Abstract
Increased energy or activity is now an essential feature of the mania of Bipolar Disorder (BD) according to DSM-5. This study examined whether objective measures of increased energy can differentiate manic BD individuals and provide greater diagnostic accuracy compared to rating scales, extending the work of previous studies with smaller samples. We also tested the relationship between objective measures of energy and rating scales. 50 hospitalized manic BD patients were compared to healthy subjects (HCS, n=39) in the human Behavioral Pattern Monitor (hBPM) which quantifies motor activity and goal-directed behavior in an environment containing novel stimuli. Archival hBPM data from 17 schizophrenia patients were used in sensitivity and specificity analyses.
Manic BD patients exhibited higher motor activity than HCS and higher novel object interactions. hBPM activity measures were not correlated with observer-rated symptoms, and hBPM activity was more sensitive in accurately classifying hospitalized BD subjects than observer ratings. Although the findings can only be generalized to inpatient populations, they suggest that increased energy, particularly specific and goal-directed exploration, is a distinguishing feature of BD mania and is best quantified by objective measures of motor activity. A better understanding is needed of the biological underpinnings of this cardinal feature.
Keywords: Bipolar disorder, Activity, Energy, DSM-5, Behavioral pattern monitor, Mania
1. Introduction
Bipolar Disorder (BD) is a highly recurrent and chronic psychiatric disorder that is characterized by states of mania. This illness represents a national health issue because it is associated with high mortality and morbidity (Post et al., 2003). While many observations suggested that elevated motor activity and increased energy appear to be a key hallmark of mania, and one that distinguishes BD from other psychiatric disorders such as schizophrenia (Cheniaux et al., 2014; Perry et al., 2009), these phenomena have only recently been incorporated as cardinal diagnostic criteria for BD. The newly released Diagnostic and Statistical Manual-5 (DSM-5) includes “increased energy” as an essential feature that must be present for most of the day to meet criteria for the manic episode.
The change to include increased energy or activity as a core symptom of the BD diagnosis is consistent with the self-report of manic patients who often describe that they have more energy than usual and are observed to be physically restless, fidgeting and changing posture and position frequently. Furthermore, the rapid control of agitation and impulsivity are primary treatment goals during a manic episode (APA, 2006). Given the importance of increased motor activity as a primary BD symptom it is surprising how few studies have measured hyperactivity in mania. The majority of studies have typically relied on subjective self-report and observer rated symptom scales. Symptom ratings may not be the most sensitive means of measuring motor hyperactivity as clinician observations and self-report of physical activity are typically unreliable (Sims et al., 1999). Therefore, there is a significant need to develop and apply objective and accurate measures that will improve and refine how we assess increased activity. Furthermore, an objective method of quantifying motor activity would allow for careful treatment studies.
Current methods available to quantitatively assess motor activity involve ambulatory monitoring with actigraphy (Krane-Gartiser et al., 2014; Teicher, 1995). We developed the human Behavioral Pattern Monitor (hBPM), a human version of the classic animal open field test, in order to obtain objective measurements of motor activity and novelty seeking in patients with a range of psychiatric conditions (Minassian et al., 2010; Perry et al., 2010). In this paradigm, subjects are placed into a novel environment that contains engaging, interactive objects intended to promote exploration. We have previously shown that small samples of patients with BD (n=12 to 15) exhibit a pattern of elevated motor activity and object interactions in the hBPM when compared to non-patients, indicating amplified novelty seeking and goal-directed behavior (Minassian et al., 2011; Perry et al., 2009). Our previous work using animal models of mania including dopamine transporter (DAT) knockdown mice and a specific DAT inhibitor, GBR12909, produced a behavioral profile consistent with observations of BD patients experiencing mania (Perry et al., 2009).
The purpose of the current study was to expand our previous work (Minassian et al., 2010, 2011; Perry, 2009) by: 1) examining whether the distinct patterns of increased activity in manic BD subjects are observed in larger samples of manic BD patients and healthy subjects. 2) In order to determine whether objectively quantified activity in the hBPM may have significant utility for classifying and discriminating between healthy and ill individuals, we also compared the sensitivity and specificity of observer-reported ratings of motor activity to hBPM measures in determining a BD diagnosis using healthy subjects and an archival group of schizophrenia patients (SCZ) as comparators. 3) Finally, using our objective quantification of motor activity, we aimed to determine whether there is a relationship between observer-rated symptom rating scales and measures obtained from the hBPM. Based on our previous work, we hypothesized that objective activity measurements would not be significantly correlated with subjective symptom rating scores (Minassian et al., 2010).
