Abstract
There is good evidence that poor sleep quality increases risk of painful temporomandibular disorder (TMD). However little is known about the course of sleep quality in the months preceding TMD onset, and whether the relationship is mediated by heightened sensitivity to pain. The Pittsburgh Sleep Quality Index was administered at enrollment into the OPPERA prospective cohort study. Thereafter the Sleep Quality Numeric Rating Scale was administered every three months to 2,453 participants. Sensitivity to experimental pressure pain and pinprick pain stimuli was measured at baseline and repeated during follow-up of incident TMD cases (n=220) and matched TMD-free controls (n=193). Subjective sleep quality deteriorated progressively, but only in those who subsequently developed TMD. A Cox proportional hazards model showed that risk of TMD was greater among participants whose sleep quality worsened during follow-up (adjusted hazard ratio=1.73, 95% confidence limits: 1.29, 2.32). This association was independent of baseline measures of sleep quality, psychological stress, somatic awareness, comorbid conditions, non-pain facial symptoms and demographics. Poor baseline sleep quality was not significantly associated with baseline pain sensitivity or with subsequent change in pain sensitivity. Furthermore the relationship between sleep quality and TMD incidence was not mediated via baseline pain sensitivity nor change in pain sensitivity.
Keywords: Epidemiology, musculoskeletal pain, pain perception, psychological stress, sleep quality
INTRODUCTION
Adults with chronic pain report a sleep debt of 42-minutes per night, exceeding the 14-minute sleep debt of adults with acute pain and the absence of sleep debt in adults with no pain.27 In addition, only 37% of adults with chronic pain rate their sleep quality as good or very good, compared to 65% of adults with no pain.27 These nationally representative findings of the 2015 Sleep in America poll add a population insight to experimental and clinical evidence that pain worsens sleep, likely via cortical arousal which interferes with sleep onset and sleep maintenance.6, 22
Just as pain disturbs sleep, sleep disturbance increases sensitivity to experimental pain,10, 19, 26, 29, 38 revealing the bidirectional nature of this relationship. In fact, napping 14 or extending sleep time 33 can reverse elevated sensitivity to pain induced by sleep deprivation. Determining the predominant direction of the sleep and pain relationship was the focus of three comprehensive reviews. These reviews examined longitudinal studies published before 2005,39 experimental studies published before 200721 and longitudinal and experimental studies published from 2006 to 2012.16 It is now clear from the more nuanced temporal analyses that sleep disturbances are stronger, more reliable predictors of pain development than are pain complaints predictors of sleep disturbance.16
An association between sleep disturbance and painful temporomandibular disorder (TMD) is well established. Sleep fragmentation, respiratory effort related arousals, 11 insomnia, 31, 40 and poor sleep quality 36 are each more common in people with TMD than pain-free controls. The limitation of all but one 31 of these studies was reliance on cross-sectional data. One contribution of the Orofacial Pain: Prospective Evaluation and Risk Assessment (OPPERA) prospective cohort study was to show that baseline assessments of obstructive sleep apnea symptoms34 and poor subjective sleep quality 35 predicted development of first-onset painful TMD among adults with no lifetime history of TMD.
Most cross-sectional analyses of variability in pain thresholds show that chronic TMD cases have lower pain thresholds than pain-free controls for a wide range of experimentally evoked noxious pain stimuli, both in the orofacial region and in extra-cranial sites.18, 23, 24, 40 Hence it is reasonable to expect that individuals with poor sleep quality may lower pain thresholds and that this effect may mediate the relationship between poor sleep quality and risk of developing TMD.
