Introduction
Neovascular eye diseases result from signals to increase the vascular supply, which can be caused by hypoxia or by inadequate metabolism for the needs of the retina[1]. Recent reports suggest that changes in the most abundant circulating adipokines such as leptin and adiponectin (APN), which are actively involved in metabolic modulation, may contribute to the development of neovascular eye diseases. For example, high levels of the adipocyte-derived hormone leptin which regulates energy homeostasis, increases vascular endothelial cell oxidative stress and contributes to endothelial cell dysfunction in retinopathy[2]. Accumulating evidence shows that APN, which is another important metabolic modulator also derived mainly from adipocytes, is associated with many retinal metabolic disorders. Circulating APN levels are correlated with the development and progression of retinopathy of prematurity [3], diabetic retinopathy [4, 5], and age-related macular degeneration[6]. Animal studies indicate that increasing circulating APN levels suppress pathological vascular proliferation in rodent models of oxygen-induced proliferative retinopathy and laser-induced choroidal neovascularization[7, 8]. We will review this emerging field of APN in retinal neovascular diseases with an emphasis on current investigations and pertinent research questions.
Adiponectin (APN)
Structure
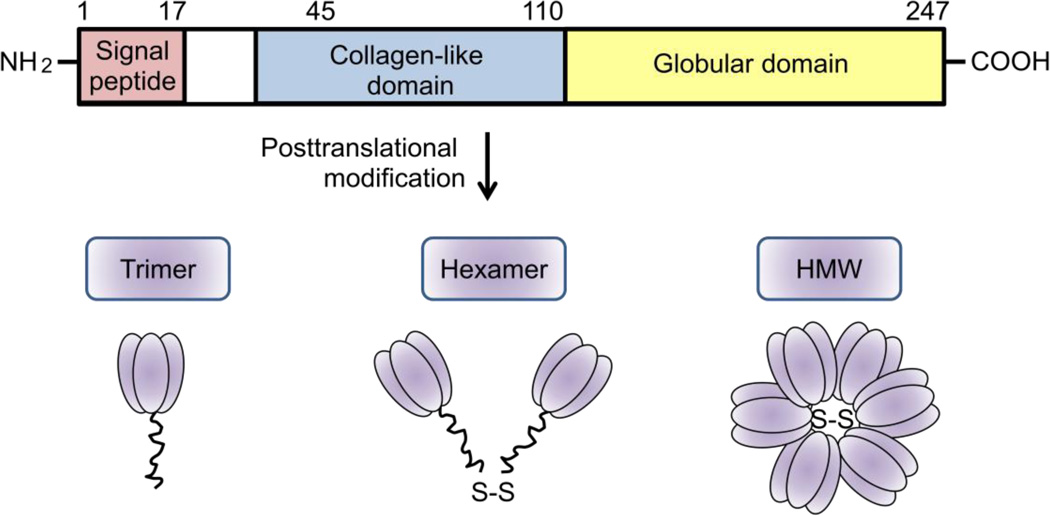
Monomeric subunits of APN (30kDa) consist of a globular C-terminal domain and a collagenous N-terminal domain (Fig. 1). The collagen domain allows APN to form trimers (~67kDa), hexamers (~120kDa), and high molecular weight polymers (HMW, >300kDa, 18–36 monomer units) in the endoplasmic reticulum prior to secretion[9]. These forms are referred to as full length APN. A small amount of processed globular APN is also reported in human plasma[10].
Figure 1.
Structure of APN. APN is composed of an N-terminal collagen-like sequence and a C-terminal globular region. APN is present as trimers, hexamers, and high molecular weight forms in circulation.
Distribution
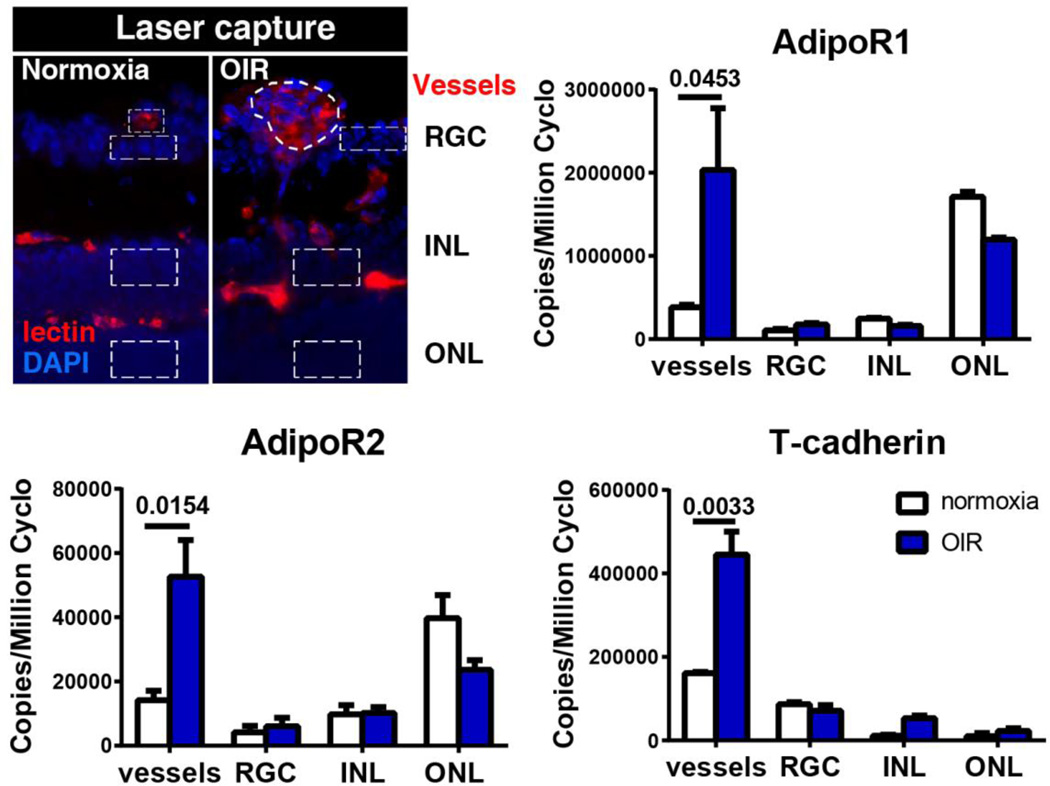
APN is most abundantly expressed in white adipose tissues[11] but has also been found in brown adipose tissue, cardiomyocytes, skeletal muscle, smooth muscle, brain, liver, osteoblasts, placenta and pituitary[12–15]. APN production is regulated by nutrition, hormones, inflammatory status and posttranslational modifications. In lean, healthy individuals, APN is the most abundant adipokine in the plasma (3–30µg/ml), accounting for 0.01% of total plasma protein[16]. All three APN receptors: APN receptor 1 (AdipoR1), APN receptor 2 (AdipoR2), and T-cadherin are expressed in the mouse retina[3]. APN and AdipoR1, AdipoR2 are detected in the human retina[17]. AdipoR1 and AdipoR2 are expressed throughout the retinal neuronal layers particularly the outer nuclear layer (rods and cones) in mouse. All the three receptors in mouse are highly induced in proliferative neovessels isolated from retinal cross-sections with laser-captured microdissection (Fig. 2)[3].
Figure 2.
Expression of APN receptors in retinal neuronal layers and blood vessels in normal and hypoxic retinas at P17, when maximal neovascularization is observed in the mouse model of oxygen-induced retinopathy. Schematic of the laser-capture microdissected retinal layers (DAPI for nuclei, blue) and blood vessels (lectin, red) is shown (outlined with dotted line). Expression of adipoR1, adipoR2, and T-cadherin was examined by using qPCR. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer.
Adapted from Fu et al, AJCN, 2015, 101 (4): 879–88
Function
APN binds to its receptors to regulate lipid/glucose metabolism and anti-inflammatory effects. HMW APN is thought to be the most bioactive form in endothelial cell protection[18] and mediates the glucose-lowering effects of thiazolidinedione in diabetic patients[19].
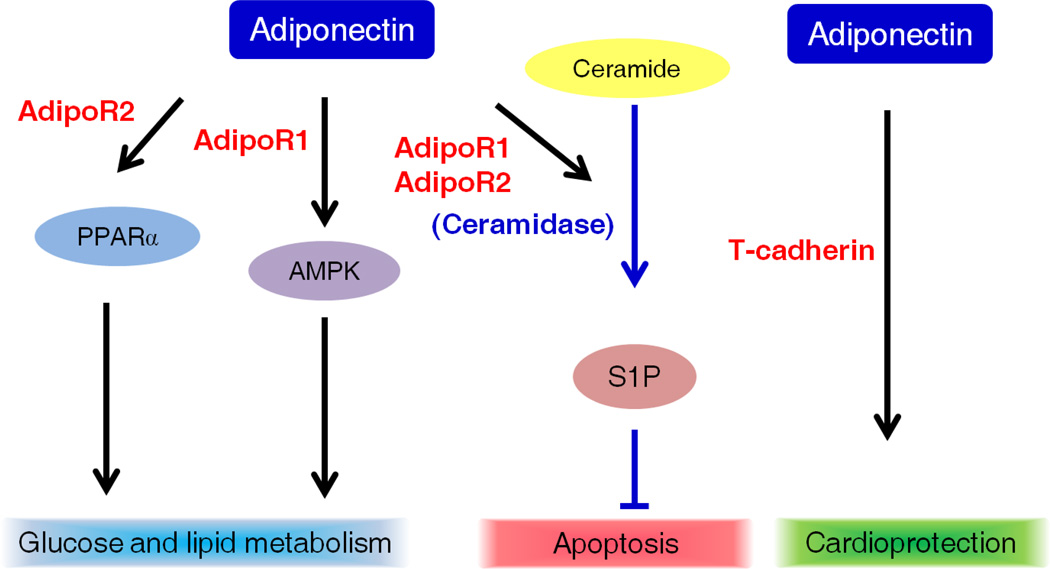
The seven-span receptors, AdipoR1 and AdipoR2 are membrane proteins with an internal N terminus and an external C terminus[9] (Fig. 3A), which bind different APN isoforms with different affinities. AdipoR1 is a high affinity receptor for globular APN and a low affinity receptor for full-length APN, while adipoR2 binds to both full-length APN and globular APN with intermediate affinity. Binding of APN with AdipoR1 increases the phosphorylation of AMP-activated protein kinase to suppress lipogenesis and gluconeogenesis[20, 21]. Interaction between APN and adipoR2 activates peroxisome proliferator-activated receptor α (PPARα) ligand activity to increase fatty acid oxidation and energy consumption[20, 21]. Therefore, APN via AdipoR1 and AdipoR2 regulates lipid and glucose metabolism to restore cellular metabolic balance under conditions of energy stress (Fig. 3B). Moreover, AdipoR1 and AdipoR2 have ceramidase activity[22] leading to decreases in serum ceramide levels, and an increase in the level of sphingosine-1-phosphate which protects cardiomyocytes and pancreatic beta cells from apoptosis[22] (Fig. 3B). Point mutations in conserved histidine residues in AdipoR1 and AdipoR2 cause a reduction in ceramidase activity[22].
