Abstract
Recent findings have revealed roles for systemic and mucosal-resident memory CD8+ T cells in the orchestration of innate immune responses critical to host defense upon microbial infection. Here we integrate these findings into the current understanding of the molecular and cellular signals controlling memory CD8+ T cell reactivation, and the mechanisms by which these cells mediate effective protection in vivo. The picture that emerges presents memory CD8+ T cells as early sensors of danger signals, mediating protective immunity both through licensing of cellular effectors of the innate immune system and via the canonical functions associated with memory T cells. We discuss implications to the development of T cell vaccines and therapies, and highlight important areas in need of further investigation.
Introduction
Three major subsets of “classical” memory CD8+ T cells have emerged over the past 15 years which include central memory T cells (TCM), effector memory T cells (TEM) and more recently the tissue-resident memory T cells (TRM) [1–4]. Other subsets of activated/memory CD8+ T cells that do not match the classical definition of memory T cells also exist, yet these are respectively generated during chronic or latent infections, and defined as “exhausted” that are largely dysfunctional [5, 6], or “inflationary” T cells [7] that remain highly functional. These subsets of memory cells are largely defined by their phenotypic and functional features and discrete expression levels of master transcriptional regulators such as T-bet, Eomesodermin, Blimp-1, Bcl-6 and others that ultimately control the identity of many of them [8].
Much is known with regards to the signals that are required to prime naive conventional T cell receptor (TCR) αβ+ CD8+ T cells [1, 9–11] but there is still substantially less knowledge and understanding of what signals orchestrate the reactivation of antigen-experienced memory T cells, namely antigenic versus cytokinic and chemokinic signals, and which cell types are most essential in providing these signals and regulating these processes in lymphoid organs and mucosal tissues. Recent work have shed new light on these mechanisms and suggest memory CD8+ T cells can act as early “innate-like” sensors and effectors initiating local and systemic immune responses.
Since antigen-specific memory CD8+ T cells are both present in higher frequencies and exhibit improved functional features compared to naive counterparts, the common view is that memory CD8+ T cells mediate potent systemic and mucosal immunity through direct recognition and killing of infected and tumor cells [12]. More recent evidence, however, suggests that memory CD8+ T cells can also orchestrate rapid and powerful host protection by recruiting and activating multiple cellular effectors of the innate immune system. These findings support the importance of non-cytolytic mechanisms in host protection, yet the contribution of antigenic versus cytokinic and chemotactic signals in regulating these processes is not well characterized.
Herein we summarize emergent evidence supporting the significance of these processes for host immunological protection, also at the mucosal surfaces, and in the context of the various subsets of memory CD8+ T cells. We also highlight key questions that we believe require further investigation going forward.
Memory CD8+ T cells can act as potent “innate-like” sensors and effector cells
While induction and maintenance of memory CD8+ T cells is extensively investigated, the cues that orchestrate memory CD8+ T cell reactivation and effector response, and the sequences of events occurring during microbial pathogen recall infection are much less defined. In addition to classical cognate antigen stimulation, several signals regulate memory CD8+ T cell reactivation, namely cytokines and chemokines that are strategically and temporally produced by specific cell subsets. These non-cognate antigenic signals are also often referred to as “bystander activation” or “alarmin” signals. Through the sensing of cytokines and chemokines early during infection, memory CD8+ T cells already initiate various cell-intrinsic expression of genes, leading to their entry into cell cycle, expression of effector functions and their trafficking.
Proliferation and effector functions occurring following “innate sensing”
Initial reports reported that ‘memory-like’ CD8+ T cells (CD44hi) could respond to non-cognate antigenic signals in vitro by sensing the activating cytokines type I IFN and IL-15 [13, 14], and initiate subsequent proliferative (BrdU+) and survival (cell numbers) programs. Yet, it is only later that this interesting observation was extended to antigen-specific memory CD8+ T cells [15]. In this study, and contrary to the widely acknowledged dogma, memory CD8+ T cells induced after immunization with various model pathogens, i.e., the intracellular bacterium Listeria monocytogenes (Lm), the Vesicular Stomatitis (VSV) and the Vaccina (VV) viruses, were shown to respond very efficiently to the inflammatory cytokines IL-12 and IL-18, and differentiate into robust IFNγ-secreting effector cells [15]. Recent series of reports using both ex vivo and in vivo gain and loss of function approaches broaden these findings and illustrated that traceable, antigen-experienced bacteria- (Lm, Salmonella thyphimurium, Yersinia pseudotuberculosis) and virus- (LCMV, Influenza) specific memory CD8+ T cells could undergo rapid activation (CD69+CD25+CD44hi), initiate cell cycle (Ki67+), differentiate into IFNγ-producing and cytolytic (Granzyme B+) effector cells in response to inflammatory cytokines such as IL-12, IL-18, type I IFN and IL-15, similarly to that of natural killer cells [16–21]. Cytokine-activated memory CD8+ T cells also expressed cell-surface receptors shared with activated NK cells (i.e., NKG2D, NK1.1). Secretion of IFNγ which occurred independently of antigen, largely accounted for the observed protective effects [15, 17–19]: adoptive transfers of purified Ovalbumin- or LCMV-specific memory CD8+ T cells induced by immunization with Lm-Ova or LCMV respectively, into naïve mice subsequently challenged with WT Lm showed modest (~0.5–1 log) but reproducible antigen-independent levels of protection [15, 17]. The memory CD8+ T cells produced IFNγ overall promoted further recruitment and activation of multiple innate immune effector cells by enhancing secretion of chemokines (CCL2, CXCL1, CXCL10 and others) and IFNγ signaling to innate myeloid and lymphoid cells [22–24]. Sensing of cytokinic signals was also proposed to allow for cell-intrinsic “pre-activation” of host memory CD8+ T cells, making them “ready to go”, e.g., to initiate proliferation and possibly other functions upon further cognate antigen encounter [17, 25].
