIDS-related cryptococcal meningitis, has become monopolized in the US with a daily cost of ∼$2000. This cost is 9000% higher than in Europe. Despite this profiteering, flucytosine remains a vital component of cryptococcal meningitis therapy.
Keywords: cryptococcal meningitis, HIV/AIDS, cost-effectiveness analysis
Abstract
Background. In the United States, cryptococcal meningitis causes approximately 3400 hospitalizations and approximately 330 deaths annually. The US guidelines recommend treatment with amphotericin B plus flucytosine for at least 2 weeks, followed by fluconazole for a minimum of 8 weeks. Due to generic drug manufacturer monopolization, flucytosine currently costs approximately $2000 per day in the United States, with a 2-week flucytosine treatment course costing approximately $28 000. The daily flucytosine treatment cost in the United Kingdom is approximately $22. Cost-effectiveness analysis was performed to determine the value of flucytosine relative to alternative regimens.
Methods. We estimated the incremental cost-effectiveness ratio (ICER) of 3 cryptococcal induction regimens: (1) amphotericin B deoxycholate for 4 weeks; (2) amphotericin and flucytosine (100 mg/kg/day) for 2 weeks; and (3) amphotericin and fluconazole (800 mg/day) for 2 weeks. Costs of care were calculated using 2015 US prices and the medication costs. Survival estimates were derived from a randomized trial and scaled relative to published US survival data.
Results. Cost estimates were $83 227 for amphotericin monotherapy, $75 121 for amphotericin plus flucytosine, and $44 605 for amphotericin plus fluconazole. The ICER of amphotericin plus flucytosine was $23 842 per quality-adjusted life-year.
Conclusions. Flucytosine is currently cost-effective in the United States despite a dramatic increase in price in recent years. Combination therapy with amphotericin and flucytosine is the most attractive treatment strategy for cryptococcal meningitis, though the rising price may be creating access issues that will exacerbate if the trend of profiteering continues.
(See the Editorial Commentary by Perfect on pages 1569–70.)
Flucytosine, an essential medicine for AIDS-related cryptococcal meningitis, has become monopolized in the United States with a daily cost of approximately $2000. This cost is 9000% higher than in Europe. Despite this profiteering, flucytosine remains a vital component of cryptococcal meningitis therapy.
Cryptococcal meningitis is one of the most prolific opportunistic infections worldwide. Cryptococcosis causes approximately 15%–20% of AIDS-related mortality [1]. The highest burden is in sub-Saharan Africa, where cryptococcosis is the most common cause of meningitis [2]. In the United States, the burden is less severe but still significant, with an estimated 3400 hospitalizations resulting in 330 deaths in 2009 [3]. The estimated incidence of cryptococcal meningitis hospitalizations, at 1.1 per 100 000 US general population, makes cryptococcosis more frequent than the estimated incidence of all causes of bacterial meningitis combined, at 0.728 per 100 000 population in 2010 [3, 4].
The 2011 World Health Organization (WHO), 2010 Infectious Diseases Society of America, and 2014 US Department of Health and Human Services guidelines recommend amphotericin B deoxycholate–based combination induction therapy [5–7]. In conjunction with amphotericin, oral flucytosine is the preferred therapy due to 2 randomized trials reporting a survival benefit and reduced rate of relapse over amphotericin alone [8, 9]. Where flucytosine is not available, guidelines recommend induction with amphotericin plus fluconazole, based on trials showing superior rates of cerebrospinal fluid clearance and non–statistically significant improved survival compared to 4 weeks of amphotericin monotherapy [8, 10]. In 2013, Day et al confirmed these findings, but the relative cost-effectiveness of the regimens has not yet been established [8].