2. Methods
2.1. Participants
The University of California, San Diego (UCSD) School of Medicine's institutional review board approved the study. Participants included 50 individuals (26 males, 24 females) who were originally diagnosed using the DSM-IV as BD, Current Episode Manic. Diagnosis was made using the Structured Clinical Interview for DSM-IV (SCID-IV) (First et al., 1994). Subjects were tested within an average of 5.02 (74.96) days of admission to the inpatient psychiatric service at UCSD Medical Center. By nature of their acute inpatient hospitalization these patients were highly symptomatic. Healthy comparison subjects (HCS) who did not have a history of psychiatric illness as determined by the SCID and did not have a first-degree relative with a bipolar disorder were recruited from the community (n=39; 15 males, 24 females). We previously established a 98% agreement for determining Axis I diagnoses using the SCID-IV (Perry et al., 2001). Potential participants were excluded for: a primary psychotic disorder as determined by the SCID, neurological illness or head trauma, unstable medical illness, drug abuse or dependence within the past 6 months, treatment with electroconvulsive therapy, or a positive result on a toxicology screen with the exception of tetrahydrocannabinol (THC). Eleven patients who tested positive for THC completed the procedures but these subjects did not meet abuse or dependence criteria and had not used THC in the 48-hour period prior to testing. A subset of the sample in this study (15 manic BD, 26 HCS) has been presented in previous reports (Minassian et al., 2010; Minassian et al., 2011; Perry et al., 2009).
Because the manic BD diagnoses were determined using DSM-IV criteria, we conducted a retrospective chart review on all manic BD subjects to determine whether their symptoms also met DSM-5 criteria. Multiple sources, including extensive hospital chart notes, the SCID, the Young Mania Rating Scale (YMRS; (Young et al., 1978)), and the Brief Psychiatric Rating Scale (BPRS; (Overall and Gorham, 1976)) were used to determine the percentage of patients who also met DSM-5 criteria for a manic episode. Endorsement of either item A93 (increased activity) or A94 (psychomotor agitation), or both, on the SCID interview was considered sufficient to meet the DSM-5 criterion of increased activity, given that the DSM-5 text includes the following statement when providing exemplars of the increased activity symptom: “They often display psychomotor agitation or restlessness (i.e., purposeless activity)” (DSM-5, 2013).
For the purposes of the sensitivity and specificity analyses, archival hBPM and YMRS data from 17 patients with SCZ were re-examined. These participants have been described previously (Minassian et al., 2010; Perry et al., 2009). Briefly, they were recruited from the UCSD inpatient psychiatric service and were tested within the first 5 days of admission. All SCZ participants met DSM-IV criteria for schizophrenia using the SCID-IV.
2.2. Procedure
Prior to entering the hBPM, participants were fitted with an ambulatory monitoring device worn on the torso that quantifies motor activity via triaxial accelerometer output (Hidalgo, 2010; Vivometrics, 2002). Encrypted data were stored on a removable memory card and subsequently extracted and analyzed with the Vivosense™ proprietary PC-based software (version 2.7). Mean acceleration values were generated from a filtered summation of movement on the x, y, and z axes while incorporating force effects due to gravity. After being fit with the monitor, individuals were placed in the hBPM for 15 min without explicit directions except to wait for the experimenter to prepare another task.
The hBPM is a 9′ by 14′ room to which the subject has not been previously exposed, containing several items of furniture placed along the periphery including small and large filing cabinets, two sets of bookshelves, but no chairs. A desk was included in the room for the majority of subjects (n=38 out of 40 BD patients and n=27 out of 39 HCS; χ=8.99, p=0.003) but later removed to discourage sitting and encourage exploration. Previous findings indicated that the absence or inclusion of this piece of furniture did not interact with group status for any of the hBPM measures, so both conditions were combined for all data analyses (Henry et al., 2013). Dispersed evenly in the hBPM are several small objects that were chosen using the criteria that they are safe, colorful, tactile, and manipulable and therefore likely to invite exploration (e.g., feather mask, stuffed animal toys, rain stick). Subjects remained in the hBPM for 15 min and were monitored by a digital video camera embedded in the ceiling. Participants were asked to wait in the room while we prepared another test, but were not provided with any other instructions.
Following the hBPM session, subjects were returned to the laboratory where other testing, including administration of symptom rating scales, was completed. Since physiologic parameters can be impacted by body weight, height and weight were collected for the calculation of Body Mass Index (BMI). Diagnostic ratings, clinical ratings, and hBPM testing were all conducted on the same day.
2.3. Data analysis
Mean acceleration in digital units was derived for each of the three 5-minute epochs of the hBPM session; mean values of acceleration are a common and accepted method with which to determine static and dynamic motor activity (Godfrey et al., 2008). The data were inspected for normality and homogeneity of variance. Mean acceleration was analyzed using analysis of covariance (ANCOVA); years of education was included as a covariate. Percentage habituation was calculated using the following formula: percentage habituation=100 (acceleration in epoch 3/acceleration in epoch 1) × 100. Object interactions in the hBPM were quantified manually by trained raters blind to group condition who evaluated participant exploration in 1-second increments during the video recording. We have previously established interrater reliability for hBPM video ratings; kappa reliability coefficients for rater-coded measures range from 0.91 to 0.96 (Perry et al., 2010). We quantified the total number of object interactions, which were defined as deliberate physical contact with a novel object with any part of the body (e.g., hand, foot).