During infections with latent gamma herpes virus 68 or the murine cytomegalovirus (MCMV), low levels of IFNγ promoted an immune activating/polarizing state allowing for sustained antimicrobial macrophage/monocyte response to unrelated microbial infections [26]. While this study suggested no involvement of T cell-derived IFNγ (systemic depletion of T cells was used), it is possible that TRM present in tissues such as lungs and salivary glands - the major sites of viral replication for these infections- accounted for these interesting findings since TRM are not eliminated using systemic depleting mAb treatment [24, 27]. CMV-based immunizations also favor the development of inflationary, highly functional effector memory CD8+ T cells [28, 29] that can populate non-lymphoid tissues and establish robust TRM in the salivary glands [30, 31], and may account for these observations.
Rapid recruitment and trafficking occurring following “innate sensing”
An effective memory response requires mobilization of resting memory CD8+ T cells to the appropriate location, either from the blood (circulating pool) or inside injured tissues (resident as well as circulating pool), so that they can sense and mediate rapid protection of the host [27, 32–34]. Memory T cell access to secondary lymphoid organs (SLOs) and to non-lymphoid tissues from the blood, and to area of active infection inside the tissues, involves distinct mechanisms, namely adhesion and chemokine-dependent migration which are regulated by secreted cytokines and chemokines sensed by the memory CD8+ T cells (See Table I).
TABLE I.
| MECHANISMS | Cytokine-dependent | Antigen-dependent | MODELS | Refs. | |
|---|---|---|---|---|---|
| Selectins: P/E/L (on inflamed endotheliums) | Leukocyte rolling via adding sialyl Lewis X glycan (sLeX) on CD43, CD44, PSGL-1 | IL-15 (via type I IFN) | No (circulating TM) | LCMV, RSV, Lm, VV | 35, 36 |
| Integrins: LFA-1/CD11a (αLβ2) VLA1/4 (α1β1, α4β1) CD103 (αEβ7), etc | CD11a, E-cadherin, VCAM-1 | Type I IFN, IFNγ | No (circulating TM) | Sendai, Influenza, LCMV | 20, 21, 23 |
| Chemotaxis: CXCR3, CCR5 etc | CXCL9, CXCL10, CCL3, CCL5 | Type I IFN, IFNγ | No (circulating TM) | Lm, LCMV, VSV | 22, 32, 37, 38, 43, 44, 45 |
From blood, leukocytes access inflammed tissues by binding the glycan motif sialyl lewis X (sLeX) added on surface proteins to the selectins expressed on endothelia [35]. IL-15 secreted upon microbial stimulation drive de novo expression of a glucosyltransferase on the memory CD8+ T cells that generates core-2 O glycans, enabling the addition of sLeX glycans to cell surface proteins. This finding provided a molecular mechanism accounting for rapid antigen-independent, cytokine-mediated recruitment of circulating memory CD8+ T cells to inflammed tissues, here the lung [36].
Memory CD8+ T cell access from blood to inflammed tissues also involves surface integrins. In a model of Sendai and Influenza viruses immunizations and heterologous challenge infections, CD11ahi memory CD8+ T cells are recruited independently of TCR stimulation after sensing of type I IFN and cell-intrinsic STAT-1 signaling [20]. In LCMV-immunized mice, virus-specific memory CD8+ T cells accumulated in the submandiblar gland (SMG) independently of cognate antigen recognition via E-cadherin [21]. In contrast, the reactivation of CD8+ TRM generated by VV or LCMV systemic immunization required cognate T cell antigen stimulation to initiate early production of IFNγ which induced subsequent cell-intrinsic and -extrinsic VCAM-1 cell-surface upregulation and recruitment of virus-unrelated memory CD8+ T cells from the circulating pool [23].
Specific sets of chemotactic receptors are also highly expressed at the surface of memory CD8+ T cell subsets -namely CXCR3, CCR5, CCR7 and others- and contribute to their trafficking inside tissues so that they may fulfill further sensing functions. For instance, CXCR3 is one of the most important memory T cell chemotactic receptors to mediates antigen-independent chemotaxis in response to IFN-induced chemokines CXCL9 and CXCL10 [32]. In the spleen of mice immunized and secondary challenged with the intracellular bacterium Lm, the memory CD8+ T cells undergo rapid recruitment and relocalization from the red and white pulp area to the marginal zones and formed “effector” clusters [22, 37, 38] exactly where blood bacteria are phagocytosed by resident macrophages (F4/80+Moma-1+) [39, 40] and CD8α+ cDCs [41, 42]. The local secretion of CXCL9 by cDCs, possibly the XCR1+ subset which express intracellular CXCL9 during recall infection [37], may initiate the formation of such clusters of IFNγ-secreting memory T and innate cells. However, sustained secretion of CXCL9 is most likely derived from the massive influx of Ly6C+ monocytes that largely outnumber cDC and closely interact with IFNγ-producing memory T cells [22, 38]. In LCMV- or VSV-infected lymph nodes, CXCR3+ CD8+ TCM were shown to relocalize very quickly in close proximity to CD169+ subcapsular macrophages via CXCL10 produced in response to antiviral type I IFN [43]. Through memory CD8+ T cell-derived IFNγ, secretion of large amounts of CXCL9 from myeloid cells is further amplified and contribute to consolidating clusters of effector cells that can physically prevent pathogen spreading [43–45]. While evidence suggest initiation of this process does not require cognate antigen, how much IFNγ and subsequent CXCL9 is produced in an antigen-dependent versus independent manner still needs further investigations.
