In human immunodeficiency virus (HIV)-hepatitis C virus-coinfected patients, 12 weeks' daclatasvir + sofosbuvir once-daily provided ≥95% sustained virologic response 12 weeks post-treatment across a broad range of antiretroviral regimens, was well tolerated and did not compromise HIV virologic control.
Keywords: daclatasvir, sofosbuvir, HIV-HCV, coinfection, antiretroviral therapy
Abstract
Background. Highly effective hepatitis C virus (HCV) direct-acting antiviral therapies that do not require modification of human immunodeficiency virus (HIV) antiretroviral regimens are needed. We evaluated the efficacy and safety of daclatasvir + sofosbuvir (DCV + SOF) for 12 weeks by antiretroviral (ARV) regimen in HIV-HCV-coinfected patients.
Methods. In the randomized, open-label ALLY-2 study, HIV-HCV-coinfected patients received 8 or 12 weeks of once-daily DCV 60 mg (dose-adjusted as-necessary for concomitant ARVs) + SOF 400 mg. Results were stratified by ARV class for the 151 patients who received 12 weeks of DCV + SOF.
Results. Fifty-one patients were HCV treatment experienced, 100 were treatment naive, 89% male and 33% black. HCV genotypes were: genotype 1a (GT1a; 69%), GT1b (15%), GT2 (8%), GT3 (6%), and GT4 (2%). Sustained virologic response 12 weeks post-treatment (SVR12) was 97% and was similar across ARV regimens (P = .774): protease inhibitor-based, 97% (95% confidence interval [CI], 90%-99.7%); nonnucleoside reverse transcriptase inhibitor-based, 100% (95% CI, 91%-100%); and integrase inhibitor based, 95% (95% CI, 83%-99.4%). SVR12 among patients receiving either tenofovir disoproxil fumarate or abacavir as part of their antiretroviral therapy regimen was 98% (95% CI, 93%-99.5%) and 100% (95% CI, 85%-100%), respectively. Age, gender, race, cirrhosis, HCV treatment history, GT , and baseline HCV RNA did not affect SVR12. No discontinuations were attributed to treatment-related adverse events.
Conclusions. DCV + SOF x12 weeks is a highly efficacious, all-oral, pan-GT HCV treatment for HIV-HCV coinfected patients across a broad range of ARV regimens.
Clinical Trials Registration. NCT02032888.
In the United States and Europe, approximately one third of all human immunodeficiency virus (HIV)–infected individuals are coinfected with hepatitis C virus (HCV), and liver disease has become the most common cause of morbidity and mortality in HIV-HCV–infected patients [1–3]. Thus, successful treatment of HCV is a priority for HIV-HCV–coinfected individuals.
Current HIV treatment guidelines recommend initiation of antiretroviral therapy (ART) in all HIV-HCV–coinfected patients [4, 5]. However, simultaneous treatment of HIV and HCV can be complicated by interactions between antiretroviral drugs and current all-oral direct-acting antivirals (DAAs) [4–9]. HIV-HCV–coinfected patients may require modification of their stable antiretroviral regimens before starting some current DAA regimens, which is neither desirable for patients with long-term HIV suppression on a specific regimen nor possible for some treatment-experienced patients with limited alternative antiretroviral options. Therefore, there is a need in HIV-HCV coinfection for all-oral DAA regimens that combine high efficacy with good tolerability, simple dosing, and few or easily manageable drug interactions with concomitant antiretrovirals.
Daclatasvir (DCV, an NS5A inhibitor) and sofosbuvir (SOF, an NS5B polymerase inhibitor) are both oral, once-daily (QD), pangenotypic DAAs with limited potential for interactions with antiretroviral drugs [10, 11]. DCV is approved for use in the United States, Europe, Japan, and multiple countries across the Americas, Middle East, and Asia-Pacific region. SOF is approved for use in the United States, Europe, and other countries. After 12 weeks of DCV + SOF, 98% of HCV treatment-naive, monoinfected patients and 97% of HIV-HCV–coinfected patients achieved sustained virologic response 12 weeks after stopping therapy (SVR12) [12, 13]. However, following 8 weeks of treatment, SVR12 rates were 76% in HIV-HCV–coinfected patients [13], suggesting that 12 weeks of DCV + SOF therapy is preferred. To investigate the impact of antiretroviral regimens on DCV + SOF efficacy and safety, we evaluated 12 weeks of DCV + SOF in HIV-HCV–coinfected patients stratified according to antiretroviral regimen.
METHODS
Patients
Eligible patients were HIV-HCV–coinfected adults who were HCV treatment naive or treatment experienced. All HCV genotypes (GTs) were allowed, but the number of patients with non-GT1 virus was capped at 20% per cohort. Compensated cirrhosis was permitted and was capped at 50% per cohort. Patients receiving ART were required to have HIV-1 RNA of <50 copies/mL and a CD4 cell count ≥100 cells/mm3 at screening. Those not receiving ART were required to have a CD4 cell count >350 cells/mm3. The following antiretroviral agents were permitted: ritonavir-boosted darunavir (DRV/r), ritonavir-boosted atazanavir (ATV/r), ritonavir-boosted lopinavir (LPV/r), efavirenz (EFV), nevirapine (NVP), rilpivirine (RPV), dolutegravir (DTG), raltegravir (RAL), enfuvirtide, maraviroc, tenofovir disoproxil fumarate (TDF), emtricitabine (FTC), abacavir (ABC), lamivudine, and zidovudine (AZT). Coadministration of a boosted protease inhibitor (PI) and a nonnucleoside reverse transcriptase inhibitor (NNRTI) was not permitted, with the exception of RPV + PI; all other combinations of permitted antiretrovirals were allowed.
