Abstract
Lesion and neuroimaging studies indicate that the insula mediates motor aspects of speech production, specifically, articulatory control. Although it has direct connections to Broca’s area, the canonical speech production region, the insula is also broadly connected with other speech and language centres, and may play a role in coordinating higher-order cognitive aspects of speech and language production. The extent of the insula’s involvement in speech and language processing was assessed using the Activation Likelihood Estimate (ALE) method. Meta-analyses of 42 fMRI studies with healthy adults were performed, comparing insula activation during performance of language (expressive and receptive) and speech (production and perception) tasks. Both tasks activated bilateral anterior insulae. However, speech perception tasks preferentially activated the left dorsal mid-insula, whereas expressive language tasks activated left ventral mid-insula. Results suggest distinct regions of the mid-insula play different roles in speech and language processing.
Keywords: activation likelihood estimate (ALE), articulation, speech production, expressive language, receptive language, functional magnetic resonance imaging (fMRI), insula
1. Introduction
Speech and language production is a complex cognitive, motor and sensory process mediated by a host of interconnected cortical and subcortical regions including the left inferior frontal gyrus (IFG; BA 44/45), primary motor cortex (BA 4), premotor cortex (BA 6), superior temporal gyrus (BA22) and basal ganglia (Guenther, 2006). The classic neuropsychological models point to the left inferior frontal gyrus, or Broca’s area, as the hub of speech and language production (Broca, 1861; Geschwind-Wernicke, 1979). However, based on lesion studies with patients with articulation difficulties, the insula has also been put forth as a brain region involved in the motor control of speech production (Cereda et al. 2002; Dronkers 1996; Duffau et al. 2001 ; Starkstein et al. 1988).
The insula shares reciprocal functional and structural connections with linguistic, motor, limbic and sensory brain areas (Augustine, 1996). Based on these findings, there has been speculation that the insula is involved in certain aspects of speech and language (Augustine, 1985). Additionally, the insula is involved in other perceptual-motor functions such as feeding behaviours, interoception (i.e., internal monitoring of bodily states), and also autonomic processes (e.g., respiratory control). The coordination and control of these functions are essential for the smooth, coordinated production of speech (Craig 2002, 2003).
The role of the insula in speech production arose from studies in patients who suffered from an apraxia of speech, usually as a consequence of stroke (Dronkers, 1996; Nagao et al., 1999; Ogar et al., 2006). Patients with articulatory dysfunction in the absence of a muscular problem (i.e., dysarthria), had lesions in the left superior tip of the precentral gyrus of the insula (Dronkers, 1996). Other studies with patients with progressive apraxia (Nestor el al., 2003), reduced fluency (Bates et al., 2003; Borovsky et al., 2007), or impairments with rapidly changing articulatory movements (Baldo et al., 2011) were also reported to have left anterior insular damage. Neuroimaging studies with healthy studies also suggested that the left precentral gyrus of the insula is involved in articulatory coordination (Murphy et al., 1997). Results indicate that the region may be preferentially involved in speech motor control, and may not be essential for language processing.
Contrary to these data is evidence from experimental neuroimaging studies with healthy adults indicating that the insula may be a core region for both speech and language processing. For example, Eickhoff and colleagues (Eickhoff, Heim, et al., 2009) performed meta-analyses of fMRI and PET studies of expressive language tasks and demonstrated a core network of activation in BA 44, anterior insula, caudate nucleus, cerebellum, pre- and primary motor cortices. In this same paper, the authors examined the effective connectivity between these areas during speech production and concluded that the insular cortex may function as a relay between the cognitive aspects of language analyzed in BA 44 and the motor preparation for vocalization in basal ganglia and cerebellum. This finding was later supported by activation likelihood estimate (ALE) meta-analyses of neuroimaging studies examining normal speech production and the comprehension of distorted speech (Adank, 2012). Left anterior insula was associated with speech production; however, bilateral anterior insulae activations were associated with distorted speech comprehension suggesting increased insular recruitment during difficult speech-language processing.
Based on these previous findings and the known anatomical connectivity of the insula, it is possible that the insula also mediates higher-order cognitive functions involved in speech and language processing. However, no single study has assessed whether the insula is primarily involved in speech motor control, or if it plays a supportive role in both speech and language processing. If the insula functions as a hub, connecting speech and language areas, it should be activated by both types of tasks; whereas, if the insula is solely involved in speech articulation, it should also be seen in cases of overt language production, but not with other types of language processing where articulation is controlled.
We tested this hypothesis using a meta-analysis of fMRI studies that reported insular activation during language (expressive and receptive) and speech (production and perception) tasks. We distinguished language and speech based on whole-word content. If whole words, or sequences of whole words were involved, this was classified in the ‘language’ category; however, if parts of words (e.g., syllables, syllable sequences or phonemes) were involved, this was classified in the ‘speech’ category. We implemented the ALE method (Eickhoff, Laird et al., 2009; Turkeltaub et al., 2002) to generate three-dimensional probability maps that described the spatial extent and localization of activation in the insula associated with language and speech processing.
2. Methods
2.1. Article Selection and Literature Search
Studies reporting language or speech-evoked activation in the insula were obtained from an initial broad search of the literature using the Web of Science (http://www.isiknowledge.com) from January 1990 to September 2012. To identify all potential studies reporting insula activation in language and speech processing as measured by fMRI, the keywords used were: functional magnetic resonance imaging, insula, language and speech. A total of 59 studies were found.
Articles were screened for specific inclusion and exclusion criteria to be analyzed using the ALE method. Inclusion criteria consisted of studies that: (1) were written in English; (2) were performed as fMRI experiments; (3) reported stereotactic coordinates; (4) used healthy adult human participants; (5) used high-quality stimuli (i.e., non-degraded); and, (6) applied a general linear model for analysis. Exclusion criteria were: (1) data from patients, case studies, or special populations, (2) functional connectivity (not localization) analyses, (3) pharmacological interventions, (4) deactivation coordinates, and (5) review articles or meta-analyses. As a result, 17 articles were excluded (tested clinical populations: 7; used reading paradigms: 3; assessed emotional tones of word stimuli: 2; assessed pitch processing: 2; completed laterality analysis: 1; presented speeded speech stimuli: 1; assessed working memory 1).
A total of 42 studies met our criteria and were included in the meta-analysis. For each article, the following information was recorded: author names, academic journal title, year of publication, number of participants, mean age of participants, gender of participants, handedness of participants, strength of MRI scanner in Tesla (T), task description, stimulus type, language of stimulation, and contrast(s) of interest.
The studies were further categorized into language and speech tasks. Studies that involved covert or overt linguistic processing (e.g., whole words, syntax, word morphology, pragmatics) were categorized as language studies; this resulted in a total of 28 articles (Table 1). These studies were divided into ‘receptive’ and ‘expressive’ language categories. The ‘receptive’ category included studies of language reception and comprehension, such as listening to words and making a semantic judgment. The ‘expressive’ category included studies of covert and overt language production. For example, this category would include studies asking participants to produce as many words possible beginning with a visually presented letter.
