Abstract
Background & Aims
Liver stiffness (LS) measured by magnetic resonance elastography (MRE) is emerging as an important biomarker in chronic liver diseases. We examined the diagnostic performance of MRE, factors associated with an increased LS and the prognostic value of LS as measured by MRE among patients with primary sclerosing cholangitis (PSC).
Methods
We performed a retrospective review of 266 patients with PSC to examine whether LS was associated with the primary endpoint of hepatic decompensation (ascites, variceal hemorrhage, and hepatic encephalopathy). The ability of MRE to differentiate stages of fibrosis was examined in a subset of patients who underwent a liver biopsy (n=20).
Results
A LS of 4.93 kPa was the optimal point to detected F4 fibrosis (sensitivity, 1.00; 95% confidence interval (CI), 0.40–1.00; specificity, 0.94; 95% CI, 0.68–1.00). While a serum alkaline phosphatase (ALP) < 1.5 times the upper limit of normal (ULN) excluded the presence of advanced LS, it was not associated with the primary endpoint (hazard ratio (HR), 0.26; 95% CI, 0.01–1.33). However, LS was associated with the development of decompensated liver disease (HR, 1.55; 95% CI, 1.41–1.70). The optimal LS thresholds which stratified patients at a low, medium and high risk for hepatic decompensation were <4.5 kPa, 4.5–6.0 kPa and >6.0 kPa (respectively).
Conclusion
MRE is able to detect cirrhosis with high specificity and an ALP < 1.5 times the ULN makes the presence of advanced LS unlikely. Moreover, LS obtained by MRE is predictive of hepatic decompensation in PSC.
Keywords: primary sclerosing cholangitis, magnetic resonance elastography, liver stiffness
Introduction
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disorder which can progress to cirrhosis and lead to complications from portal hypertension.1 Effective medical therapy for PSC is lacking. The reasons for this are multifactorial but are in part due to the variable progression of this disease, which makes stratifying patients in clinical trials challenging. Hence, it is important to identify and utilize surrogate markers that can accurately predict clinically relevant events.
Liver stiffness (LS) is a surrogate marker for hepatic fibrosis and can be quantified using elastography. LS has been shown to correlate with the stage of fibrosis and it has been associated with hepatic decompensation and survival.2–5 Transient elastography (TE) and magnetic resonance elastography (MRE) are the two principle elastography techniques most commonly employed. Among patients with PSC, TE is able to differentiate between stages of fibrosis and link the baseline LS and rates of LS progression to patient outcomes.6 However, the use of MRE may offer several key advantages when determining the LS in PSC patients when compared to TE. First, MRE can be performed at the same time as magnetic resonance cholangiography (MRC), a test commonly performed in PSC patients, without adding a significant amount of time or cost to the examination. This approach also allows for the identification of dominant strictures which is important because the presence of a biliary obstruction may increase the LS irrespective of the degree of fibrosis.7–9 Second, fibrosis in PSC can be patchy and MRE can characterize a larger volume of the liver when compared to TE.10, 11 Third, among other causes of chronic liver disease, MRE has been shown to have a better diagnostic performance and lower failure rate when compared to TE.5, 12–14 Finally, MRE (unlike TE) is not influenced by obesity.11
To date, the use of MRE among a dedicated cohort of PSC patients has not been explored. It is important to improve our understanding of the performance of MRE among PSC patients and factors that can influence LS measurements because the accurate determination of LS can guide clinical decisions and serve as a tool to risk stratify PSC patients in clinical trials. Consequently, we examined the diagnostic performance of MRE, factors associated with an increased LS and the prognostic (short-term) value of LS in a large PSC cohort.
Materials and Methods
Patients
This study was approved by the institutional review board at Mayo Clinic, Rochester, MN. A retrospective review was conducted among individuals who met the following criteria: i) typical features of PSC on cholangiography or liver biopsy;15 and ii) underwent a MRE at our institution between January 1, 2007 and December 31, 2013. Patients were excluded if any of the following were present: i) had concurrent chronic liver disease with the exception of overlap syndrome with autoimmune hepatitis (PSC-AIH); or ii) history of decompensated liver disease (ascites, variceal hemorrhage or hepatic encephalopathy) or liver transplant prior to undergoing a MRE.
Magnetic Resonance Elastography
During a MRE exam, mechanical shear waves are delivered to a fasting patient via an acoustic driver and its propagation through the liver is imaged with a special MRI sequence. An inversion algorithm is then used to process the acquired data from the wave images and generate elastograms (stiffness maps). Regions of interest (ROI) are typically drawn to obtain the average LS values, reported in kilopascals (kPa), by the reviewing radiologist.11
To ensure consistency across the dataset, raw images from all the exams were re-processed with the most recent MRE inversion algorithm. To avoid inter- and intra-reader discrepancies caused by subjective manual selection of ROIs for the stiffness measurement, a fully automated segmentation algorithm was used to generate all the ROIs and calculate the average LS for this study. When compared to the correlation between 2 expert readers, this method has superior reproducibility.16 The automated ROIs were reviewed for errors by a single expert reader (BD) and none needed to be adjusted manually.