Although flucytosine is on the WHO essential medicines list, flucytosine is currently unavailable in all low- and middle-income countries where the disease burden is largest [11]. Where available, flucytosine can be prohibitively expensive. Since 2009, flucytosine has dramatically increased in cost in the United States to $70.46 per 250-mg tablet. When dosed at 100 mg/kg/day, this equates to a cost of $1973 per day for a 70-kg adult, making a 2-week course approximately $28 000 (December 2015 wholesale US cost). In contrast, the United Kingdom National Health Service price for flucytosine 500-mg tablet is £1.05 (US$1.52), making the US flucytosine cost 90-fold higher (9000%) for an off-patent generic medicine discovered in 1957 (Dr Angela Loyse, personal communication). To this end, a cost-effectiveness analysis was carried out using December 2015 US prices and the Day et al relative survival data, scaled to published US survival outcomes of 90% [3].
METHODS
A decision analysis was conducted for cryptococcal meningitis treatment for those living in the United States to estimate the incremental cost-effectiveness ratio (ICER) of 3 recommended induction treatment regimens for cryptococcosis: (1) amphotericin B deoxycholate (1 mg/kg/day) for 4 weeks, followed by oral fluconazole (400 mg/day); (2) amphotericin (1 mg/kg/day) and flucytosine (100 mg/kg/day) for 2 weeks, followed by oral fluconazole (400 mg/day) for 8 weeks; and (3) amphotericin (1 mg/kg/day) and fluconazole (800 mg/day) for 2 weeks, followed by fluconazole (400 mg/day) for 8 weeks.
Costs
Costs for each strategy are derived from wholesale US drug prices as of 28 December 2015. To reflect US standard of care, the cost of amphotericin was taken as the current price of liposomal amphotericin B, and efficacy was assumed to be equivalent to that of amphotericin B deoxycholate [12, 13]. Liposomal amphotericin B was $163.74 per 50-mg dose, oral flucytosine was $70.46 per 250-mg tablet, and oral fluconazole was $2.08 per 200-mg tablet, based on actual wholesale US prices available at the University of Minnesota Medical Center. Inpatient stay was priced at $2157 per day, based on national estimates [14]. One flucytosine-monitoring test was assumed for the flucytosine-containing regimen, priced at $236. All other hospitalization, laboratory, and personnel costs, as well as costs accrued postinduction, were assumed to be equal, with the exception that the amphotericin monotherapy regimen required 2 additional weeks of inpatient stay. This assumption was based on the finding by Day et al that adverse events did not vary significantly between treatment strategies [8]. Estimated costs per person were $83,227.46 for amphotericin monotherapy, $75,121.30 for amphotericin plus flucytosine, and $44,604.56 for amphotericin plus fluconazole. Patients who died in the interval from 2 to 10 weeks were assumed to die, on average, at week 6, and not incur additional cost thereafter.
For comparison, an identical analysis was conducted using amphotericin B deoxycholate, currently priced at $33.11 per 50-mg dose, in place of liposomal amphotericin B. This reduces the cost of a 2-week amphotericin course from $14,120.94 to $713.85.
Effectiveness
Data on the effectiveness of the treatment strategies is taken from the Day et al randomized clinical trial, which had a 2-week mortality of approximately 20% [8]. The estimated US survival outcomes were assumed to be 50% better, based on published in-hospital US mortality of 10.5% for human immunodeficiency virus (HIV)–infected persons [3], and 66% better between 2 weeks and 26 weeks based on better availability of supportive care and emergency services (Table 1).
Table 1.
Estimated Clinical Outcomes by Cryptococcal Meningitis Induction Treatment Regimen
| Induction Regimen | 2-Week Survival Probability (95% CI) | 10-Week Survival Probability (95% CI) | 26-Week Survival Probability (95% CI) |
|---|---|---|---|
| Amphotericin B | 0.75 (.67–.84) | 0.56 (.47–.66) | 0.46 (.37–.57) |
| US estimate | 0.875 (.835–.920) | 0.812 (.768–.860) | 0.778 (.735–.830) |
| Amphotericin B + 5FC | 0.85 (.78–.92) | 0.69 (.61–.79) | 0.65 (.56–.75) |
| US estimate | 0.925 (.890–.960) | 0.872 (.833–.917) | 0.858 (.817–.903) |
| Amphotericin B + fluconazole | 0.80 (.73–.88) | 0.67 (.58–.77) | 0.55 (.45–.65) |
| US estimate | 0.90 (.865–.940) | 0.857 (.815–.903) | 0.817 (.772–.863) |
Source: Day et al [8].