Table 1.
Summary of included studies.
| Study | Year | Imaging | N | Task | Language | Stimulus Type | Modality |
|---|---|---|---|---|---|---|---|
| Receptive Language | |||||||
| Anderson et al. | 2010 | fMRI 3.0 | 15 | auditory-phase recognition | English | phrase & sentence | sound |
| Butchweitz et al. | 2012 | fMRI 3.0 | 12 | dual-tasking | English | sentence | sound |
| Carreiras et al. | 2006 | fMRI 1.5 | 16 | lexical decision | Spanish | word | visual |
| Fiebach et al. | 2003 | fMRI 1.5 | 12 | lexical decision | German | noun & pseudo word | visual & sound |
| Gandour et al. | 2007 | fMRI 1.5 | 10 | sentence comprehension | Chinese English | sentence | sound |
| Hernandez et al. | 2004 | fMRI 3.0 | 9 | grammatical gender decision | Spanish | noun | visual |
| Hesling et al. | 2005 | fMRI 3.0 | 12 | passive listening | English | sentence | sound |
| Isel et al. | 2010 | fMRI 3.0 | 20 | semantic categorization | French German | noun | visual |
| Mason & Just | 2007 | fMRI 1.5 | 12 | lexical ambiguity | English | sentence | visual |
| Noesselt et al. | 2003 | fMRI 1.5 | 10 | passive listening semantic categorization |
German | noun | sound |
| Sass et al. | 2009 | fMRI 1.5 | 15 | lexical decision | English | word | visual & sound |
| Schmidt & Seger | 2009 | fMRI 3.0 | 10 | metaphor comprehension | English | literal sentence metaphorical sentence non-word sentence |
visual |
| Thompson et al. | 2010 | fMRI 3.0 | 7 | sentence comprehension | English | sentence & picture | sound visual |
| Zempleni et al. | 2006 | fMRI 3.0 | 17 | relatedness judgement task | Dutch | figurative sentence ambiguous sentence |
visual |
| Expressive Language | |||||||
| Abel et al. | 2012 | fMRI 3.0 | 19 | picture-word interference | German | drawing | visual |
| Chee et al. | 2004 | fMRI 3.0 | 30 | phonological working memory | French | word & pseudo word | sound |
| Christoffels et al. | 2007 | fMRI 3.0 | 14 | overt picture naming | Dutch | white-on-black drawing | visual |
| Gauthier et al. | 2009 | fMRI 1.5 | 44 | covert verbal fluency | French | letter | sound |
| Grande et al. | 2012 | fMRI 3.0 | 18 | picture description | German | line drawing | visual |
| Haller et al. | 2005 | fMRI 1.5 | 15 | sentence generation | German | three word | visual |
| Heim et al. | 2002 | fMRI 3.0 | 12 | picture naming | German | line drawing | visual |
| Loevenbruck et al. | 2005 | fMRI 3.0 | 16 | speech production | French | sentence | visual |
| Lurito et al. | 2000 | fMRI 1.5 | 5 | covert word generation | English | letter | visual |
| Mechelli et al. | 2007 | fMRI 3.0 | 20 | reading & picture naming | English | word pair | visual |
| Richter et al. | 2008 | fMRI 1.5 | 8 | word stem completion | German | word stem | visual |
| Riecker et al. | 2000 | fMRI 1.5 | 18 | overt speech | German | symbol | visual |
| Shuster et al. | 2005 | fMRI 1.5 | 10 | overt word production | English | noun | sound |
| Voets et al. | 2006 | fMRI 3.0 | 12 | covert phonemic fluency covert semantic fluency |
English | letter category name |
visual |
| Speech Perception | |||||||
| Aleman et al. | 2005 | fMRI 1.5 | 6 | metrical stress evaluation | Dutch | bisyllabic word | sound |
| Benson et al. | 2001 | fMRI 1.5 | 12 | passive listening | English | nonsense syllable (V, CV, CVC) | sound |
| Falkenberg et al. | 2011 | fMRI 3.0 | 40 | dichotic listening | English | CV syllable | sound |
| Golestani & Zatorre | 2004 | fMRI 1.5 | 10 | phonetic contrast identification | English | CV syllable | sound |
| Marvel & Desmond | 2012 | fMRI 3.0 | 16 | verbal working memory | English | letter | visual |
| Szenkovits et al. | 2012 | fMRI 3.0 | 21 | active listening | English | CVC pseudo word | sound |
| Speech Production | |||||||
| Bohland & Guenther | 2006 | fMRI 3.0 | 13 | CV syllable production | English | three syllable sequence | visual |
| Brendel et al. | 2010 | fMRI 1.5 | 18 | syllable (/ta/) production | German | acoustic click | sound |
| Dogil et al. | 2002 | fMRI 1.5 | 18 | syllable production | German | syllable | visual |
| Golestani & Pallier | 2007 | fMRI 3.0 | 21 | phoneme production | French Persian | CV syllable bisyllabic non-word |
sound |
| Moser et al. | 2009 | fMRI 3.0 | 30 | speech repetition | English | multisyllabic non-word | sound |
| Riecker et al. | 2008 | fMRI 3.0 | 9 | syllable production | German | bisyllabic pseudo word | visual |
| Riecker et al. | 2006 | fMRI 1.5 | 8 | syllable (/pa/) production | English | click series | sound |
| Soros et al. | 2006 | fMRI 3.0 | 9 | syllable (/pataka/) production | English | verbal cue | sound |
Fourteen studies used phonetic, syllabic, or non-word stimuli and were categorized as speech studies (Table 1). The studies were then divided into speech ‘perception’ and ‘production’ categories. The ‘perception’ category included studies that presented simple and complex speech stimuli. For example, this would include studies asking participants to passively listen to nonsense syllables, or studies requiring subjects to classify phonemes or speech sounds. The ‘production’ category included articles where subjects produced a speech sound or sounds. This would include studies involving the pronunciation of syllable sequences; however, full words were not produced.
2.2. Meta-analytic method
Activation foci reported for each study were entered into four separate meta-analyses (receptive language, expressive language, speech perception, and speech production). To account for spatial disparities between MNI and Talairach brain templates used across studies, coordinates reported in MNI space were converted to Talairach space using the Lancaster transform, icbm2tal, within the GingerALE software package (Laird et al., 2007; Lancaster et al., 2007; Talairach & Tournoux, 1988). All further analyses were conducted in Talairach space, and figures are presented in TAL space.