Data Collection and Key Definitions
Data was collected from an electronic medical record. Baseline features, body mass index (BMI), laboratory values and presence of comorbid conditions were abstracted at the time of the MRE. The revised Mayo PSC risk and model for end-stage liver disease (MELD) scores, and aminoaspartate transferase (AST) to platelet ratio (APRI) were also determined at the time of the MRE. The presence of splenomegaly (typically defined as a length greater than 12 cm along the longitudinal axis) was determined by the radiologist reviewing the MRE.17 Thrombocytopenia was defined as a platelet value less than 150 × 109/L. The serum alkaline phosphatase (ALP) was reported as the ratio of the laboratory value and the upper limit of normal (ULN) for the assay. We also determined if patients had a persistent ALP less than 1.5 times the ULN on 3 separate occasions (at least 6 months apart) prior to the MRE.18 An untreated dominant stricture was defined as the presence of a dominant stricture and a bilirubin 2.0 mg/dL or greater at the time of the MRE.19 Liver biopsies of patients with PSC who did not have a concurrent untreated dominant stricture were reviewed by a single blinded pathologist (TCS) if they were performed within one year of the MRE. The stage of fibrosis (F0-F4) was determined for each specimen in accordance with the Batts-Ludwig staging system.15
Statistical Analysis
Statistical analysis was performed with JMP and SAS software (SAS Institute; Cary, NC). All tests were 2-sided with a level of significance of p <0.05. Categorical data were compared using the Pearson chi-squared test and continuous variables were compared using the nonparametric Wilcoxon test. Categorical data are presented as numbers (percentages) while continuous variables are expressed as medians, interquartile ranges (IQR) unless otherwise stated.
We estimated receiver operating characteristic curves and the area under the curve (AUC) of LS for the histologic stage of fibrosis and laboratory parameters associated with increased LS. In addition, the sensitivity, specificity, positive and negative predictive values (PPV and NPV) with respective 95% confidence intervals (CI) were determined. Spearman’s rank correlation coefficient was performed to examine the relationship between LS and the stage of fibrosis. Linear regression was utilized to determine which variables were associated with elevated LS while adjusting for the presence of an untreated dominant stricture.
The prognostic value of LS was also examined. Follow up was determined from the time of the MRE (baseline) to either the development of the primary endpoint (hepatic decompensation defined as the development of ascites, hepatic encephalopathy or variceal hemorrhage) or at the time of censoring (liver transplantation for an indication not associated with portal hypertension or the last clinical encounter). Cox proportional hazards regression analysis were employed to examine associations between covariates and the primary endpoint, and the results were expressed as hazard ratios (HR). The Mayo PSC risk score is a tool that is predictive of survival among PSC patients.20 Consequently, the LS value, Mayo PSC risk score plus another covariate significant in the univariable analyses was incorporated into a series of multivariable models given the limited number of expected events. The cumulative incidence of hepatic decompensation was determined by the Kaplan-Meier method. Log rank statistics were used to determine the optimal unbiased LS cut-offs which defined LS thresholds for 3 prognostic groups of patients (low, medium and high) at risk for developing hepatic decompensation.21
Results
Patients
Two-hundred and seventy-four PSC patients were reviewed for this study and 8 were excluded because the MRE was performed after the primary endpoint. Ultimately, 266 individuals were included and followed for a median (IQR) of 2.05 (1.43–2.74) years. Between 2007and 2011 the majority of PSC patients underwent a MRE due to a clinical suspicion for advanced fibrosis (n=62). In 2012, the Cholestatic Liver Disease Clinic began to perform MRE’s on all PSC patients (regardless if there was a concern for cirrhosis) who also underwent a concurrent MRC for their annual cholangiocarcinoma screening (n=204). The baseline features at the time of the MRE are illustrated in Table 1.
Table 1.
Baseline Characteristics of Cohort
| Age (years) | 46.12 (33.02–59.40) |
| Female | 30% (81/266) |
| BMI (kg/m2) | 25.96 (23.10–29.63) |
| IBD Present1 | 81% (215/266) |
| PSC Duration (years) | 5.84 (1.09–12.05) |
| Large Duct | 94% (249/266) |
| Small Duct | 6% (17/266) |
| PSC & Autoimmune Hepatitis | 5% (13/266) |
| Untreated Dominant Stricture | 8% (21/266) |
| Cholangiocarcinoma2 | 5% (13/266) |
| Splenomegaly3 | 22% (58/263) |
| Nonbleeding Varices | 9% (24/266) |
| Ascites4 | 4% (10/266) |
| Platelets (×109/L) | 240.00 (186.25–297.00) |
| Platelets < 150 × 109/L | 14% (37/261) |
| Features of Portal Hypertension5 | 27% (73/266) |
| AST (U/L) | 48.00 (30.00–90.00) |
| APRI | 0.47 (0.28–0.95) |
| Alkaline Phosphatase (U/L)/ULN | 1.60 (0.90–2.79) |
| Alkaline Phosphatase < 1.5 × ULN6 | 26% (57/219) |
| Total Bilirubin (mg/dL) | 0.80 (0.50–1.43) |
| MELD | 7.00 (6.00–10.00) |
| Mayo PSC Risk Score | −0.09 (−0.84–0.66) |
Continuous variables are expressed as median (interquartile range). Categorical variables are expressed as numbers and percentages.
Abbreviations: BMI (body mass index); IBD (inflammatory bowel disease); PSC (primary sclerosing cholangitis); AST (aspartate aminotransferase); APRI (AST to platelet ratio index); ULN (upper limit of normal); MELD (model end stage liver disease).