Figure 3.
Structure of APN receptors and downstream pathways of APN A). The N terminus of AdipoR1 and AdipoR2 (67% identity in amino acids to adipoR1) is internal of the cell membrane and the C terminus is external. B). Binding of APN to adipoR1 activates the AMP-activated protein kinase (AMPK) while binding of APN to adipoR2 activates the PPARα. The activation of these pathways results in reduced gluconeogenesis and increased fatty acid oxidation. In addition, AdipoR1 and AdipoR2 possess ceramidase activity. Adiponectin via AdipoR1 and AdipoR2 decreases ceramide, increases sphingosine-1-phosphate (S1P), to possess the anti-apoptotic effects. T-cadherin is critical for APN’s cardioprotective effects on mice.
The third APN receptor T-cadherin (truncated) binds to hexameric and HMW APN isoforms[23]. Instead of ligand-binding and intracellular signaling capability, T-cadherin docks APN in responsive tissues such as blood vessels, smooth muscle and heart. T-cadherin is essential in mediating APN’s cardio-protective effects[24].
APN in retinal metabolic diseases
Metabolic pathways influence retinal angiogenesis
Retina (in particular photoreceptors) is the most metabolically demanding tissue in the body and photoreceptors have the highest number of mitochondria of any cell[25]. Blood vessels supply oxygen and nutrition for the retina and early loss of blood vessels leads to hypoxia and fuel deficiency, major driving forces for angiogenesis in retinopathy. Under hypoxic conditions, the activity of prolyl-hydroxylase, which rapidly degrades hypoxia-inducible factor (HIF)-1 protein under normal oxygen tension, is decreased. Accumulation of HIF-1 protein induces the expression of angiogenic factors like vascular endothelial growth factor A (VEGFA). VEGFA expression can also be modulated by HIF-1 independent pathways that are metabolically driven[26]. VEGFA promotes blood vessel proliferation, in an attempt to re-establish the oxygen and nutritional supply to the retina. However, these newly formed vessels are abnormal and leaky, leading to retinal damage and even blindness in severe cases.
Hyperglycemia and dyslipidemia as well as mitochondrial abnormalities, lead to retinal vascular dysfunction. 6-Phosphofructo-2-kinase/fructose-2, 6-bisphosphatase isoform 3, a key activator of glycolysis, plays a critical role in angiogenesis[27]. Modulation of the key enzyme in the polyol pathway of glucose metabolism protects the retina against neovascularization and retinal dysfunction[28, 29]. In addition, blocking the rate-limiting enzyme in fatty acid oxidation, carnitine palmitoyltransferase 1, inhibits vessel sprouting[30].
Moreover, a cholesterol-enriched diet causes age-related macular degeneration-like pathology in rabbit retina[31] and 7-ketocholesterol, formed by the auto-oxidation of cholesterol and cholesterol esters present in lipoprotein deposits, induces inflammation and angiogenesis in rat eyes[32]. Liver X receptors (LXRα, LXRβ) are important modulators of cholesterol homeostasis and activation of LXR protects against N-methyl-D-aspartate-induced retinal damages in mice[33]. Cholesterol-mediated activation of acid sphingomyelinase disrupts retinal pigment epithelial autophagy, which is reversed with drugs to remove excess cholesterol[34]. Acid sphingomyelinase converts sphingomyelin to ceramide and phosphatidylcholine. Alterations in acid sphingomyelinase lead to retinal abnormalities[35]. APN activates LXRα to increase cholesterol efflux and attenuate lipid accumulation in macrophages isolated from type 2 diabetic patients[36]. Activation of AdipoR1 and AdipoR2 converts the cell apoptosis-promoting ceramide to cell protective sphingosine-1-phosphate[16]. Therefore, APN may modulate retinal lipid metabolism by removing excess cholesterol, attenuating acid sphingomyelinase activation or converting ceramide to sphingosine-1-phosphate.
Retinal very long-chain polyunsaturated fatty acids (VLC-PUFAs) are important for photoreceptor function and longevity[37]. Photoreceptor elongation of very long chain fatty acids protein-4 is a critical factor for the synthesis of phosphatidylcholinecontaining sn-1 VLC-PUFAs and vision[38]. AdipoR1 promotes docosahexaenoic acid (DHA) uptake that enables its elongation in photoreceptors and retinal pigment epithelium[39]. Loss of AdipoR1 reduces retinal phosphatidylcholine-VLC-PUFA levels despite a lack of change in the expression of elongation of very long chain fatty acids protein-4[39]. Therefore, the APN pathway plays a key role in local retinal VLC-PUFA production, contributing to photoreceptor function and survival.
APN is involved in systemic lipid metabolism and APN deficiency decreases key lipogenic gene expression in liver[40]. Therefore APN may have an impact on the systemic lipid profile, which can also affect retinal vasculature and function.
Dietary supplementation of ω-3 LCPUFA, DHA and eicosapentaenoic acid (EPA), which are major lipid constituents of retina[41], protects against neovascularization in the animal models of retinopathy of prematurity, diabetic retinopathy and age-related macular degeneration[42–45]. In humans, ω-3 LCPUFA intake is associated with a reduced risk of retinopathy of prematurity[46] and age-related macular degeneration[47]. There may be a minimum level of ω-3 LCPUFA required for the maintenance of retinal stability and supplementing above that level may not increase benefit[48]. Mitochondrial dysfunction accompanied by induced oxidative stress is seen in retinal diseases like retinopathy of prematurity, diabetic retinopathy and age-related macular degeneration [49]. Understanding vascular responses to hypoxia and fuel deficiency cues will improve our knowledge of vessel loss and vessel proliferation.
Retinopathy of Prematurity
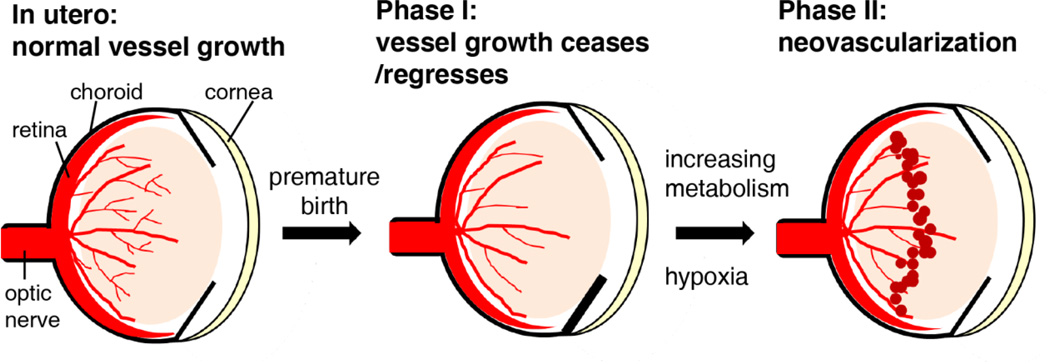
Retinopathy of prematurity was first described as a complication of premature birth in the 1940s[50], after the introduction of unregulated supplemental oxygen, and is still a leading cause of blindness in children. Retinopathy of prematurity begins after preterm birth with the cessation and in some cases also regression of normal retinal vascular development that would have occurred in utero (Phase 1) (Fig. 4). With increasing metabolic demand as the infant’s neural retina matures postnatally, areas of avascular retina become hypoxic and nutrient deprived, which generates hypoxia- and non-hypoxia-regulated vascular growth factors. These factors promote abnormal proliferation of blood vessels (phase 2 or proliferative retinopathy of prematurity, starting around postmenstrual age 30–32 weeks). Supplemental oxygen (which exacerbates vessel loss of phase I), low gestational age (which initiates the process secondary to incomplete retinal vascularization at birth) and low birth weight are major risk factors for retinopathy of prematurity[51]. Increasing evidence also shows that a high perinatal glucose level, indicating dysregulated metabolism, in the first few weeks after birth, independently correlates with the later development of all stages of proliferative retinopathy of prematurity[52].
Figure 4.