To determine the sensitivity and specificity of YMRS and hBPM measures in distinguishing BD subjects from healthy subjects and patients with schizophrenia, participants from all groups were categorized on two dimensions: a) whether their score on Item 2 of the YMRS (Increased Motor Activity/Energy) was 3 or greater (signifies “excessive energy, hyperactive, can be calmed”); and b) whether the subject's acceleration or number of object interactions was greater than 2 standard deviations above the mean of comparison subjects. Two standard deviations, which is a commonly used cut-off that accounts for 95% of values in normally distributed data, was chosen in an effort to capture markedly increased motor activity and energy, as does the “3″ rating on the YMRS. For each dimension, participants were classified as either “true positive” (fulfills the dimension and has BD), “false positive” (fulfills the dimension but is a comparison or SCZ subject), “false negative” (does not fulfill the dimension but has BD) and “true negative” (does not have the dimension and is a comparison subject or SCZ).
Pearson r correlation coefficients measured the relationship between motor activity and symptom scores. Statistical analyses were conducted with SPSS version 22.0 (IBM Corporation, 2013).
3. Results
3.1. Demographics and diagnostic criteria
A total of 50 patients met the DSM-IV criteria for either a manic (n=47) or mixed episode (n=3). HCS and BD participants were not significantly different in age [t(87)=1.79, NS] or BMI [t(85)=1.78, NS]. The HCS had slightly more years of education than BD patients [mean difference =1.20 years, t (87)=3.01, p=0.003]. The majority of BD patients were taking an antipsychotic medication plus a mood stabilizer [n=32 (64.0%)] or an antipsychotic medication alone [n=12 (24%)]. Four patients (8%) were prescribed a mood stabilizer alone and two patients were unmedicated at the time of testing (4%). See Table 1 for participant demographic information. The SCZ patients used in the sensitivity and specificity analyses have been described previously (Minassian et al., 2010; Perry et al., 2009).
Table 1.
Demographic information, clinical features and medications for manic bipolar disorder (BD) subjects and healthy comparison subjects (HCS).
| BD patients | HCS | Group differences | |
|---|---|---|---|
| Gender | 26M, 24F | 15M, 24F | ns |
| Age (years) | 34.2 (12.7) | 29.6 (10.7) | ns |
| Years of education | 13.8 (2.1) | 15.0 (1.6) | t (87) = 3.01, p = .003 |
| Body Mass Index | 27.4 (7.4)a | 25.0 (5.0) | ns |
| Clinical features | |||
| Age of onset of illness | 22.7 (8.4)b | - | - |
| Duration of illness (years) | 11.1 (10.0)b | - | - |
| Number of days in treatment | 5.02 (4.96) | - | - |
| YMRS total score | 26.0 (10.3) | - | - |
| BPRS total score | 37.0 (12.9) | - | - |
| Medication at time of testing | |||
| Antipsychotic alone | n = 12 (24%) | - | - |
| Mood stabilizer alone | n = 4 (8%) | - | - |
| Antipsychotic plus mood stabilizer | n = 32 (64.0%) | - | - |
| Not medicated | n = 2 (4%) | - | - |
| Additional Medications | |||
| Benzodiazepine | n = 24 (48%) | - | - |
| Antidepressant | n = 1 (2%) | - | - |
For all variables except gender and medications at time of testing values in the first column represent means and standard deviations.
BD = Bipolar Disorder, HCS = healthy comparison subject, M = male, F = female, ns = not significant, YMRS = Young Mania Rating Scale, BPRS = Brief Psychiatric Rating Scale.
Weight and height data were not available for two patients.
Age of onset and duration of illness could not be determined for three patients due to disorganized symptoms at the time of testing.
When the full DSM-5 criteria were applied in that either item A93 (increased activity) or item A94 (psychomotor agitation) was endorsed, 49 out of 50 patients (98%) continued to meet the criteria for mania, including two patients with mixed features (manic episode n=47, mixed features n=2), leaving only one patient (2%) who no longer met the criteria for a manic episode. Fifteen patients had item A94 endorsed but not item A93, thus 30% of DSM-IV-diagnosed manic BD patients evidenced psychomotor agitation without observable increased goal-directed activity during the SCID interview.