Ulcerative colitis n=189; Crohn’s Disease n=22; Indeterminate Colitis n=4.
Thirteen patients had CCA detected on baseline elastography and 4 additional patients ultimately developed CCA at a later date.
Three patients had prior splenectomy.
Ascites was detected for the first time in 10 patients at time of elastography.
Had one or more signs suggestive of underlying portal hypertension at the time of the MRE (splenomegaly n=36, thrombocytopenia n=8, nonbleeding varices n=5, new onset ascites detected on MRE n=2, or a combination of these features n=22).
Forty-seven patients were excluded from this analysis since they had alkaline phosphatase < 1.5 upper limit of normal at time of elastography but did not have at least 3 separate measurements at least 6 months apart.
Diagnostic Performance of Magnetic Resonance Elastography & Hepatic Fibrosis
Twenty patients (8%) with PSC underwent a liver biopsy (F0 n=4; F1 n=3; F2 n=6, F3 n=3, F4 n=4). The median (IQR) time between liver biopsy and MRE was 24 (2–220) days and the median (IQR) number of portal tracts was 11 (9–16). An association between the fibrosis stage and LS was detected (Spearman’s correlation 0.84, p<0.001). The LS median (range) for each stage is depicted in Figure 1. Despite having adequate tissue on liver biopsy, 3 (50%) patients with F2 fibrosis had signs of portal hypertension on their MRE and median (IQR) LS of 4.39 (3.80–4.93) kPa. The respective optimal LS cut-off for any fibrosis (F1 or higher), significant fibrosis (F2 or higher) or cirrhosis (F4) was 2.41 kPa, 3.26 kPa and 4.93 kPa. The sensitivity and specificity for detection of cirrhosis was 1.00 (95% CI, 0.40–1.00) and 0.94 (95% CI, 0.68–1.00), respectively (Table 2).
Figure 1.
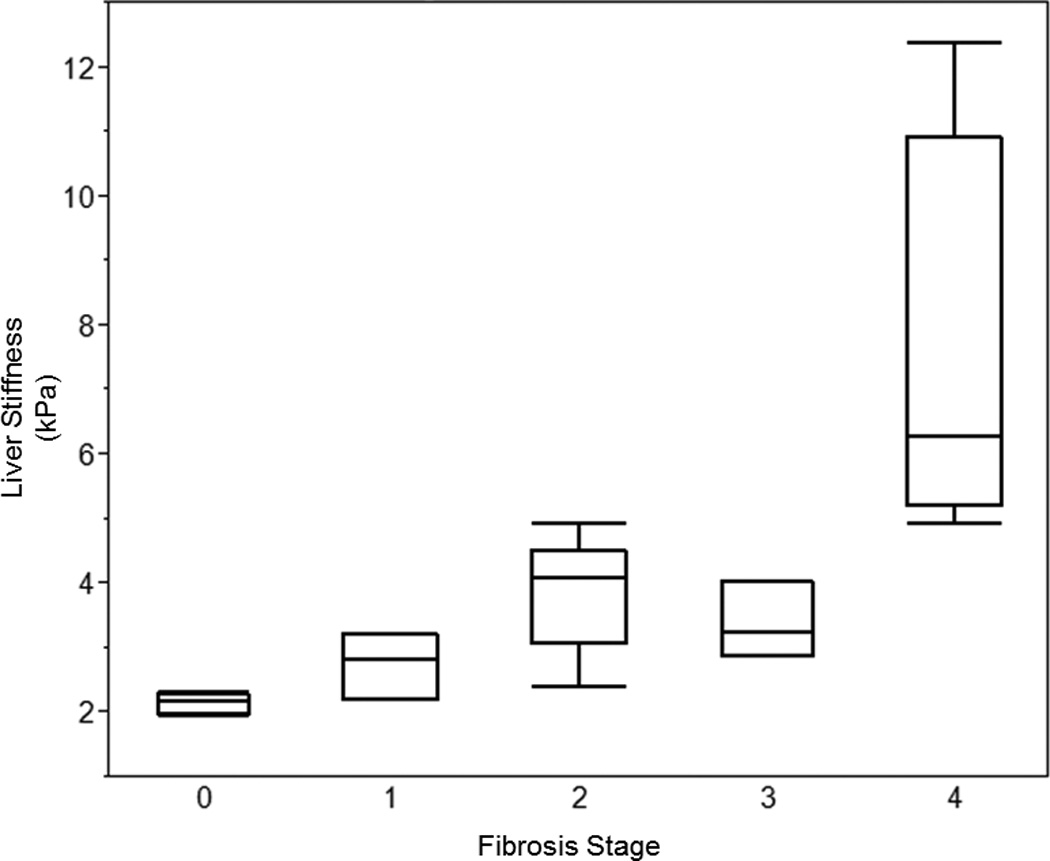
Box plot of Liver Stiffness for Each Stage of Fibrosis.
Table 2.