What has yet to be characterized is the longitudinal trajectory of sleep quality prior to development of painful TMD. It is not clear whether sleep quality is stable, fluctuating or worsening in the months prior to first-onset TMD. Consequently our study had two aims. First we examined the temporal dynamics of sleep quality in a cohort of initially TMD-free adults followed over time, comparing sleep quality trajectories of incident TMD cases with those of matched TMD-free controls in the cohort. We evaluated the contribution of sleep quality trajectories to risk of developing first-onset TMD. Second we estimated the potential mediation of heightened sensitivity to experimental pain in the pathway between poor sleep quality and TMD development. We hypothesized that poor sleep quality has a hyperalgesic effect which, in turn, increases risk of developing TMD.
METHODS AND MATERIALS
Institutional review boards at each study site approved the study procedures, and signed, informed consent was obtained from each participant. This article complies with recommendations made for the Reporting of Observational Studies in Epidemiology (STROBE).42
Study Design
This study utilized two studies designs, both of which drew on the OPPERA study. OPPERA is an acronym for Orofacial Pain: Prospective Evaluation and Risk Assessment (OPPERA) project. Essentially OPPERA is a series of community-based epidemiologic studies designed to characterize the etiology and persistence of painful TMD. This analysis draws upon two of OPPERA's studies. These are the prospective cohort study and its nested case control study (described below). The advantage of the nested case control study is that it combines the efficiency and comparison group of a conventional case control study. Meanwhile the strength of the prospective cohort study is its longitudinal design in which exposure is ascertained prior to TMD onset.
Setting, Study Participants and Enrollment
OPPERA recruited community-based volunteers into its prospective cohort between May 2006 and November 2008. Its four study sites are located at Baltimore, Maryland; Buffalo, New York; Chapel Hill, North Carolina; and Gainesville, Florida. Initially, potential participants were screened for eligibility. Those who were aged between 18 to 44 years, with no significant history of TMD symptoms, no significant medical illnesses or recent history of facial injury or surgery, not pregnant or nursing, ≤4 headaches per month within the preceding 3 months, not receiving orthodontic treatment, never diagnosed with TMD, and no use of a night-guard occlusal splint were invited to come to a clinic appointment. There they were clinically examined using Research Diagnostic Criteria for TMD (RDC/TMD).12 A total of 3,263 were confirmed as TMD-free were enrolled and followed for up to 5.2 years (median follow-up = 2.8-years).
Baseline Assessment of Subjective Sleep Quality and Experimental Pain Sensitivity
At enrollment, OPPERA participants completed standardized questionnaires with well-established psychometric properties. Habitual sleep quality and sleep disturbance in the past month was assessed using the 19-item Pittsburgh Sleep Quality Index (PSQI).7 The PSQI has seven subscales that assess subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction. Each subscale is weighted equally, scored from 0–3, summing to a global score (range, 0–21). Higher scores denote worse sleep quality and a global score greater than 5 has diagnostic sensitivity of 89.6% and specificity of 86.5% in distinguishing poor from good sleep.7 In people who have TMD, the PSQI is a unidimensional construct. 32 Hence, in this analysis we used the PSQI's single global score.
Methods for quantitative sensory testing (QST) of thermal pain, pressure pain and mechanical pain in OPPERA have been described in detail.18 In OPPERA, QST assessed pressure pain, mechanical cutaneous (pricking) pain, and heat pain. Of these three sensory domains, pressure pain thresholds (PPTs) were most strongly associated with chronic TMD.18 PPTs were assessed bilaterally at the center of the temporalis muscle; the center of the masseter muscle; the temporomandibular joint; the center of the trapezius muscle; and the lateral epicondyle. PPT was determined using a pressure algometer and recorded in kilopascals (kPa).
Mechanical cutaneous (pinprick) pain sensitivity was assessed using weighted probes of 0.2mm diameter that exerted a force of 512 mN applied to the dorsum of digits 2 to 4. Participants rated pain using a 0–100 numeric rating scale reported immediately following a series of 10 stimuli (wind-up protocol) and 15 seconds later. This current analysis was confined to assessment of PPTs at the trapezius muscle site. Being anatomically remote from the temporomandibular joint region, sensitivity to experimental pain of the trapezius muscle is unlikely to be conflated with TMD pain in cases, and more likely to point to a generalized upregulation of nociceptive processing. The study reports findings for sensitivity to experimental pain and change from baseline in experimental pain sensitivity for pressure pain sensitivity and mechanical cutaneous pain. We do not report results for thermal pain in this study because there was a larger number of missing observations for thermal pain measures.