Study Design
This was a phase 3, randomized, open-label study (ALLY-2). Patients were randomized to receive either 8 or 12 weeks of DCV 60 mg + SOF 400 mg (both QD). On the basis of initial pharmacokinetic drug–drug interaction data [11], the standard 60-mg dose of DCV was adjusted to 30 mg in patients receiving ritonavir-boosted PIs and to 90 mg in those receiving EFV or NVP. Patients who had received 12 weeks of DCV +SOF were stratified into PI, NNRTI, or integrase inhibitor (INSTI) regimen groups based on their ART. Patients receiving more than 1 class of antiretroviral agent were listed as follows: PI regimen if receiving any boosted PI (PI/r); NNRTI if receiving an NNRTI but not a PI/r; INSTI if receiving an INSTI but not a PI/r or NNRTI; and nucleosides only if receiving combination nucleoside therapy without any other antiretroviral class. Two patients who were not receiving ART were excluded from this analysis. Patients were also evaluated according to nucleoside reverse transcriptase inhibitor (NRTI) backbone, categorized as tenofovir based or ABC based. Further details on study design and data from the 2 patients who were not receiving ART and from those who received DCV + SOF for 8 weeks have been described previously [13].
Study Endpoints
The primary efficacy endpoint was SVR (HCV RNA < lower limits of quantification; <25 IU/mL) at post-treatment week 12 (SVR12) among previously untreated patients with GT1 infection treated for 12 weeks. Secondary endpoints included efficacy among HCV treatment-experienced patients and overall safety. Exploratory endpoints included the proportion of patients receiving ART who maintained HIV-1 suppression (<50 copies/mL at end of DCV + SOF treatment) or experienced HIV virologic failure (defined as a confirmed or last available measurement of HIV-1 RNA ≥400 copies/mL), and the change in absolute CD4 cell count throughout the treatment phase.
Efficacy and Safety Assessments
Serum HCV and HIV-1 RNA and CD4 cell counts were assessed at screening, baseline, and weeks 1, 2, 4, 6, 8, and 12. HCV RNA was measured using the Roche COBAS TaqMan HCV Test v2.0. Virologic response was defined as undetectable HCV RNA (<20 IU/mL) during the treatment period and as unquantifiable HCV RNA post-treatment (<25 IU/mL). Adverse events (AEs) and laboratory abnormalities were graded using National Institutes of Health Division of AIDS criteria (v1.0 2009 clarification).
Resistance Monitoring
Polymorphisms associated with DCV resistance in the HCV NS5A region (at positions M28, Q30, L31, and Y93) were assessed in all patients at baseline and at the time of virologic failure (when HCV RNA was ≥1000 IU/mL) using population-based (≥20%) sequencing of plasma samples. NS5B resistance testing was performed on each sample obtained from patients with virologic failure that could be evaluated (plus a matched baseline comparator) and in comparator samples from 2 patients who achieved SVR.
Statistical Analyses
Safety and antiviral activity were assessed for all treated patients using descriptive and exploratory analyses. For the primary endpoint, SVR12 was summarized for patients in each cohort (PI, NNRTI, and INSTI regimens). The primary statistical objective was to determine whether the SVR12 rate among previously untreated patients with HCV GT1 was higher after 12 weeks of DCV + SOF than the historical response rate of 29% observed in similar patients after 48 weeks of treatment with peginterferon–ribavirin [14].
RESULTS
Study Population
Overall, 153 patients were treated with DCV + SOF for 12 weeks, of whom 151 were receiving concomitant ART (100 were HCV treatment naive and 51 were HCV treatment experienced). Patient demographics and HCV and HIV disease characteristics are summarized in Table 1. Median age was 53 years, 49/151 (32.5%) patients were black and 96/151 (63.6%) white, 23/151 (15.2%) had cirrhosis, and 24/151 (15.9%) were infected with non-GT1 HCV (12/151 [7.9%], GT2; 9/151 [6.0%], GT3; and 3/151 [2%], GT4). Approximately half (70/151 [46.4%]) of the patients receiving ART were on a PI regimen; 40/151 (26.5%) were on an NNRTI regimen, 39/151 (25.8%) were on an INSTI regimen, and 2/151 (1.3%) were taking nucleosides only. Sixteen patients (10.5%) were on a complex antiretroviral regimen that contained 3 or more antiretroviral classes (among PI, NNRTI, INSTI, and NRTI). In total, 120/151 (79.5%) patients were receiving TDF as part of their NRTI backbone (5 [4.2%] received both ABC and TDF). An additional 23 of 151 patients (15.2%) were receiving ABC without TDF, 4 patients (2.7%) were receiving other NRTIs (FTC ± AZT), and 4 patients (2.7%) were on ART that did not include an NRTI. Two patients (1.3%) were not receiving any ART (and were thus excluded from this analysis). Patients with HCV GT1a were evenly distributed across antiretroviral groups. Overall, 141 of 149 patients (95%) on ART with available data had HIV RNA <50 copies/mL at baseline, which was similar between treatment groups.
Table 1.