The ALE method (Eickhoff, Laird, et al., 2009; Laird, et al., 2005; Turkeltaub et al., 2002) was used to create probabilistic maps that describe the spatial location and extent of activation in response to each of the four categories of interest. Reported foci were treated as the centre of probability distributions within a standard image space (GingerALE v 2.1.1 - http://brainmap.org/ale/). Data were smoothed using a Gaussian blurring kernel. The size of each kernel’s full-width at half maximum (FWHM) was calculated based on the number of participants in each individual study. For each voxel in the brain, the probability of activation for all reported foci was computed, resulting in an ALE value. A permutation test was then utilized to distinguish real convergence across studies from random convergence or “noise”. This process compared each study’s ALE values to a null-distribution of randomly generated ALE values. The creation of the ALE null-distribution values involved the sampling of a random voxel from each study 2 ×1010 times. Data were corrected for multiple comparisons using the false discovery rate (FDR) with q=0.001 (Laird, et al., 2005). The resultant ALE maps contain voxels that have a significant probability of activation. To view these results, the ALE maps were overlaid on a template MRI (Colin1 in Talairach space) using the program mricron (http://www.mccauslandcenter.sc.edu/mricro/mricron/). Anatomical areas within the insular cortex were categorized according to Craig (2009).
3. Results
3.1. Demographic Information
The 44 studies of speech and language tasks included 639 participants (58.5% male). The median age across studies was computed from the reported mean age in each study, and was 28.5 yrs (SD=3.2). The majority (88.9%) of the studies assessed handedness. Most studies tested right-handed individuals with only a few including left-handed or ambidextrous individuals. Details of the studies included in the meta-analyses are listed in Table 1.
3.2 Receptive language stimuli
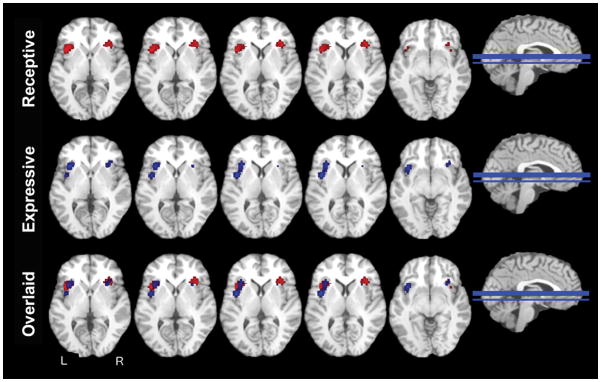
A total of 64 foci were extracted from 14 studies reporting activation in the insula associated with receptive language tasks. Activations occurred in the accessory gyrus of the anterior insula, bilaterally. A portion of the left-sided cluster was observed in the region of the dorsal dysgranular zone of the anterior short insular gyrus as seen in the bottom left corner of Figure 1 (Table 2).
Figure 1.
ALE meta-analyses across language studies. Multi-slice ALE maps for receptive language stimuli displayed in red (top row), expressive language stimuli displayed in blue (middle row), and overlaid language stimuli (bottom row). ALE maps are overlaid on axial slices from a template in TAL space. These figures show that for receptive language, activations occurred in the accessory gyrus of the anterior insula bilaterally. For expressive language, activations were most likely to activate the left accessory gyrus in the territory of the dorsal dysgranular zone.
Table 2.
Results from meta-analyses for all speech and language stimuli. Activation foci obtained from insula responses to language and speech stimuli. Activation likelihood estimate (ALE) values range from a minimum of 0 to a maximum of 1. Greater likelihood of activation in response to stimuli is indicated by higher ALE values. Significant clusters of voxels are listed in order from largest to smallest. Coordinates are in Talairach space (Talairach and Tournoux, 1988).
| Stimuli | Side | Insula Region | Cluster # | x | y | z | Volume (mm3) | ALE Value | P value |
|---|---|---|---|---|---|---|---|---|---|
| Receptive Language | Left | anterior | 1 | −36 | 16 | 5 | 4056 | 0.02 | <0.000001 |
| Right | anterior | 2 | 33 | 21 | 3 | 2168 | 0.02 | <0.000001 | |
| Right | mid | 3 | 38 | −3 | 16 | 408 | 0.01 | <0.000001 | |
| Left | anterior | 4 | −38 | −13 | 11 | 320 | 0.01 | <0.000001 | |
| Expressive Language | Left | anterior | 1 | −35 | 11 | 3 | 3896 | 0.02 | <0.000001 |
| Right | anterior | 2 | 31 | 20 | −1 | 936 | 0.02 | <0.000001 | |
| Speech Perception | Left | mid | 1 | −35 | 7 | 10 | 1896 | 0.03 | <0.000001 |
| Right | anterior | 2 | 38 | 9 | 12 | 184 | 0.02 | <0.000001 | |
| Right | anterior | 3 | 48 | 27 | 21 | 48 | 0.01 | <0.000001 | |
| Speech Production | Left | anterior | 1 | −35 | 14 | 5 | 3176 | 0.06 | <0.000001 |
| Right | anterior | 2 | 35 | 20 | 8 | 440 | 0.03 | <0.000001 | |
| Left | anterior | 3 | −36 | 6 | 10 | 8 | 0.02 | <0.000001 |
3.3. Expressive language stimuli
Expressive language functions were associated with 41 foci from 14 studies. These stimuli were most likely to activate the left accessory gyrus in the territory of the dorsal dysgranular zone. Activation was observed in the region of the left anterior short insular gyrus that extended into the ventral mid-insula (Figure 1; Table 2). A small non-significant peak was located in the right anterior insula.
3.4. Speech perception stimuli
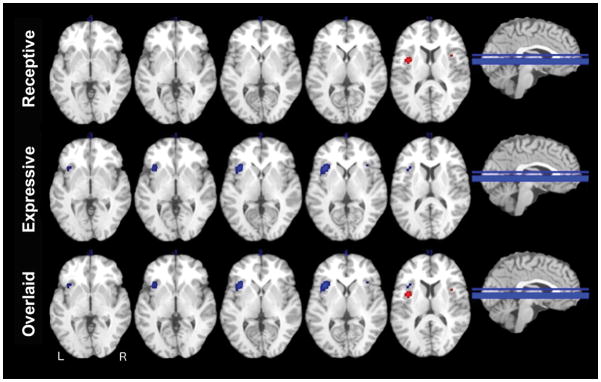
Speech perception stimuli were associated with 18 foci from 6 studies. In response to these stimuli, left-sided activations occurred in the region of the granular zone of the dorsal middle short insular gyrus. Right-sided activations were more likely to occur in a slightly more anterior region, that is, in the ventral granular zone of the anterior short insular gyrus (Figure 2; Table 2).
Figure 2.
ALE meta-analyses across speech studies. Multi-slice ALE maps for speech perception stimuli displayed in red (top row), speech production stimuli displayed in blue (middle row), and overlaid speech stimuli (bottom row). ALE maps are overlaid on axial slices from a template in TAL space. These figures show that for speech perception, activations occurred in the granular zone of the left dorsal middle short insular gyrus. For speech production, activations were predominately in the left accessory gyrus and left anterior short insular gyrus.