Progression of retinopathy of prematurity. Retinal blood vessels grow normally in utero (low oxygen tension). After premature birth, the retinal vasculature is incomplete and vascularization is inhibited (Phase 1), due to the hyperoxic exposure that maintains the adequate circulating oxygen levels to keep the infants survive. As the retina matures, metabolic demand is increased, leading to the localized hypoxia which in turn stimulates the oxygen-regulated angiogenic factors to induce retinal neovascularization.
Current treatments for retinopathy of prematurity are destructive
Current treatment of retinopathy of prematurity relies on blocking angiogenic factors that are over expressed in phase 2 of retinopathy of prematurity by either ablating (with laser) the avascular retina which produces the angiogenic factors or by intravitreal injections of antibodies to block VEGF, a major angiogenic growth factor stimulated by the non-vascularized hypoxic and nutrient deficient retina. However, laser treatment leads to visual field defects and loss of peripheral vision. It destroys potentially viable retina and is also, although to a lesser extent, associated with iris and lens burns, corneal edema, cataract formation, intraocular hemorrhage, and choroidal rupture[51]. The use of anti-VEGF agents has been rapidly increasing in recent years with the anticipation of less destructive retinal outcomes versus laser treatment in the short term[53]. However, the potential systemic long-term adverse effects related to VEGF inhibition include leakage of the antibody into the systemic circulation resulting in systemic suppression of VEGF with inhibition of vascular growth in brain, gut and internal organs as well as inhibition of retinal neural development[54]. Alternative treatments to prevent phase 1 retinopathy of prematurity with better metabolic control are therefore of great interest.
Loss of factors from the maternal supply affects metabolism and retinopathy of prematurity progression
Prevention of retinopathy of prematurity depends on restoring the metabolic health of the retina in phase 1 to prevent the release of angiogenic factors in phase 2. After preterm birth there is a loss of factors (such as insulin growth factor (IGF)-1, ω-3 LCPUFA and APN) that are normally provided by the maternal/placental interface. This loss contributes to the metabolic disruption in preterm infants as does an immature insulin system and insulin resistance. Exogenously increased IGF-1 and HMW APN restore insulin action when mutations in the insulin receptor gene occur[55].
There is a strong association between early-postnatal low IGF-1 levels, poor postnatal weight gain and later development of proliferative retinopathy of prematurity[51]. Experimentally, low IGF-1 contributes to suppression of normal retinal vascular development and exogenous IGF-1 improves vessel survival and inhibits neovascularization in the mouse model of oxygen-induced retinopathy[56]. However, the timing of IGF-1 intervention may be important: a minimal level of IGF-1 is required for maximal activation of Akt and mitogen-activated protein kinases by VEGFA, needed for endothelial cell survival and proliferation. Increasing IGF-1 in phase 1 promotes normal vessel growth to prevent retinopathy of prematurity development; later intervention in phase 2 with high VEGFA levels, might potentially promote retinal neovascularization[51] but might also inhibit neovascularization if IGF-1 acts as a neurotrophic factor to stabilize stressed VEGF producing retinal cells. Studies of the effect of IGF-1 treatment in phase II are needed.
Serum APN levels in preterm infants are significantly lower than those in term infants despite a marked increase in the first three weeks after premature birth and an overall increase from birth to term-equivalent age[3]. Decreased circulating APN levels correlate with hyperglycemia in very preterm newborns[57], suggesting that APN may contribute to the regulation of glucose metabolism and increasing endogenous production of APN or supplying exogenous APN could potentially be of benefit.
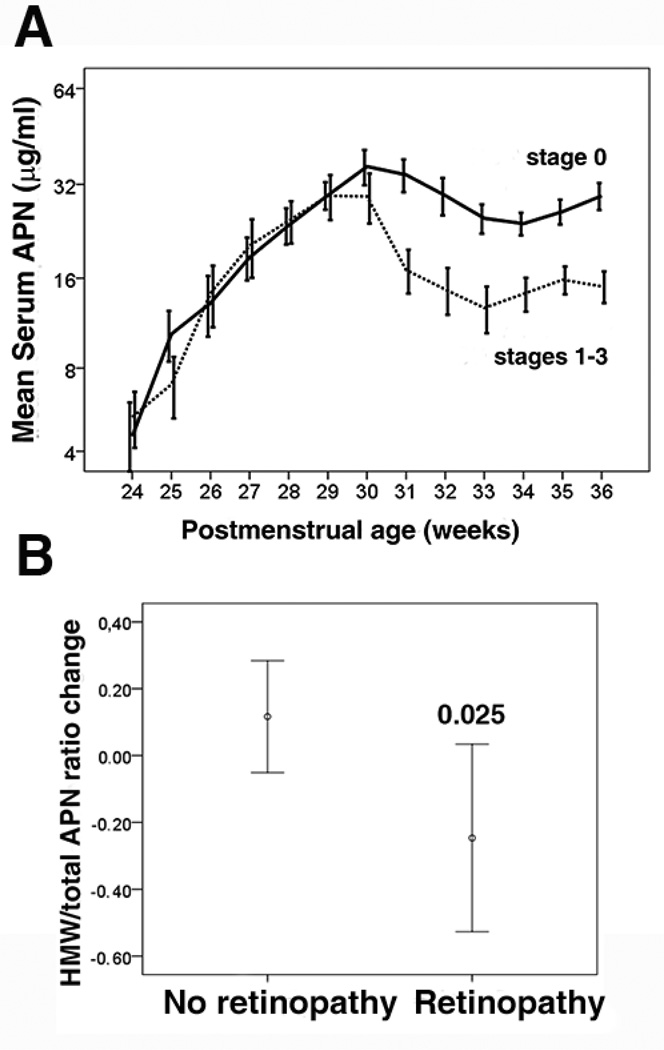
APN levels also positively correlate with weight gain after preterm birth[58], which in turn is associated with less retinopathy of prematurity progression. Persistently low circulating levels of APN especially during postmenstrual age 30–36 weeks (phase 2 of retinopathy of prematurity) is observed in premature infants with retinopathy (Fig. 5)[3]. The ratio of HMW APN to total APN is significantly lower in premature infants with versus those without retinopathy of prematurity (Fig. 5)[3]. An inverse correlation between IGF-1 and APN is suggested[59]. It is postulated that increasing APN in phase 2 of retinopathy of prematurity, could improve insulin sensitivity and benefit the normal growth of premature infants without stimulating proliferative retinopathy. Further investigation is required to understand the relationship between IGF-1 and APN in premature infants as well as in retinopathy of prematurity progression.
Figure 5.
Serum APN and retinopathy of prematurity development in premature infants. A) Correlation of the longitudinal serum APN concentrations in preterm infants (gestational age <29 weeks from birth to postmenstrual age 36 weeks) with retinopathy of prematurity development. Solid line, no retinopathy of prematurity; dotted line, retinopathy of prematurity (stages 1–3, log scale on the y axis). B) The change of HMW to total APN ratio between postmenstrual age 31 and 36 weeks in preterm infants (gestational age <26 weeks). t test for Equality of Means was used comparison of means.
Adapted from Fu et al, AJCN, 2015, 101 (4): 879–88.
APN protects against retinopathy of prematurity in animal models
Clinical data correlates low APN levels with retinopathy of prematurity but no interventional studies have been conducted to show causality. Much of our understanding about the underlying mechanism of retinopathy of prematurity pathogenesis with respect to APN comes from the use of animal models with genetic loss of APN or intervention with exogenous APN. Many animals particularly mice and rats have incompletely vascularized retinas at birth and resemble the immature retinal vascular development of premature infants. The mouse model of oxygen-induced retinopathy (Fig.6) has been useful in the investigation of neovascular eye diseases[60]. In the mouse retina, there are three layers of retinal vessels: the superficial layer forms at postnatal day (P)1–P10, next the deep layer at P8–P12 and finally the intermediate layer at P14–P20[61]. In oxygen-induced retinopathy, neonatal mice are exposed to 75% oxygen from P7–12. Hyperoxia leads to the vessel regression and cessation of the normal vessel growth (phase I retinopathy of prematurity). When returned to room air (21% oxygen), the non-perfused retina becomes hypoxic and nutrient starved, resulting in the induction of angiogenic factors and formation of retinal neovascular tufts (phase II retinopathy of prematurity). The areas of vaso-obliteration and neovascularization can be quantified in the retinal whole mounts.
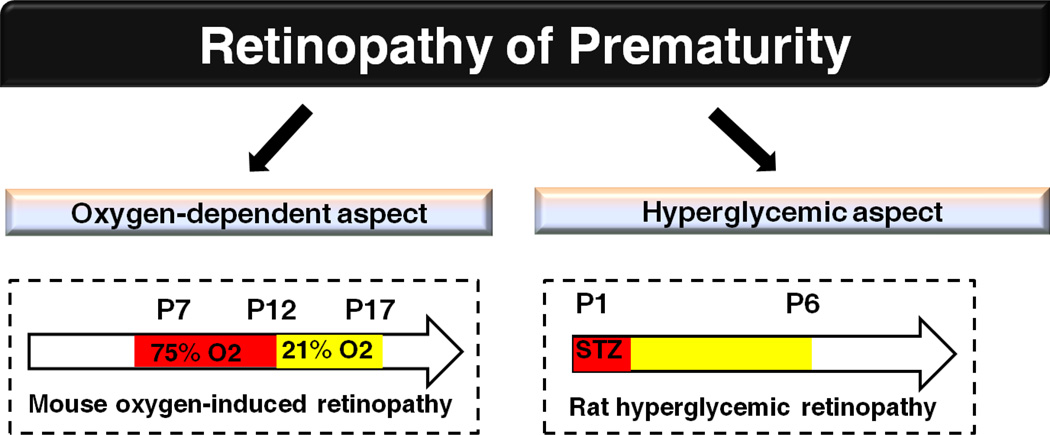
Figure 6.