A recent report [46] using parabiosis experiments quite convincingly suggested the existence of a subset of SLOs CD69+ CD8+ TRM positioned at common antigen entry sites, e.g., the marginal zone and red pulp of the spleen and the subcapsular and medullary sinuses of LNs. These cells did not redistribute in response to “alarmin” signals, consistent with a role as contributors to the early IFNγ-dependent immune response and subsequent chemotaxis of other responsive immune cells.
Sentinel cells initiating memory CD8+ T cell sensing and effector response
While memory CD8+ T cells can directly sense cytokines and chemokines, and subsequently produce signals that amplify the immune response, production of original “alarmins” come from strategically localized tissue-resident sentinel cells as introduced above. The key initial study [47] conducted in the CD11c depleter mouse model [48] in which CD11c+ cells can be depleted upon diphtheria toxin (DT) injection, made the unexpected observation that CD11chi conventional dendritic cells (cDCs) control the reactivation of memory CD8+ T cells in vivo in Lm, VSV and VV models of immunizations and challenge infections. This finding could be extended to viral reactivation of latent Herpes Simplex Virus (HSV) in sensory dorsal root ganglia (DRG), in which DCs derived from circulating Gr1hi monocytes also controlled the reactivation of HSV-specific memory CD8+ T cells [49]. Both of these studies revealed the role of “DCs” of different origins in reactivating memory CD8+ T cells. While the latter report demonstrated cognate antigen requirement, neither of these works investigated the contribution of the various signals provided by the DCs.
More recent evidence established a direct link between antigen-presenting cells and both cytokinic and antigenic signals provided to the T cells [16, 17]. Production of activating cytokines requires innate sensing intrinsic to specific subsets of myeloid cells [16, 17, 37, 43, 44]. cDCs [16] including XCR1+ DCs [37], blood-derived inflammatory Ly6C+ monocytes [17], and F4/80hi and/or CD169+ tissue-resident macrophages may all provide the reactivating signals to the memory CD8+ T cells during challenge infections in vivo. cDC, and notably CD8α+ cDC, can act as Troyan horses carrying pathogens inside the spleen [41, 42, 50] thus may represent key initiators, while Ly6C+ monocytes and tissue-macrophages can quickly amplify and sustain the signals and an effective immune response. Pathogen capture by DC or other tissue-resident myeloid cells is documented in many distinct infections (such as Salmonella, VSV, VV, MCMV), therefore such scenario may be further extended. Mechanistically, the production of activating cytokines involves the inflammasomes [16, 17] (IL-18 production), MyD88 (IL-12) [51] and IRF3 [17] (type I IFN and subsequent IL-15) sensing pathways in these various innate cell types. The importance of cytokine-mediated-activation of memory CD8+ T cells was confirmed in multiple experimental systems including Lm, LCMV and VSV [16, 17, 43, 44], but also in the context of infections taking place in mucosal tissues (Sendai, Influenza, HSV) [20, 21, 36, 37, 52, 53]. Altogether these results support a model in which systemic circulating memory CD8+ T cells are the ones mostly responding to such cytokines. While current studies suggest TRM need antigen sensing to initiate the immune reaction [23, 24, 54], whether CD8+ TRM can also mount a response to inflammatory cues only and to which extent remains to be determined.
The role of antigenic signals
Sentinel cells act as initiators and not only provide cytokinic and chemotactic signals but also present cognate antigen to the memory CD8+ T cells. While most reports did not discriminate the contribution of antigen versus inflammatory signals given to the T cells, in the presence of the same antigen load in vivo, inflammation can boost effector [55] and memory [56] CD8+ T cell expansion and promotes their differentiation into robust effector cells (KLRG1hi). These works used either transferred peptide-pulsed DCs with or without adjuvant, or boosting pathogens providing all inflammatory stimuli except the cognate antigenic peptide as a result of a select mutation disrupting T cell epitope presentation by the pathogen.
While antigen-independent mechanisms initiate and enhance memory CD8+ T cell activation, it is clear that the presence of cognate antigen is necessary to achieve pathogen-specific host protection and sterilizing immunity. Despite evidence that NKG2D+ memory CD8+ T cells may contribute to lysis of damaged/stressed RAE1ε+ cells [57], antigen-independent activation, that include adhesion, recruitment and initial effector differentiation, most likely contribute to an early “innate-like” protective reponse that make the antigen-dependent memory response “fitter, faster and better” [17, 25]. Without cognate antigen, memory CD8+ T cells can initiate T cell proliferation [18, 21, 25], but the extent of their expansion remains limited and antigen is necessary to achieve full T cell expansion [17, 34, 37, 49]. In the initial hours following memory T cell reactivation and prior to T cell robust expansion, cognate antigen triggering allows both systemic (SLO) and tissue-resident (skin) memory CD8+ T cells to arrest and establish stable interactions with virus-infected cells [44, 52]. Antigen recognition also leads to sustained and prolonged secretion of cytokines such as IFNγ from CD8+ (and CD4+) TRM [24, 58], and the production of specific chemokines such as CCL3 [59–61], CCL4, CCL5 [23, 24, 60–63], that overall contribute to the recruitment and activation of multiple effectors of the immune system. For instance, antigen-dependent production of the chemokine CCL3 by memory CD8+ T cells leads to heterologous host protection against the parasite Leishmania major [60]. Recent studies [23, 54, 62] illustrated further such concept and the existence of an “antiviral state” in various models of viral immunizations and challenge infections. Using HSV, LCMV and VV as models, TRM (CD8+ and CD4+) initiated rapid pathogen sensing in the vaginal mucosa or the skin of vaccinated mice undergoing a secondary challenge infection. In these experimental systems, early antigen-dependent production of IFNγ by TRM led to rapid mobilization of both adaptive (T, B) and innate effector cells (NK cells, macrophages) which mediated comparable levels (~4 logs) of host protection against heterologous and homologous viral pathogen challenges. While the identity of the mucosa-resident sentinel cells initiating the response needs further investigations, tissue-resident macrophages clustering with virus-specific memory T cells in the vaginal mucosa of vaccinated mice may play such role [62]. These local clusters are constituted by CD11b+ CD64+ macrophages and lymphocytes -termed Myeloid Lymphocyte Clusters or “MLC”-inside the vaginal mucosa of vaccinated hosts. The MLCs were proposed to be maintained by low levels of TRM-derived IFNγ-here CD4+ TRM- that induces CCL5 expression in the resident macrophages, allowing to stabilize the MLCs. The discovery of such “physical” structures in mucosal tissues of vaccinated mice underlines the necessity to further understand the regulation of chemotactic mechanisms and how they are orchestrating memory T cell behaviors in situ. Also, whether local inflammatory signals, e.g., type I IFN, IL-12, IL-18 and IL-15 as found in SLOs and in the lung airways [20] [16, 17, 37], contribute to the reactivation of TRM in general and promote their sensing function remains unknown.