3.5 Speech production stimuli
The ALE maps for speech production were created from 38 foci from 8 studies. Activations were localized predominately on the left side, in the accessory gyrus and anterior short insular gyrus. A small right-sided cluster of activation was observed in the region of the dorsal dysgranular accessory gyrus (Figure 2; Table 2).
4. Discussion
Using an ALE-based meta-analysis of existing functional neuroimaging data, we provide information on the spatial location and extent of activation in the insula during various speech and language tasks. Our results are striking in that it is evident that receptive language, expressive language and speech production activate very similar regions of the anterior insula while activation in response to the perception of speech was processed separately in the left dorsal mid insula. As well, expressive language further showed activations in the left ventral mid-insula. Moreover, all tasks activated bilateral regions in the insula; the language tasks were more equally bilaterally represented while the speech tasks tended to show more left-hemisphere dominance.
4.1. Receptive and Expressive Language
For the language tasks, activations were similar for both receptive and expressive language stimuli. These tasks were most likely to activate bilateral anterior regions of the insula, specifically, the accessory insular gyrus; and in the left hemisphere, the spatial extent of the likelihood of activation spread to the anterior short insular gyrus. The only distinction between the receptive and expressive language tasks was a discrete ALE peak of activation in left mid-insula for the expressive language task.
Activation of the left anterior insula with these language tasks was not surprising as this area is known to have a direct anatomical connection to inferior frontal and lateral frontal regions (Kalab et al., 2012). Further, the left anterior insula has extensive and reciprocal functional connections with frontal areas involved in language processing, such as the prefrontal cortex and frontal operculum (Augustine, 1996). Specifically, IFG activation has been associated with semantic retrieval, lexical decisions, and the generation of semantic categories. Finally, neuroimaging studies have implicated the IFG in syntactic processing (e.g., Friederici et al., 2006; Kaan and Swaab, 2002; Roder et al., 2002). These classic areas are known to be involved in language processing; and, the IFG has been long associated with the semantic, phonological and syntactic aspects of language (e.g., Bedny et al., 2008; Kemeny et al., 2005; Rodd et al., 2010). Studies reported that the frontal operculum is involved in sentence comprehension (Friederici et al., 2003; Friederici et al., 2006) and syntactic speech (Friederici et al., 2000). This activation was observed predominantly in the left hemisphere and emphasizes that the left frontal cortex is associated with the processing of syntactic information.
Of note, while the classic Broca-Wernicke model of language separates expressive and receptive language into frontal and temporal foci, respectively, there is now substantial empirical evidence that the language system is much more complex. In a meta-analysis of 485 neuroimaging studies, Liakasis, Nicket, and Seitz (2011) showed that the IFG (canonical Broca’s area) is involved in both the perceptive and productive aspects of language. In fact, studies have found that bilateral insular damage may produce total auditory agnosia (Bamiou et al., 2003). A functional imaging study showed that the insula was involved in several key processes such as auditory attention allocation, auditory tuning to novel stimuli, temporal processing, phonological processing, and auditory-visual integration (Bamiou et al., 2003). Thus, it is possible that the anterior insulae serves as an integration hub for auditory stimuli and plays a role in auditory processing as well (Bamiou et al., 2003).
Another interesting finding is that the right anterior insula was activated by both language tasks. While language is considered to be left-hemisphere dominant, substantial evidence indicates that the right hemisphere is also important for language processing. Therefore, activity in the right anterior insula could be heightened during language tasks due to the existence of functional connections with frontal right-hemisphere regions that support language functioning. These were the conclusions of a PET study, which found bilateral activation in anterior insulae elicited by sentence stimuli (Wong et al., 2002). Right hemisphere frontal areas offer resources to maintain language function and this points to the high likelihood of a pre-existing language network in the right hemisphere that can be recruited when needed (Perani et al, 2003; Voets et al., 2006; Winhuisen et al., 2005). For example, a number of reports indicate that language dominance shifts to homologous right-hemisphere regions following a disabling left hemisphere brain injury (Lazar et al., 2000; Staudt et al., 2002; Tivarus et al., 2012). However, the role of the right insula in language processing requires further study to determine its specificity of function as the activation could be related to other cognitive processes subserving irrelevant task features.
Although syntactic and semantic processes are supported primarily by the left-hemisphere, the processing of prosody is primarily a function of the right-hemisphere (Gandour et al., 2004; Pell et al., 2006; Friederici et al., 2002). More specifically, frontal brain regions in the right hemisphere play a role in the comprehension and production of affective prosody. George et al. (1996) used PET to examine the brain regions involved in understanding emotional prosody versus content-based emotion. An increase in activity in the right prefrontal cortex was observed during the detection of emotional prosody of verbal sentences. On the other hand, left prefrontal activation was observed when participants were asked to concentrate on the content of sentences instead of the prosody. More recently, an fMRI study investigating neural responses to sentences varying in pitch information (normal intonation versus synthesized flattened speech) and syntactic information (normal speech versus synthesized, de-lexicalized speech) found right fronto-lateral activation in response to prosodic cues. The right-superior temporal region and fronto-opercular cortex were identified as being more involved in processing prosodic information (Meyer et al., 2003). These findings suggest that the right hemisphere frontal lobe support prosodic comprehension. Finally, in an experiment by Pell (1999), subjects with right hemisphere brain damage were found to employ fewer prosodic cues than healthy controls in a sentence elicitation task, resulting in a reduction of emotional inflection as perceived by the listeners. While we did not specifically assess prosody within the current study, potentially some tasks included in the meta-analyses used prosodic stimuli that evoked activity in the right hemisphere. The results of our meta-analysis provide support for the hypothesis that right-hemisphere areas, in particular the anterior insula, mediate the production and perception of prosodic elements of language. Future work in this area should examine activation in the insula in response to tasks with and without prosodic linguistic stimuli to clarify its contribution to these processes.
The left ventral mid-insula showed preferential activation in response to language production tasks. The role of the ventral mid-insula in language processing remains unclear although this region looks similar to the insular region implicated in patients with apraxia of speech (Dronkers, 1996; Nestor et al., 2003). These regions need to be directly compared for confirmation. The right mid-insula has been implicated in viscerosensory processing, that is, processing regarding the state of one’s own body (Menon and Uddin, 2010), particularly linking representations of the outside world with the body’s internal state (Farb et al., in press). Therefore the left mid-insula may serve the function of monitoring the body’s internal state, but focuses particularly on language impact and relations. This is purely speculative, however, and requires empirical testing.