3.2. Motor activity
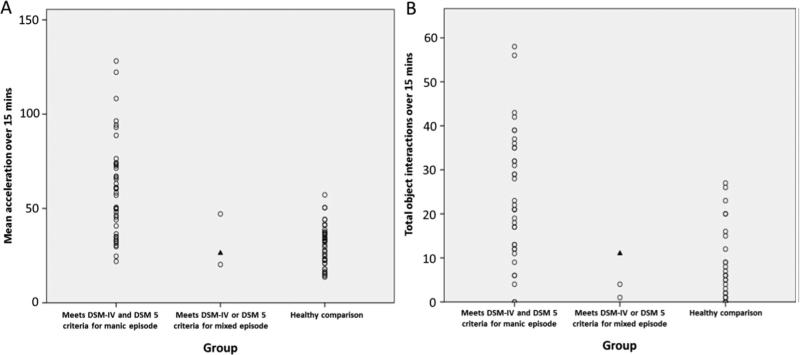
Five BD patients declined to complete the entire duration of hBPM testing. Acceleration data from one HCS was greater than 2 standard deviations from the group mean; 15-minute and 5-minute epoch acceleration data for this patient were replaced by the Winsorized mean in motor activity analyses. Manic BD subjects had significantly higher motor activity than HCS [F=30.55, df(1,79), p<0.001, partial η2=0.28] (Table 2). BD patients who met criteria for a mixed episode appeared to exhibit less motor activity than those who met criteria for a manic episode without mixed characteristics (Fig. 1(A)); however, there were insufficient numbers in the mixed episode group to compare patient group means.
Table 2.
Motor activity and object interactions in the hBPM for manic BD subjects and healthy comparison subjects.
| hBPM measures | BD (n = 44) | HCS (n = 38) | Group differences |
||
|---|---|---|---|---|---|
| Accelerationa | M (SE) | M (SE) | F (df) | p-value | partial η2 |
| Epochs 1-3b | 57.77 (3.17) | 31.34 (3.38) | 30.55 (1,79) | < 0.001 | 0.28 |
| Epoch 1c | 57.87 (3.41) | 35.69 (3.41) | 21.18 (1,79) | < 0.001 | 0.21 |
| Epoch 2d | 61.36 (4.10) | 29.62 (4.37) | 26.41 (1,79) | < 0.001 | 0.25 |
| Epoch 3e | 55.27 (4.07) | 29.62 (4.34) | 17.42 (1,79) | < 0.001 | 0.18 |
| % Habituation | −6.54 (10.97) | 6.15 (11.70) | 0.59 (1,79) | 0.45 | 0.01 |
| Object interactions | BD (n = 38) | HCS (n = 38) | Group differences |
||
|---|---|---|---|---|---|
| F (df) | p-value | partial η2 | |||
| Epochs 1-3b | 23.11 (2.01) | 5.84 (1.99) | 35.23 (1,73) | < 0.001 | 0.32 |
| Epoch 1c | 10.80 (1.00) | 2.56 (0.99) | 32.27 (1,73) | < 0.001 | 0.30 |
| Epoch 2d | 8.93 (0.90) | 1.69 (0.89) | 30.87 (1,73) | < 0.001 | 0.29 |
| Epoch 3e | 4.98 (0.82) | 1.80 (0.81) | 7.42 (1,73) | 0.01 | 0.09 |
Analysis of covariance (ANCOVA) conducted with years of education as a covariate. BD = Bipolar Disorder, hBPM = human Behavioral Pattern Monitor, HCS = healthy comparison subjects, M = estimated marginal mean, SE = standard error.
Acceleration measured in digital units (estimated marginal mean, standard error).
Epochs 1-3 = 1-15 min.
Epoch 1 = 1-5 min.
Epoch 2 = 5-10 min.
Epoch 3 = 10-15 min.
Fig. 1.
Motor activity (acceleration) and object interactions in the human Behavioral Pattern Monitor (hBPM) across diagnostic categories. A: Motor activity in the hBPM over 15 min for each diagnostic category; BD patients who met criteria for a manic episode (n=42), BD patients who met criteria for a mixed episode (n=3) and healthy comparison subjects (n=38). B: Object interactions in the hBPM for each diagnostic category; BD patients who met 5 criteria for a manic or mixed episode (n=36), BD patients who met criteria for a mixed episode (n=3) and healthy comparison subjects (n=38). Note: One patient met the DSM-IV but not DSM 5 criteria for a mixed episode, indicated by the ▲ symbol in both graphs.
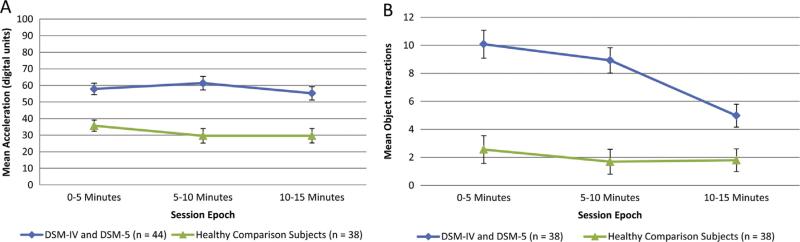
Planned comparisons indicated that manic BD patients exhibited more activity than HCS during each of the three 5-minute epochs (partial η2=0.18–0.25, p<0.001; Table 2 and Fig. 2(A)). The mixed ANCOVA on motor activity revealed a strong main effect of group [F=30.97, df (1,79), p<0.001, partial η2=0.28 but no effect of epoch or group-by-epoch interaction. There was no significant group difference in habituation, or percentage decrease of acceleration, over the three epochs.