Rodent models of retinopathy of prematurity. In mouse model of oxygen-induced retinopathy, mouse pups are exposed to hyperoxia from P7–12 and then relative hypoxia starting from P12, retinal vasculature will be examined at P17. In rat model of hyperglycemic retinopathy, 50mg/kg STZ is administrated intraperitoneally at P1, sustained increase of blood glucose from P2/3–6 is observed and retinal vascular formation is inhibited at P6.
In oxygen-induced retinopathy in mice, APN deficiency worsens the pathological vessel proliferation in the retina[7] and APN intervention attenuates the retinal neovascular area[7]. APN and ω-3 LCPUFA are inter-related. The loss of the maternally provided essential lipid ω-3 LCPUFA (DHA), which is not provided by total parenteral nutrition, affects retinal vascular development in preterm infants[51]. Dietary ω-3 versus ω-6 LCPUFA promotes normal revascularization of avascular retina and independently directly reduces pathological retinal neovascularization by ~50% in mouse oxygen-induced retinopathy through PPARγ[62]. In mice, APN mediates the protective effects of ω-3 LCPUFA on reducing retinal neovascularization[3] and preserving retinal function[39]. In mouse oxygen-induced retinopathy, ω-3 LCPUFA protects against neovascularization with an associated reduction in TNF-α[62] and APN ameliorates oxygen-induced retinopathy via TNF-α[7]. Therefore, ω-3 LCPUFA upregulates APN levels to attenuate retinal TNF-α production and reduce neovascularization. Activation of adipoR1 is also essential for DHA uptake and elongation, to restore photoreceptor morphology and function[39]. These observations suggest that APN may also contribute to the modulation of lipid metabolism for the prevention of retinopathy of prematurity.
Hyperglycemia in the early postnatal period is strongly associated with later proliferative retinopathy of prematurity[52, 63], indicating the influence of abnormal glucose metabolism in retinopathy. Studies of the influence of hyperglycemia in phase 1 retinopathy of prematurity have been limited by lack of appropriate animal models. Although mouse oxygen-induced retinopathy models the oxygen-dependent aspect of vessel loss and the proliferative phase in retinopathy of prematurity, it is difficult to assess the influence of hyperglycemia on early aspects of retinopathy of prematurity. Streptozotocin treatment of neonatal rodents destroys pancreatic beta cells to induce high circulating glucose levels. A novel neonatal hyperglycemic retinopathy model in rat with streptozotocin has been recently developed[64] (Fig. 6), providing a pronounced phenotype for the study of pathogenesis of retinopathy influenced by hyperglycemia. Replication of the rat model in mice will further facilitate the investigation of APN in hyperglycemia-associated retinopathy of prematurity.
Diabetic Retinopathy
Diabetic retinopathy is a retinal neovascular disease and a significant diabetic complication. Diabetic retinopathy development is strongly linked with hyperglycemia, dyslipidemia[65] and mitochondrial dysfunction accompanied by induced oxidative stress[49] and is associated with abnormal APN levels. Clinically, diabetic retinopathy can be classified into non-proliferative retinopathy (phase 1) and proliferative retinopathy (phase 2) similar to retinopathy of prematurity. Hyperglycemia through metabolic changes leads to retinal vascular loss (phase 1). The incompletely vascularized retina is deprived of nutrition and oxygen, inducing pathological angiogenesis (phase 2). APN modifies these primary drivers of diabetic retinopathy. Abnormalities in the APN pathway result in increased insulin resistance[66] and APN gene polymorphisms are associated with retinopathy in diabetic patients[67].
APN improves insulin sensitivity and vascular abnormalities in diabetic patients
Insulin sensitivity is reduced in type 1 diabetic patients versus non-diabetic controls[68] and higher levels of APN positively correlate with increased insulin sensitivity in each group. In type 2 diabetic patients, APN controls insulin sensitivity by modulating glycogen synthesis in human skeletal muscle[69]. Serum APN levels in type 1 diabetes positively correlate with plasma total antioxidant status[70]. Increased APN levels are associated with less renal disease[71], suggesting possible protective effects of APN with respect to other type 1 diabetes complications including diabetic retinopathy. In type 2 diabetic patients, circulating APN levels are positively correlated with retinal blood flow in male patients with early-phase diabetic retinopathy, possibly through increased blood velocity and dilated vessels[5]. Low APN levels may stimulate vascular nicotinamide adenine dinucleotide phosphate-oxidase activity in the human arterial wall, contributing to the development of diabetic retinopathy[72]. These observations suggest that APN might protect against diabetic complications such as diabetic retinopathy in type 1 diabetes and type 2 diabetes.
However, interpreting studies in diabetic patients linking levels of APN with diabetic retinopathy or diabetes complications is difficult as elevated APN may be a compensatory and beneficial response. In type 1 diabetic patients, elevated APN levels are associated with retinopathy and nephropathy[73]. However in type 2 diabetic patients versus healthy controls, especially in patients with diabetic retinopathy, plasma APN levels are decreased[74]. In type 2 diabetic patients, high aqueous humor APN levels are observed with proliferative diabetic retinopathy versus non-diabetic controls[4] possibly due to increased permeability of the blood-retinal-barrier with diabetic retinopathy progression. Much lower brain and aqueous humor APN levels are reported compared with circulating APN levels, suggesting a role of the blood brain barrier and blood retinal barrier in APN homeostasis[4]. There is also the possibility of induction of local APN expression in the diabetic retina.
Although some reports indicate that high serum APN levels correlate with the severity of diabetic retinopathy progression especially in the proliferative phase[75], there is increasing evidence suggesting that APN improves insulin-resistance and supports vascular maintenance indicating a potentially protective role of APN in diabetic retinopathy in type 2 diabetic patients. However, there is limited knowledge of the effect of administering APN in diabetic retinopathy.
APN modulates glucose/lipid metabolic alterations in diabetic models
Research in diabetic retinopathy pathogenesis is limited by lack of adequate models in mice (needed for genetic manipulation[76]) Current models rarely if ever develop proliferative diabetic retinopathy (phase 2), and take months (or years) to develop chronic retinal vessel degeneration (phase 1)[76]. The rat model of neonatal hyperglycemic retinopathy with disrupted stability of retinal vascular formation[64] (Fig. 6) may be useful for the investigation of phase 1 diabetic retinopathy in type 1 diabetes. In STZ-induced diabetes in adult mice, hyperglycemia causes retinal gliosis, and inflammation contributing to endothelial cell loss[76]. STZ-induced neonatal hyperglycemia impairs retinal vascular stability accompanied by inflammation and gliosis[64], suggesting that the mechanisms behind the disturbed stability of vascular formation in neonates and vascular maintenance in adults may be similar. Therefore, investigations of the APN effects on neonatal hyperglycemic retinopathy may help the elucidation of its role in the pathogenesis of diabetic retinopathy in type 1 diabetes. To model the neovascular phase 2 of diabetic retinopathy, the non-diabetic oxygen-induced retinopathy model is widely used[60]. APN in mouse oxygen-induced retinopathy protects against retinal neovascularization[3, 7].
Although diabetic retinopathy animal models are limited, studies in these systems suggest that APN is protective metabolically. APN governs lipid deposition in non-adipose tissues and reduces the accumulation of ceramide, a cytotoxic and insulin desensitizing lipid metabolite formed when excessive lipids are transferred into peripheral tissues[77]. APN is a key mediator of fibroblast growth factor 21, which reduces ceramide and controls energy expenditure in obese mice[78]. In the rodent models of type 1 diabetes, APN supplementation modulates glucose and lipid metabolic dysregulation and increases the levels of high-density lipoprotein, leading to reduced inflammation and a reduction of hepatic diseases[79]. In addition, APN protects against pancreatic beta-cell apoptosis and significantly reduces plasma triglycerides and glucose levels in high-fat-diet fed STZ-induced diabetic mice[80]. The AdipoR1 and AdipoR2 receptor agonist AdipoRon ameliorates insulin resistance and prolongs a mouse lifespan in type 2 diabetes models[81], suggesting a promising therapeutic approach for the treatment of type 2 diabetes and potentially its complications. In the rodent models of type 2 diabetes, APN restores endothelial cell function with attenuation of oxidative stress[82]. Thus experimentally, APN is associated with improved mitochondrial function, improved insulin sensitivity and anti-inflammatory effects indicating that APN may protect against diabetic complications like diabetic retinopathy.
Age-related macular degeneration
Age-related macular degeneration is a leading cause of vision loss in the elderly (age >50) affecting the macula and central vision. Aging is associated with declining mitochondrial function. Age-related macular degeneration is categorized as non-neovascular (also known as dry or nonexudative) or neovascular (wet or exudative)[83]. Mitochondrial morphological changes and translocation to the nucleus occurs in degenerating macular cones[84]. Mitochondrial DNA damage in retinal pigment epithelial cells and photoreceptors contributes to age-related macular degeneration progression[85].
Dysregulated glucose and lipid metabolism in photoreceptors (a dual shortage of glucose/lipid fuel) results in the reduction of α-ketoglutarate (a Krebs cycle intermediate) in a mouse model of wet age-related macular degeneration. Low levels of α- ketoglutarate promote HIF-1α stabilization and induce VEGFA secretion, leading to the formation of neovessels to supply fuel[1]. Therefore, the modulation of nutritional supply and energy metabolic pathways may provide novel therapeutic approaches for agerelated macular degeneration treatment. APN, with its significant effects on energy metabolic modulation and anti-apoptosis, may be a promising target in the prevention of age-related macular degeneration progression.