4.2. Speech perception and production
For the speech tasks, our meta-analysis revealed some distinct differences. While both speech perception and production involved strong left-hemisphere anterior insula activation, the ALE clusters were in separate anatomical locations. Speech production activated bilateral accessory gyri of the insula and the left anterior short gyrus of the insula. Speech perception activated only the short gyrus of the insula, in the superior middle portion in the left hemisphere, and the anterior portion in the right hemisphere. Although both speech perception and production activated both left and right insula, speech production was more left dominant, and speech perception is more equally bilateral.
The activation in the anterior short insula in response to both speech and language tasks provides important information on the function of this region in linguistic processing. While previous evidence from clinical and neuroimaging studies would support the role of the anterior insula in solely motor speech production (see Ackermann and Riecker, 2010, for a review) our findings would indicate that this region serves a broader function beyond motor speech control, and is in fact a crucial region that mediates language function.
Finally, our results showed bilateral anterior insula involvement in speech perception, in locations distinct from those that have been involved in the language and speech production tasks. This finding was expected, as the perception of speech relies on slightly different cognitive and sensory processes than those implicated in language perception. However, in future studies, meta-analytic methods could be used to compare speech perception with tone and melody perception to ascertain whether they share common brain regions.
Activation in the insula in response to speech perception is likely due to this region’s extensive connections with the auditory cortex, temporal pole, and superior temporal sulcus (Augustine, 1996). A number of neuroimaging studies have indicated the involvement of the insula in auditory processing (for a review, see Bamiou et al., 2003). A more recent clinical report has suggested that the insula may be involved in temporal processing, as insular damage was correlated with temporal resolution and sequencing impairments (Bamiou et al., 2006). Neuroimaging studies in control populations, using different stimuli and paradigms showed that increased task difficulty on a temporal discrimination task (Tregellas et al., 2006) and increased frequency of click trains in a passive-listening task (Ackermann et al., 2001; Steinbrick, 2009) were associated with robust activation in bilateral anterior insula. Potentially, our finding of bilateral anterior insula activation in response to speech stimuli raises the possibility of insular recruitment in the encoding and processing of temporal information within the auditory system.
4.3 Summary and future research
Meta-analysis is a robust tool that permits the determination of contiguous brain regions showing reliable activation in response to particular tasks or cognitive functions. This technique summarizes findings across studies and can reduce the impact of confounds inherent in single fMRI studies such as image artifacts, head motion, few subjects, low signal-to-noise ratio, type I and II errors, and contamination of unassociated task features (Eickhoff et al., 2012, Price et al., 2005, Raemaekers et al., 2007). Additionally it aids in overcoming assumptions that experimental and control tasks solely represent particular cognitive functions {Price, 2005}.
In the current studies, we performed meta-analysis of neuroimaging data from a variety of speech and language experimental paradigms and did not take into account differences in tasks or stimuli.. The resulting probability maps are indicative of reliable activation in the insula in response to different experimental paradigms and provide information on the spatial extent and localization of general speech and language processes.
Our finding of common left-sided activation in the anterior insula in response to speech production and language tasks may point to this region as being a functional hub for canonical and non-canonical language areas. Furthermore, our finding of bilateral activation, even with explicit language stimuli, points to the right hemisphere as an important mediator of speech and language function. Future research should examine the functional and structural organization of the insula relative to the entire speech and language processing connectome to better understand outcomes seen after damage to this region as a result of neurological injury or disease.
Acknowledgments
This work was partially funded by a CIHR Operating Grant (MOP-89961) to the last author. The authors would like to thank Alexandra Trelle and Jessica Chan for their help with the literature review and data entry.
References
- Abel S, Dressel K, Weiller C, Huber W. Enhancement and suppression in a lexical interference fMRI-paradigm. Brain Behav. 2012;2(2):109–127. doi: 10.1002/brb3.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann H, Riecker A. The contribution(s) of the insula to speech production: a review of the clinical and functional imaging literature. Brain Struct Funct. 2010;214(5–6):419–433. doi: 10.1007/s00429-010-0257-x. [DOI] [PubMed] [Google Scholar]
- Ackermann H, Riecker A, Mathiak K, Erb M, Grodd W, Wildgruber D. Rate-dependent activation of a prefrontal-insular-cerebellar network during passive listening to trains of click stimuli: an fMRI study. Neuroreport. 2001;12(18):4087–4092. doi: 10.1097/00001756-200112210-00045. [DOI] [PubMed] [Google Scholar]
- Adank P. The neural bases of difficult speech comprehension and speech production: Two Activation Likelihood Estimation (ALE) meta-analyses. Neuroimage. 2012;122:42–54. doi: 10.1016/j.bandl.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Aleman A, Formisano E, Koppenhagen H, Hagoort P, de Haan EH, Kahn RS. The functional neuroanatomy of metrical stress evaluation of perceived and imagined spoken words. Cereb Cortex. 2005;15(2):221–228. doi: 10.1093/cercor/bhh124. [DOI] [PubMed] [Google Scholar]
- Anderson JS, Lange N, Froehlich A, DuBray MB, Druzgal TJ, Froimowitz MP, et al. Decreased left posterior insular activity during auditory language in autism. AJNR Am J Neuroradiol. 2010;31(1):131–139. doi: 10.3174/ajnr.A1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine JR. The insular lobe in primates including humans. Neurol Res. 1985;7(1):2–10. doi: 10.1080/01616412.1985.11739692. [DOI] [PubMed] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22(3):229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Bamiou DE, Musiek FE, Luxon LM. The insula (Island of Reil) and its role in auditory processing. Literature review. Brain Res Brain Res Rev. 2003;42(2):143–154. doi: 10.1016/s0165-0173(03)00172-3. [DOI] [PubMed] [Google Scholar]