Collectively, current literature reveals that inflammatory and chemotactic signals from tissue-resident sentinel cells can promote reactivation of memory CD8+ T cells in vivo and their rapid “sensing and effector” functions in the absence of cognate T cell-antigen stimulation. The existence of various subsets of memory CD8+ T cells adds to the complexity of these processes and underlines the necessity to further dissect the regulation of memory CD8+ T cell activation and their subsets in the course microbial infections in vivo. Several scenarios need to be considered depending on the tissue, and whether host immunity involves TRM and/or the local recruitment of circulating memory CD8+ T cells. The identity of the myeloid cell subsets or other cell types producing the activating cytokines has only been studied in a limited number of experimental systems.
Memory CD8+ T cells can act as potent “orchestrators” and license cellular effectors of the innate immune system for protection
Memory CD8+ T cells mediate host protection through a variety of mechanisms that have been studied extensively. The major canonical mechanism by which CD8+ T cells are classically thought to operate is direct cytolysis and elimination of infected and tumor cells [12]. Production of effector cytokines (IFNγ, TNFα) and chemokines (CCL3, CCL4, CCL5, XCL1) is known to occur but the contribution of such “indirect” effector mechanisms is always considered to be of lesser importance when discussing of CD8+ T cells. Recent work however, challenge this view and highlight the importance of the coordinated implication of the cellular effectors of the innate immune system in mediating effective memory CD8+ T cell protection.
Early evidence for the importance of such indirect mechanism in effective host memory CD8+ T cell recall responses came from the study of the Lm infection model, in which protection of vaccinated hosts undergoing a secondary infection fully depends on memory CD8+ T cells [61, 64]. Yet memory CD8+ T cell-mediated protection neither required perforin nor Fas cytolysis of infected cells, but rather non-CD8-derived TNF [65]. The key role of TNF was confirmed using either TNF-neutralizing antibody-based reagents or a TNF conditional knockout mouse model in Lysozyme M+ cells (macrophages, monocytes and neutrophils) [66–68]. The major source of TNF was further identified to come from activated Ly6Chi monocytes, and to a lower extent from neutrophils [60]. Importantly, in this system, control of bacterial growth occurred within only a few hours (6–8 hours) post challenge infection, thus prior to memory CD8+ T cell clonal expansion [61], supporting the idea that memory cells, still in relatively low numbers, should act as early orchestrators of protective innate immune responses rather than as direct killers. Upon reactivation, memory CD8+ T cells quickly relocalized to the marginal zone area of the spleen which concentrates filtered blood bacteria, and form clusters with newly recruited innate immune effector cells including Ly6C+ monocytes, neutrophils and NK cells [22, 38] which help preventing bacterial spreading. Subsequent advanced dynamic studies visualizing memory versus naive CD8+ T cell-trafficking behavior in the lymph nodes of mice immunized with VV, VSV or LCMV, provided formal demonstration of the rapid relocalization of memory CD8+ T cells next to the sites of pathogen entry where CD169+ myeloid cells engulf pathogens, altogether preventing pathogen dissemination to the tissues [43–45]. The accumulation of innate effector cells in these clusters, as also described in the Lm model 15, represent an essential determinant of effective protection and pathogen clearance. Not only do these cells, and in particular Ly6Chi monocytes, secrete important amounts of essential chemotactic cues (CXCL9) and effector cytokines (TNF), but also do they express robust microbicidal effector functions such as reactive nitric and oxygen species (RNS, ROS) [22, 60, 61]. Autocrine and paracrine TNF enhances ROS production by monocytes and neutrophils, and antimicrobial autophagy. The adoptive transfers of WT but not of ROS deficient (Phox47−/−) phagocytes could rescue protection of Lm-vaccinated ROS-deficient mice [60]. While Ly6Chi monocytes emigration from the bone-marrow to the bloodstream is controlled by CCL2 produced by bone-marrow mesenchymal stem cells [69, 70], access to the spleen and the marginal zone clusters involved other mechanisms, possibly CX3CR1 [71], CCR1 [60] or other chemotactic cues not yet defined.
Production of IFNγ by “sensing” memory CD8+ T cells, initiated even in the absence of cognate antigen stimulation in SLOs, appears as one of the major key signals that switch on recall responses and the cellular effectors of the immune system [22–24, 45], both during systemic [16, 17] and mucosal infections [23, 24, 62] with bacteria or viruses. Most convincingly, IFNγ signaling to Ly6Chi monocytes and to some extend neutrophils, macrophages and subsets of DCs, directly control their capacity to secrete the key mediators of effective protection such as TNF and CXCL9 [22], which subsequently promote microbicidal response (ROS, autophagy) and effector cell recruitment. Lack of IFNγ receptor signaling in these subsets of myeloid cells or lack of memory T cell-derived IFNγ impairs protective recall response in the Lm model of vaccination and challenge infection. Perhaps in support of this concept, and contrasting to the established view [72], a recent study suggested that the rate of effector CD8+ T cell-killing during acute viral infection by intravital time-lapse biphoton microscopy, was only 2–16 target cells per day [73], a finding that suggest that direct killing in acute infections may not be the major mechanism of host protection. Collectively, this body of work highlights novel evidence for the importance of non-cytolytic, memory CD8+ T cell-dependent mechanisms of protection in vaccinated hosts.