Fig. 2.
Motor Activity (acceleration) and object interactions across the hBPM session for BD subjects and healthy comparison subjects. A: Motor activity by epoch in the hBPM for BD patients who met criteria for a manic or mixed episode (n=44) and healthy comparison subjects (n=38). B: Object interactions by epoch in the hBPM for BD patients who met criteria for a manic or mixed episode (n=38) and healthy comparison subjects (n=38). Data are estimated marginal means with years of education as a covariate.
3.3. Object interactions
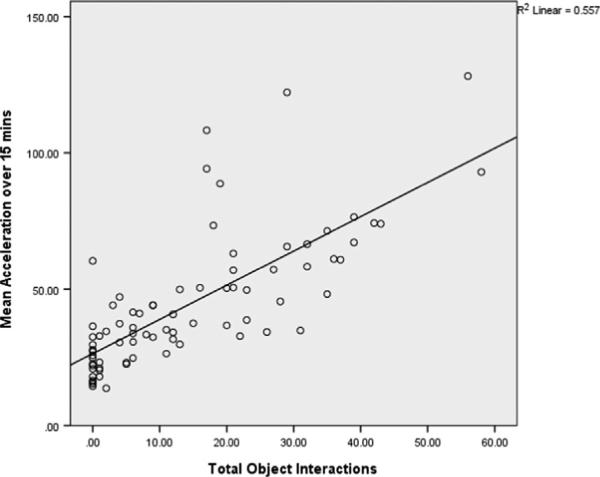
Object interaction data was not available for five BD patients. Manic BD patients had a significantly higher number of object interactions than HCS [mean difference =17.27, SE =2.91, F=35.23, df(1,73), p<0.001] (Table 2). BD patients who met criteria for a mixed episode appear to have had fewer object interactions than those who met criteria for a manic episode; however there were insufficient numbers in the mixed episode group to compare patient group means (Fig. 1(B)). There was a significant positive correlation between total object interactions and mean acceleration (n=77, r=0.75 p<0.001; Fig. 3).
Fig. 3.
Relationship between motor activity (acceleration) and object interactions in manic BD subjects. Positive correlation between motor activity and object interactions in the hBPM; BD patients who met criteria for a manic or mixed episode (n=38), one BD patient who only met criteria for a mixed episode (n=1) and healthy comparison subjects (n=38), r=0.75, p<0.001.
BD patients who met criteria for a manic or mixed episode exhibited more object interactions than HCS during each of the three 5-minute epochs (partial η2=0.09–0.30, p<0.01; Table 2 and Fig. 2(B)). The mixed ANCOVA on object interactions revealed a strong main effect of group [F=38.46, df (1,73), p<0.001, partial η2=0.35] but no effect of epoch or group-by-epoch interaction.
3.4. Sensitivity and specificity
A YMRS Item 2 score of 3 or greater correctly classified 39.1% of BD subjects (“true positive” ). Of the HCS, 100% had a YMRS Item 2 score of less than 3, while 81% of SCZ had a YMRS Item 2 score of less than 3 (“true negative”). An acceleration or object interaction value greater than 2 standard deviations from the mean of HCS resulted in correct classification of 61.9% of BD subjects (“true positive”). 92.3% of the HCS and 59% of SCZ had acceleration and object interaction values within 2 standard deviations of the mean (“true negative” ). Thus, while high YMRS Item 2 scores are highly specific to the diagnosis of BD, use of hBPM activity measures resulted in higher sensitivity to the presence of BD than did the YMRS item.
3.5. Correlation with YMRS scores
Total YMRS scores
Total YMRS scores did not significantly correlate with hBPM measures of mean acceleration (r=0.03, p=0.83, n=45), percentage habituation (r=–0.04, p=0.81, n=45), and total object interactions (r=–0.17, p=0.32, n=39).
YMRS Item 2 – Increased Motor Activity/Energy
YMRS Item 2 did not significantly correlate with hBPM measures of mean acceleration (r=0.01, p=0.95, n=45), percentage habituation (r=0.04, p=0.79, n=45), and total object interactions (r=–0.09, p=0.59, n=39).
Correlation with BPRS Scores
BPRS scores did not significantly correlate with hBPM measures of mean acceleration (r=0.03, p=0.83, n=45), percentage habituation (r=0.01, p=0.93, n=45), and total object interactions (r= 0.14, p=0.39, n=39).
