Abstract
Conclusion
The intraoperative application of glucocorticoid-loaded hydrogels seems to cause a reduction in neutrophil infiltration. No beneficial effect on hearing thresholds was detected.
Objectives
To evaluate the application of dexamethasone- and triamcinolone-acetonide- loaded hydrogels for effects on hearing-preservation and foreign-body reaction in a guinea pig model for cochlear implantation.
Methods
48 guinea pigs (n= 12/group) were implanted with a single channel electrode and intraoperatively treated with 50 µl of a 20% w/v poloxamer 407 hydrogel loaded with 6% dexamethasone or 30% triamcinolone-acetonide, a control hydrogel, or physiological saline. Click- and tone burst-evoked compound action potential thresholds were determined pre- and directly postoperatively as well as on days 3, 7, 14, 21 and 28. At the end of the experiment, temporal bones prepared for histological evaluation by a grinding/polishing technique with the electrode in situ. Three ears per treatment group were serially sectioned and evaluated for histological alterations.
Results
The intratympanic application of glucocorticoid-loaded hydrogels did not improve the preservation of residual hearing in this cochlear implant model. The foreign body reaction to the electrode appeared reduced in the glucocorticoid treated animals. No correlation was found between the histologically described trauma to the inner ear and the resulting hearing threshold-shifts.
Keywords: Cochlear Implants, Glucocorticoids, Hearing Preservation, Residual Hearing, Guinea Pig
Introduction
The auditory benefits of electric-acoustic stimulation raised interest in the preservation of residual hearing during cochlear implantation [1, 2]. Therefore, considerable efforts have been put into improved electrode designs and surgical techniques [3]. Nevertheless, the occurrence of hearing loss days to months after surgery [4] and in regions apically to the electrode position highlights the need for pharmacological interventions to further improve outcomes in candidates for electric-acoustic stimulation. Possible causative factors for delayed hearing loss include inflammation, oxidative stress and apoptosis, all of which are influenced by glucocorticoids [5, 6]. Because of these well-known effects this class of medication plays an important role in surgical protocols aiming at hearing preservation during and after cochlear implantation [7].
The intratympanic delivery of glucocorticoids has gained popularity in recent years in this setting, because it results in higher drug levels in perilymph as compared to systemic administration. This advantage of topical application protocols has been demonstrated in humans and in animal models [8, 9]. Furthermore, various studies provided evidence that the application of thermoreversible poloxamer 407 hydrogels can prolong therapeutic glucocorticoid levels in the perilymph [10, 11]. Even though it has been shown that an earlier application of glucocorticoids leads to better hearing preservation rates [12], the intraoperative time-point for the hydrogel application, directly after the electrode insertion and sealing of the cochleostomy, was chosen for this trial, because it could be easily implemented into cochlear implantation surgery protocols.
The current study focused on the evaluation of hearing thresholds as well as on the histological evaluation of the implanted inner ears.
Material and methods
Experimental animals
Animal experiments were approved by the local animal welfare committee and the Austrian Federal Ministry for Science and Research (BMWF-66.009/0159-II/3b/2011). In total, 48 male and female pigmented guinea pigs, bred in the Department of Biomedical Research and weighing 350 grams or more were used. Animals were equally distributed into four experimental groups (n = 12): 1) physiological saline (NaCl) control, 2) poloxamer 407 hydrogel (POX) control, 3) 6% dexamethasone (DEX) hydrogel, 4) and 30% triamcinolone-acetonide (TAAc) hydrogel. Glucocorticoid concentrations were selected with regard to the published literature on glucocorticoid hydrogels for intratympanic application [10] and the known glucocorticoid-potencies after systemic application. For each animal, the side for cochlear implantation was randomly chosen.
Hydrogel preparation
The thermoreversible 20% w/v poloxamer 407 hydrogel (BASF SE, Ludwigshafen, Germany) was prepared using the cold-method and loaded with 6% dexamethasone (Gatt-Koller, Absam, Austria) or 30% triamcinolone-acetonide (Fagron, Barsbüttel, Germany), as previously described [13].