Baseline Demographic and Disease Characteristics
| Parameter | PI Regimena N = 70 | NNRTI Regimena N = 40 | INSTI Regimena N = 39 | Total N = 151b |
|---|---|---|---|---|
| Median age (range), y | 54.0 (27, 71) | 54.0 (24, 65) | 49.7 (24, 64) | 53.0 (24, 71) |
| Male, n (%) | 63 (90.0) | 38 (95.0) | 31 (79.5) | 134 (88.7) |
| Race, n (%) | ||||
| White | 41 (58.6) | 23 (57.5) | 30 (76.9) | 96 (63.6) |
| Black | 26 (37.1) | 14 (35.0) | 9 (23.1) | 49 (32.5) |
| Other | 3 (4.3) | 3 (7.5) | … | 6 (4.0) |
| HCV disease characteristics | ||||
| HCV genotype, n (%) | ||||
| GT1a | 45 (64.3) | 28 (70.0) | 30 (76.9) | 104 (68.9) |
| GT1b | 18 (25.7) | 2 (5.0) | 3 (7.7) | 23 (15.2) |
| GT2 | 3 (4.3) | 5 (12.5) | 3 (7.7) | 12 (7.9) |
| GT3 | 4 (5.7) | 4 (10.0) | 1 (2.6) | 9 (6.0) |
| GT4 | … | 1 (2.5) | 2 (5.1) | 3 (2.0) |
| HCV RNA, median (range), log10 IU/mL | 6.76 (3.9, 7.9) | 6.36 (3.3, 7.6) | 6.69 (4.1, 7.8) | 6.71 (3.3, 7.9) |
| Cirrhosis, n (%) | 11 (15.7) | 6 (15.0) | 6 (15.4) | 23 (15.2) |
| HIV-1 disease characteristics | ||||
| HIV-1 RNA < 50 copies/mL, n (%)c | 63 (90.0) | 39 (97.5) | 39 (100.0) | 141 (94.6) |
| Median CD4 cells/mm3 (range) | 523.0 (122, 1115) | 605.5 (143, 1087) | 571.0 (212, 1318) | 557.0 (122, 1318) |
| HIV-1 treatment, n (%) | ||||
| PI regimen | ||||
| Atazanavir/r | 31 (44.3) | … | … | 31 (20.5) |
| Darunavir/r | 30 (42.9) | … | … | 30 (19.9) |
| Lopinavir/r | 9 (12.9) | … | … | 9 (6.0) |
| NNRTI regimen | ||||
| Efavirenz | … | 26 (65.0) | … | 26 (17.2) |
| Nevirapine | … | 8 (20.0) | … | 8 (5.3) |
| Rilpivirine | … | 6 (15.0) | … | 6 (4.0) |
| INSTI regimen | ||||
| Raltegravir | … | … | 32 (82.1) | 32 (21.2) |
| Dolutegravir | … | … | 7 (17.9) | 7 (4.6) |
Abbreviations: GT, genotype; HCV, hepatitis C virus; HIV, human immunodeficiency virus; INSTI, integrase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; r, ritonavir-boosted.
a Patients listed as PI regimen if receiving any boosted PI; as NNRTI regimen if receiving an NNRTI but not a PI/r; as INSTI regimen if receiving an INSTI but not a PI/r or NNRTI. Patients could have received antiretrovirals from multiple classes, except for the combination of PI + efavirenz or PI + nevirapine.
b Two patients were taking nucleoside reverse transcriptase inhibitors only.
c Patients on antiretroviral therapy with available baseline HIV-1 RNA data; n = 149.
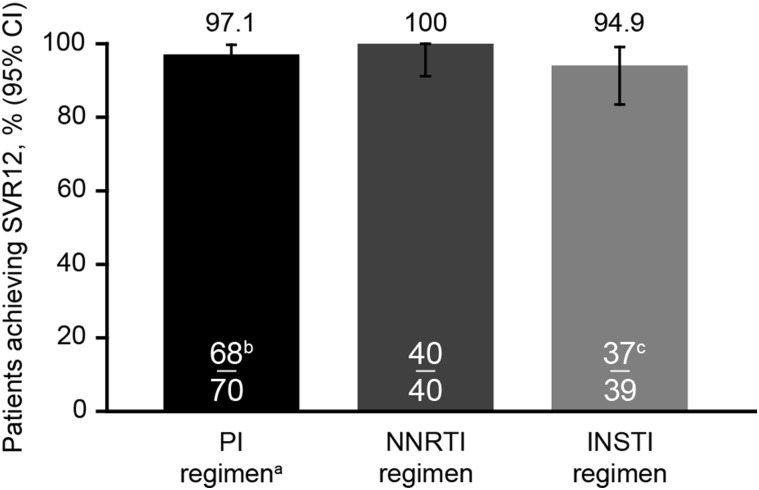
Efficacy
Following 12 weeks of DCV + SOF treatment, SVR12 rates were similarly high across all groups, regardless of concomitant ART (Figure 1, Table 2). SVR12 was achieved by 97.1% (95% confidence interval [CI], 90%–99.7%), 100% (95% CI, 91%–100%), and 94.9% (95% CI, 83%–99.4%) of patients receiving PI, NNRTI, and INSTI therapy, respectively. Within the PI group, SVR12 was achieved by all 31 (100%) patients receiving ATV/r, 28/30 (93.3%) receiving DRV/r, and all 9 (100%) of those receiving LPV/r. Within the NNRTI group, SVR12 was achieved by all 26 patients (100%) receiving EFV, all 8 (100%) receiving NVP, and all 6 (100%) receiving RPV. In the INSTI group, 30/32 (93.8%) of patients receiving RAL and 7/7 (100%) receiving DTG achieved SVR12. In total, 117/120 (97.5% [95% CI, 92.9%–99.5%]) of patients receiving TDF and all 23 patients (100% [95% CI, 85.2%–100%]) receiving ABC (without TDF) achieved SVR12. Of the patients receiving a complex antiretroviral regimen containing 3 or more antiretroviral classes, 15/16 (93.8%) achieved SVR12; the one participant with virologic failure on complex ART was taking DRV/r, RAL, tenofovir/emtricitabine.