4. Discussion
The aims of this study were: 1) to determine whether objective quantification of increased energy, now a required symptom for the diagnosis of mania, is consistently exhibited in manic BD patients, building on earlier studies that were limited by very small cohort size (Minassian et al., 2011; Perry et al., 2009); 2) To examine the relative sensitivity and specificity of hBPM activity in identifying manic BD patients compared to HCS and schizophrenia patients; and 3) to assess whether there was a relationship between the categorical rating scales of motor activity based on observation and the objective activity measures in the hBPM. The results confirm that the majority of hospitalized patients with BD exhibited increased activity, object interaction (a proxy for specific exploration), and a persistent level of activity with a relative lack of habituation. It is important to note however that this pattern of results, while representative of this sample of hospitalized BD patients, was not observed in all subjects. Furthermore, in our previous published study with a small subset of the same subjects (n=15), we found the BD subjects evidenced decreases in motor activity across the epochs of the hBPM (Perry, 2009). The reason for these discrepant findings on habituation are not entirely clear, other than the most parsimonious explanation that the addition of subjects has allowed for a more valid characterization of the motor activity phenotype of mania. In further support of the assumption that increased energy is a core feature of the mania of BD (Cheniaux et al., 2014), almost all manic BD subjects who met DSM-IV criteria for a manic episode also met DSM-5 criteria. Thus there is a high degree of convergence between the DSM-IV and DSM-5 with respect to the defining feature of bipolar disorder. Interestingly, for one-third of the DSM-IV-diagnosed patients, psychomotor agitation was the primary activity-related symptom observed during the SCID interview, suggesting that in acutely hospitalized manic BD individuals agitation/restlessness may be a more overt and more easily-observed symptom than is goal-directed activity.
While most manic BD patients were observed to have either increased goal-directed activity or energy, there was no correlation between YMRS Item 2 (increased motor activity/energy) and hBPM measures of acceleration. This finding suggests that while behavioral observations are useful for detecting the presence or absence of a symptom, in general, rating scales (e.g., YMRS) may not allow for the accurate and sensitive quantification of these behaviors. This point is underscored by the observation that high hBPM activity resulted in higher sensitivity to differentiating between BD versus SCZ and comparison subjects than did a high rating on the activity item of the YMRS. The findings may also suggest that increased energy is to some extent context-dependent. In contrast to responses to a structured interview where psychomotor agitation may be more easily observed than is goal-directed activity, the hBPM is a novel environment that elicits exploration and activity and thus may be a more valid proxy for real-world novel situations where increased energy may manifest and result in functionally adverse consequences. It is interesting to note that increased hBPM activity was not highly specific to BD, rather a notable proportion of SCZ patients also demonstrated increased activity primarily in the form of acceleration but not object interactions. We have previously observed that a subgroup of people with SCZ are more motorically active in the hBPM, specifically they demonstrate less predictable movement patterns and less habituation of motor activity, and these may also be the patients who are more excitable and agitated on symptom-rating scales (Perry, 2009). Again, what appears to distinguish BD in both the manic and euthymic phases is the engagement in specific exploration and goal-directed behavior.
The importance of motor activity in BD has been long appreciated but understudied. For example, Klein and colleagues found that patients with remitted bipolar illness on lithium maintenance therapy who underwent placebo-controlled lithium discontinuation would experience a relapse into mania within the first 3 months after lithium discontinuation (Klein et al., 1992). The use of wrist-worn actigraphs before and after lithium discontinuation revealed that patients who relapsed had higher baseline levels of daytime motor activity than patients without relapse. They suggested that motor activity is a sensitive marker of subclinical manic tendencies and can be used to detect early relapse. Swann and colleagues found that some critical cognitive impairments of mania, such as impulsivity, were related more strongly to measures of motor activity than to mood states (Swann et al., 2008). Most recently, Krane-Gartiser and colleagues demonstrated distinctly different activity patterns in patients with BD in episodes of mania and depression, assessed by actigraphy and analyzed with linear and nonlinear mathematical methods (Krane-Gartiser et al., 2014). Collectively, these studies as well as work from our laboratory highlight the importance of studying motor activity.
A further advantage of studying hyperactive locomotor activity in mania is the development of adequate animal models to illuminate neurobiological systems and provide a direction for potential therapeutic targets (Young et al., 2010). Unlike grandiosity and irritability, which do not lend themselves to being studied in rodents, motor activity can be readily quantified. However, for motor activity to have acceptable translational potential, a paradigm must be used that objectively quantifies motor activity in humans and is sensitive to potentially subtle alterations in activity levels. Finally, having an objective means of quantifying motor activity offers promise for treatment studies.