Anesthesia and perioperative management
All surgical procedures were performed under general anesthesia using medetomidine (0.3 mg/kg), midazolam (1 mg/kg), fentanyl (0.03 mg/kg) s.c. and ketamin (10 mg/kg) i.m‥ Lidocaine (4 mg/kg) was used for local anesthesia. At the end of surgery, anesthesia was partially antagonized by atipamezole (1 mg/kg) s.c. to aid recovery. All animals received carprofen (4 mg/kg) and enrofloxacin (7 mg/kg) s.c. before surgery and on the following two days. Throughout the cochlear implantation procedure, heart rate as well as vascular pO2 were measured using a pulse oximeter. Body temperature was maintained at 38°C with a heating plate during surgery and audiometries.
Surgical procedure
After the induction of surgical anesthesia, the bony bulla was opened via a postauricular approach, and a teflon-insulated gold wire (Goodfellow, Bad Nauheim, Germany) was fixed to the bony ridge of the round window niche using histoacryl glue (Braun Melsungen, Melsungen, Germany). Using this wire preoperative compound action potential (CAP) input/output functions of the cochlear nerve were determined before the drilling of the cochleostomy. The cochleostomy – drilled using a 0.8 mm diamond burr - was placed at an approximate distance of 1 mm from the round window niche. A single electrode array was inserted for 3 mm into the cochlea and fixed to the bony bulla using histoacryl glue. After sealing of the cochleostomy with temporalis muscle, 50 µl of the POX-hydrogels or physiological saline were placed to the round window niche. The hydrogel was applied as empty control or loaded with 6% DEX or 30% TAAc. The transcutaneous pin of the electrode was positioned at the vertex of the animals using two 4 mm stainless steel screws and denture resin (Paladur, Heraeus Kulzer, Hanau, Germany). An additional pin was welded to the gold-wire for future CAP-measurements. Surgical wounds were closed using 4-0 Vicryl sutures (Ethicon, Norderstedt, Germany) and animals were allowed to recover under a heating lamp.
Electrophysiology
Auditory brainstem responses (ABRs) and CAPs were recorded in a soundproof chamber (Industrial Acoustics Company; mac-2). To generate the sound field, a DT-48 speaker (Beyerdynamic, Heilbronn, Germany) was positioned 3 cm from the tested ear and a K2 microphone (Sennheiser, Wedemark, Germany) was placed at the pinna of the animal for calibration. For acoustic isolation the contralateral ear was filled with a wax earplug (Ohropax, Werheim, Germany). A custom-made setup, including a PC system running a multifunction I/O card (National Instruments, Austin, TX, USA) and AudiologyLab software (Otoconsult, Frankfurt am Main, Germany), was used for the measurement of auditory potentials. Stimuli were presented alternatingly and included clicks and tone bursts (3 ms duration; 1 ms rise/fall) presented in the frequency range of 0.5–32 kHz with 1 step/octave for the ABR- and 3 steps/octave for the CAP-measurements. Sound pressure was presented in 2-dB steps for the determination of click thresholds and in 5-dB steps for the tone bursts. Signals were amplified (80 dB), band-pass filtered between 10 Hz and 10 kHz or 200 Hz and 5 kHz, and averaged (128× or 32×) for the measurement of ABRs and CAPs, respectively. CAP-thresholds were evaluated before and immediately after the surgery as well as on postoperative days 3, 7, 14, 21 and 28. ABR-thresholds were determined approximately 1 week before surgery to exclude animals with impaired hearing from the studies and after 7, 14, 21 & 28 days to ensure that the gold-wire used for the CAP-measurements was still in place. For the determination of hearing thresholds CAP results were used.