Figure 1.
Sustained virologic response 12 weeks post-treatment (SVR12) by antiretroviral therapy. (a) The 60-mg standard dose of daclatasvir (DCV) was adjusted to 30 mg with ritonavir-boosted (/r) protease inhibitors. Recent data suggest that a 60-mg dose of DCV should be coadministered with darunavir/r or lopinavir/r [15]. (b) Two patients on protease inhibitors had hepatitis C virus (HCV) relapse. (c) One patient was lost to follow-up at week 6 due to incarceration; 1 nonadherent patient with detectable HCV RNA at end of treatment received approximately 1 week of treatment. Abbreviations: CI, confidence interval; INSTI, integrase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Table 2.
Sustained Virologic Response 12 Weeks Post-Treatment According to Human Immunodeficiency Virus Type 1 Antiretroviral Regimen
| Antiretroviral Regimen | Proportion of Patients Achieving Sustained Virologic Response 12 Weeks Post-Treatment n/N (%) |
|---|---|
| Protease inhibitor regimen | |
| Atazanavir/r | 31/31 (100) |
| Darunavir/r | 28/30 (93)a |
| Lopinavir/r | 9/9 (100) |
| Nonnucleoside reverse transcriptase inhibitor regimen | |
| Efavirenz | 26/26 (100) |
| Nevirapine | 8/8 (100) |
| Rilpivirine | 6/6 (100) |
| Integrase inhibitor | |
| Raltegravir | 30/32 (94)b |
| Dolutegravir | 7/7 (100) |
| Nucleoside reverse transcriptase inhibitor | |
| Tenofovir disoproxil fumarate | 117/120 (97.5) |
| Abacavir (without tenofovir disoproxil fumarate) | 23/23 (100) |
| Complex antiretroviral regimen (≥3 classes) | 15/16 (93.8) |
Abbreviation: r, ritonavir-boosted.
a Two patients who did not achieve sustained virologic response 12 weeks post-treatment had a detectable NS5A-related variant at baseline and/or failure. One patient, a white male with genotype (GT)1a, who was hepatitis C virus (HCV) treatment-naive and cirrhotic, and had a baseline HCV viral load ≥10 × 106 IU/mL experienced HCV relapse with Y93N at baseline and at the time of failure. The second patient was a black male with GT1a HCV, who was HCV-treatment-experienced with cirrhosis and baseline viral load ≥10 × 106 IU/mL. This patient, who also experienced HCV relapse, had no NS5A polymorphism at baseline but relapsed with an emergent Q30R mutation detected at time of failure. This patient was receiving a complex human immunodeficiency virus regimen that included a boosted-protease inhibitor along with 2 nucleoside reverse transcriptase inhibitors and an integrase inhibitor (darunavir/ritonavir, tenofovir/emtricitabine [FCT] and raltegravir [RAL]).
b Two patients did not achieve sustained virologic response 12 weeks post-treatment. Neither patient had any NS5A-related polymorphisms (at M28, Q30, L31, or Y93). One treatment-naive patient with GT1a, without cirrhosis and with baseline viral load of approximately 1 × 106 IU/mL on integrase inhibitor (INSTI)–based combined antiretroviral therapy (RAL plus zidovudine and lamivudine) had on-treatment failure (HCV RNA < lower limits of quantification; target detected at end of treatment) after early discontinuation due to noncompliance. One GT1a treatment-naive patient without cirrhosis and with baseline viral load approximately 1 × 106 IU/mL on INSTI-based combined antiretroviral therapy (RAL plus tenofovir and FCT) was lost to follow-up after having undetectable HCV RNA at end of treatment.
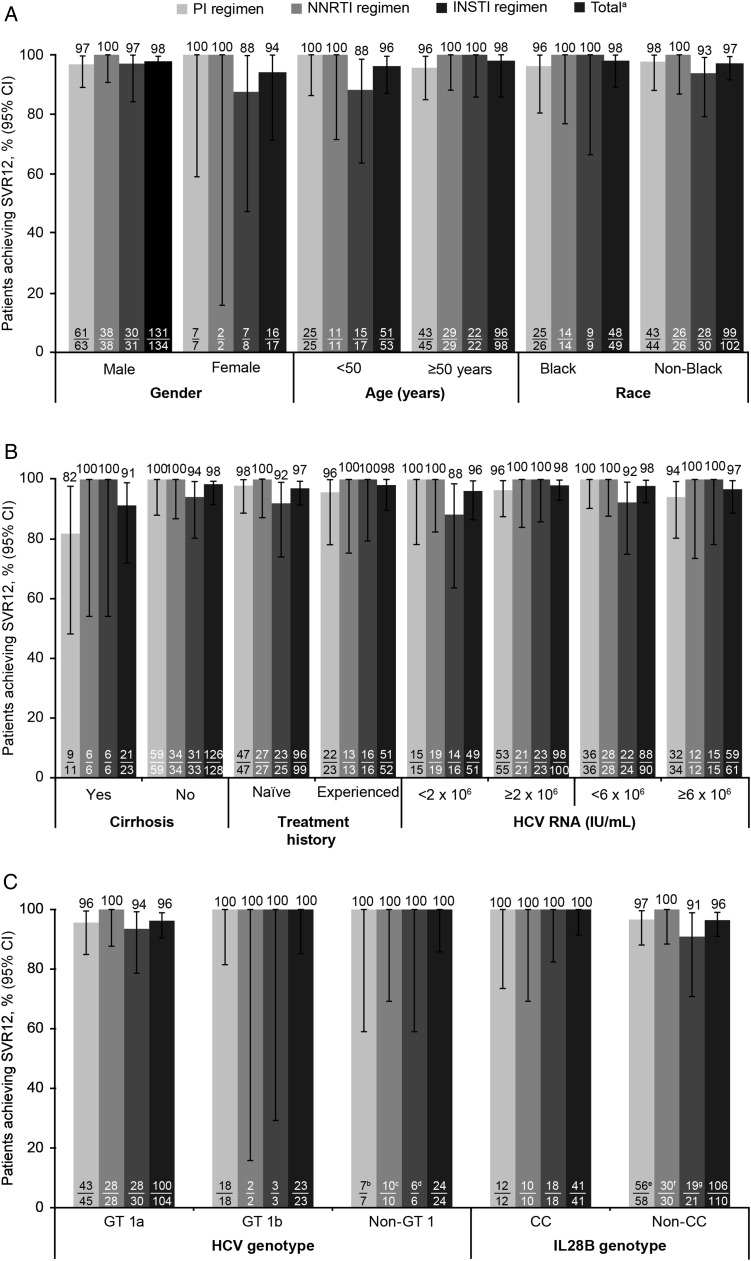