Concluding Remarks and Future Directions
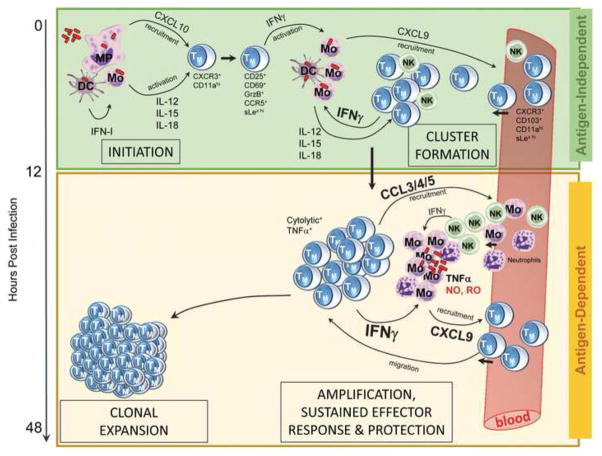
The concepts discussed in this review challenge the classical dogmas by which systemic and tissue-resident memory CD8+ T cells undergo reactivation and mediate protective immunity in vaccinated hosts. We present evidence suggesting that early activation of memory CD8+ T cells by cytokinic and chemotactic signals makes them act as true “innate sensors” that provides a rapid and efficient window of “innate immune” protection to infected hosts. We also discuss that TCR stimulation though cognate antigen recognition endows memory CD8+ T cells with additional functional features that are required to achieve full protection “indirectly”, by orchestrating innate immune cell antimicrobial effector response. We discuss what is known about the various sequences of events that need to take place and the different signals that control each of these steps (See Figure).
Figure 1. Illustration of memory CD8+ T cell-reactivation and protective immune response.
Schematic representation of the distinct phases and signals regulating pathogen-specific memory CD8+ T cell-reactivation and effective protection. Illustrated steps occur in secondary lymphoid organs, and for most part can be extended to mucosal tissues (See Review for specifics).
One important take home message is that memory CD8+ T cell “indirect” mechanisms of protection may indeed represent a major process by which memory CD8+ T cells mediate host defense and sterilizing immunity. CD8+ TRM [2–4] residing at various mucosal barriers (skin, vagina, lung and possibly others) were recently reported to orchestrate effective protection by promoting an “antiviral state”, which may obey similar cues as systemic circulating memory CD8+ T cells. A comparable network of cytokine and chemotactic “innate sensing” signals are likely to regulate TRM activation. Further investigations are needed to precisely understand how the various subsets of memory T cells respond to local and systemic signals of reactivation in vivo.
Amongst important unanswered questions are whether memory CD4+ T cells also respond to cytokine-mediated activation as a general functional feature. This has been shown during active Salmonella and Chlamydia infections [74, 75], but the extent of such characteristics for memory CD4+ T cells still remains to be determined. Prior work [53, 76] demonstrated essential roles for CD4+ TRM in orchestrating the protective mucosal response, but exact underlying mechanisms and whether activating cytokines and chemokines are contributing is not known. A recent report from the Kaech group [77] in a model of lung Influenza virus infection reported an essential role for IFNγ from effector CD4+ T cells in promoting establishment and localization of CD8+ TRM to the lung airways, a key location for effective recall response. As IFNγ does not directly act on CD8+ TRM, it may involve IFNγ-induced CXCL9 or CCL5 and similar mechanism may be at work for CD4+ TRM.
Lastly, another interesting concept outlined by this review is the acquisition of “non-cognate” memory CD8+ T cell functional features, which is particularly appealling, especially if one could induce populations of memory cells that can mediate broad, non-pathogen specific protection but very little is know as to how to drive -and potentiate?- such differentiation program in the memory T cells. A related question is whether such innate features are also acquired by memory CD4+ T cells.
BOX. Outline of limitations associated with the various experimental models.
We present below experimental systems used to establish the major concepts underpinning the focus of this review, and underline the limitations of the various models to study memory T cell functions. We also discuss how to move forward and which experimental systems should be used in the future in order to achieve next level of understanding of these complex processes.
Three major approaches have been utilized to study the signals that control memory CD8+ T cell reactivation and ability to protect:
First, adoptive transfers of TCR transgenic CD8+ T cells are traceable, have known antigenic specificity, and can form stable memory upon primary immunization. Such memory cells can be isolated from vaccinated hosts and retransferred into recipient mice to study cell-intrinsic features of these memory cells [17]. However, many potentials flaws are to be noted, in particular (i) loss of cells associated with isolation and purification steps, (ii) strategic localization of the memory cells most commonly isolated from SLOs of vaccinated hosts that may not traffic back to the same area of SLOs or tissue, (iii) skewing of results depending on the source of isolated memory cells -for instance a growing number of memory T cell subsets are described such as TRM, and it is conceivable that their isolation from SLOs is preferentially skewing the pool of isolated memory T cells towards central memory cells, (iv) the whole sequence of events leading to a protective recall response in a vaccinated host is very different than that of a naive host in which most of these experiments were conducted, and (v) the use of a single clonal T cell specificity is unlikely to mimic the behaviors of T cells with distinct antigenic specificities. The use of parabionts in many studies [46, 62], in particular to define TRM systemic behavior has provided great advances eliminating most of these flaws. Next steps should include the tracking of polyclonal T cells, using advanced tetramer enrichment and staining methods [78].