Histology
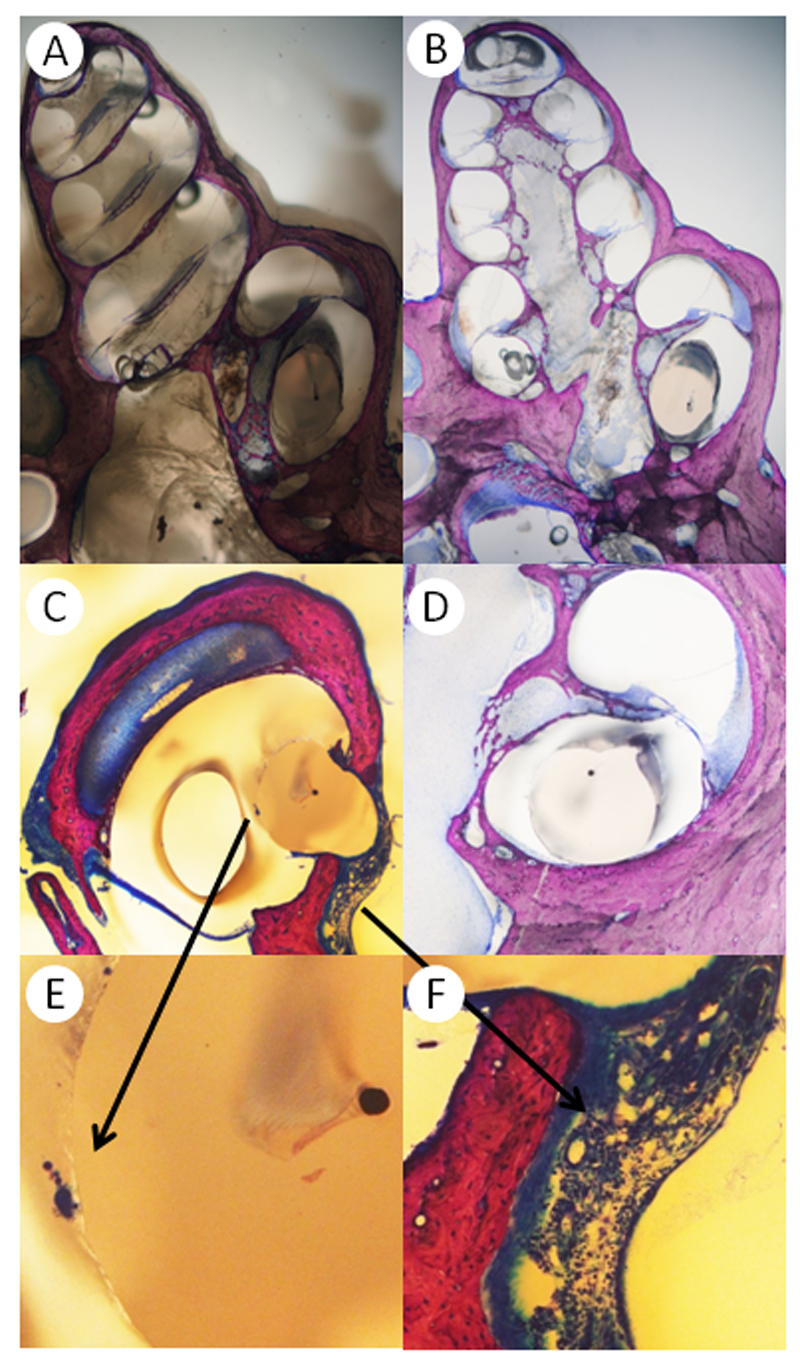
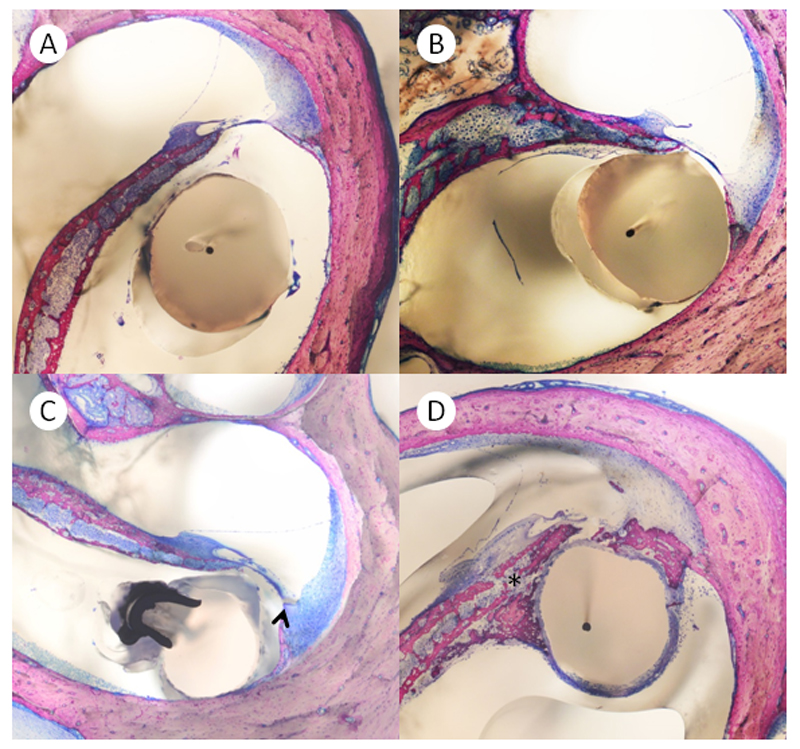
Directly after the determination of hearing thresholds on day 28, animals were euthanized by the intracardial injection of pentobarbital (600 mg/kg). Bullae were rapidly dissected and fixed in Schaffer’s solution (ethanol 80% and formalin 37%, mixed 2:1). After fixation, three randomly selected implanted cochleae per group were prepared for the histological evaluation with the electrode in situ and without decalcification, using a previously described technique [14]. In short, the fixed temporal bones were dehydrated with an ascending series of alcohols (70–100% ethanol) prior to embedding in polymethylmethacrylate at room temperature. For correct sectioning, the blocks were positioned parallel to the cochlear axis and serial 100 µm sections were prepared. The special grinding–polishing technique used allowed the sectioning of the temporal bones with the electrodes in situ, thereby avoiding potential additional damage caused by electrode-removal (Figure 3 A, B). Sections were then examined by two of the authors (L.D.L. and H.P.).