When stratified by antiretroviral regimen, SVR12 rates were similar regardless of gender, age, or race (Figure 2A); HCV treatment history or baseline HCV RNA levels (Figure 2B); or HCV GT or IL28B GT (Figure 2C). SVR12 was achieved by 21/23 (91.3%) and 126/128 (98.4%) of patients with and without cirrhosis, respectively (Figure 2C).
Figure 2.
A, Sustained virologic response 12 weeks post-treatment (SVR12) by patient demographics. B, SVR12 by baseline disease characteristics. C, SVR12 by hepatitis C virus (HCV) genotype (GT) at baseline. (a) Two patients were taking nucleoside reverse transcriptase inhibitors only. (b) GT2, n = 3; GT3, n = 4. (c) GT2, n = 5; GT3, n = 4; GT4, n = 1. (d) GT2, n = 4; GT3, n = 1; GT4, n = 2. (e) IL28B TT: 16/16. (f) IL28B TT: 12/12. (g) IL28B TT: 6/6. Abbreviations: CI, confidence interval; INSTI, integrase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Resistance-Associated Variants
Of the 4 patients who did not achieve SVR12 (Table 2), 3 had no DCV resistance-associated variants at baseline. One of these 3 patients relapsed with an emergent Q30R mutation detected at time of failure. The fourth patient had a detectable DCV polymorphism (Y93N mutation) both at baseline and at time of HCV relapse.
Safety
On-treatment safety is summarized in Table 3. A similar number of patients experienced ≥1 AE across groups (70.7%–77.5%). Common AEs (experienced by ≥10% in any group) were fatigue, nausea, headache, diarrhea, and rash. Similar proportions of patients reported these AEs across groups and were similar to those observed in DCV + SOF-treated HCV-monoinfected patients [16]. No patient discontinued treatment for AEs. Serious AEs (SAEs) during treatment included priapism in a patient receiving medication for erectile dysfunction, presyncope plus chest pain, drug abuse plus pulmonary embolism, and syncope plus hypertensive crisis. No SAE was considered related to study treatment. There was 1 death among patients in the 12-week treatment groups: a 53-year-old black male died of cardiomyopathy and multiorgan failure at post-treatment day 194. There was 1 additional death across the entire study: a 52-year-old white male in the 8-week treatment group suffered cardiac arrest at post-treatment day 40. Neither death was judged by the investigator to be related to study treatment.
Table 3.
Summary of Adverse Events During Treatment
| Event, n (%) | Protease Inhibitor Regimen N = 70 |
Nonnucleoside Reverse Transcriptase Inhibitor Regimen N = 40 |
Integrase Inhibitor Regimen N = 39 |
Total N = 151a |
|---|---|---|---|---|
| Patients with at least 1 AE | 50 (71.4) | 31 (77.5) | 29 (74.7) | 110 (72.8) |
| Serious AEsb | 3 (4.3) | 0 | 1 (2.6) | 4 (2.6) |
| Death | 0 | 0 | 0 | 0 |
| AEs leading to discontinuation | 0 | 0 | 0 | 0 |
| Common AEs while on treatment (≥10% in any treatment group) | ||||
| Fatigue | 12 (17.1) | 7 (17.5) | 9 (23.1) | 28 (18.5) |
| Nausea | 8 (11.4) | 4 (10.0) | 9 (23.1) | 21 (13.9) |
| Headache | 13 (18.6) | 2 (5.0) | 5 (12.8) | 20 (13.2) |
| Diarrhea | 9 (12.9) | 3 (7.5) | 2 (5.1) | 14 (9.3) |
| Rash | 3 (4.3) | 1 (2.5) | 5 (12.8) | 9 (6.0) |
| Insomnia | 2 (2.9) | 2 (5.0) | 4 (10.3) | 8 (5.3) |
| Treatment-emergent grade 3–4 laboratory abnormalitiesc | ||||
| International normalized ratio ≥ 2.1 × ULNd | 2 (2.9) | 0 | 0 | 2 (1.3) |
| Total bilirubin ≥2.6 × ULNe | 8 (11.4) | 0 | 0 | 8 (5.3) |
| Lipase ≥3.1 × ULNf | 2 (2.9) | 3 (7.5) | 1 (2.6) | 6 (4.0) |
Abbreviations: AE, adverse event; ULN, upper limit of normal.