Laser-induced choroidal neovascularization (in which a laser burns through Bruch’s membrane provoking choroidal blood vessel proliferation), is commonly used to model age-related macular degeneration-associated choroidal neovascularization. Administration of recombinant APN protein decreases VEGF levels and inhibits choroidal neovascularization[8], suggesting a potential therapeutic role of APN in the treatment of wet age-related macular degeneration. More investigation is required for the elucidation of the role of APN in age-related macular degeneration regarding mitochondrial disruption and the contribution of metabolic abnormalities to macular degeneration, as the laser-induced choroidal neovascularization model is one of injury and inflammation rather than one of mitochondrial dysfunction. Clinically, gene polymorphisms in the APN pathway are linked to age-related macular degeneration risk. In a Chinese population[86], APN genetic variant rs822396 is associated with advanced age-related macular degeneration. In addition, the AdipoR1 variant rs10753929 is a genetic risk factor for severe age-related macular degeneration in a Finnish population[6]. More advanced studies correlating APN levels with age-related macular degeneration status in patients will help us understand APN influences on age-related macular degeneration progression.
Activation of APN pathway
Overall, activation of the APN pathway may benefit the retina under stress conditions. Upregulation of endogenous APN production or activation of APN receptors may be beneficial. Dietary intervention with ω-3 LCPUFA or treatment with fibric acid derivatives and PPARγ agonists increases circulating APN levels.
ω-3 LCPUFA
Dietary supplementation of ω-3 LCPUFA increases circulating APN levels in both premature infants and diabetic patients. In premature infants, circulating levels of APN correlate with ω-3 LCPUFA DHA[3]. In mouse oxygen-induced retinopathy, dietary ω-3 LCPUFA increases serum total and HMW APN levels produced in stressed subcutaneous fat to decrease retinopathy[3]. Accumulation of subcutaneous fat predicts APN levels in preterm infants at term-equivalent age[87]. Similarly in diabetic patients, ω-3 LCPUFA modulates circulating APN levels. Increases in EPA/ arachidonic acid (AA) ratio or supplementation of ω3 LCPUFA are associated with increased APN and improved arterial stiffness in obese patients with dyslipidemia[88]. Dietary inclusion of fish rich in ω-3 LCPUFA increases APN levels, reduces triglycerides and lowers TNFα in serum in dyslipidemic middle-aged Chinese women[89]. ω-3 LCPUFA supplements lower plasma triglyceride levels in the elderly[90]. ω-3 LCPUFA supplementation increases APN levels, reduces pro-inflammatory cytokines expression, and improves insulin sensitivity in high-fat-diet-induced diabetic mice[91]. ω-3 LCPUFA (predominantly DHA) increases APN secretion from white adipocytes in vitro and exerts anti-inflammatory actions[92].
Fibric acid derivatives
Fenofibrate therapy is associated with less retinopathy progression in type 2 diabetes [93]. Clinically, fenofibrate, together with other fibric acid derivatives such as bezafibrate, is correlated with increased circulating APN levels in patients with either dyslipidemia[94] or metabolic syndrome[95]. Although the response to fenofibrate-induced APN levels increases may be genetically dependent[96], clinically available fibric acid derivatives may be used to increase APN levels to benefit eye diseases.
PPARγ agonists
Drugs that activate PPARγ are also positively linked with increased circulating APN levels[97]. In both humans and mice, APN promoters contain a putative PPARγ-recognition site, and point mutations at this site result in reduced basal levels and PPARγ agonist thiazolidinediones-induced APN promoter transactivation[98].
APN receptor agonist
APN receptors AdipoR1, AdipoR2 and T-cadherin mediate anti-inflammatory, anti-angiogenic and insulin-sensitizing effects. Therefore, active synthetic small-molecule AdipoR agonist, AdipoRon (which binds both AdipoR1 and AdipoR2 in vitro), may be clinically useful in patients with low APN levels. In type 2 diabetes, AdipoRon administration improves insulin resistance and glucose intolerance in obese mice[81], providing a promising therapeutic approach for the treatment of diabetes and diabetes-related complications. However, some studies also suggest that AdipoR1 and AdipoR2 have divergent roles in mediating vascular signaling and energy metabolism[99]. AdipoR2, but not AdipoR1, improves ischemia-induced vascular changes[99]. AdipoR1-deficient mice are vulnerable to high-fat diet-induced obesity with increased adiposity, decreased glucose tolerance and spontaneous locomotor activity, while adipoR2-deficient mice are resistant to these metabolic dysfunctions[99, 100]. Therefore, further understanding of the functions of each APN receptor and the chemical synthesis of more specific receptor agonists might be helpful to optimize clinical application.
Thus, activation of the APN pathway through dietary supplementation of ω-3 LCPUFA, fibric acid derivatives, PPARγ agonists increasing APN levels or direct activation of APN receptors may be clinical strategies to protect against retinal abnormalities.
Conclusions
Altered circulating APN levels or APN variant distributions are associated with the development of retinopathy of prematurity, diabetic retinopathy and age-related macular degeneration. Experimentally, APN inhibits retinal and choroidal neovascularization. As a key glucose and lipid modulator, APN may re-establish metabolic balance. Intervention with ω-3 LCPUFA, and fibric acid derivatives which activate the nuclear receptor PPARγ increases circulating APN levels. Additional studies are needed to clarify the role of APN and its individual receptors in retinopathy as well as the underlying mechanisms both clinically and experimentally.
Highlights.
Clinical and experimental studies suggest that adiponectin protects against retinopathy.
Adiponectin modulates retinal metabolic alterations in retinopathy.
ω3LCPUFA and fibric acid derivatives activate adiponectin pathways.
Acknowledgments
Financial Support:
LS is supported by NIH EY024864, EY017017, EY022275, P01 HD18655, Lowy Medical Research Institute, European Commission FP7 project 305485 PREVENT-ROP AH is supported by PREVENTROP, the Swedish Medical Research Council (2011-2432), Swedish government grants (ALFGB2770).
CL is supported by PREVENTROP, De Blindas Vänner
ZF is supported by Knights Templar Eye Foundation and Bernadotte foundation
Abbreviations
- AA
Arachidonic acid
- APN
Adiponectin
- DHA
Docosahexaenoic acid
- EPA
Eicosapentaenoic acid
- HIF
Hypoxia-inducible factor
- HMW APN
High molecular weight adiponectin
- IGF-1
Insulin-like growth factor 1
- LCPUFA
Long-chain polyunsaturated fatty acids
- LXRs
Liver X receptors
- PPAR
Peroxisome proliferator-activated receptor
- TNFα
Tumor necrosis factor α
- VEGFA
Vascular endothelial growth factor A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- 1.Joyal JS, Sun Y, Gantner ML, Shao Z, Evans LP, Saba N, Fredrick T, Burnim S, Kim JS, Patel G, Juan AM, Hurst CG, Hatton CJ, Cui Z, Pierce KA, Bherer P, Aguilar E, Powner MB, Vevis K, Boisvert M, Fu Z, Levy E, Fruttiger M, Packard A, Rezende FA, Maranda B, Sapieha P, Chen J, Friedlander M, Clish CB, Smith LE. Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1. Nature medicine. 2016 doi: 10.1038/nm.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahmouni K, Haynes WG. Endothelial effects of leptin: implications in health and diseases. Current diabetes reports. 2005;5:260–266. doi: 10.1007/s11892-005-0020-5. [DOI] [PubMed] [Google Scholar]
- 3.Fu Z, Lofqvist CA, Shao Z, Sun Y, Joyal JS, Hurst CG, Cui RZ, Evans LP, Tian K, SanGiovanni JP, Chen J, Ley D, Hansen Pupp I, Hellstrom A, Smith LE. Dietary omega-3 polyunsaturated fatty acids decrease retinal neovascularization by adipose-endoplasmic reticulum stress reduction to increase adiponectin. The American journal of clinical nutrition. 2015;101:879–888. doi: 10.3945/ajcn.114.099291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao D, Peng H, Li Q, Wang J, Li P, Hu K, Zhang X, Lei B. Aqueous humor and plasma adiponectin levels in proliferative diabetic retinopathy patients. Current eye research. 2012;37:803–808. doi: 10.3109/02713683.2012.676700. [DOI] [PubMed] [Google Scholar]
- 5.Omae T, Nagaoka T, Yoshida A. Relationship Between Retinal Blood Flow and Serum Adiponectin Concentrations in Patients With Type 2 Diabetes Mellitus. Investigative ophthalmology & visual science. 2015;56:4143–4149. doi: 10.1167/iovs.15-16447. [DOI] [PubMed] [Google Scholar]
- 6.Kaarniranta K, Paananen J, Nevalainen T, Sorri I, Seitsonen S, Immonen I, Salminen A, Pulkkinen L, Uusitupa M. Adiponectin receptor 1 gene (ADIPOR1) variant is associated with advanced age-related macular degeneration in Finnish population. Neuroscience letters. 2012;513:233–237. doi: 10.1016/j.neulet.2012.02.050. [DOI] [PubMed] [Google Scholar]