Abbreviations: 5FC, flucytosine; CI, confidence interval.
To measure the efficacy of the treatment regimens, persons were divided into 4 categories: those who died within 2 weeks of initiating treatment, those who died before the end of the 10-week treatment program, those who died between 10 and 26 weeks after initiation, and those who survived past 26 weeks. For measurement purposes, persons who died within 2 weeks did not accrue any additional life-years. Those who died before the end of the 10-week treatment program and those who died between 10 and 26 weeks after initiation were credited with 6 and 18 weeks of life, respectively, the midpoint of those intervals. Life during the treatment program was discounted at 50%, due to patients’ need for constant medical care, and a large portion reporting significant disability. Life after 10 weeks was discounted at 95%, based on a Ugandan cryptococcal cohort that had a mean Karnofsky score of 95 at 12 and 24 weeks [15]. Persons who lived past 26 weeks were assumed to live out their full life expectancy in line with prior longer-term cryptococcal outcomes [16–18]. Life expectancy was assumed to be 33.0, consistent with the life expectancy for HIV-infected persons receiving HIV therapy at 35 years of age [18, 19]. A further 3% annual discount rate was not used as the accrued costs and survival benefits were immediate in the first 2 weeks of antifungal therapy, unlike most health interventions, which have an upfront investment and delayed impact [20].
Analysis
The treatment strategies were compared through a cost-effectiveness analysis with the primary goal of calculating the ICER. The ICER was calculated as the additional cost compared to amphotericin B plus fluconazole divided by the additional expected quality-adjusted life-years (QALYs). A probabilistic sensitivity analysis was also performed using TreeAge Pro 2014 software (TreeAge, Williamstown, Massachusetts) to account for variations in cost and outcomes. The sensitivity analysis included statistical uncertainty in mortality means (95% confidence interval [CI]) used as well as medicine costs [8].
RESULTS
Survival Benefit
For the US scenario, assuming a 33-year life expectancy, treatment with amphotericin plus flucytosine provides an additional 26.90 QALYs gained, making it the most effective cryptococcal induction regimen studied. Amphotericin in conjunction with high-dose fluconazole was the second most effective treatment strategy, yielding 25.62 QALYs gained. Amphotericin monotherapy for 4 weeks was the least effective of the 3 strategies, yielding 24.40 QALYs. The number needed to treat to save 1 additional life over amphotericin monotherapy is 11.11 for amphotericin plus fluconazole, and 5.26 for amphotericin plus flucytosine. The number needed to treat for adjunctive flucytosine over adjunctive fluconazole is 24.4 per additional US life saved.
Cost-effectiveness Analysis
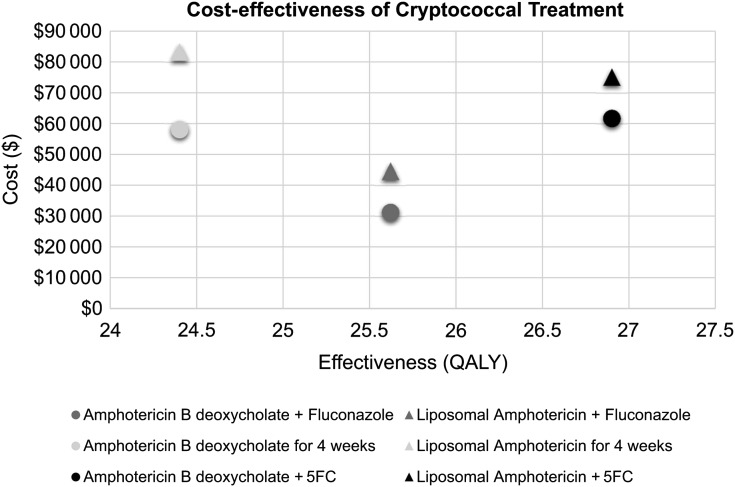
Four weeks of amphotericin monotherapy costs more and yields less benefit than the amphotericin plus fluconazole and amphotericin plus flucytosine induction regimens, so amphotericin monotherapy is dominated. Compared to amphotericin plus fluconazole, treatment with amphotericin plus flucytosine costs $30 517 more and provides an additional 1.28 QALYs, resulting in an ICER of $23 842 (Table 2). As the US 2014 gross domestic product per capita is $54 629, amphotericin plus flucytosine could be considered a “highly cost-effective” upgrade over amphotericin plus fluconazole using WHO criteria (Figure 1) [21].