a Two patients were taking nucleoside reverse transcriptase inhibitors only.
b Serious AEs while on protease inhibitor regimen: priapism, drug abuse/pulmonary embolism, and hypertensive crisis/syncope; while on integrase inhibitor regimen: chest pain/presyncope. Some patients presented with multiple serious AEs; none were related to treatment.
c No grade 3–4 alanine aminotransferase or aspartate aminotransferase elevations were detected.
d One patient had a history of aortic valve replacement and was receiving anticoagulation therapy and 1 patient had an isolated grade 3 elevation at week 6 that was within normal limits on repeat testing at week 8.
e Each event was an indirect hyperbilirubinemia in patients receiving concomitant ritonavir-boosted atazanavir.
f Transient hyperlipasemia without reported pancreatitis.
Treatment-emergent increases in the international normalized ratio of ≥ 2.1 × upper limit of normal (ULN) were observed in 2/70 (2.9%) patients in the PI group: 1 patient had a history of aortic valve replacement and was receiving anticoagulation therapy and 1 patient had an isolated grade 3 elevation at week 6 that was within normal limits on repeat testing at week 8. Treatment-emergent increases in total bilirubin of ≥2.6 × ULN were observed among patients receiving ATV/r only; 8 patients (5.3%) presented with this laboratory abnormality. Overall, transient grade 3–4 increases in lipase (≥3.1 × ULN) were observed in 4% of patients, but there were no reported cases of clinical pancreatitis.
Renal Safety
A low proportion of patients reported AEs relating to renal function in the PI (3/70 [4.3%]) and NNRTI (1/40 [2.5%]) groups; none were reported in the INSTI group (Table 4). Three patients receiving TDF had a change in baseline creatinine that was ≥0.4 mg/dL. There was minimal change in mean creatinine clearance (calculated using the Cockcroft–Gault equation) through week 12 across all groups, regardless of NRTI backbone. Three patients altered their antiretroviral regimen during the treatment period due to progression of tenofovir-related safety concerns. Of these, two patient with reported history of chronic renal insufficiency had an increase in their serum creatinine levels (serum creatinine = 1.44 mg/dL [grade 1] and 1.47 mg/dL [grade 1] at baseline to 1.85 mg/dL [grade 2] at week 6 and 1.56 mg/dL [grade 1] at week 2, respectively) and 1 patient with a history of osteoporosis had progressive bone loss on a dual-energy X-ray absorptiometry scan.
Table 4.
Summary of Renal Safety During Treatment
| Event, n (%) | Protease Inhibitor Regimen N = 70 | Nonnucleoside Reverse Transcriptase Inhibitor Regimen N = 40 | Other N = 41 | Total N = 151 |
|---|---|---|---|---|
| Grade 1–4 renal urinary disorders | 3 (4.3) | 1 (2.5) | 0 | 4 (2.6) |
| Hematuria | 1 (1.4) | 1 (2.5) | 0 | 2 (1.3) |
| Dysuria | 1 (1.4) | 0 | 0 | 1 (0.7) |
| Nocturia | 1 (1.4) | 0 | 0 | 1 (0.7) |
| Grade 3–4 creatinine | 0 | 0 | 0 | 0 |
| Mean creatinine clearance (mL/min) with Cockcroft–Gault formula at week 12 | 89.8a | 85.7b | 99.6c | 91.4d |
| Change from baseline | –4.8 | –3.0 | –1.2 | –3.3 |
a n = 69.
b n = 38.
c n = 39.
d n = 146.
HIV-1 Suppression
Overall, 142/151 (94.0%) patients on ART had HIV-1 RNA < 50 copies/mL through the end of treatment (Table 5). The proportions of patients maintaining <50 copies/mL were 64/70 (91.4%) of those on a PI regimen, 40/40 (100%) of those on a NNRTI regimen, and 36/39 (92.3%) of those on an INSTI regimen. Of the 9 patients who had ≥50 copies/mL at the end of treatment, 6 had <50 copies/mL on repeat testing without a change in ART, 2 had ≥50 copies/mL on repeat testing (1 had 59 copies/mL and 1 had 66 copies/mL), and 1 was lost to follow up before repeat testing could be completed. HIV-1 virologic failure (confirmed or last available HIV-1 RNA ≥400 copies/mL) was observed in 2/151 (1.3%) of patients.
Table 5.
Proportion of Patients on Antiretroviral Therapy With Human Immunodeficiency Virus Type 1 RNA < 50 copies/mL at Baseline and End of Treatment
| Event, n (%) | Protease Inhibitor Regimen N = 70 | Nonnucleoside Reverse Transcriptase Inhibitor Regimen N = 40 | Integrase Inhibitor Regimen N = 39 | Total N = 151a |
|---|---|---|---|---|
| HIV-1 RNA < 50 copies/mL, n (%) at baseline | 63 (90.0) | 39 (97.5) | 39 (100.0) | 141 (94.6)b |
| HIV-1 RNA < 50 copies/mL, n (%) at end of treatment | 64 (91.4) | 40 (100) | 36 (92.3) | 142 (94.0)b |
Abbreviation: HIV-1, human immunodeficiency virus type 1.
a Two patients were taking nucleoside reverse transcriptase inhibitors only.
b Patients on antiretroviral therapy with available data; baseline n = 149; end of treatment n = 151.