Second, the selective and conditional depletion of immune cell subsets rely on the use of monoclonal depleting antibody and “depleter” systems in which the human or simian diphtheria toxin receptor (DTR) is expressed under a “cell-specific” promoter such as CD11c, CCR2, XCR1 and others [37, 48, 79, 80]. Flaws include (i) non-specific effects related to massive mAb injections (often mg), (ii) lack of true cell subset specificity for almost all of these models as a result of the absence of markers specific for each population and (iii) toxicity associated with DT treatment and deletion of unexpected DTR+ cell subsets.
Third, the cell-specific deletion of a pathway or effector function that involves (i) the use of mixed bone-marrow chimeras in which lethally irradiated recipient wild type mice are reconstituted with a mix of bone-marrow cells isolated from a given depleter mouse and a knockout mouse of interest. Upon DT treatment only knockout cells are left [22]. Same limitations apply as with the regular depleter systems. Moreover having a proportion of the hematopoietic system lacking important pathways or effector mechanism can significantly affect outcomes and interpretation. A “cleaner” system (ii) includes the use of Cre/LoxP models in which the Cre recombinase is expressed under the control of a cell-specific promoter and crossed to mice modified to bear loxP excision sites flanking a gene of interest. Flaws still include cell-specificity of Cre expression and efficiency of Cre-mediated recombination that can be highly variable. More advanced systems to address these questions will need to take advantage of time-controlled promoters (inducible promoters, using tamoxifen or tetracyclin) which limits non-specific effects associated with cells lacking important functions during the duration of the experiment. Recent development of dual specificity depleter models in which only cells co-expressing two marker genes (here LysM and Cd115) will express the DTR and become sensitive to DT represent novel promising alternatives [81]. Finally, it is possible to engineer inducible CRISPR/Cas9 system [82] with chosen guided RNA of interest as a possibly faster alternative to knocking out genes of interest.
TREND BOX.
Memory CD8+ T cell can act as powerfull “sensors” of inflammatory cytokines (type I interferon, IL-15, IL-12, IL-18) released by sentinel cells upon pathogen invasion.
Memory CD8+ T cells can undergo potent differentiation into robust IFNγ and cytolytic effector cells in response to non-antigenic cytokinic signals and mediate modest but significant IFNγ-dependent protection.
Memory CD8+ T cells are initially recruited to infected tissues in a cognate antigen-independent manner. Inflammatory cytokines enhance expression of selectin ligands (sialyl lewis X) and integrins (CD11a, CD103, VLA1/4) allowing circulating memory T cell adhesion to endotheliums, and both TM and TRM chemotaxis (via CXCR3, CCR5) local infectious foci.
Memory CD8+ T cells achieve sustained antigen-dependent full protection by local recruitment (via CXCL9, CCL3, CCL5) and IFNγ-mediated activation of microbicidal functions (TNF, reactive nitric and oxygen intermediates) in multiple subsets of innate immune cells including Ly6C+ monocytes, neutrophils and NK cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cui W, Kaech SM. Generation of effector CD8+ T cells and their conversion to memory T cells. Immunol Rev. 2010;(236):151–66. doi: 10.1111/j.1600-065X.2010.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity. 2014;41(6):886–97. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin H, Iwasaki A. Tissue-resident memory T cells. Immunol Rev. 2013;255(1):165–81. doi: 10.1111/imr.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carbone FR. Tissue-Resident Memory T Cells and Fixed Immune Surveillance in Nonlymphoid Organs. J Immunol. 2015;195(1):17–22. doi: 10.4049/jimmunol.1500515. [DOI] [PubMed] [Google Scholar]
- 5.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–9. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 6.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–99. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Hara GA, et al. Memory T cell inflation: understanding cause and effect. Trends Immunol. 2012;33(2):84–90. doi: 10.1016/j.it.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Chang JT, Wherry EJ, Goldrath AW. Molecular regulation of effector and memory T cell differentiation. Nat Immunol. 2014;15(12):1104–15. doi: 10.1038/ni.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haring JS, V, Badovinac P, Harty JT. Inflaming the CD8+ T cell response. Immunity. 2006;25(1):19–29. doi: 10.1016/j.immuni.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;(25):171–92. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 11.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;(18):593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 12.Harty JT, Tvinnereim AR, White DW. CD8+ T cell effector mechanisms in resistance to infection. Annu Rev Immunol. 2000;(18):275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, et al. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8(5):591–9. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 14.Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272(5270):1947–50. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 15.Berg RE, et al. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J Exp Med. 2003;198(10):1583–93. doi: 10.1084/jem.20031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kupz A, et al. NLRC4 inflammasomes in dendritic cells regulate noncognate effector function by memory CD8(+) T cells. Nat Immunol. 2012 doi: 10.1038/ni.2195. [DOI] [PubMed] [Google Scholar]