- Bamiou DE, Musiek FE, Stow I, Stevens J, Cipolotti L, Brown MM, et al. Auditory temporal processing deficits in patients with insular stroke. Neurology. 2006;67(4):614–619. doi: 10.1212/01.wnl.0000230197.40410.db. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Wilkins DP, Ogar J, Willock S, Dronkers NF. Role of the precentral gyrus of the insula in complex articulation. Cortex. 2011;47:800–7. doi: 10.1016/j.cortex.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson S, Saygin AP, Dick F, Sereno M, Knight RT, Dronkers NF. Voxel-based lesion-symptom mapping. Nature Neuroscience. 2003;6(5):448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Bedny M, Caramazza A, Grossman E, Pascual-Leone A, Saxe R. Concepts are more than percepts: the case of action verbs. J Neurosci. 2008;28(44):11347–11353. doi: 10.1523/JNEUROSCI.3039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson RR, Whalen DH, Richardson M, Swainson B, Clark VP, Lai S, et al. Parametrically dissociating speech and nonspeech perception in the brain using fMRI. Brain Lang. 2001;78(3):364–396. doi: 10.1006/brln.2001.2484. [DOI] [PubMed] [Google Scholar]
- Bohland JW, Guenther FH. An fMRI investigation of syllable sequence production. Neuroimage. 2006;32(2):821–841. doi: 10.1016/j.neuroimage.2006.04.173. [DOI] [PubMed] [Google Scholar]
- Borovsky A, Saygin AP, Bates E, Dronkers N. Lesion correlates of conversational speech production deficits. Neuropsychologica. 2007;(45):2525–2533. doi: 10.1016/j.neuropsychologia.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendel B, Hertrich I, Erb M, Lindner A, Riecker A, Grodd W, Ackermann H. The contribution of mesiofrontal cortex to the preparation and execution of repetitive syllable productions: an fMRI study. Neuroimage. 2010;50:1219–1230. doi: 10.1016/j.neuroimage.2010.01.039. [DOI] [PubMed] [Google Scholar]
- Broca MP. Perte de la parole, ramolissement chronique et destruction partielle du lobe anterieur gauche du cerveau. Bull Soc Anthropol Paris. 1861;2:235–238. [Google Scholar]
- Buchweitz A, Keller TA, Meyler A, Just MA. Brain activation for language dual-tasking: listening to two people speak at the same time and a change in network timing. Hum Brain Mapp. 2012;33(8):1868–1882. doi: 10.1002/hbm.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreiras M, Mechelli A, Price CJ. Effect of word and syllable frequency on activation during lexical decision and reading aloud. Hum Brain Mapp. 2006;27(12):963–972. doi: 10.1002/hbm.20236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereda C, Ghika J, Maeder P, Bogousslavsky J. Strokes restricted to the insular cortex. Neurology. 2002;59:1950–1955. doi: 10.1212/01.wnl.0000038905.75660.bd. [DOI] [PubMed] [Google Scholar]
- Chee MWL, Soon CS, Lee HL, Pallier C. Left insula activation: A marker for language attainment in bilinguals. Proc Natl Acad Sci U S A. 2004;101(42):15265–15270. doi: 10.1073/pnas.0403703101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffels IK, Formisano E, Schiller NO. Neural correlates of verbal feedback processing: an fMRI study employing overt speech. Hum Brain Mapp. 2007;28(9):868–879. doi: 10.1002/hbm.20315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2002;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel-now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Duffau H, Bauchet L, Lehéricy S, Capelle L. Functional compensation of the left dominant insula for language. Neuroreport. 2001;12:2159–2163. doi: 10.1097/00001756-200107200-00023. [DOI] [PubMed] [Google Scholar]
- Dogil G, Ackermann H, Grodd W, Haider H, Kamp H, Mayer J, Riecker A, Wildgruber D. The speaking brain: A tutorial introduction to fMRI experiments in the production of speech, prosody, and syntax. J Neurolinguist. 2002;15:59–90. [Google Scholar]
- Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384(6605):159–161. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012 Feb 1;59(3):2349–61. doi: 10.1016/j.neuroimage.2011.09.017. Epub 2011 Sep 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K. A systems perspective on the effective connectivity of overt speech production. Philos Trans R Soc Lond A. 2009;367(1896):2399–2421. doi: 10.1098/rsta.2008.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30(9):2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenberg LE, Specht K, Westerhausen R. Attention and cognitive control networks assessed in a dichotic listening fMRI study. Brain Cogn. 2011;76(2):276–285. doi: 10.1016/j.bandc.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Farb NAS, Segal ZV, Anderson AK. Attentional modulation of primary interoceptive and exteroceptive cortiex. Cerebral Cortex. doi: 10.1093/cercor/bhr385. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebach CJ, Friederici AD, Muller K, von Cramon DY, Hernandez AE. Distinct brain representations for early and late learned words. Neuroimage. 2003;19(4):1627–1637. doi: 10.1016/s1053-8119(03)00227-1. [DOI] [PubMed] [Google Scholar]
- Friederici AD. Towards a neural basis of auditory sentence processing. Trends Cogn Sci. 2002;6(2):78–84. doi: 10.1016/s1364-6613(00)01839-8. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Bahlmann J, Heim S, Schubotz RI, Anwander A. The brain differentiates human and non-human grammars: functional localization and structural connectivity. Proc Natl Acad Sci U S A. 2006;103(7):2458–2463. doi: 10.1073/pnas.0509389103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD, Kotz SA. The brain basis of syntactic processes: functional imaging and lesion studies. Neuroimage. 2003;20(Suppl 1):S8–17. doi: 10.1016/j.neuroimage.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Meyer M, von Cramon DY. Auditory language comprehension: an event-related fMRI study on the processing of syntactic and lexical information. Brain Lang. 2000;74(2):289–300. doi: 10.1006/brln.2000.2313. [DOI] [PubMed] [Google Scholar]
- Gandour J, Tong Y, Talavage T, Wong D, Dzemidzic M, Xu Y, et al. Neural basis of first and second language processing of sentence-level linguistic prosody. Hum Brain Mapp. 2007;28(2):94–108. doi: 10.1002/hbm.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandour J, Tong Y, Wong D, Talavage T, Dzemidzic M, Xu Y, et al. Hemispheric roles in the perception of speech prosody. Neuroimage. 2004;23(1):344–357. doi: 10.1016/j.neuroimage.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Gauthier CT, Duyme M, Zanca M, Capron C. Sex and performance level effects on brain activation during a verbal fluency task: a functional magnetic resonance imaging study. Cortex. 2009;45(2):164–176. doi: 10.1016/j.cortex.2007.09.006. [DOI] [PubMed] [Google Scholar]
- George MS, Parekh PI, Rosinsky N, Ketter TA, Kimbrell TA, Heilman KM, et al. Understanding emotional prosody activates right hemisphere regions. Arch Neurol. 1996;53(7):665–670. doi: 10.1001/archneur.1996.00550070103017. [DOI] [PubMed] [Google Scholar]