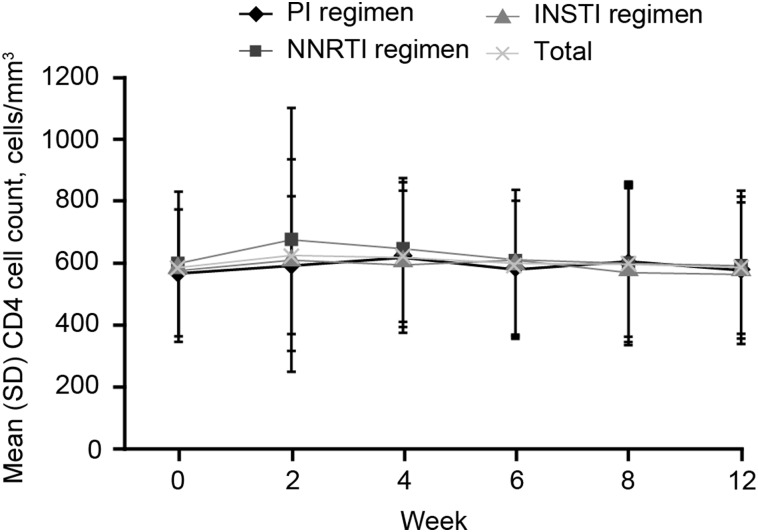
One patient receiving a PI-based regimen (DRV/r, RAL, ABC/3TC) had HIV-1 RNA 4442 copies/mL at the end-of-treatment (week 12) visit. HIV virologic failure was confirmed at the next visit (post-therapy follow-up week 4; HIV-1 RNA 1755 copies/mL). The patient's HIV RNA was resuppressed to <40 copies/mL at the post-treatment week 12 visit without adjusting the patient's antiretroviral regimen. This patient achieved SVR12. The second patient receiving TDF/FTC/RAL had an HIV RNA of 418 copies/mL at screening but <40 copies/mL at day 1. The patient discontinued study medications early due to incarceration (lost to follow-up) following the week 6 visit (HIV-1 RNA was 629 copies/mL at this treatment visit). For both patients, HIV resistance testing did not demonstrate any evidence of resistance (data not shown). Mean CD4 cell counts were similar between groups across the 12-week treatment period (Figure 3).
Figure 3.
CD4 cell counts during treatment. Abbreviations: INSTI, integrase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; SD, standard deviation.
DISCUSSION
Twelve weeks of DCV + SOF resulted in high SVR12 rates (97%), comparable with SVR rates observed in similar trials of other combinations of DAAs in HIV and HCV GT1-coinfected patients [16–18]. Participants were on a wide range of antiretrovirals, and SVR rates were high across all antiretroviral classes, including complex antiretroviral regimens that contained drugs from 3 or more antiretroviral classes. The efficacy of DCV + SOF was similarly high in patients receiving TDF vs those receiving ABC (without TDF), with SVR12 rates achieved by 98% and 100% of patients, respectively. Thus, DCV + SOF represents an effective HCV treatment option that does not require modification for many of the common antiretroviral regimens, including 4 of 5 Department of Health and Human Services–recommended and 5 of the 6 European AIDS Clinical Society-recommended first-line regimens [4, 5]. Importantly, DCV + SOF treatment in HIV-HCV–coinfected patients did not compromise HIV control. CD4 cell counts remained stable and HIV RNA remained suppressed for the majority of participants throughout the study.
No differences in efficacy were observed in patients when stratified by gender, age, or race. HCV treatment history, baseline HCV RNA levels, cirrhosis, HCV GT, and IL28B GT also had no effect on SVR12 rates. Patients with any of the 6 HCV GTs were allowed to enroll in the study. However, due to the small number of HIV-HCV–coinfected patients with HCV GTs other than GT1, definitive data on response rates across all GTs cannot be provided.
Based on previous pharmacokinetic data with ATV/r [11], the requirement to reduce the dose of DCV to 30 mg was extrapolated to other boosted PIs (ie, DRV/r or LPV/r). However, more recent pharmacokinetic data suggest DRV/r and LPV/r have more limited effects on systemic DCV exposures (1.41- and 1.15-fold increases, respectively) compared to the effect observed during ATV/r coadministration (2.1-fold increase in DCV levels) [15,19]. However, the dose adjustment of DCV to 30 mg in this current study did not appear to have a clinically meaningful effect on efficacy, as SVR12 was achieved by 93% and 100% of patients receiving DRV/r and LPV/r, respectively.
The DCV + SOF regimen was generally well tolerated, with no treatment discontinuations due to intolerance and no treatment-related SAEs. Grade 3–4 hyperbilirubinemia was only observed in patients receiving ATV/r and occurred at a rate consistent with the known safety profile of ATV [20]. While healthy volunteer studies have not identified any clinically significant interactions between DCV and TDF or between SOF and TDF [11, 21], evaluation of renal function in patients receiving TDF-based regimens and DCV + SOF is important due to the complex nature of the antiretroviral regimens of some of the patients in this study and because of known interactions between TDF and other HCV DAAs [9]. Three patients taking TDF were required to modify HIV treatment due to TDF-induced complications that were not attributed to DCV + SOF coadministration.
The main strength of this study was that a broad range of antiretrovirals, including ritonavir-boosted PIs, NRTIs, NNRTIs, INSTIs, CCR5 antagonists, and fusion inhibitors, were permitted in the ALLY-2 study. In conclusion, this comprehensive evaluation of DCV + SOF when coadministered with a broad range of antiretrovirals demonstrates that this combination was highly efficacious and generally well tolerated.