Table 2.
Results of a Cost-effectiveness Analysis of 3 Cryptococcal Meningitis Induction Regimens for the United States, Using Amphotericin B, Referencing a Common Baseline
| Induction Regimen | Cost ($) | Incremental Cost ($) | Effectiveness (QALY) | Incremental Efficiency (QALY) | ICER ($/QALY) | C/E ($/QALY) |
|---|---|---|---|---|---|---|
| Amphotericin B deoxycholate + fluconazole | $31 197 | Reference | 25.62 | Reference | Reference | $1217 |
| Liposomal amphotericin + fluconazole | $44 605 | $13 408 | 25.62 | 0 | NA | $1741 |
| Amphotericin B deoxycholate for 4 weeks | $58 089 | $26 891 | 24.40 | −1. 22 | –$21 990 | $2380 |
| Liposomal amphotericin for 4 weeks | $83 227 | $52 030 | 24.40 | −1. 22 | –$31 584 | $3411 |
| Amphotericin B deoxycholate + 5FC | $61 714 | $30 517 | 26.90 | 1.28 | $23 842 | $2293 |
| Liposomal amphotericin + 5FC | $75 121 | $43 924 | 26.90 | 1.28 | $34 315 | $2792 |
Abbreviations: 5FC, flucytosine; C/E, cost effectiveness; ICER, incremental cost-effectiveness ratio; NA, not applicable; QALY, quality-adjusted life-year.
Figure 1.
Cost-effectiveness of cryptococcal meningitis induction regimens in the United States, showing cost (in US dollars) of induction and consolidation therapy for cryptococcal meningitis in the United States vs the effectiveness as measured by quality-adjusted life-years (QALYs) saved. Amphotericin monotherapy is dominated in this analysis, meaning that this regimen is both more expensive and less effective than alternative therapy choices. Abbreviation: 5FC, flucytosine.
Using amphotericin B deoxycholate in place of liposomal amphotericin B significantly improves the cost-effectiveness of all 3 regimens by reducing cost by >$13 000 without affecting efficacy. Choice of liposomal versus deoxycholate formulations does not, however, affect the incremental cost-effectiveness of amphotericin plus flucytosine relative to amphotericin plus fluconazole, because the 2 strategies require the same amphotericin duration.
Sensitivity Analysis
To account for uncertainty in costs and outcomes, a sensitivity analysis was conducted to validate the conclusion that amphotericin plus flucytosine should be considered a “highly cost-effective” upgrade over amphotericin plus fluconazole. A standard deviation of 5% of the value was assumed for all cost variables. A probabilistic sensitivity analysis including 1 million iterations was conducted; 76.3% of iterations produced ICERs less than the “highly cost-effective” threshold of $54 629 per QALY and an additional 9.9% falling under the “cost-effective” range, confirming the base-case analysis.
DISCUSSION
This analysis suggests that amphotericin plus flucytosine is a highly desirable upgrade over amphotericin plus fluconazole for the treatment of cryptococcal meningitis. Amphotericin monotherapy is dominated in the analysis, as it is less effective than either of the combination regimens and more expensive due to a longer amphotericin induction period. Induction with amphotericin plus flucytosine is strongly recommended, and where flucytosine is not available induction with amphotericin plus fluconazole should be preferred to amphotericin monotherapy.