Figure 3.
A) Preparation of a cochlea with the electrode in situ. B) Mid-modiolar cut of the same specimen. C) Cochleostomy of a control-animal. The foreign body response includes neutrophil granulocytes, lymphocytes, foreign body giant cells and neovascularization. D) CI-electrode in the basal turn of a TAAc treated animal, no foreign body reaction can be detected on this slide. E) A foreign body giant cell in higher magnification. F) The foreign body reaction at the cochleostomy at higher magnification. Neutrophil granulocytes, lymphocytes and newly formed vessels are visible.
Statistical analysis
CAP-data are presented as means ± SD. Linear mixed models were used to compare threshold shifts between treatment groups accounting for the frequency and the time of measurement and including the postoperative measurement as a continuous covariate. Furthermore, the animal factor was considered as a random effect. P-values < 0.05 were considered as indicating statistical significance. All statistical analyses were performed with SAS software (Version 9.4, SAS Institute Inc., Cary, NC, USA 2002-2012). Descriptive statistics (Microsoft Excel) were used for the analysis of the histology results.
Results
CAP thresholds
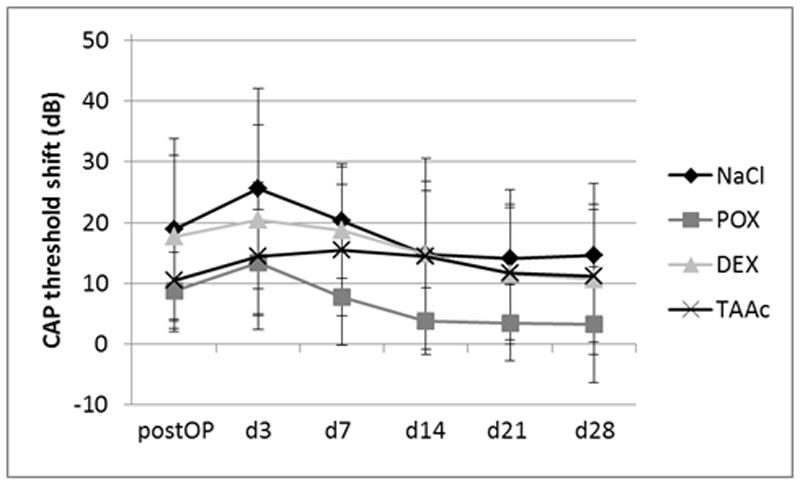
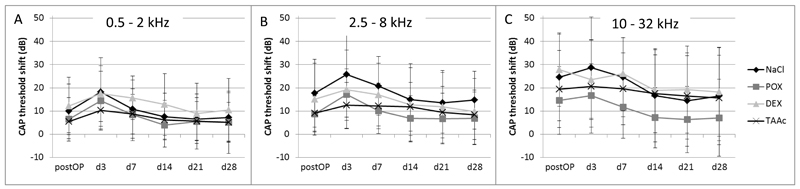
The intraoperative application of neither 6% DEX nor 30% TAAc hydrogel did result in a statistically significant improvement of residual hearing as compared to the control groups when the points in time were evaluated separately (Figures 1 and 2). Analysis of the d3, d7, d14, d 21 and d28 click-measurements in a single model showed a statistically significant lower recovery of the TAAc treated ears as compared to the POX-treated controls (P= 0.038; Figure 1), which was not found between the other groups or in the frequency specific measurements (Figure 2). Postoperative click threshold-shifts ranged from 8.8 dB (POX) to 18.8 dB (NaCl). Threshold shifts further increased until postoperative day 3 in all groups and started to recover from then on (Figure 1). A similar pattern was observed in the CAP responses to tone bursts. Postoperative CAP-threshold shifts increased starting from the apical region, where they ranged from 5.4 dB (TAAc) to 12.2 dB (DEX) (Figure 2A) to the base of the cochlea (ranging from 14.6 dB (POX) to 27.8 dB (DEX)) (Figure 2C). The increase in threshold shifts from postoperative measurements to day 3 assessments tended to be less pronounced in the glucocorticoid-treated groups, but the difference did not reach statistical significance.