Diagnostic Performance of Magnetic Resonance Elastography for Hepatic Fibrosis in Primary Sclerosing Cholangitis
| Fibrosis Stage |
True Positive |
True Negative |
Cutoff (kPa) |
AUC | Sensitivity (95% CI) |
Specificity (95% CI) |
PPV (95% CI) |
NPV (95%CI) |
|---|---|---|---|---|---|---|---|---|
| ≥F1 | 15 | 4 | 2.41 | 0.97 | 0.94 (0.68–1.00) |
1.00 (0.40–1.00) |
1.00 (0.75–1.00) |
0.80 (0.30–0.99) |
| ≥F2 | 11 | 7 | 3.26 | 0.97 | 0.85 (0.54–0.97) |
1.00 (0.56–1.00) |
1.00 (0.68–1.00) |
0.78 (0.40–0.96) |
| F4 | 4 | 15 | 4.93 | 0.99 | 1.00 (0.40–1.00) |
0.94 (0.68–1.00) |
0.80 (0.30–0.99) |
1.00 (0.75–1.00) |
Abbreviations: kPa (kilopascals); AUC (area under the curve); CI (confidence interval); PPV (positive predictive value); NPV (negative predictive value (NPV).
Features Associated with an Increased Liver Stiffness
The median (range) LS for the entire cohort was 2.88 (1.27–13.56) kPa. Patients with an untreated dominant stricture (n=21) had a higher median (IQR) LS compared to patients without, 5.36 (4.23–7.56) kPa vs 2.82 (2.41–3.61) kPa, p<0.001. Among those 21 individuals, 4 underwent a subsequent MRE within 1 year of endoscopic therapy and 3 patient’s LS decreased by a median (range) of 1.25 (0.48–2.56) kPa.
Table 3 depicts the relationship between LS and other clinical covariates after adjusting for the presence of an untreated dominant stricture. The presence of an untreated dominant stricture continued to be associated with increased LS after adjustment for the other variables in Table 3 (adjusted slope estimates ranged from 0.92–3.49, p<0.05). This model revealed several additional factors associated with elevated LS: longer PSC duration, higher BMI, signs of underlying portal hypertension (splenomegaly, nonbleeding varices or ascites), lower platelet count, or an elevated APRI, ALP, MELD and Mayo PSC risk score. The ability of these laboratory parameters to predict LS values which are clinically significant and suggest cirrhosis (4.93 kPa or greater) were examined. A persistent ALP more than 1.5 times the ULN (sensitivity, 1.00; 95% CI, 0.89–1.00; specificity, 0.32; 95% CI, 0.25–0.39) and a single ALP value of 1.46 times the ULN (sensitivity, 0.98; 95% CI, 0.86–1.00; specificity, 0.52; 95% CI, 0.45–0.59) were the most sensitive predictors of advanced liver stiffness (Supplementary Table 1).
Table 3.
Predictors of Increased Liver Stiffness in Primary Sclerosing Cholangitis After Adjusting for Untreated Dominant Stricture
| Slope Estimate (95% CI) | P value | |
|---|---|---|
| Age (years)1 | 0.01 (0.001–0.03) | 0.25 |
| Female | 0.002 (−0.46–0.46) | 0.82 |
| BMI (kg/m2) | 0.05 (0.02–0.10) | <0.01 |
| IBD Present | −0.30 (−0.85–0.24) | 0.28 |
| PSC Duration (years)2 | 0.03 (0.01–0.06) | 0.02 |
| Large Duct (vs Small Duct) | 0.18 (−0.69–1.05) | 0.68 |
| PSC & Autoimmune Hepatitis (vs PSC alone) | 0.32 (−0.69–1.28) | 0.55 |
| Cholangiocarcinoma Present | 0.69 (−0.40–1.78) | 0.22 |
| Splenomegaly Present | 1.92 (1.45–2.38) | <0.001 |
| Nonbleeding Varices at time of MRE | 2.39 (1.71–3.07) | <0.001 |
| Ascites on MRE | 2.64 (1.57–3.71) | <0.001 |
| Platelets (×109/L) | −0.01 (−0.01– −0.003) | <0.001 |
| APRI | 0.65 0.47–0.83) | <0.001 |
| ALP (U/L)/ULN | 0.10 (0.01–0.21) | 0.03 |
| ALP < 1.5 × ULN | −1.19 (−1.7– −0.64) | <0.01 |
| Total Bilirubin (mg/dL) | 0.03 (−0.05–0.10) | 0.51 |
| MELD | 0.10 (0.03–0.17) | <0.01 |
| Mayo PSC Risk Score | 0.92 (0.73–1.12) | <0.001 |
Also adjusted for disease duration.
Also adjusted for age.
Abbreviations: CI (confidence interval); BMI (body mass index); IBD (inflammatory bowel disease); PSC (primary sclerosing cholangitis); MRE (magnetic resonance elastography); AST (aspartate aminotransferase); APRI (AST to platelet ratio index);ALP (alkaline phosphatase); ULN (upper limit of normal); MELD (model end stage liver disease).
Prognostic Value of Magnetic Resonance Elastography
Among the 266 patients, 36 (14%) were diagnosed with the primary endpoint of hepatic decompensation (ascites n=20, hepatic encephalopathy n=5, variceal hemorrhage n=3, multiple n=8). The median (IQR) LS among those with and without hepatic decompensation was 5.99 (4.86–7.27) kPa and 2.76 (2.39–3.40) kPa (p<0.001), respectively. LS was not associated with a diagnosis of cholangiocarcinoma (unadjusted HR, 1.16; 95% CI, 0.94–1.35) among the 17 individuals ultimately diagnosed with biliary cancer.