Notes
Acknowledgments. The authors thank the participants and their families for their support and dedication and the investigators and research staff at all study sites. The authors acknowledge the following personnel at Bristol-Myers Squibb (BMS) for their contribution to this study: Patricia Mrowiec, Nancy Beckert, Lisa Jones, Nicole Brini, Fiona McPhee, Vincent Vellucci, Joseph Ueland, Dennis Hernandez, William O′Brien, and Eric Y Wong. Editorial support was provided by Kerry Garza, PhD, at MediTech Media, funded by BMS.
Financial support. This study was funded by BMS.
Potential conflicts of interest. C. M. reports personal fees from BMS during the conduct of the study and personal fees from Gilead, Merck, Viiv, and Janssen outside the submitted work. A. F. L. reports grants from BMS during the conduct of the study and grants from Abbvie, Gilead, Pfizer, and Merck outside the submitted work. S. N. reports employment/stockholder from BMS, during the conduct of the study; employment/stockholder from BMS, stockholder/former employee from Merck/Schering-Plough, stockholder from J&J, outside the submitted work. P. A. reports employment/stockholder from BMS, Squibb, during the conduct of the study; employment/stockholder from BMS, outside the submitted work. M. R. reports Research BMS, Gilead, Viiv, Speaker BMS, Gilead, Merck, Ad Boards - Viiv, Gilead. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Bica I, McGovern B, Dhar R et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis 2001; 32:492–7. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. HIV and viral hepatitis factsheet. 2014. Available at: www.cdc.gov/hiv/pdf/library_factsheets_HIV_and_viral_Hepatitis.pdf Accessed August 2015.
- 3.Sulkowski MS, Benhamoi Y. Therapeutic issues in HIV/HCV-coinfected patients. J Viral Hepat 2007; 14:371–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Available at: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf Updated April 2015. Accessed November 2015.
- 5.European AIDS Clinical Society. Guidelines Version 8.0. October 2015. Available at: http://www.eacsociety.org/files/guidelines-8.0-english.pdf Accessed November 2015.
- 6.European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2015. J Hepatol 2015; 63:199–236. [DOI] [PubMed] [Google Scholar]
- 7.Cope R, Pickering A, Glowa T et al. Majority of HIV/HCV patients need to switch antiretroviral therapy to accommodate direct acting antivirals. AIDS Patient Care STDS 2015; 29:379–83. [DOI] [PubMed] [Google Scholar]
- 8.Viekira Pak® (ombitasvir, paritaprevir and ritonavir tablets; dasabuvir tablets). US prescribing information. Abbvie 2014. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2014/206619lbl.pd Accessed October 2015.
- 9.Harvoni® (ledipasvir and sofosbuvir). US prescribing information. Foster City, CA: Gilead Sciences, 2015. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/205834s001lbl.pdf Accessed December 2015. [Google Scholar]
- 10.Sovaldi® (sofosbuvir) US prescribing information. Foster City, CA: Gilead Sciences, 2013. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/204671s000lbl.pdf Accessed December 2015. [Google Scholar]
- 11.Bifano M, Hwang C, Oosterhuis B et al. Assessment of pharmacokinetic interactions of the HCV NS5A replication complex inhibitor daclatasvir with antiretroviral agents: ritonavir-boosted atazanavir, efavirenz and tenofovir. Antivir Ther 2013; 18:931–40. [DOI] [PubMed] [Google Scholar]
- 12.Sulkowski MS, Gardiner DF, Rodriguez-Torres M et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med 2014; 370:211–21. [DOI] [PubMed] [Google Scholar]
- 13.Wyles DL, Ruane PJ, Sulkowski MS et al. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med 2015; 373:714–25. [DOI] [PubMed] [Google Scholar]
- 14.Torriani FJ, Rodriguez-Torres M, Rockstroh JK et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med 2004; 351:438–50. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi Y, Adamczyk R, Wang R et al. Assessment of drug–drug interactions between daclatasvir and darunavir/ritonavir or lopinavir/ritonavir. Rev Antiviral Ther Infect Dis 2015; 4:Abstract 80 Available at: http://regist2.virology-education.com/abstractbook/2015_4.pdf Accessed September 2015. [Google Scholar]
- 16.Zeuzem S, Ghalib R, Reddy KR et al. Grazoprevir-elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic hepatitis c virus genotype 1, 4, or 6 infection: a randomized trial. Ann Intern Med 2015; 163:1–13. [DOI] [PubMed] [Google Scholar]
- 17.Naggie S, Cooper C, Saag M et al. Ledipasvir and sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med 2015; 373:705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sulkowski MS, Eron JJ, Wyles D et al. Ombitasvir, paritaprevir co-dosed with ritonavir, dasabuvir, and ribavirin for hepatitis C in patients co-infected with HIV-1: a randomized trial. JAMA 2015; 313:1223–31. [DOI] [PubMed] [Google Scholar]
- 19.Eley T, You X, Wang R et al. Daclatasvir: overview of drug-drug interactions with antiretroviral agents and other common concomitant drugs. GAJ 2014; 10(suppl 1):54–5. [Google Scholar]
- 20.Reyataz® (atazanavir). US prescribing information. Princeton, NJ, Bristol Myers Squibb, 2014. Available at: http://www.reyataz.com/ Accessed October 2015. [Google Scholar]
- 21.Kirby B, Mathias A, Rossi S et al. No clinically significant pharmacokinetic drug interactions between sofosbuvir (GS-7977) and HIV antiretrovirals atripla, rilpivirine, darunavir/ritonavir, or raltegravir in healthy volunteers. AASLD 2012. Abstract: 1877. [Google Scholar]