Figure 1.
Click-evoked CAP threshold shifts after cochlear implantation and intraoperative application of 50 µl of NaCl, 20% w/v POX, 6% DEX/POX or 30% TAAc/POX hydrogels. The analysis of all measurements following the postoperative hearing evaluation using a statistical model showed a significantly lower recovery of CAP-thresholds in the TAAc treated animals as compared to the POX-controls. This difference was not evident when each point in time was analyzed separately.
Figure 2.
Mean CAP threshold shifts after cochlear implantation in A) the low (0.5-2 kHz), B) the middle (2.5-8 kHz) and C) the high (10-32 kHz) frequency regions. Threshold shifts were lager in the high frequencies (area of electrode insertion), but no significant differences between the treatment-groups were observed.
Histology
Three randomly selected implanted ears per group were evaluated in 100 µm sections with the electrode in situ using a grinding/polishing technique. The parameters evaluated included the inflammatory and foreign body reaction (FBR) evoked by the electrode, osteoneogenesis, alterations of the round window membrane and in the area of the cochleostomy, as well as the electrode insertion trauma.
Neutrophil granulocytes were detected in 20% of the sections of the NaCl controls, but only in 5.9%, 0% and 5.4% of the POX, the DEX and the TAAc groups, respectively (Figure 3 C, F and Table 1). Lymphocytes were found in 100% of the control, DEX and TAAc sections. Foreign body giant cells (FBGCs) and neovascularization were found in 100% of the control and DEX slides and in 90.5% of the sections after TAAc treatment (Figure 3 C-E).
Table I.
Inflammation and foreign body reaction (found in % of sections)
| NaCl | POX | 6% DEX | 30% TAAc | |
|---|---|---|---|---|
| Neutrophils | 20 | 5.9 | 0 | 5.4 |
| Lymphocytes | 100 | 100 | 100 | 100 |
| FBGCs | 100 | 100 | 100 | 90.5 |
| Neovascularization | 100 | 100 | 100 | 90.5 |
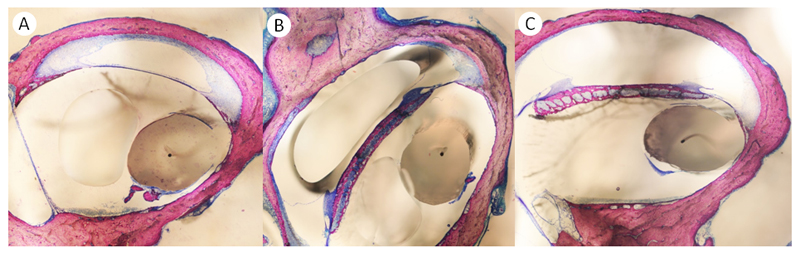
Osteoneogenesis was found in almost all sections evaluated (Table 2). A classification system, differentiating between pronounced (>25% of the FBR), moderate (<25% of the FBR), minimal (Figure 4 A-C) and no osteoneogenesis was used. The percentage of sections with pronounced osteoneogenesis was highest in the DEX group (65.2%) and lowest in the TAAc animals (31.1%) (Table 2).
Table II.
Osteoneogenesis (% of sections)
| NaCl | POX | 6% DEX | 30% TAAc | |
|---|---|---|---|---|
| >25% FBR | 31.3 | 48.5 | 65.2 | 31.1 |
| <25% of FBR | 16.3 | 7.3 | 5.6 | 4.1 |
| minimal | 52.5 | 44.1 | 28.1 | 64.9 |
| none | 0 | 0 | 1.1 | 0 |
Figure 4.