In the unadjusted analysis, LS was associated with the hepatic decompensation (HR, 1.55; 95% CI, 1.41–1.70) (Supplementary Table 2). In the adjusted analysis, both LS and Mayo PSC risk score remained significant and the point estimates remained similar across all of the models (Table 4). Splenomegaly, nonbleeding varices, platelets and total bilirubin were also associated with the primary endpoint in the multivariable analysis. However, when ALP was examined in the multivariable model it did not remain significant after adjusting for the LS and Mayo PSC risk score (Table 4). Notably, LS continued to provide prognostic information among individuals who did not have features of advanced liver disease at the time of the MRE. For example, LS continued to be associated with hepatic decompensation after excluding individuals who had ascites detected for the first time on the MRE (unadjusted HR, 1.69; 95% CI, 1.51–1.92) or had any signs of portal hypertension at the time of the MRE (unadjusted HR, 1.82; 95% CI, 1.49–2.35).
Table 4.
Predictors of Hepatic Decompensation (Adjusted)
| Model | Variable | HR (95% CI) | P value |
|---|---|---|---|
| Model 1 | LS (per unit) | 1.29 (1.15–1.45) | <0.001 |
| Mayo Risk Score (per unit) | 2.69 (1.76–3.90) | <0.001 | |
| Untreated Dominant Stricture | 0.48 (0.18–5.47) | 0.11 | |
| Model 2 | LS (per unit) | 1.28 (1.12–1.45) | <0.001 |
| Mayo Risk Score (per unit) | 2.39 (1.70–3.36) | <0.001 | |
| Splenomegaly | 3.33 (1.70–6.85) | <0.001 | |
| Model 3 | LS (per unit change) | 1.27 (1.12–1.43) | <0.001 |
| Mayo Risk Score (per unit) | 2.56 (1.81–3.62) | <0.001 | |
| Platelets (per unit) | 0.99 (0.98–0.99) | <0.001 | |
| Model 4 | LS (per unit) | 1.31 (1.15–1.46) | <0.001 |
| Mayo Risk Score (per unit) | 2.37 (1.68–3.32) | <0.001 | |
| APRI (per unit) | 0.99 (0.81–1.15) | 0.90 | |
| Model 5 | LS (per unit) | 1.30 (1.15–1.46) | <0.001 |
| Mayo Risk Score (per unit) | 2.42 (1.68–3.47) | <0.001 | |
| ALP/ULN (per unit) | 0.97 (0.83–1.09) | 0.68 | |
| Model 6 | LS (per unit) | 1.29 (1.14–1.43) | <0.001 |
| Mayo Risk Score (per unit) | 2.21 (1.56–3.10) | <0.001 | |
| ALP < 1.5 × ULN1 | 0.26 (0.01–1.33) | 0.12 | |
| Model 7 | LS (per unit) | 1.24 (1.17–1.41) | <0.01 |
| Mayo Risk Score (per unit) | 3.22 (2.07–5.23) | <0.001 | |
| Total Bilirubin (per unit) | 0.91 (0.80–0.99) | 0.03 | |
| Model 8 | LS (per unit) | 1.28 (1.13–1.43) | <0.001 |
| Mayo Risk Score (per unit) | 3.90 (1.98–4.93) | <0.001 | |
| MELD (per unit) | 0.91 (0.82–1.01) | 0.06 | |
| Model 9 | LS (per unit) | 1.28 (1.12–1.45) | <0.001 |
| Mayo Risk Score (per unit) | 2.35 (1.69–3.27) | <0.001 | |
| PSC Duration (per year) | 1.02 (0.98–1.06) | 0.38 | |
| Model 10 | LS (per unit) | 1.30 (1.15–1.46) | <0.001 |
| Mayo Risk Score (per unit) | 2.42 (1.68–3.47) | <0.001 | |
| History of Nonbleeding Varices | 2.82 (1.32–5.90) | <0.01 | |
Abbreviations: LS (liver stiffness); ULN (upper limit of normal); ALP (alkaline phosphatase) MELD (model end stage liver disease).
Includes patients with a persistent alkaline phosphatase < 1.5 times the upper limit of normal at the time of the MRE and on 3 or more occasions (at least 6 months apart) before the MRE was performed.
The optimal LS thresholds for low, medium and high risk groups for the development of hepatic decompensation was determined to be less than 4.5 kPa, 4.5–6.0 kPa and greater than 6.0 kPa (respectively). Patients with a LS of less than 4.5 kPa, 4.5–6.0 kPa and greater than 6.0 kPa had a 1-year cumulative incidence (95% CI) of hepatic decompensation which was 0.50% (0.01–3.96%), 25.19% (14.06–40.95%) and 54.00% (34.63–71.49%), respectively (Figure 2). The cumulative incidence remained similar for each group after excluding individuals with an untreated dominant stricture (Supplementary Figure 1). After excluding individuals who had hepatic decompensation within 30 days of the MRE (n=17), the 1-year cumulative incidence (95%) CI of hepatic decompensation for a LS less than 4.5 kPa, 4.5–6.0 kPa, or greater than 6.0 kPa was 0.57% (0.08–3.96%), 10.00% (3.26–26.81%) and 25.96% (10.06–52.37%), respectively (Supplementary Figure 2).
Figure 2.