- Geschwind N. The organization of language and the brain. Science. 1970;170(961):940–944. doi: 10.1126/science.170.3961.940. [DOI] [PubMed] [Google Scholar]
- Golestani N, Pallier C. Anatomical correlates of foreign speech sound production. Cereb Cortex. 2007;17:929–934. doi: 10.1093/cercor/bhl003. [DOI] [PubMed] [Google Scholar]
- Golestani N, Zatorre RJ. Learning new sounds of speech: reallocation of neural substrates. Neuroimage. 2004;21(2):494–506. doi: 10.1016/j.neuroimage.2003.09.071. [DOI] [PubMed] [Google Scholar]
- Grande M, Meffert E, Schoenberger E, Jung S, Frauenrath T, Huber W, et al. From a concept to a word in a syntactically complete sentence: an fMRI study on spontaneous language production in an overt picture description task. Neuroimage. 2012;61(3):702–714. doi: 10.1016/j.neuroimage.2012.03.087. [DOI] [PubMed] [Google Scholar]
- Guenther HF. Cortical interactions underlying the production of speech sounds. J Commun Disord. 2006;39(5):350–365. doi: 10.1016/j.jcomdis.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Haller S, Radue EW, Erb M, Grodd W, Kircher T. Overt sentence production in event-related fMRI. Neuropsychologia. 2005;43(5):807–814. doi: 10.1016/j.neuropsychologia.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Heim S, Opitz B, Friederici AD. Broca’s area in the human brain is involved in the selection of grammatical gender for language production: evidence from event-related functional magnetic resonance imaging. Neurosci Lett. 2002;328(2):101–104. doi: 10.1016/s0304-3940(02)00494-9. [DOI] [PubMed] [Google Scholar]
- Hernandez AE, Kotz SA, Hofmann J, Valentin VV, Dapretto M, Bookheimer SY. The neural correlates of grammatical gender decisions in Spanish. Neuroreport. 2004;15(5):863–866. doi: 10.1097/00001756-200404090-00026. [DOI] [PubMed] [Google Scholar]
- Hesling I, Clement S, Bordessoules M, Allard M. Cerebral mechanisms of prosodic integration: evidence from connected speech. Neuroimage. 2005;24(4):937–947. doi: 10.1016/j.neuroimage.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Isel F, Baumgaertner A, Thran J, Meisel JM, Buchel C. Neural circuitry of the bilingual mental lexicon: effect of age of second language acquisition. Brain Cogn. 2010;72(2):169–180. doi: 10.1016/j.bandc.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Kaan E, Swaab TY. The brain circuitry of syntactic comprehension. Trends Cogn Sci. 2002;6(8):350–356. doi: 10.1016/s1364-6613(02)01947-2. [DOI] [PubMed] [Google Scholar]
- Kalab J, Molnár PP, Bogner P, Béres M, Berényi EI. Connectivity-based parcellation reveals interhemispheric differences in the insula. Brain Topography. 2012;25:264–271. doi: 10.1007/s10548-011-0205-y. [DOI] [PubMed] [Google Scholar]
- Kemeny S, Ye FQ, Birn R, Braun AR. Comparison of continuous overt speech fMRI using BOLD and arterial spin labeling. Hum Brain Mapp. 2005;24(3):173–183. doi: 10.1002/hbm.20078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, et al. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25(1):155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Robinson JL, McMillan KM, Tordesillas-Gutierrez D, Moran ST, Gonzales SM, Ray KL, Franklin C, Glahn DC, Fox PT, Lancaster JL. Comparison of the disparity between Talairach and MNI coordinates in functional neuroimaging data: Validation of the Lancaster transform. Neuroimage. 2010;51:677–683. doi: 10.1016/j.neuroimage.2010.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar RM, Marshall RS, Pile-Spellman J, Duong HC, Mohr JP, Young WL, et al. Interhemispheric transfer of language in patients with left frontal cerebral arteriovenous malformation. Neuropsychologia. 2000;38(10):1325–1332. doi: 10.1016/s0028-3932(00)00054-3. [DOI] [PubMed] [Google Scholar]
- Liakakis G, Nickel J, Seitz RJ. Diversity of the inferior frontal gyrus--a meta-analysis of neuroimaging studies. Behav Brain Res. 2011;225(1):341–347. doi: 10.1016/j.bbr.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Loevenbruck H, Baciu M, Segebarth C, Abry C. The left inferior frontal gyrus under focus: an fMRI study of the production of deixis via syntactic extraction and prosodic focus. J Neuroling. 2005;18:237–258. [Google Scholar]
- Lurito JT, Kareken DA, Lowe MJ, Chen SH, Mathews VP. Comparison of rhyming and word generation with FMRI. Hum Brain Mapp. 2000;10(3):99–106. doi: 10.1002/1097-0193(200007)10:3<99::AID-HBM10>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvel CL, Desmond JE. From storage to manipulation: How the neural correlates of verbal working memory reflect varying demands on inner speech. Brain Lang. 2012;120(1):42–51. doi: 10.1016/j.bandl.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RA, Just MA. Lexical ambiguity in sentence comprehension. Brain Res. 2007;1146:115–127. doi: 10.1016/j.brainres.2007.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Josephs O, Lambon Ralph MA, McClelland JL, Price CJ. Dissociating stimulus-driven semantic and phonological effect during reading and naming. Hum Brain Mapp. 2007;28(3):205–217. doi: 10.1002/hbm.20272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Alter K, Friederici AD. Functional MR imaging exposes differential brain responses to syntax and prosody during auditory sentence comprehension. J Neurolinguist. 2003;16:277–300. [Google Scholar]
- Moser D, Fridriksson J, Bonilha L, Healy EW, Baylis G, Baker JM, et al. Neural recruitment for the production of native and novel speech sounds. NeuroImage. 2009;46(2):549–557. doi: 10.1016/j.neuroimage.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Corfield DR, Guz A, Fink GR, Wise RJS, Harrison J, Adams L. Cerebral areas associated with motor control of speech in humans. J Appl Physiol. 1997;83:1438–1447. doi: 10.1152/jappl.1997.83.5.1438. [DOI] [PubMed] [Google Scholar]
- Nagels A, Kircher T, Dietsche B, Backes H, Marquetand J, Krug A. Neural processing of overt word generation in healthy individuals: the effect of age and word knowledge. Neuroimage. 2012;61(4):832–840. doi: 10.1016/j.neuroimage.2012.04.019. [DOI] [PubMed] [Google Scholar]
- Nagao M, Takeda K, Komori T, Isozaki E, Hirai S. Apraxia of speech associated with an infarct in the precentral gyrus of the insula. Neuroradiology. 41(5):356–357. doi: 10.1007/s002340050764. [DOI] [PubMed] [Google Scholar]
- Nestor P, Graham N, Fryer T, Williams G, Patterson K, Hodges J. Progressive non-fluent aphasia is associated with hypometaboism centred on the left anterior insula. Brain. 2003;126(11):2406– 2418. doi: 10.1093/brain/awg240. [DOI] [PubMed] [Google Scholar]
- Noesselt T, Shah NJ, Jancke L. Top-down and bottom-up modulation of language related areas--an fMRI study. BMC Neurosci. 2003;4:13. doi: 10.1186/1471-2202-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogar JM, Dronkers NF, Brambati SM, Miller BL, Gorno-Tempini ML. Progressive nonfluent aphasia and its characteristic motor speech deficits. Alzheimer Dis Assoc Disord. 2007;21(4):S23–30. doi: 10.1097/WAD.0b013e31815d19fe. [DOI] [PubMed] [Google Scholar]