- 7.Higuchi A, Ohashi K, Kihara S, Walsh K, Ouchi N. Adiponectin suppresses pathological microvessel formation in retina through modulation of tumor necrosis factor-alpha expression. Circulation research. 2009;104:1058–1065. doi: 10.1161/CIRCRESAHA.109.194506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyzogubov VV, Tytarenko RG, Bora NS, Bora PS. Inhibitory role of adiponectin peptide I on rat choroidal neovascularization. Biochimica et biophysica acta. 2012;1823:1264–1272. doi: 10.1016/j.bbamcr.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocrine reviews. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 10.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. The Journal of biological chemistry. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 12.Fujimoto N, Matsuo N, Sumiyoshi H, Yamaguchi K, Saikawa T, Yoshimatsu H, Yoshioka H. Adiponectin is expressed in the brown adipose tissue and surrounding immature tissues in mouse embryos. Biochimica et biophysica acta. 2005;1731:1–12. doi: 10.1016/j.bbaexp.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Kaser S, Moschen A, Cayon A, Kaser A, Crespo J, Pons-Romero F, Ebenbichler CF, Patsch JR, Tilg H. Adiponectin and its receptors in non-alcoholic steatohepatitis. Gut. 2005;54:117–121. doi: 10.1136/gut.2003.037010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bluher M, Bullen JW, Jr, Lee JH, Kralisch S, Fasshauer M, Kloting N, Niebauer J, Schon MR, Williams CJ, Mantzoros CS. Circulating adiponectin and expression of adiponectin receptors in human skeletal muscle: associations with metabolic parameters and insulin resistance and regulation by physical training. The Journal of clinical endocrinology and metabolism. 2006;91:2310–2316. doi: 10.1210/jc.2005-2556. [DOI] [PubMed] [Google Scholar]
- 15.Psilopanagioti A, Papadaki H, Kranioti EF, Alexandrides TK, Varakis JN. Expression of adiponectin and adiponectin receptors in human pituitary gland and brain. Neuroendocrinology. 2009;89:38–47. doi: 10.1159/000151396. [DOI] [PubMed] [Google Scholar]
- 16.Hebbard L, Ranscht B. Multifaceted roles of adiponectin in cancer. Best practice & research. Clinical endocrinology & metabolism. 2014;28:59–69. doi: 10.1016/j.beem.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin T, Qiu Y, Liu Y, Mohan R, Li Q, Lei B. Expression of adiponectin and its receptors in type 1 diabetes mellitus in human and mouse retinas. Molecular vision. 2013;19:1769–1778. [PMC free article] [PubMed] [Google Scholar]
- 18.Torigoe M, Matsui H, Ogawa Y, Murakami H, Murakami R, Cheng XW, Numaguchi Y, Murohara T, Okumura K. Impact of the high-molecular-weight form of adiponectin on endothelial function in healthy young men. Clinical endocrinology. 2007;67:276–281. doi: 10.1111/j.1365-2265.2007.02876.x. [DOI] [PubMed] [Google Scholar]
- 19.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. The Journal of biological chemistry. 2004;279:12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 20.Yamauchi T, Kadowaki T. Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell metabolism. 2013;17:185–196. doi: 10.1016/j.cmet.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nature medicine. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 22.Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, Wade MR, Tenorio VM, Kuo MS, Brozinick JT, Zhang BB, Birnbaum MJ, Summers SA, Scherer PE. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nature medicine. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denzel MS, Scimia MC, Zumstein PM, Walsh K, Ruiz-Lozano P, Ranscht B. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. The Journal of clinical investigation. 2010;120:4342–4352. doi: 10.1172/JCI43464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoang QV, Linsenmeier RA, Chung CK, Curcio CA. Photoreceptor inner segments in monkey and human retina: mitochondrial density, optics, and regional variation. Visual neuroscience. 2002;19:395–407. doi: 10.1017/s0952523802194028. [DOI] [PubMed] [Google Scholar]
- 26.Lee DC, Sohn HA, Park ZY, Oh S, Kang YK, Lee KM, Kang M, Jang YJ, Yang SJ, Hong YK, Noh H, Kim JA, Kim DJ, Bae KH, Kim DM, Chung SJ, Yoo HS, Yu DY, Park KC, Yeom YI. A lactate-induced response to hypoxia. Cell. 2015;161:595–609. doi: 10.1016/j.cell.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 27.De Bock K, Georgiadou M, Schoors S, Kuchnio A, Wong BW, Cantelmo AR, Quaegebeur A, Ghesquiere B, Cauwenberghs S, Eelen G, Phng LK, Betz I, Tembuyser B, Brepoels K, Welti J, Geudens I, Segura I, Cruys B, Bifari F, Decimo I, Blanco R, Wyns S, Vangindertael J, Rocha S, Collins RT, Munck S, Daelemans D, Imamura H, Devlieger R, Rider M, Van Veldhoven PP, Schuit F, Bartrons R, Hofkens J, Fraisl P, Telang S, Deberardinis RJ, Schoonjans L, Vinckier S, Chesney J, Gerhardt H, Dewerchin M, Carmeliet P. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013;154:651–663. doi: 10.1016/j.cell.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 28.Fu ZJ, Li SY, Kociok N, Wong D, Chung SK, Lo AC. Aldose reductase deficiency reduced vascular changes in neonatal mouse retina in oxygen-induced retinopathy. Investigative ophthalmology & visual science. 2012;53:5698–5712. doi: 10.1167/iovs.12-10122. [DOI] [PubMed] [Google Scholar]
- 29.Fu Z, Nian S, Li SY, Wong D, Chung SK, Lo AC. Deficiency of aldose reductase attenuates inner retinal neuronal changes in a mouse model of retinopathy of prematurity. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2015;253:1503–1513. doi: 10.1007/s00417-015-3024-0. [DOI] [PubMed] [Google Scholar]
- 30.Schoors S, Bruning U, Missiaen R, Queiroz KC, Borgers G, Elia I, Zecchin A, Cantelmo AR, Christen S, Goveia J, Heggermont W, Godde L, Vinckier S, Van Veldhoven PP, Eelen G, Schoonjans L, Gerhardt H, Dewerchin M, Baes M, De Bock K, Ghesquiere B, Lunt SY, Fendt SM, Carmeliet P. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature. 2015;520:192–197. doi: 10.1038/nature14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dasari B, Prasanthi JR, Marwarha G, Singh BB, Ghribi O. Cholesterol-enriched diet causes age-related macular degeneration-like pathology in rabbit retina. BMC ophthalmology. 2011;11:22. doi: 10.1186/1471-2415-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amaral J, Lee JW, Chou J, Campos MM, Rodriguez IR. 7-Ketocholesterol induces inflammation and angiogenesis in vivo: a novel rat model. PloS one. 2013;8:e56099. doi: 10.1371/journal.pone.0056099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng S, Yang H, Chen Z, Zheng C, Lei C, Lei B. Activation of liver X receptor protects inner retinal damage induced by N-methyl-D-aspartate. Investigative ophthalmology & visual science. 2015;56:1168–1180. doi: 10.1167/iovs.14-15612. [DOI] [PubMed] [Google Scholar]
- 34.Toops KA, Tan LX, Jiang Z, Radu RA, Lakkaraju A. Cholesterol-mediated activation of acid sphingomyelinase disrupts autophagy in the retinal pigment epithelium. Molecular biology of the cell. 2015;26:1–14. doi: 10.1091/mbc.E14-05-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aslan M, Basaranlar G, Unal M, Ciftcioglu A, Derin N, Mutus B. Inhibition of neutral sphingomyelinase decreases elevated levels of inducible nitric oxide synthase and apoptotic cell death in ocular hypertensive rats. Toxicology and applied pharmacology. 2014;280:389–398. doi: 10.1016/j.taap.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 36.Wang M, Wang D, Zhang Y, Wang X, Liu Y, Xia M. Adiponectin increases macrophages cholesterol efflux and suppresses foam cell formation in patients with type 2 diabetes mellitus. Atherosclerosis. 2013;229:62–70. doi: 10.1016/j.atherosclerosis.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 37.Bennett LD, Brush RS, Chan M, Lydic TA, Reese K, Reid GE, Busik JV, Elliott MH, Anderson RE. Effect of reduced retinal VLC-PUFA on rod and cone photoreceptors. Investigative ophthalmology & visual science. 2014;55:3150–3157. doi: 10.1167/iovs.14-13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harkewicz R, Du H, Tong Z, Alkuraya H, Bedell M, Sun W, Wang X, Hsu YH, Esteve-Rudd J, Hughes G, Su Z, Zhang M, Lopes VS, Molday RS, Williams DS, Dennis EA, Zhang K. Essential role of ELOVL4 protein in very long chain fatty acid synthesis and retinal function. The Journal of biological chemistry. 2012;287:11469–11480. doi: 10.1074/jbc.M111.256073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rice DS, Calandria JM, Gordon WC, Jun B, Zhou Y, Gelfman CM, Li S, Jin M, Knott EJ, Chang B, Abuin A, Issa T, Potter D, Platt KA, Bazan NG. Adiponectin receptor 1 conserves docosahexaenoic acid and promotes photoreceptor cell survival. Nature communications. 2015;6:6228. doi: 10.1038/ncomms7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Q, Yuan B, Lo KA, Patterson HC, Sun Y, Lodish HF. Adiponectin regulates expression of hepatic genes critical for glucose and lipid metabolism. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:14568–14573. doi: 10.1073/pnas.1211611109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. The American journal of clinical nutrition. 