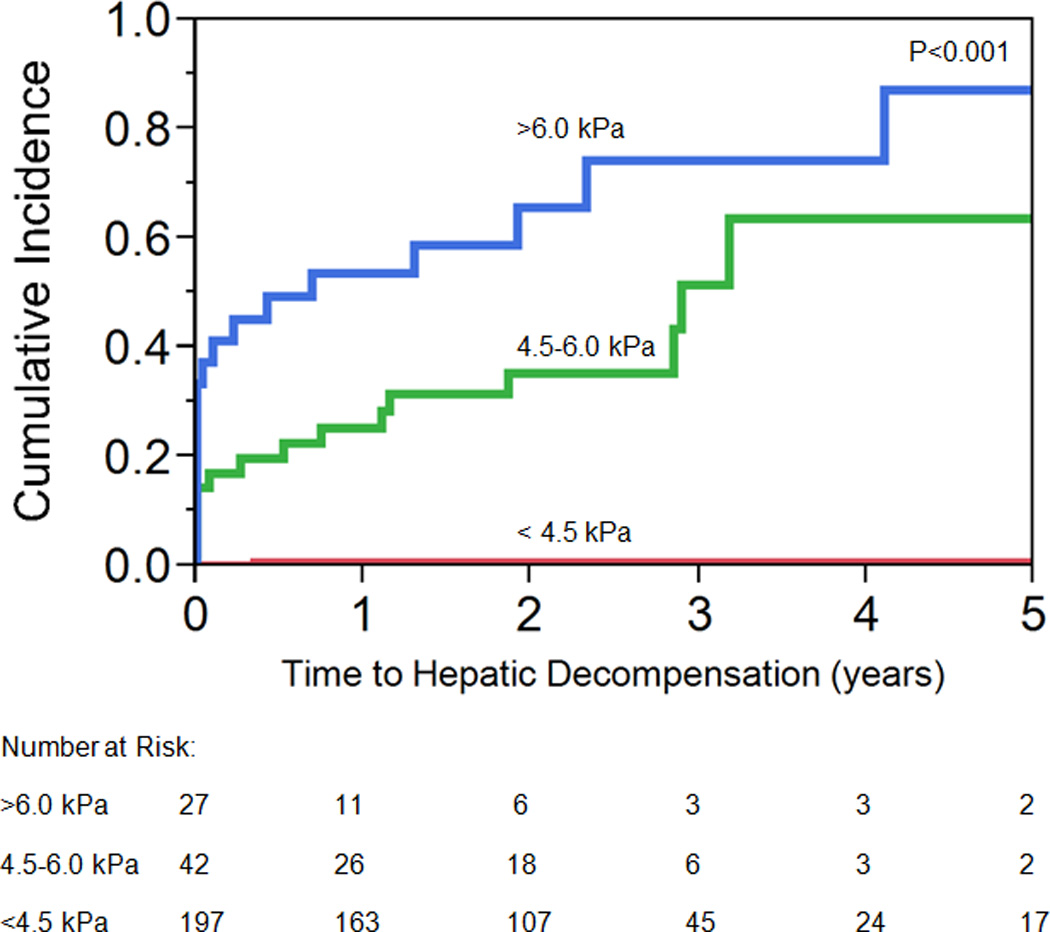
Time to Hepatic Decompensation
Discussion
This large study, the first published to date with a dedicated cohort of PSC patients who underwent MRE testing, shows that MRE is able to accurately distinguish the presence of cirrhosis and predict patient outcomes. Furthermore, our results provide additional insights into factors associated with elevated LS, which can guide clinicians when determining whether to order an MRE and how to interpret the results.
MRE accurately distinguished the presence of any fibrosis (F1 or greater) and cirrhosis (F4 or greater) (Table 2). Hence, clinicians should institute screening measures, such as assessment for varices, once a LS of 4.93 kPa is encountered or if the MRE reveals an elevated LS with other signs consistent with cirrhosis. Our results also highlight that a liver biopsy is an imperfect gold standard. For example, 3 patients with F2 fibrosis on biopsy had features of portal hypertension and elevated median LS, suggesting they had more advanced fibrosis than was detected on the biopsy sample. Indeed, among these 3 individuals, there were regional areas of decreased LS (compared to the rest of the liver) which could have been in the vicinity of tissue acquisition. However, we were unable to retrospectively determine the exact site of the liver biopsies. Importantly, a liver biopsy samples approximately 1:50,000th of the liver and can be prone to sampling error.21 In contrast, MRE examines a liver volume 9000 times higher than a liver biopsy and 1000 times larger than TE.22 The patchy distribution of fibrosis seen in PSC has the potential to compound this issue and is one reason why MRE could be more advantageous when compared to liver biopsy or TE.10
Several clinical features are associated with increased LS. An untreated dominant stricture is associated with higher LS. This observation has been previously described with TE and it reinforces that LS can be influenced by biliary obstruction.7–9 This is important for PSC patients since an estimated 10–30% of patients may develop symptomatic dominant strictures.24, 25 Consequently, LS should not be assessed until a dominant stricture is treated. In addition to a dominant stricture, a longer duration of PSC and an increasing BMI were associated with increasing LS. The relationship between BMI and LS could be secondary to the concurrent presence of nonalcoholic fatty liver disease as the accuracy of LS measurements obtained by MRE has consistently been shown to be independent of the patients BMI.5 As previously described in other studies, we confirmed that several laboratory tests and prognostic scores were associated with LS (Table 3).6, 26, 27 Patients with ALP persistently less than 1.5 times the ULN and a single ALP level 1.46 times the ULN were unlikely to have advanced LS associated with cirrhosis. Therefore, ALP can be utilized in clinical practice to triage patients who are unlikely to benefit from a MRE.