Representative sections demonstrating A) pronounced (asterisk) B) moderate (arrow) and C) minimal (arrowhead) osteoneogenesis.
A foreign body reaction of the round window membrane to the hydrogel, which was defined as a thickening of the membrane, was noted in 1/3 of the NaCl control-animals, in 3/3 animals of the POX controls and in 0/3 of the DEX- and TAAc- treated guinea pigs (see Figure 3 C for a representative slide, showing also the RWM).
In the area of the cochleostomy, membranous connective tissue, woven bone and bone fragments were found in every single specimen evaluated (Figure 3 C). In addition a swelling of the electrode, most likely due to the embedding process, was noted. The preparation of consecutive 100 µm sections also allowed for the evaluation of the electrode insertion trauma. No trauma was detected in seven animals, one animal showed an elevation and one animal a rupture of the basilar membrane. In three animals the osseous spiral lamina (OSL) was fractured. Interestingly, there was no significant correlation between the observed trauma and the click CAP-threshold shifts in the postoperative measurements (Table 3).
Table III.
Trauma and resulting postoperative click
| threshold-shifts | ||
| Treatment | Trauma | Threshold-shift (dB) |
|---|---|---|
| NaCl | Elevation of the BM | 32 |
| Fracture of the OSL | 20 | |
| Fracture of the OSL | 4 | |
| POX | No trauma | 8 |
| No trauma | 18 | |
| No trauma | 0 | |
| DEX | Fracture of the OSL | 22 |
| No trauma | 22 | |
| No trauma | 8 | |
| TAAc | No trauma | 6 |
| No trauma | 18 | |
| Rupture of the BM | 16 |
Discussion
The data presented herein demonstrate that the intraoperative application of 6% dexamethasone and 30% triamcinolone-acetonide hydrogels does not improve postoperative residual hearing in a guinea pig model of cochlear implantation, even though some inflammatory parameters were influenced. The slower postoperative recovery of click-CAP thresholds in the TAAc treated animals as compared to the POX treated controls is in contrast to previously published studies, which showed a protective effect of the topical application of TAAc and DEX [15, 16]. These differing findings can most likely be explained by the varying application protocols. While preoperative glucocorticoid-loading and direct injection of the drug into the cochlea prior to cochlear implantation may help to prepare the cochlea for the upcoming insult of electrode insertion, local application of a high dose glucocorticoid after electrode insertion might slow wound healing and could thereby increase the time needed for a complete sealing of the cochleostomy site. This could result in a prolonged leakage of perilymph and thereby explain the slower click-CAP recovery in TAAc treated animals we described. Even though the reduced tissue growth caused by the TAAc hydrogel might slow hearing-recovery in the short term, it could still be beneficial in the long-term, resulting in reduced impedances. To evaluate potential long term-benefits additional studies will be necessary.
Even though high glucocorticoid perilymph-levels have been described one day after the application of dexamethasone and triamcinolone-acetonide loaded poloxamer407-hydrogels [10, 11], changes in the permeability of the round window membrane after drilling of a cochleostomy and insertion of the electrode cannot be excluded. This could lead to lower glucocorticoid-concentrations in the inner ear and thereby reduce the otoprotective potential of these drugs.
We also observed a relatively high variability of postoperative threshold shifts in our study, which is most likely not dependent on the glucocorticoid-application. We believe so, because the drugs were delivered after the drilling of the cochleostomy and the insertion of the cochlear implant electrode. Therefore, a major influence of the glucocorticoid-loaded hydrogels is to be expected only on the delayed threshold shifts, which occur hours or days after the implantation.