- Ogar JM, Willock S, Baldo J, Wilkins D, Ludy C, Dronkers N. Clinical and anatomical correlates of apraxia of speech. Brain Lang. 2006;97(3):343–350. doi: 10.1016/j.bandl.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Pell MD. Fundamental frequency encoding of linguistic and emotional prosody by right hemisphere-damaged speakers. Brain Lang. 1999;69(2):161–192. doi: 10.1006/brln.1999.2065. [DOI] [PubMed] [Google Scholar]
- Pell MD. Cerebral mechanisms for understanding emotional prosody in speech. Brain Lang. 2006;96(2):221–234. doi: 10.1016/j.bandl.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Perani D, Cappa SF, Tettamanti M, Rosa M, Scifo P, Miozzo A, et al. A fMRI study of word retrieval in aphasia. Brain Lang. 2003;85(3):357–368. doi: 10.1016/s0093-934x(02)00561-8. [DOI] [PubMed] [Google Scholar]
- Price CJ, Devlin JT, Moore CJ, Morton C, Laird AR. Meta-analyses of object naming: effect of baseline. Hum Brain Mapp. 2005;25:70–82. doi: 10.1002/hbm.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raemaekers M, Vink M, Zandbelt B, van Wezel RJ, Kahn RS, Ramsey NF. Test-retest reliability of fMRI activation during prosaccades and antisaccades. Neuroimage. 2007 doi: 10.1016/j.neuroimage.2007.03.061. [DOI] [PubMed] [Google Scholar]
- Richter M, Miltner WH, Straube T. Association between therapy outcome and right-hemispheric activation in chronic aphasia. Brain. 2008;131(Pt 5):1391–1401. doi: 10.1093/brain/awn043. [DOI] [PubMed] [Google Scholar]
- Riecker A, Ackermann H, Wildgruber D, Dogil G, Grodd W. Opposite hemispheric lateralization effects during speaking and singing at motor cortex, insula and cerebellum. Neuroreport. 2000;11(9):1997–2000. doi: 10.1097/00001756-200006260-00038. [DOI] [PubMed] [Google Scholar]
- Riecker A, Brendel B, Ziegler W, Erb M, Ackermann H. The influence of syllable onset complexity and syllable frequency on speech motor control. Brain Lang. 2008;107(2):102–113. doi: 10.1016/j.bandl.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Riecker A, Kassubek J, Groschel K, Grodd W, Ackermann H. The cerebral control of speech tempo: opposite relationship between speaking rate and BOLD signal changes at striatal and cerebellar structures. Neuroimage. 2006;29(1):46–53. doi: 10.1016/j.neuroimage.2005.03.046. [DOI] [PubMed] [Google Scholar]
- Rodd JM, Johnsrude IS, Davis MH. The role of domain-general frontal systems in language comprehension: evidence from dual-task interference and semantic ambiguity. Brain Lang. 2010;115(3):182–188. doi: 10.1016/j.bandl.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Roder B, Stock O, Neville H, Bien S, Rosler F. Brain activation modulated by the comprehension of normal and pseudo-word sentences of different processing demands: a functional magnetic resonance imaging study. Neuroimage. 2002;15(4):1003–1014. doi: 10.1006/nimg.2001.1026. [DOI] [PubMed] [Google Scholar]
- Sass K, Krach S, Sachs O, Kircher T. Lion - tiger - stripes: Neural correlates of indirect semantic priming across processing modalities. Neuroimage. 2009;45(1):224–236. doi: 10.1016/j.neuroimage.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Schmidt GL, Seger CA. Neural correlates of metaphor processing: the roles of figurativeness, familiarity and difficulty. Brain Cogn. 2009;71(3):375–386. doi: 10.1016/j.bandc.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster LI, Lemieux SK. An fMRI investigation of covertly and overtly produced mono- and multisyllabic words. Brain Lang. 2005;93(1):20–31. doi: 10.1016/j.bandl.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Soros P, Sokoloff LG, Bose A, McIntosh AR, Graham SJ, Stuss DT. Clustered functional MRI of overt speech production. NeuroImage. 2006;32:376–387. doi: 10.1016/j.neuroimage.2006.02.046. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Berthier M, Leiguarda R. Bilateral opercular syndrome and crossed aphemia due to a right insular lesion: a clinicopathological study. Brain Lang. 1988;34:253–261. doi: 10.1016/0093-934x(88)90137-x. [DOI] [PubMed] [Google Scholar]
- Staudt M, Lidzba K, Grodd W, Wildgruber D, Erb M, Krageloh-Mann I. Right-hemispheric organization of language following early left-sided brain lesions: functional MRI topography. Neuroimage. 2002;16(4):954–967. doi: 10.1006/nimg.2002.1108. [DOI] [PubMed] [Google Scholar]
- Steinbrink C, Ackermann H, Lachmann T, Riecker A. Contribution of the anterior insula to temporal auditory processing deficits in developmental dyslexia. Hum Brain Mapp. 2009;30(8):2401–2411. doi: 10.1002/hbm.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szenkovits G, Peelle JE, Norris D, Davis MH. Individual differences in premotor and motor recruitment during speech perception. Neuropsychologia. 2012;50(7):1380–1392. doi: 10.1016/j.neuropsychologia.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; NY: 1988. [Google Scholar]
- Thompson CK, den Ouden DB, Bonakdarpour B, Garibaldi K, Parrish TB. Neural plasticity and treatment-induced recovery of sentence processing in agrammatism. Neuropsychologia. 2010;48(11):3211–3227. doi: 10.1016/j.neuropsychologia.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tivarus ME, Starling SJ, Newport EL, Langfitt JT. Homotopic language reorganization in the right hemisphere after early left hemisphere injury. Brain Lang. 2012;123(1):1–10. doi: 10.1016/j.bandl.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Davalos DB, Rojas DC. Effect of task difficulty on the functional anatomy of temporal processing. Neuroimage. 2006;32(1):307–315. doi: 10.1016/j.neuroimage.2006.02.036. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16(3 Pt 1):765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Voets NL, Adcock JE, Flitney DE, Behrens TE, Hart Y, Stacey R, et al. Distinct right frontal lobe activation in language processing following left hemisphere injury. Brain. 2006;129(Pt 3):754–766. doi: 10.1093/brain/awh679. [DOI] [PubMed] [Google Scholar]
- Winhuisen L, Thiel A, Schumacher B, Kessler J, Rudolf J, Haupt WF, et al. Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia: a combined repetitive transcranial magnetic stimulation and positron emission tomography study. Stroke. 2005;36(8):1759–1763. doi: 10.1161/01.STR.0000174487.81126.ef. [DOI] [PubMed] [Google Scholar]
- Wong D, Pisoni DB, Learn J, Gandour JT, Miyamoto RT, Hutchins GD. PET imaging of differential cortical activation by monaural speech and nonspeech stimuli. Hear Res. 2002;166(1–2):9–23. doi: 10.1016/s0378-5955(02)00311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zempleni MZ, Haverkort M, Renken R, LAS Evidence for bilateral involvement in idiom comprehension: An fMRI study. Neuroimage. 2007;34(3):1280–1291. doi: 10.1016/j.neuroimage.2006.09.049. [DOI] [PubMed] [Google Scholar]