LS obtained by MRE is predictive of hepatic decompensation in PSC. These observations persisted after excluding individuals with signs of advanced liver disease or were diagnosed with hepatic decompensation shortly after the baseline MRE. Patients with LS greater than 6 kPa were at the highest risk for hepatic decompensation. This threshold is beyond the value anticipated for F4 fibrosis and indicates that LS can continue to provide prognostic information among patients expected to have cirrhosis. If these thresholds are confirmed, it would be advantageous to utilize such LS cut-offs to stratify PSC patients in clinical trials. A persistent ALP less than 1.5 times the ULN has been reported to be associated with an improved prognosis in PSC.18 However, ALP did not have a prognostic value after adjusting for LS and the Mayo PSC risk score. This suggests that stratifying patients based on their LS rather than ALP alone would be preferable in future therapeutic trials. While previous studies have shown that increased LS is associated with hepatocellular carcinoma, it was not associated with the development of cholangiocarcinoma in PSC patients.2
Our study has several limitations. First, this was a retrospective study conducted at a single academic medical center with a limited duration of patient follow up. Consequently, our findings should be confirmed. Our data highlights that LS as measured by MRE can provide useful short term prognostic information even among patients who lack other features of advanced liver disease. Because LS changes over time it is likely that a single LS value is more reflective of the risk of short term outcomes when compared to longer term outcomes. However, it will be important for future studies to determine if longitudinal changes in LS can better predict outcomes. Second, we only had limited numbers of patients with liver biopsies. Yet, it is noteworthy that the optimal LS cut-off for cirrhosis (4.93 kPa) is similar to what has been reported in a meta-analysis that examined MRE in a large sample of patients with other chronic liver diseases.5 In addition, the cut-off value for cirrhosis when TE was investigated in PSC was nearly identical to the figure reported in the present study (recognizing that shear-based MRE measurements can be compared to Young’s modulus based TE measurements by dividing by a conversion factor of 3).6
In conclusion, this study indicates that MRE has both diagnostic and prognostic value for patients with PSC. Based on our data and the cut-offs reported elsewhere, LS value of 4.93 kPa is consistent with cirrhosis among patients with PSC. However, LS measurements should be interpreted with caution if an untreated dominant stricture is present. A low ALP suggests PSC patients are unlikely to have LS associated with cirrhosis. Finally, LS as measured by MRE has a robust association with hepatic decompensation. These attributes make MRE an attractive tool for both routine patient management and as a potential method for patient stratification in clinical trials.
Supplementary Material
Supplementary Figure 1. Time to Hepatic Decompensation after Excluding Those with Untreated Dominant Stricture
Supplementary Figure 2. Time to Hepatic Decompensation after Excluding Those with Hepatic Decompensation within 30 days of Elastography
Acknowledgements
None
Grant Support: This work was supported by the following NIH grants: R01DK 084960 (KNL), R01DK 59427 (GJG), R01EB 01981 (RLE), R01DK 57993 (NFL).The Mayo Clinic and RLE have intellectual property rights and a financial interest related to Magnetic Resonance Elastography, a technology used in this study.
Abbreviations
- LS
liver stiffness
- MRE
magnetic resonance elastography
- PSC
primary sclerosing cholangitis
- kPa
kilopascals
- CI
confidence interval
- SAP
serum alkaline phosphatase
- ULN
upper limit of normal
- HR
hazard ratio
- TE
transient elastography
- MRC
magnetic resonance cholangiography
- ROI
regions of interest
- AIH
autoimmune hepatitis
- BMI
body mass index
- MELD
model for end-stage liver disease
- AST
aminoaspartate transferase
- APRI
AST to platelet ratio index
- IQR
interquartile range
- AUC
area under the curve
- PPV
positive predictive value
- NPV
negative predictive value
Footnotes
Transcript Profile: None.
Writing Assistance: Not utilized.
Conflicts of Interests & Disclosure Statement: The remaining coauthors have nothing else to disclose.