2006;83:1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 42.Gong Y, Li J, Sun Y, Fu Z, Liu CH, Evans L, Tian K, Saba N, Fredrick T, Morss P, Chen J, Smith LE. Optimization of an Image-Guided Laser-Induced Choroidal Neovascularization Model in Mice. PloS one. 2015;10:e0132643. doi: 10.1371/journal.pone.0132643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shao Z, Fu Z, Stahl A, Joyal JS, Hatton C, Juan A, Hurst C, Evans L, Cui Z, Pei D, Gong Y, Xu D, Tian K, Bogardus H, Edin ML, Lih F, Sapieha P, Chen J, Panigrahy D, Hellstrom A, Zeldin DC, Smith LE. Cytochrome P450 2C8 omega3-long-chain polyunsaturated fatty acid metabolites increase mouse retinal pathologic neovascularization--brief report. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:581–586. doi: 10.1161/ATVBAHA.113.302927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sapieha P, Chen J, Stahl A, Seaward MR, Favazza TL, Juan AM, Hatton CJ, Joyal JS, Krah NM, Dennison RJ, Tang J, Kern TS, Akula JD, Smith LE. Omega-3 polyunsaturated fatty acids preserve retinal function in type 2 diabetic mice. Nutrition & diabetes. 2012;2:e36. doi: 10.1038/nutd.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D, Hellstrom A, Kang JX, Chew EY, Salem N, Jr, Serhan CN, Smith LE. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nature medicine. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pawlik D, Lauterbach R, Walczak M, Hurkala J, Sherman MP. Fish-Oil Fat Emulsion Supplementation Reduces the Risk of Retinopathy in Very Low Birth Weight Infants: A Prospective, Randomized Study, JPEN. Journal of parenteral and enteral nutrition. 2013 doi: 10.1177/0148607113499373. [DOI] [PubMed] [Google Scholar]
- 47.Tan JS, Wang JJ, Flood V, Mitchell P. Dietary fatty acids and the 10-year incidence of age-related macular degeneration: the Blue Mountains Eye Study. Archives of ophthalmology. 2009;127:656–665. doi: 10.1001/archophthalmol.2009.76. [DOI] [PubMed] [Google Scholar]
- 48.Kabasawa S, Mori K, Horie-Inoue K, Gehlbach PL, Inoue S, Awata T, Katayama S, Yoneya S. Associations of cigarette smoking but not serum fatty acids with age-related macular degeneration in a Japanese population. Ophthalmology. 2011;118:1082–1088. doi: 10.1016/j.ophtha.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 49.Barot M, Gokulgandhi MR, Mitra AK. Mitochondrial dysfunction in retinal diseases. Current eye research. 2011;36:1069–1077. doi: 10.3109/02713683.2011.607536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Terry TL. Fibroblastic Overgrowth of Persistent Tunica Vasculosa Lentis in Infants Born Prematurely: II. Report of Cases-Clinical Aspects. Transactions of the American Ophthalmological Society. 1942;40:262–284. [PMC free article] [PubMed] [Google Scholar]
- 51.Hellstrom A, Smith LE, Dammann O. Retinopathy of prematurity. Lancet. 2013;382:1445–1457. doi: 10.1016/S0140-6736(13)60178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Au SC, Tang SM, Rong SS, Chen LJ, Yam JC. Association between hyperglycemia and retinopathy of prematurity: a systemic review and meta-analysis. Scientific reports. 2015;5:9091. doi: 10.1038/srep09091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leskov I, Mukai S. Laser Therapy Versus Anti-VEGF Agents for Treatment of Retinopathy of Prematurity. International ophthalmology clinics. 2015;55:81–90. doi: 10.1097/IIO.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 54.Jalali S, Balakrishnan D, Zeynalova Z, Padhi TR, Rani PK. Serious adverse events and visual outcomes of rescue therapy using adjunct bevacizumab to laser and surgery for retinopathy of prematurity. The Indian Twin Cities Retinopathy of Prematurity Screening database Report number 5, Archives of disease in childhood. Fetal and neonatal edition. 2013;98:F327–F333. doi: 10.1136/archdischild-2012-302365. [DOI] [PubMed] [Google Scholar]
- 55.Hojlund K, Beck-Nielsen H, Flyvbjerg A, Frystyk J. Characterisation of adiponectin multimers and the IGF axis in humans with a heterozygote mutation in the tyrosine kinase domain of the insulin receptor gene. European journal of endocrinology / European Federation of Endocrine Societies. 2012;166:511–519. doi: 10.1530/EJE-11-0790. [DOI] [PubMed] [Google Scholar]
- 56.Lofqvist C, Chen J, Connor KM, Smith AC, Aderman CM, Liu N, Pintar JE, Ludwig T, Hellstrom A, Smith LE. IGFBP3 suppresses retinopathy through suppression of oxygen-induced vessel loss and promotion of vascular regrowth. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10589–10594. doi: 10.1073/pnas.0702031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oberthuer A, Donmez F, Oberhauser F, Hahn M, Hoppenz M, Hoehn T, Roth B, Laudes M. Hypoadiponectinemia in extremely low gestational age newborns with severe hyperglycemia--a matched-paired analysis. PloS one. 2012;7:e38481. doi: 10.1371/journal.pone.0038481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saito M, Nishimura K, Nozue H, Miyazono Y, Kamoda T. Changes in serum adiponectin levels from birth to term-equivalent age are associated with postnatal weight gain in preterm infants. Neonatology. 2011;100:93–98. doi: 10.1159/000322654. [DOI] [PubMed] [Google Scholar]
- 59.Kanazawa I, Yamaguchi T, Sugimoto T. Serum insulin-like growth factor-I is negatively associated with serum adiponectin in type 2 diabetes mellitus. Growth hormone & IGF research : official journal of the Growth Hormone Research Society and the International IGF Research Society. 2011;21:268–271. doi: 10.1016/j.ghir.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 60.Connor KM, Krah NM, Dennison RJ, Aderman CM, Chen J, Guerin KI, Sapieha P, Stahl A, Willett KL, Smith LE. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nature protocols. 2009;4:1565–1573. doi: 10.1038/nprot.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dorrell MI, Friedlander M. Mechanisms of endothelial cell guidance and vascular patterning in the developing mouse retina. Progress in retinal and eye research. 2006;25:277–295. doi: 10.1016/j.preteyeres.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 62.Stahl A, Sapieha P, Connor KM, Sangiovanni JP, Chen J, Aderman CM, Willett KL, Krah NM, Dennison RJ, Seaward MR, Guerin KI, Hua J, Smith LE. Short communication: PPAR gamma mediates a direct antiangiogenic effect of omega 3-PUFAs in proliferative retinopathy. Circulation research. 2010;107:495–500. doi: 10.1161/CIRCRESAHA.110.221317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chavez-Valdez R, McGowan J, Cannon E, Lehmann CU. Contribution of early glycemic status in the development of severe retinopathy of prematurity in a cohort of ELBW infants. Journal of perinatology : official journal of the California Perinatal Association. 2011;31:749–756. doi: 10.1038/jp.2011.19. [DOI] [PubMed] [Google Scholar]
- 64.Kermorvant-Duchemin E, Pinel AC, Lavalette S, Lenne D, Raoul W, Calippe B, Behar-Cohen F, Sahel JA, Guillonneau X, Sennlaub F. Neonatal hyperglycemia inhibits angiogenesis and induces inflammation and neuronal degeneration in the retina. PloS one. 2013;8:e79545. doi: 10.1371/journal.pone.0079545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang YC, Wu WC. Dyslipidemia and diabetic retinopathy. The review of diabetic studies : RDS. 2013;10:121–132. doi: 10.1900/RDS.2013.10.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lian K, Du C, Liu Y, Zhu D, Yan W, Zhang H, Hong Z, Liu P, Zhang L, Pei H, Zhang J, Gao C, Xin C, Cheng H, Xiong L, Tao L. Impaired adiponectin signaling contributes to disturbed catabolism of branched-chain amino acids in diabetic mice. Diabetes. 2015;64:49–59. doi: 10.2337/db14-0312. [DOI] [PubMed] [Google Scholar]
- 67.Zietz B, Buechler C, Kobuch K, Neumeier M, Scholmerich J, Schaffler A. Serum levels of adiponectin are associated with diabetic retinopathy and with adiponectin gene mutations in Caucasian patients with diabetes mellitus type 2, Experimental and clinical endocrinology & diabetes : official journal. German Society of Endocrinology [and] German Diabetes Association. 2008;116:532–536. doi: 10.1055/s-2008-1058086. [DOI] [PubMed] [Google Scholar]
- 68.Pereira RI, Snell-Bergeon JK, Erickson C, Schauer IE, Bergman BC, Rewers M, Maahs DM. Adiponectin dysregulation and insulin resistance in type 1 diabetes. The Journal of clinical endocrinology and metabolism. 2012;97:E642–E647. doi: 10.1210/jc.2011-2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yokoyama H, Emoto M, Mori K, Araki T, Teramura M, Koyama H, Shoji T, Inaba M, Nishizawa Y. Plasma adiponectin level is associated with insulin-stimulated nonoxidative glucose disposal. The Journal of clinical endocrinology and metabolism. 2006;91:290–294. doi: 10.1210/jc.2004-2549. [DOI] [PubMed] [Google Scholar]
- 70.Prior SL, Tang TS, Gill GV, Bain SC, Stephens JW. Adiponectin, total antioxidant status, and urine albumin excretion in the low-risk "Golden Years" type 1 diabetes mellitus cohort. Metabolism: clinical and experimental. 2011;60:173–179. doi: 10.1016/j.metabol.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 71.Yuan F, Liu YH, Liu FY, Peng YM, Tian JW. Intraperitoneal administration of the globular adiponectin gene ameliorates diabetic nephropathy in Wistar rats. Molecular medicine reports. 2014;9:2293–2300. doi: 10.3892/mmr.2014.2133. [DOI] [PubMed] [Google Scholar]