The biological underpinnings of increased energy and motor activity are being studied across a variety of domains. Investigations using DAT inhibitors administered to non-patient participants while completing the hBPM paradigm are ongoing. Magnetic resonance imaging (MRI) and positron emission tomography (PET) studies performed in individuals with attention deficit hyperactivity disorder (ADHD) suggest that low DAT expression in the striatum is the primary source of neurobiological dysfunction resulting in increased motor activity (Diamond, 2005; Fusar-Poli et al., 2012; Jucaite et al., 2005). To the best of our knowledge, neuroimaging studies that aim to determine the source of increased energy in BD patients have not been conducted. Future studies should aim to identify structural and functional correlates of increased energy, measured using objective paradigms such as the hBPM, in BD mania.
The main limitation of this naturalistic study is that medication status was not controlled for; it is conceivable that psychotropic medications may impact motor activity and exploration patterns. It is important to note that the majority of BD patients were treated with antipsychotic and/or mood stabilizing medications at the time of testing, yet effect sizes for group differences between BD patients and comparison subjects were large. Still, if our sample were comprised of non-medicated manic BD patients, we may have expected that the sensitivity of the hBPM measures in correctly identifying mania may have been higher than the 62% sensitivity observed here. We have previously reported that, in euthymic BD subjects, treatment with a mood stabilizer attenuates motor activity but not object interactions (Henry et al., 2013), a pattern not dissimilar to the effects of chronic valproate treatment in a mouse model of mania (van Enkhuizen et al., 2013). Rodent studies hold some promise in illuminating the specific mechanisms of mood stabilizing agents with respect to these cardinal features of mania. The generalizability of these findings is limited to hospitalized inpatients. A further limitation is the relative restriction of range of YMRS item scores; this narrow range likely contributed to the weakness of the relationship between the increased activity item score and the hBPM measures. Finally, a larger sample size including multiple recruitment sites and inclusion of other states of BD (euthymic, hypomanic, depressed) as well as other psychiatric conditions could have broadened the generalizability of these findings.
In conclusion, increased energy and motor activity are prominent and co-occur with the cardinal mood symptoms of a manic episode in hospitalized patients; furthermore the objective quantification of motor activity is a sensitive means of detecting manic BD and may offer advantages when compared to symptom-rating scales. These findings underscore the value of motor activity when attempting to model mania in animals and highlight the relevance of physiological and ambulatory monitoring over reliance on patient reports and clinician impressions.
Acknowledgments
The authors thank Dustin Kreitner, M.S., for her contributions to this manuscript.
Role of funding source
This study was supported by two grants from the National Institute of Mental Health (R01 MH071916 and R21 MH097112).
Footnotes
Conflict of interest
The authors have no conflict of interest to declare.
References
- APA . Quick reference to the American Psychiatric Association practice guidelines for the treatment of psychiatric disorders: compendium 2006. American Psychiatric Pub.; 2006. [Google Scholar]
- Cheniaux E, Filgueiras A, Silva Rde A, Silveira LA, Nunes AL, Landeira-Fernandez J. Increased energy/activity, not mood changes, is the core feature of mania. J. Affect. Disord. 2014;152–154:256–261. doi: 10.1016/j.jad.2013.09.021. [DOI] [PubMed] [Google Scholar]
- Diamond A. Attention-deficit disorder (attention-deficit/hyperactivity disorder without hyperactivity): a neurobiologically and behaviorally distinct disorder from attention-deficit/hyperactivity disorder (with hyperactivity). Dev. Psychopathol. 2005;17(3):807–825. doi: 10.1017/S0954579405050388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DSM-5 . Diagnostic and Statistical Manual of Mental Health Disorders: DSM-5. 5th ed. American Psychiatric Publishing; Washington, DC.: 2013. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM-IV Disorders (SCID) Psychiatric Press; Washington D.C.: 1994. [Google Scholar]
- Fusar-Poli P, Rubia K, Rossi G, Sartori G, Balottin U. Striatal dopamine transporter alterations in ADHD: pathophysiology or adaptation to psychostimulants? A meta-analysis. Am. J. Psychiatry. 2012;169(3):264–272. doi: 10.1176/appi.ajp.2011.11060940. [DOI] [PubMed] [Google Scholar]