- 17.Soudja SM, et al. Inflammatory Monocytes Activate Memory CD8(+) T and Innate NK Lymphocytes Independent of Cognate Antigen during Microbial Pathogen Invasion. Immunity. 2012;37(3):549–62. doi: 10.1016/j.immuni.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raue HP, et al. Cytokine-mediated programmed proliferation of virus-specific CD8(+) memory T cells. Immunity. 2013;38(1):131–9. doi: 10.1016/j.immuni.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruiz AL, et al. NK1.1+ CD8+ T cells escape TGF-beta control and contribute to early microbial pathogen response. Nat Commun. 2014;5:5150. doi: 10.1038/ncomms6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohlmeier JE, et al. Type I interferons regulate cytolytic activity of memory CD8(+) T cells in the lung airways during respiratory virus challenge. Immunity. 2010;33(1):96–105. doi: 10.1016/j.immuni.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofmann M, Pircher H. E-cadherin promotes accumulation of a unique memory CD8 T-cell population in murine salivary glands. Proc Natl Acad Sci U S A. 2011;108(40):16741–6. doi: 10.1073/pnas.1107200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soudja SM, et al. Memory-T-Cell-Derived Interferon-gamma Instructs Potent Innate Cell Activation for Protective Immunity. Immunity. 2014;40(6):974–88. doi: 10.1016/j.immuni.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schenkel JM, et al. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346(6205):98–101. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schenkel JM, et al. Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol. 2013;14(5):509–13. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richer MJ, et al. Inflammatory IL-15 is required for optimal memory T cell responses. J Clin Invest. 2015;125(9):3477–90. doi: 10.1172/JCI81261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barton ES, et al. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature. 2007;447(7142):326–9. doi: 10.1038/nature05762. [DOI] [PubMed] [Google Scholar]
- 27.Slutter B, et al. Lung airway-surveilling CXCR3(hi) memory CD8(+) T cells are critical for protection against influenza A virus. Immunity. 2013;39(5):939–48. doi: 10.1016/j.immuni.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snyder CM, et al. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity. 2008;29(4):650–9. doi: 10.1016/j.immuni.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karrer U, et al. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J Immunol. 2003;170(4):2022–9. doi: 10.4049/jimmunol.170.4.2022. [DOI] [PubMed] [Google Scholar]
- 30.Smith CJ, et al. Murine CMV Infection Induces the Continuous Production of Mucosal Resident T Cells. Cell Rep. 2015;13(6):1137–48. doi: 10.1016/j.celrep.2015.09.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thom JT, et al. The Salivary Gland Acts as a Sink for Tissue-Resident Memory CD8(+) T Cells, Facilitating Protection from Local Cytomegalovirus Infection. Cell Rep. 2015;13(6):1125–36. doi: 10.1016/j.celrep.2015.09.082. [DOI] [PubMed] [Google Scholar]
- 32.Guarda G, et al. L-selectin-negative CCR7- effector and memory CD8+ T cells enter reactive lymph nodes and kill dendritic cells. Nat Immunol. 2007;8(7):743–52. doi: 10.1038/ni1469. [DOI] [PubMed] [Google Scholar]
- 33.Kohlmeier JE, et al. The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections. Immunity. 2008;29(1):101–13. doi: 10.1016/j.immuni.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ely KH, et al. Nonspecific recruitment of memory CD8+ T cells to the lung airways during respiratory virus infections. J Immunol. 2003;170(3):1423–9. doi: 10.4049/jimmunol.170.3.1423. [DOI] [PubMed] [Google Scholar]
- 35.Nolz JC, Starbeck-Miller GR, Harty JT. Naive, effector and memory CD8 T-cell trafficking: parallels and distinctions. Immunotherapy. 2011;3(10):1223–33. doi: 10.2217/imt.11.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nolz JC, Harty JT. IL-15 regulates memory CD8+ T cell O-glycan synthesis and affects trafficking. J Clin Invest. 2014;124(3):1013–26. doi: 10.1172/JCI72039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alexandre YO, et al. XCR1+ dendritic cells promote memory CD8+ T cell recall upon secondary infections with Listeria monocytogenes or certain viruses. J Exp Med. 2015 doi: 10.1084/jem.20142350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bajenoff M, et al. Visualizing early splenic memory CD8+ T cells reactivation against intracellular bacteria in the mouse. PLoS One. 2010;5(7):e11524. doi: 10.1371/journal.pone.0011524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aoshi T, et al. The cellular niche of Listeria monocytogenes infection changes rapidly in the spleen. Eur J Immunol. 2009;39(2):417–25. doi: 10.1002/eji.200838718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muraille E, et al. Distinct in vivo dendritic cell activation by live versus killed Listeria monocytogenes. Eur J Immunol. 2005;35(5):1463–71. doi: 10.1002/eji.200526024. [DOI] [PubMed] [Google Scholar]
- 41.Neuenhahn M, et al. CD8alpha+ dendritic cells are required for efficient entry of Listeria monocytogenes into the spleen. Immunity. 2006;25(4):619–30. doi: 10.1016/j.immuni.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 42.Edelson BT, et al. CD8alpha(+) Dendritic Cells Are an Obligate Cellular Entry Point for Productive Infection by Listeria monocytogenes. Immunity. 2011;35(2):236–48. doi: 10.1016/j.immuni.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sung JH, et al. Chemokine guidance of central memory T cells is critical for antiviral recall responses in lymph nodes. Cell. 2012;150(6):1249–63. doi: 10.1016/j.cell.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kastenmuller W, et al. Peripheral Prepositioning and Local CXCL9 Chemokine-Mediated Guidance Orchestrate Rapid Memory CD8(+) T Cell Responses in the Lymph Node. Immunity. 2013 doi: 10.1016/j.immuni.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kastenmuller W, et al. A spatially-organized multicellular innate immune response in lymph nodes limits systemic pathogen spread. Cell. 2012;150(6):1235–48. doi: 10.1016/j.cell.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schenkel JM, Fraser KA, Masopust D. Cutting edge: resident memory CD8 T cells occupy frontline niches in secondary lymphoid organs. J Immunol. 2014;192(7):2961–4. doi: 10.4049/jimmunol.1400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zammit DJ, et al. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005;22(5):561–70. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jung S, et al. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17(2):211–20. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wakim LM, et al. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008;319(5860):198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 50.Dalod M, et al. Dendritic cell responses to early murine cytomegalovirus infection: subset functional specialization and differential regulation by interferon alpha/beta. J Exp Med. 2003;197(7):885–898. doi: 10.1084/jem.20021522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Serbina NV, et al. Sequential MyD88-independent and -dependent activation of innate immune responses to intracellular bacterial infection. Immunity. 2003;19(6):891–901. doi: 10.1016/s1074-7613(03)00330-3. [DOI] [PubMed] [Google Scholar]