- 72.Antonopoulos AS, Margaritis M, Coutinho P, Shirodaria C, Psarros C, Herdman L, Sanna F, De Silva R, Petrou M, Sayeed R, Krasopoulos G, Lee R, Digby J, Reilly S, Bakogiannis C, Tousoulis D, Kessler B, Casadei B, Channon KM, Antoniades C. Adiponectin as a link between type 2 diabetes and vascular NADPH oxidase activity in the human arterial wall: the regulatory role of perivascular adipose tissue. Diabetes. 2015;64:2207–2219. doi: 10.2337/db14-1011. [DOI] [PubMed] [Google Scholar]
- 73.Hadjadj S, Aubert R, Fumeron F, Pean F, Tichet J, Roussel R, Marre M. Increased plasma adiponectin concentrations are associated with microangiopathy in type 1 diabetic subjects. Diabetologia. 2005;48:1088–1092. doi: 10.1007/s00125-005-1747-x. [DOI] [PubMed] [Google Scholar]
- 74.Yilmaz MI, Sonmez A, Acikel C, Celik T, Bingol N, Pinar M, Bayraktar Z, Ozata M. Adiponectin may play a part in the pathogenesis of diabetic retinopathy. European journal of endocrinology / European Federation of Endocrine Societies. 2004;151:135–140. doi: 10.1530/eje.0.1510135. [DOI] [PubMed] [Google Scholar]
- 75.Jung CH, Kim BY, Mok JO, Kang SK, Kim CH. Association between serum adipocytokine levels and microangiopathies in patients with type 2 diabetes mellitus. Journal of diabetes investigation. 2014;5:333–339. doi: 10.1111/jdi.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lai AK, Lo AC. Animal models of diabetic retinopathy: summary and comparison. Journal of diabetes research. 2013;2013:106594. doi: 10.1155/2013/106594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tao C, Sifuentes A, Holland WL. Regulation of glucose and lipid homeostasis by adiponectin: effects on hepatocytes, pancreatic beta cells and adipocytes, Best practice & research. Clinical endocrinology & metabolism. 2014;28:43–58. doi: 10.1016/j.beem.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Holland WL, Adams AC, Brozinick JT, Bui HH, Miyauchi Y, Kusminski CM, Bauer SM, Wade M, Singhal E, Cheng CC, Volk K, Kuo MS, Gordillo R, Kharitonenkov A, Scherer PE. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell metabolism. 2013;17:790–797. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Long W, Hui Ju Z, Fan Z, Jing W, Qiong L. The effect of recombinant adeno-associated virus-adiponectin (rAAV2/1-Acrp30) on glycolipid dysmetabolism and liver morphology in diabetic rats. General and comparative endocrinology. 2014;206:1–7. doi: 10.1016/j.ygcen.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 80.Jian L, Su YX, Deng HC. Adiponectin-induced inhibition of intrinsic and extrinsic apoptotic pathways protects pancreatic beta-cells against apoptosis. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2013;45:561–566. doi: 10.1055/s-0033-1341500. [DOI] [PubMed] [Google Scholar]
- 81.Okada-Iwabu M, Yamauchi T, Iwabu M, Honma T, Hamagami K, Matsuda K, Yamaguchi M, Tanabe H, Kimura-Someya T, Shirouzu M, Ogata H, Tokuyama K, Ueki K, Nagano T, Tanaka A, Yokoyama S, Kadowaki T. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature. 2013;503:493–499. doi: 10.1038/nature12656. [DOI] [PubMed] [Google Scholar]
- 82.Wong WT, Tian XY, Xu A, Yu J, Lau CW, Hoo RL, Wang Y, Lee VW, Lam KS, Vanhoutte PM, Huang Y. Adiponectin is required for PPARgamma-mediated improvement of endothelial function in diabetic mice. Cell metabolism. 2011;14:104–115. doi: 10.1016/j.cmet.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 83.Yonekawa Y, Miller JW, Kim IK. Age-Related Macular Degeneration: Advances in Management and Diagnosis. Journal of clinical medicine. 2015;4:343–359. doi: 10.3390/jcm4020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Litts KM, Messinger JD, Freund KB, Zhang Y, Curcio CA. Inner Segment Remodeling and Mitochondrial Translocation in Cone Photoreceptors in Age-Related Macular Degeneration With Outer Retinal Tubulation. Investigative ophthalmology & visual science. 2015;56:2243–2253. doi: 10.1167/iovs.14-15838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barron MJ, Johnson MA, Andrews RM, Clarke MP, Griffiths PG, Bristow E, He LP, Durham S, Turnbull DM. Mitochondrial abnormalities in ageing macular photoreceptors. Investigative ophthalmology & visual science. 2001;42:3016–3022. [PubMed] [Google Scholar]
- 86.Cao G, Chen Y, Zhang J, Liu Y, Zhang M, Zhang K, Su Z. Effects of adiponectin polymorphisms on the risk of advanced age-related macular degeneration. Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals. 2015;20:266–270. doi: 10.3109/1354750X.2015.1068857. [DOI] [PubMed] [Google Scholar]
- 87.Nakano Y, Itabashi K, Sakurai M, Aizawa M, Dobashi K, Mizuno K. Accumulation of subcutaneous fat, but not visceral fat, is a predictor of adiponectin levels in preterm infants at term-equivalent age. Early human development. 2014;90:213–217. doi: 10.1016/j.earlhumdev.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 88.Ito R, Satoh-Asahara N, Yamakage H, Sasaki Y, Odori S, Kono S, Wada H, Suganami T, Ogawa Y, Hasegawa K, Shimatsu A. An increase in the EPA/AA ratio is associated with improved arterial stiffness in obese patients with dyslipidemia. Journal of atherosclerosis and thrombosis. 2014;21:248–260. doi: 10.5551/jat.19976. [DOI] [PubMed] [Google Scholar]
- 89.Zhang J, Wang C, Li L, Man Q, Meng L, Song P, Froyland L, Du ZY. Dietary inclusion of salmon, herring and pompano as oily fish reduces CVD risk markers in dyslipidaemic middle-aged and elderly Chinese women. The British journal of nutrition. 2012;108:1455–1465. doi: 10.1017/S0007114511006866. [DOI] [PubMed] [Google Scholar]
- 90.Olza J, Mesa MD, Aguilera CM, Moreno-Torres R, Jimenez A, Perez de la Cruz A, Gil A. Influence of an eicosapentaenoic and docosahexaenoic acid-enriched enteral nutrition formula on plasma fatty acid composition and biomarkers of insulin resistance in the elderly. Clin Nutr. 2010;29:31–37. doi: 10.1016/j.clnu.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 91.Kuda O, Jelenik T, Jilkova Z, Flachs P, Rossmeisl M, Hensler M, Kazdova L, Ogston N, Baranowski M, Gorski J, Janovska P, Kus V, Polak J, Mohamed-Ali V, Burcelin R, Cinti S, Bryhn M, Kopecky J. n-3 fatty acids and rosiglitazone improve insulin sensitivity through additive stimulatory effects on muscle glycogen synthesis in mice fed a high-fat diet. Diabetologia. 2009;52:941–951. doi: 10.1007/s00125-009-1305-z. [DOI] [PubMed] [Google Scholar]
- 92.Prostek A, Gajewska M, Kamola D, Balasinska B. The influence of EPA and DHA on markers of inflammation in 3T3-L1 cells at different stages of cellular maturation. Lipids in health and disease. 2014;13:3. doi: 10.1186/1476-511X-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Keech AC, Mitchell P, Summanen PA, O'Day J, Davis TM, Moffitt MS, Taskinen MR, Simes RJ, Tse D, Williamson E, Merrifield A, Laatikainen LT, d'Emden MC, Crimet DC, O'Connell RL, Colman PG. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370:1687–1697. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- 94.Buldak L, Dulawa-Buldak A, Labuzek K, Okopien B. Effects of 90-day hypolipidemic treatment on insulin resistance, adipokines and proinflammatory cytokines in patients with mixed hyperlipidemia and impaired fasting glucose. International journal of clinical pharmacology and therapeutics. 2012;50:805–813. doi: 10.5414/CP201735. [DOI] [PubMed] [Google Scholar]
- 95.Rosenson RS. Effect of fenofibrate on adiponectin and inflammatory biomarkers in metabolic syndrome patients. Obesity (Silver Spring) 2009;17:504–509. doi: 10.1038/oby.2008.530. [DOI] [PubMed] [Google Scholar]
- 96.Aslibekyan S, An P, Frazier-Wood AC, Kabagambe EK, Irvin MR, Straka RJ, Tiwari HK, Tsai MY, Hopkins PN, Borecki IB, Ordovas JM, Arnett DK. Preliminary evidence of genetic determinants of adiponectin response to fenofibrate in the Genetics of Lipid Lowering Drugs and Diet Network. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2013;23:987–994. doi: 10.1016/j.numecd.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hulsmans M, Geeraert B, Arnould T, Tsatsanis C, Holvoet P. PPAR agonist-induced reduction of Mcp1 in atherosclerotic plaques of obese, insulin-resistant mice depends on adiponectin-induced Irak3 expression. PloS one. 2013;8:e62253. doi: 10.1371/journal.pone.0062253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu M, Liu F. Transcriptional and post-translational regulation of adiponectin. The Biochemical journal. 2010;425:41–52. doi: 10.1042/BJ20091045. [DOI] [PubMed] [Google Scholar]
- 99.Parker-Duffen JL, Nakamura K, Silver M, Zuriaga MA, MacLauchlan S, Aprahamian TR, Walsh K. Divergent roles for adiponectin receptor 1 (AdipoR1) and AdipoR2 in mediating revascularization and metabolic dysfunction in vivo. The Journal of biological chemistry. 2014;289:16200–16213. doi: 10.1074/jbc.M114.548115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bjursell M, Ahnmark A, Bohlooly YM, William-Olsson L, Rhedin M, Peng XR, Ploj K, Gerdin AK, Arnerup G, Elmgren A, Berg AL, Oscarsson J, Linden D. Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes. 2007;56:583–593. doi: 10.2337/db06-1432. [DOI] [PubMed] [Google Scholar]