- Godfrey A, Conway R, Meagher D, OLaighin G. Direct measurement of human movement by accelerometry. Med. Eng. Phys. 2008;30(10):1364–1386. doi: 10.1016/j.medengphy.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Henry BL, Minassian A, Patt VM, Hua J, Young JW, Geyer MA, Perry W. Inhibitory deficits in euthymic bipolar disorder patients assessed in the human behavioral pattern monitor. J. Affect. Disord. 2013;150(3):948–954. doi: 10.1016/j.jad.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo . Equivital lifemonitor. Cambridgeshire, UK.: 2010. [Google Scholar]
- IBM Corporation . IBM SPSS Statistics for Windows, 22.0. IBM Corp.; Armonk, NY.: 2013. [Google Scholar]
- Jucaite A, Fernell E, Halldin C, Forssberg H, Farde L. Reduced midbrain dopamine transporter binding in male adolescents with attention-deficit/hyperactivity disorder: association between striatal dopamine markers and motor hyperactivity. Biol. Psychiatry. 2005;57(3):229–238. doi: 10.1016/j.biopsych.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Klein E, Lavie P, Meiraz R, Sadeh A, Lenox R. Increased motor activity and recurrent manic episodes: predictors of rapid relapse in remitted bipolar disorder patients after lithium discontinuation. Biol. Psychiatry. 1992;31(3):279–284. doi: 10.1016/0006-3223(92)90051-z. [DOI] [PubMed] [Google Scholar]
- Krane-Gartiser K, Henriksen TEG, Morken G, Vaaler A, Fasmer OB. Actigraphic assessment of motor activity in acutely admitted inpatients with bipolar disorder. PLoS One. 2014;9:2. doi: 10.1371/journal.pone.0089574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Henry BL, Geyer MA, Paulus MP, Young JW, Perry W. The quantitative assessment of motor activity in mania and schizophrenia. J. Affect. Disord. 2010;120(1–3):200–206. doi: 10.1016/j.jad.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Henry BL, Young JW, Masten V, Geyer MA, Perry W. Repeated assessment of exploration and novelty seeking in the human behavioral pattern monitor in bipolar disorder patients and healthy individuals. PLoS One. 2011;6(8):e24185. doi: 10.1371/journal.pone.0024185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall J, Gorham D. ECDEU Assessment Manual for Psychopharmacology (Revised Edition) USNIH, Psychopharmacology Research Branch; Rockville, MD: 1976. Brief psychiatric rating scale (BPRS) 1962. pp. 158–169. [Google Scholar]
- Perry W, Heaton RK, Potterat E, Roebuck T, Minassian A, Braff DL. Working memory in schizophrenia: transient “online” storage versus executive functioning. Schizophr. Bull. 2001;27(1):157–176. doi: 10.1093/oxfordjournals.schbul.a006854. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Henry B, Kincaid M, Young JW, Geyer MA. Quantifying over-activity in bipolar and achizophrenia patients in a human open field paradigm. Psychiatry Res. 2010;178(1):84–91. doi: 10.1016/j.psychres.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry W, Minassian A, Paulus MP, Young JW, Kincaid MJ, Ferguson EJ, Henry BL, Zhuang X, Masten VL, Sharp RF, Geyer MA. A reverse-translational study of dysfunctional exploration in psychiatric disorders: from mice to men. Arch. Gen. Psychiatry. 2009;66(10):1072–1080. doi: 10.1001/archgenpsychiatry.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post RM, Denicoff KD, Leverich GS, Altshuler LL, Frye MA, Suppes TM, Rush AJ, Keck Jr PE, McElroy SL, Luckenbaugh DA. Morbidity in 258 bipolar outpatients followed for 1 year with daily prospective ratings on the NIMH life chart method. J. Clin. Psychiatry. 2003;64(6):680–690. doi: 10.4088/jcp.v64n0610. [DOI] [PubMed] [Google Scholar]
- Sims J, Smith F, Duffy A, Hilton S. The vagaries of self-reports of physical activity: a problem revisited and addressed in a study of exercise promotion in the over 65s in general practice. Fam. Pract. 1999;16(2):152–157. doi: 10.1093/fampra/16.2.152. [DOI] [PubMed] [Google Scholar]
- Swann AC, Steinberg JL, Lijffijt M, Moeller FG. Impulsivity: differential relationship to depression and mania in bipolar disorder. J. Affect. Disord. 2008;106(3):241–248. doi: 10.1016/j.jad.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH. Actigraphy and motion analysis: new tools for psychiatry. Harv. Rev. Psychiatry. 1995;3(1):18–35. doi: 10.3109/10673229509017161. [DOI] [PubMed] [Google Scholar]
- van Enkhuizen J, Geyer MA, Kooistra K, Young JW. Chronic valproate attenuates some, but not all, facets of mania-like behaviour in mice. Int. J. Neuropsychopharmacol. 2013;16(5):1021–1031. doi: 10.1017/S1461145712001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivometrics . The LifeShirt System™. Ventura, CA.: 2002. [Google Scholar]
- Young JW, Goey AKL, Minassian A, Perry W, Paulus MP, Geyer MA. The mania-like exploratory profile in genetic dopamine transporter mouse models is diminished in a familiar environment and reinstated by subthreshold psychostimulant administration. Pharmacol. Biochem. Behav. 2010;96(1):7–15. doi: 10.1016/j.pbb.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R, Biggs J, Ziegler V, Meyer D. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry. 1978;133(5):429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]