It would be interesting to evaluate the effects of glucocorticoids in cochlear implant models, which mimic electric-acoustic stimulation patients more closely. An animal model using noise-exposure to create a high-frequency hearing loss prior to cochlear implantation was recently published and used to evaluate reductions in the cochlear microphonic as potential indicators for intracochlear damage during electrode insertion [17]. In this study only relatively small changes in the cochlear microphonic and minor histological alterations were observed after the insertion of a flexible electrode array. This model, maybe combined with a slightly more traumatic electrode, could also be used for the evaluation of glucocorticoids for otoprotective effects. The grinding technique used together with the preparation of 100 µm sections allowed for a more detailed evaluation of the foreign body reaction and cochlear trauma than the typically performed evaluation of mid-modiolar sections only [18]. Comparable to the data published by Burghard et al. [19], a pronounced foreign body reaction and osteoneogenesis were evident in the area of the cochleostomy. The amount of connective tissue formation in the scala tympani decreased from the cochleostomy site, so that in the mid-modiolar sections of most animals only a thin fibrous sheet covering the electrode was present. This is in contrast to the data published by O’Leary et al., who reported a considerably higher amount of connective tissue formation in these sections [18]. Surprisingly, we did not find a correlation between the electrode insertion trauma, which would have been clearly underestimated if only mid-modiolar cuts had been performed, and the postoperative hearing threshold shifts. Even though we still believe that an atraumatic electrode insertion is a major factor contributing to the preservation of residual hearing during cochlear implantation, our data suggests that there are other factors important for the postoperative hearing outcome. Potential candidates are the induction of apoptosis in the neurosensory system or inflammatory processes and the generation of reactive oxygen species [18].
The published release kinetics of DEX- and TAAc-loaded hydrogels [10, 11] in synopsis with the reported improved hearing preservation rates after preoperative glucocorticoid application [12] tempt speculations about potential benefits of the intratympanic injection of such hydrogels on the preoperative day or even at earlier time-points. A prolonged glucocorticoid-exposure using such gels could not only be easily translated into clinical practice, it might also help to decrease the pronounced perilymph-gradients after short term glucocorticoid application [20] and thereby increase the chance of hearing preservation in the clinically relevant low frequency regions.
In conclusion, even though changes in the foreign body reaction were observed, we were unable to protect residual hearing by the intraoperative application of glucocorticoid-loaded hydrogels, which might be explained with the findings of other publications, which described improved hearing preservation after prolonged preoperative exposure to the topically applied glucocorticoids. Even though the application of a depot formulation cannot compensate a delayed delivery, the use of glucocorticoid loaded poloxamer 407 hydrogels in the setting of hearing preserving cochlear implantation is still promising, because it could be used for a preoperative “glucocorticoid-loading” of the cochlea.
Figure 5.
Footnotes
Conflicts of Interest and Source of Funding:
Christoph Arnoldner is currently receiving a grant from the Austrian Science Fund (FWF; Project No. P 24260-B19) and funding from MED-EL Corporation, Innsbruck, Austria. Clemens Honeder and Elisabeth Engleder are financed by these grants. For the remaining authors none were declared.
References
- 1.Gstoettner W, Kiefer J, Baumgartner WD, Pok S, Peters S, Adunka O. Hearing preservation in cochlear implantation for electric acoustic stimulation. Acta Otolaryngol. 2004;124:348–52. doi: 10.1080/00016480410016432. [DOI] [PubMed] [Google Scholar]
- 2.Usami S, Moteki H, Tsukada K, Miyagawa M, Nishio SY, Takumi Y, et al. Hearing preservation and clinical outcome of 32 consecutive electric acoustic stimulation (EAS) surgeries. Acta Otolaryngol. 2014;134:717–27. doi: 10.3109/00016489.2014.894254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helbig S, Settevendemie C, Mack M, Baumann U, Helbig M, Stover T. Evaluation of an electrode prototype for atraumatic cochlear implantation in hearing preservation candidates: preliminary results from a temporal bone study. Otol Neurotol. 2011;32:419–23. doi: 10.1097/MAO.0b013e31820e75d9. [DOI] [PubMed] [Google Scholar]