The desirability of flucytosine, even at the current monopolized cost, motivates an exploration of whether or not flucytosine will actually be available to the patients who need it. With a 306% price increase in just the last 2 years, flucytosine may be nearing the point of being prohibitively expensive. The “highly cost-effective” status conferred in this analysis should be taken primarily as a testament to the essentiality of flucytosine for cryptococcal meningitis treatment. The accelerating trend of drug monopolization and price inflation has been well documented [22]. Such profiteering behavior can dramatically raise the price of healthcare while providing no added benefit to patients and is thus toxic to health system efficiency. The nearly 100-fold higher cost in the United States by Valeant Pharmaceuticals than in Europe for a medicine discovered in 1957 lacks common-sense justification. At this time, flucytosine in conjunction with amphotericin B is in theory still a desirable upgrade over fluconazole. However, increasing prices are likely already limiting access and will eventually render flucytosine inviable if the trend continues.
In many low- and middle-income countries, including those places where cryptococcal meningitis burden is greatest, flucytosine is completely unavailable as of January 2016. In addition to the widespread unavailability of flucytosine, healthcare providers in many impoverished areas are poorly equipped to deliver a full 14-day course of amphotericin. In these cases, recent research has shown that 5- to 7-day courses of amphotericin in conjunction with fluconazole 1200 mg/day may be an attractive compromise [20, 23, 24].
Application of these results to other nations should be done with caution, as the costs of medications vary widely internationally. There are also limitations concerning the validity of using survival data from low-income countries to assess the value of treatment regimens in the United States. Higher all-around standards of care and better access to medical resources will likely improve all outcomes, and while overall survival outcomes have been adjusted to match US cryptococcal survival rates, it is possible that higher standards of care may affect the various strategies differently.
Cryptococcal meningitis causes significant morbidity and mortality in the United States, with an estimated 3400 hospitalizations and 330 in-hospital deaths in 2009 [3]. The WHO, Infectious Diseases Society of America, and US Department of Health and Human Services all recommend treatment with amphotericin B deoxycholate and flucytosine as the preferred induction therapy for cryptococcal meningitis [5–7]. This analysis has shown this to be the most attractive amphotericin-based treatment regimen, and it should be considered the primary treatment option for cryptococcal meningitis in the United States. Yet, the explosive cost of flucytosine reflects larger dysfunction.
Notes
Acknowledgments. This work was supported by the National Institutes of Health, National Institute of Neurological Disorders and Stroke (grant number R01NS086312).
Potential conflict of interest. Both authors: No reported conflicts. Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 2009; 23:525–30. [DOI] [PubMed] [Google Scholar]
- 2.Durski KN, Kuntz KM, Yasukawa K, Virnig BA, Meya DB, Boulware DR. Cost-effective diagnostic checklists for meningitis in resource-limited settings. J Acquir Immune Defic Syndr 2013; 63:e101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pyrgos V, Seitz AE, Steiner CA, Prevots DR, Williamson PR. Epidemiology of cryptococcal meningitis in the US: 1997–2009. PLoS One 2013; 8:e56269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castelblanco RL, Lee M, Hasbun R. Epidemiology of bacterial meningitis in the USA from 1997 to 2010: a population-based observational study. Lancet Infect Dis 2014; 14:813–9. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Rapid advice: diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children. Available at: www.who.int/hiv/pub/cryptococcal_disease2011 Accessed 1 November 2015. [PubMed] [Google Scholar]