- 52.Ariotti S, et al. Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. Proc Natl Acad Sci U S A. 2012;109(48):19739–44. doi: 10.1073/pnas.1208927109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gebhardt T, et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477(7363):216–9. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- 54.Ariotti S, et al. T cell memory. Skin-resident memory CD8(+) T cells trigger a state of tissue-wide pathogen alert. Science. 2014;346(6205):101–5. doi: 10.1126/science.1254803. [DOI] [PubMed] [Google Scholar]
- 55.Busch DH, Kerksiek KM, Pamer EG. Differing roles of inflammation and antigen in T cell proliferation and memory generation. J Immunol. 2000;164(8):4063–70. doi: 10.4049/jimmunol.164.8.4063. [DOI] [PubMed] [Google Scholar]
- 56.Wirth TC, et al. Secondary CD8+ T-cell responses are controlled by systemic inflammation. Eur J Immunol. 2011;41(5):1321–33. doi: 10.1002/eji.201040730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Markiewicz MA, et al. RAE1epsilon Ligand Expressed on Pancreatic Islets Recruits NKG2D Receptor-Expressing Cytotoxic T Cells Independent of T Cell Receptor Recognition. Immunity. 2012;36(1):132–41. doi: 10.1016/j.immuni.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakanishi Y, et al. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462(7272):510–3. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cook DN, et al. Requirement of MIP-1 alpha for an inflammatory response to viral infection. Science. 1995;269(5230):1583–5. doi: 10.1126/science.7667639. [DOI] [PubMed] [Google Scholar]
- 60.Narni-Mancinelli E, et al. Memory CD8+ T cells mediate antibacterial immunity via CCL3 activation of TNF/ROI+ phagocytes. J Exp Med. 2007;204(9):2075–87. doi: 10.1084/jem.20070204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Narni-Mancinelli E, et al. Inflammatory Monocytes and Neutrophils Are Licensed to Kill During Memory Responses In Vivo. PLoS Pathog. 2011:29. doi: 10.1371/journal.ppat.1002457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iijima N, Iwasaki A. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science. 2014;346(6205):93–8. doi: 10.1126/science.1257530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dorner BG, et al. MIP-1alpha, MIP-1beta, RANTES, and ATAC/lymphotactin function together with IFN-gamma as type 1 cytokines. Proc Natl Acad Sci U S A. 2002;99(9):6181–6. doi: 10.1073/pnas.092141999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lauvau G, et al. Priming of memory but not effector CD8 T cells by a killed bacterial vaccine. Science. 2001;294(5547):1735–9. doi: 10.1126/science.1064571. [DOI] [PubMed] [Google Scholar]
- 65.White DW, Harty JT. Perforin-deficient CD8+ T cells provide immunity to Listeria monocytogenes by a mechanism that is independent of CD95 and IFN-gamma but requires TNF-alpha. J Immunol. 1998;160(2):898–905. [PubMed] [Google Scholar]
- 66.Neighbors M, et al. A critical role for interleukin 18 in primary and memory effector responses to Listeria monocytogenes that extends beyond its effects on Interferon gamma production. J Exp Med. 2001;194(3):343–54. doi: 10.1084/jem.194.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Samsom JN, et al. Tumour necrosis factor, but not interferon-gamma, is essential for acquired resistance to Listeria monocytogenes during a secondary infection in mice. Immunology. 1995;86(2):256–62. [PMC free article] [PubMed] [Google Scholar]
- 68.Grivennikov SI, et al. Distinct and nonredundant in vivo functions of TNF produced by t cells and macrophages/neutrophils: protective and deleterious effects. Immunity. 2005;22(1):93–104. doi: 10.1016/j.immuni.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 69.Shi C, et al. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34(4):590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7(3):311–7. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 71.Auffray C, et al. CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J Exp Med. 2009;206(3):595–606. doi: 10.1084/jem.20081385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barber DL, Wherry EJ, Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J Immunol. 2003;171(1):27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
- 73.Halle S, et al. In Vivo Killing Capacity of Cytotoxic T Cells Is Limited and Involves Dynamic Interactions and T Cell Cooperativity. Immunity. 2016;44(2):233–45. doi: 10.1016/j.immuni.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Srinivasan A, et al. Innate immune activation of CD4 T cells in salmonella-infected mice is dependent on IL-18. J Immunol. 2007;178(10):6342–9. doi: 10.4049/jimmunol.178.10.6342. [DOI] [PubMed] [Google Scholar]
- 75.O’Donnell H, et al. Toll-like receptor and inflammasome signals converge to amplify the innate bactericidal capacity of T helper 1 cells. Immunity. 2014;40(2):213–24. doi: 10.1016/j.immuni.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature. 2012;491(7424):463–7. doi: 10.1038/nature11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laidlaw BJ, et al. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity. 2014;41(4):633–45. doi: 10.1016/j.immuni.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moon JJ, et al. Tracking epitope-specific T cells. Nat Protoc. 2009;4(4):565–81. doi: 10.1038/nprot.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hohl TM, et al. Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell Host Microbe. 2009;6(5):470–81. doi: 10.1016/j.chom.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hildner K, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322(5904):1097–100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schreiber HA, et al. Intestinal monocytes and macrophages are required for T cell polarization in response to Citrobacter rodentium. J Exp Med. 2013;210(10):2025–39. doi: 10.1084/jem.20130903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dow LE, et al. Inducible in vivo genome editing with CRISPR-Cas9. Nat Biotechnol. 2015;33(4):390–4. doi: 10.1038/nbt.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]