References
- 1.Eaton JE, Talwalkar JA, Lazaridis KN, Gores GJ, Lindor KD. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology. 2013;145:521–536. doi: 10.1053/j.gastro.2013.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh S, Fujii LL, Murad MH, et al. Liver stiffness is associated with risk of decompensation, liver cancer, and death in patients with chronic liver diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1573–1584. e1–e2. doi: 10.1016/j.cgh.2013.07.034. quiz e88-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castera L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Corpechot C, El Naggar A, Poujol-Robert A, et al. Assessment of biliary fibrosis by transient elastography in patients with PBC and PSC. Hepatology. 2006;43:1118–1124. doi: 10.1002/hep.21151. [DOI] [PubMed] [Google Scholar]
- 5.Singh S, Venkatesh SK, Wang Z, et al. Diagnostic Performance of Magnetic Resonance Elastography in Staging Liver Fibrosis: A Systematic Review and Meta-analysis of Individual Participant Data. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corpechot C, Gaouar F, El Naggar A, et al. Baseline values and changes in liver stiffness measured by transient elastography are associated with severity of fibrosis and outcomes of patients with primary sclerosing cholangitis. Gastroenterology. 2014;146:970–979. doi: 10.1053/j.gastro.2013.12.030. quiz e15-6. [DOI] [PubMed] [Google Scholar]
- 7.Millonig G, Reimann FM, Friedrich S, et al. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718–1723. doi: 10.1002/hep.22577. [DOI] [PubMed] [Google Scholar]
- 8.Ehlken H, Lohse AW, Schramm C. Transient elastography in primary sclerosing cholangitis-the value as a prognostic factor and limitations. Gastroenterology. 2014;147:542–543. doi: 10.1053/j.gastro.2014.04.058. [DOI] [PubMed] [Google Scholar]
- 9.Attia D, Pischke S, Negm AA, et al. Changes in liver stiffness using acoustic radiation force impulse imaging in patients with obstructive cholestasis and cholangitis. Dig Liver Dis. 2014;46:625–631. doi: 10.1016/j.dld.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 10.Scheuer PJ. Ludwig Symposium on biliary disorders--part II. Pathologic features and evolution of primary biliary cirrhosis and primary sclerosing cholangitis. Mayo Clin Proc. 1998;73:179–183. doi: 10.4065/73.2.179. [DOI] [PubMed] [Google Scholar]
- 11.Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J Magn Reson Imaging. 2013;37:544–555. doi: 10.1002/jmri.23731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castera L, Foucher J, Bernard PH, et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010;51:828–835. doi: 10.1002/hep.23425. [DOI] [PubMed] [Google Scholar]
- 13.Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135:32–40. doi: 10.1053/j.gastro.2008.03.076. [DOI] [PubMed] [Google Scholar]
- 14.Ichikawa S, Motosugi U, Morisaka H, et al. Comparison of the diagnostic accuracies of magnetic resonance elastography and transient elastography for hepatic fibrosis. Magn Reson Imaging. 2015;33:26–30. doi: 10.1016/j.mri.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Eaton JE, Talwalkar JA, Lazaridis KN, Gores GJ, Lindor KD. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology. 2013;145:521–536. doi: 10.1053/j.gastro.2013.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dzyubak B, Glaser K, Yin M, et al. Automated liver stiffness measurements with magnetic resonance elastography. J Magn Reson Imaging. 2013;38:371–379. doi: 10.1002/jmri.23980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrews MW. Ultrasound of the spleen. World J Surg. 2000;24:183–187. doi: 10.1007/s002689910031. [DOI] [PubMed] [Google Scholar]
- 18.Al Mamari S, Djordjevic J, Halliday JS, Chapman RW. Improvement of serum alkaline phosphatase to <1.5 upper limit of normal predicts better outcome and reduced risk of cholangiocarcinoma in primary sclerosing cholangitis. J Hepatol. 2012 doi: 10.1016/j.jhep.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660–678. doi: 10.1002/hep.23294. [DOI] [PubMed] [Google Scholar]
- 20.Kim WR, Therneau TM, Wiesner RH, et al. A revised natural history model for primary sclerosing cholangitis. Mayo Clin Proc. 2000;75:688–694. doi: 10.4065/75.7.688. [DOI] [PubMed] [Google Scholar]
- 21.Contal C, O'Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data An. 1999;30:253–270. [Google Scholar]
- 22.Sanai FM, Keeffe EB. Liver biopsy for histological assessment: The case against. Saudi J Gastroenterol. 2010;16:124–132. doi: 10.4103/1319-3767.61244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shire NJ, Yin M, Chen J, et al. Test-retest repeatability of MR elastography for noninvasive liver fibrosis assessment in hepatitis C. J Magn Reson Imaging. 2011;34:947–955. doi: 10.1002/jmri.22716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaya M, Petersen BT, Angulo P, et al. Balloon dilation compared to stenting of dominant strictures in primary sclerosing cholangitis. Am J Gastroenterol. 2001;96:1059–1066. doi: 10.1111/j.1572-0241.2001.03690.x. [DOI] [PubMed] [Google Scholar]
- 25.Stiehl A, Rudolph G, Kloters-Plachky P, Sauer P, Walker S. Development of dominant bile duct stenoses in patients with primary sclerosing cholangitis treated with ursodeoxycholic acid: outcome after endoscopic treatment. J Hepatol. 2002;36:151–156. doi: 10.1016/s0168-8278(01)00251-3. [DOI] [PubMed] [Google Scholar]
- 26.Recio E, Macias J, Rivero-Juarez A, et al. Liver stiffness correlates with Child-Pugh-Turcotte and MELD scores in HIV/hepatitis C virus-coinfected patients with cirrhosis. Liver Int. 2012;32:1031–1032. doi: 10.1111/j.1478-3231.2012.02782.x. [DOI] [PubMed] [Google Scholar]
- 27.Fung J, Lai CL, Fong DY, Yuen JC, Wong DK, Yuen MF. Correlation of liver biochemistry with liver stiffness in chronic hepatitis B and development of a predictive model for liver fibrosis. Liver Int. 2008;28:1408–1416. doi: 10.1111/j.1478-3231.2008.01784.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Time to Hepatic Decompensation after Excluding Those with Untreated Dominant Stricture
Supplementary Figure 2. Time to Hepatic Decompensation after Excluding Those with Hepatic Decompensation within 30 days of Elastography