- 6.Perfect JR, Dismukes WE, Dromer F et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2010; 50:291–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masur H, Brooks JT, Benson CA, Holmes KK, Pau AK, Kaplan JE. Prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: updated guidelines from the Centers for Disease Control and Prevention, National Institutes of Health, and HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58:1308–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Day JN, Chau TT, Wolbers M et al. Combination antifungal therapy for cryptococcal meningitis. N Engl J Med 2013; 368:1291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Horst CM, Saag MS, Cloud GA et al. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. N Engl J Med 1997; 337:15–21. [DOI] [PubMed] [Google Scholar]
- 10.Loyse A, Wilson D, Meintjes G et al. Comparison of the early fungicidal activity of high-dose fluconazole, voriconazole, and flucytosine as second-line drugs given in combination with amphotericin B for the treatment of HIV-associated cryptococcal meningitis. Clin Infect Dis 2012; 54:121–8. [DOI] [PubMed] [Google Scholar]
- 11.Loyse A, Thangaraj H, Easterbrook P et al. Cryptococcal meningitis: improving access to essential antifungal medicines in resource-poor countries. Lancet Infect Dis 2013; 13:629–37. [DOI] [PubMed] [Google Scholar]
- 12.Hamill RJ. Amphotericin B formulations: a comparative review of efficacy and toxicity. Drugs 2013; 73:919–34. [DOI] [PubMed] [Google Scholar]
- 13.Hamill RJ, Sobel JD, El-Sadr W et al. Comparison of 2 doses of liposomal amphotericin B and conventional amphotericin B deoxycholate for treatment of AIDS-associated acute cryptococcal meningitis: a randomized, double-blind clinical trial of efficacy and safety. Clin Infect Dis 2010; 51:225–32. [DOI] [PubMed] [Google Scholar]
- 14.Henry J. Kaiser Family Foundation. Hospital adjusted expenses per inpatient day (2013). Available at: kff.org/other/state-indicator/expenses-per-inpatient-day/ Accessed 22 October 2015. [Google Scholar]
- 15.Boulware DR, Bonham SC, Meya DB et al. Paucity of initial cerebrospinal fluid inflammation in cryptococcal meningitis is associated with subsequent immune reconstitution inflammatory syndrome. J Infect Dis 2010; 202:962–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butler EK, Boulware DR, Bohjanen PR, Meya DB. Long term 5-year survival of persons with cryptococcal meningitis or asymptomatic subclinical antigenemia in Uganda. PLoS One 2012; 7:e51291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boulware DR, Meya DB, Muzoora C et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med 2014; 370:2487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mills EJ, Bakanda C, Birungi J et al. Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from Uganda. Ann Intern Med 2011; 155:209–16. [DOI] [PubMed] [Google Scholar]
- 19.Wada N, Jacobson LP, Cohen M, French A, Phair J, Munoz A. Cause-specific life expectancies after 35 years of age for human immunodeficiency syndrome-infected and human immunodeficiency syndrome-negative individuals followed simultaneously in long-term cohort studies, 1984–2008. Am J Epidemiol 2013; 177:116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajasingham R, Rolfes MA, Birkenkamp KE, Meya DB, Boulware DR. Cryptococcal meningitis treatment strategies in resource-limited settings: a cost-effectiveness analysis. PLoS Med 2012; 9:e1001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Bank. GDP per capita (current US$). Available at: data.worldbank.org/indicator/NY.GDP.PCAP.CD Accessed 22 October 2015. [Google Scholar]
- 22.US Government Accountability Office. Brand-name prescription drug pricing: lack of therapeutically equivalent drugs and limited competition may contribute to extraordinary price increases. GAO-10–201 . Washington, DC: GAO, 2009:40. [Google Scholar]
- 23.Muzoora CK, Kabanda T, Ortu G et al. Short course amphotericin B with high dose fluconazole for HIV-associated cryptococcal meningitis. J Infect 2012; 64:76–81. [DOI] [PubMed] [Google Scholar]
- 24.Jackson AT, Nussbaum JC, Phulusa J et al. A phase II randomized controlled trial adding oral flucytosine to high-dose fluconazole, with short-course amphotericin B, for cryptococcal meningitis. AIDS 2012; 26:1363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]