- 4.Gstoettner WK, Helbig S, Maier N, Kiefer J, Radeloff A, Adunka OF. Ipsilateral electric acoustic stimulation of the auditory system: results of long-term hearing preservation. Audiol Neurootol. 2006;11(Suppl 1):49–56. doi: 10.1159/000095614. [DOI] [PubMed] [Google Scholar]
- 5.Hoang KN, Dinh CT, Bas E, Chen S, Eshraghi AA, Van De Water TR. Dexamethasone treatment of naive organ of Corti explants alters the expression pattern of apoptosis-related genes. Brain Res. 2009;1301:1–8. doi: 10.1016/j.brainres.2009.08.097. [DOI] [PubMed] [Google Scholar]
- 6.Cope D, Bova R. Steroids in otolaryngology. Laryngoscope. 2008;118:1556–60. doi: 10.1097/MLG.0b013e31817c0b4d. [DOI] [PubMed] [Google Scholar]
- 7.Kiefer J, Gstoettner W, Baumgartner W, Pok SM, Tillein J, Ye Q, et al. Conservation of low-frequency hearing in cochlear implantation. Acta Otolaryngol. 2004;124:272–80. doi: 10.1080/00016480310000755a. [DOI] [PubMed] [Google Scholar]
- 8.Bird PA, Murray DP, Zhang M, Begg EJ. Intratympanic versus intravenous delivery of dexamethasone and dexamethasone sodium phosphate to cochlear perilymph. Otol Neurotol. 2011;32:933–6. doi: 10.1097/MAO.0b013e3182255933. [DOI] [PubMed] [Google Scholar]
- 9.Parnes LS, Sun AH, Freeman DJ. Corticosteroid pharmacokinetics in the inner ear fluids: an animal study followed by clinical application. Laryngoscope. 1999;109:1–17. doi: 10.1097/00005537-199907001-00001. [DOI] [PubMed] [Google Scholar]
- 10.Piu F, Wang X, Fernandez R, Dellamary L, Harrop A, Ye Q, et al. OTO-104: a sustained-release dexamethasone hydrogel for the treatment of otic disorders. Otol Neurotol. 2011;32:171–9. doi: 10.1097/MAO.0b013e3182009d29. [DOI] [PubMed] [Google Scholar]
- 11.Honeder C, Engleder E, Schöpper H, Gabor F, Reznicek G, Wagenblast J, et al. Sustained Release of Triamcinolone Acetonide from an Intratympanically Applied Hydrogel Designed for the Delivery of High Glucocorticoid Doses. Audiol Neurootol. 2014;19:193–202. doi: 10.1159/000358165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang A, Eastwood H, Sly D, James D, Richardson R, O’Leary S. Factors influencing the efficacy of round window dexamethasone protection of residual hearing post-cochlear implant surgery. Hear Res. 2009;255:67–72. doi: 10.1016/j.heares.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Engleder E, Honeder C, Klobasa J, Wirth M, Arnoldner C, Gabor F. Preclinical evaluation of thermoreversible triamcinolone acetonide hydrogels for drug delivery to the inner ear. Int J Pharm. 2014;471:297–302. doi: 10.1016/j.ijpharm.2014.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plenk H., Jr . The microscopic evaluation of hard tissue implants. In: Williams DF, editor. Techniques of biocompatibility Testing. I CRC Press; 1986. [Google Scholar]
- 15.Braun S, Ye Q, Radeloff A, Kiefer J, Gstoettner W, Tillein J. Protection of inner ear function after cochlear implantation: compound action potential measurements after local application of glucocorticoids in the guinea pig cochlea. ORL J Otorhinolaryngol Relat Spec. 2011;73:219–28. doi: 10.1159/000329791. [DOI] [PubMed] [Google Scholar]
- 16.James DP, Eastwood H, Richardson RT, O’Leary SJ. Effects of round window dexamethasone on residual hearing in a Guinea pig model of cochlear implantation. Audiol Neurootol. 2008;13:86–96. doi: 10.1159/000111780. [DOI] [PubMed] [Google Scholar]
- 17.Choudhury B, Adunka OF, Awan O, Pike JM, Buchman CA, Fitzpatrick DC. Electrophysiologic consequences of flexible electrode insertions in gerbils with noise-induced hearing loss. Otol Neurotol. 2014;35:519–25. doi: 10.1097/MAO.0b013e31829bdf2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Leary SJ, Monksfield P, Kel G, Connolly T, Souter MA, Chang A, et al. Relations between cochlear histopathology and hearing loss in experimental cochlear implantation. Hear Res. 2013;298:27–35. doi: 10.1016/j.heares.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Burghard A, Lenarz T, Kral A, Paasche G. Insertion site and sealing technique affect residual hearing and tissue formation after cochlear implantation. Hear Res. 2014;312:21–7. doi: 10.1016/j.heares.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Plontke SK, Biegner T, Kammerer B, Delabar U, Salt AN. Dexamethasone concentration gradients along scala tympani after application to the round window membrane. Otol Neurotol. 2008;29:401–6. doi: 10.1097/MAO.0b013e318161aaae. [DOI] [PMC free article] [PubMed] [Google Scholar]