Follow-up Visit for Ascertainment of Incident TMD and Selection of Matched Control Subjects
During follow-up study participants completed the Quarterly Health Update questionnaire every three months. Its purpose was to screen for TMD pain symptoms. Follow-up continued in this way either until clinically-determined TMD developed, the study ended (in May 2011), or until participants were lost to follow-up. Participants who had experienced orofacial pain symptoms were invited to a follow-up clinic visit. At that appointed the RDC/TMD examination was repeated to determine the presence or absence of TMD. Some TMD incident cases may have had joint-meniscus problems, although those conditions were not classified by examiners. As incident cases were identified, one control participant at random was matched to the incident case. Controls were cohort members who had not developed TMD. They were matched to the case by study site, time in study, and sex. The study participant selected as matched control was likewise invited to a follow-up clinic visit where examiners verified absence of clinical TMD. Experimental pain procedures were repeated at the follow-up visit, both for incident cases and matched controls. The median period between enrollment and the second examination was 17 months (interquartile range = 10–26 months).
Assessment of Subjective Sleep Quality during Observation Period
The Quarterly Health Update also monitored subjective sleep quality using the Sleep Quality Numeric Rating Scale (NRS). Participants were instructed to rate their sleep quality over the preceding three months. An anchor value of 0 represented “worst sleep imaginable” while the anchor value of 10 represented “best sleep imaginable”. The Sleep Quality NRS is brief, simple to administer, and easy to understand and complete.25 Its psychometric properties of reproducibility, convergent validity, and responsiveness to treatment are established in individuals with chronic pain in two independent clinical trials.8, 25 The Sleep Quality NRS is also sensitive to detecting change in response to intervention. This was demonstrated in a review of 12 clinical trials of pregabalin versus placebo to treat pain and sleep disturbance in fibromyalgia patients. Improvements in sleep quality—assessed with the Sleep Quality NRS—were observed in 11 of the 12 trials in as little as one or two days of treatment.3 In this analysis, the Sleep Quality NRS values were reverse coded for directional consistency with the PSQI in which higher scores denote worse sleep quality.
The number of completed Quarterly Health Updates completed per person varied accordingly to the length of time they were enrolled in the study. We standardized these reporting periods in the following way. The first quarter refers to the three months following enrollment into the OPPERA cohort. The final quarter refers to the three months preceding the follow-up visit.
The penultimate quarter refers to the three months preceding the final quarter, and the intermediate quarters are the pooled periods that fall between the first and penultimate quarters.
Covariates
In addition to study site, age, sex, race and ethnicity, multivariable analysis adjusted for the four strongest predictors of incident TMD in OPPERA 5 to eliminate their potential confounding effects. These were: non-painful facial symptoms (stiffness or tightness, cramping, fatigue, pressure, soreness or tenderness, ache or dull ache); score for the Pennebaker Inventory of Limbic Languidness (PILL);30 score for the Perceived Stress Scale; 9 and a checklist of 20 comorbid health conditions, which included psychological conditions, painful conditions, and sleep disorders.
Sample size determination
The planned sample size of the OPPERA prospective cohort study (N=3,200) was based on an expected yield 196 first-onset TMD cases. This sample size would have 80% statistical power to detect risk ratios of at least 1.8 for risk predictors with as few as 15% of people in the high-risk category.4 In fact, the actual number of first-onset cases (n=260) slightly exceeded the estimated number of 196, providing adequate power to detect an effect of poor sleep quality, even after adjustment for potential confounding (adjusted hazard ratio for sleep quality during follow-up=1.73, 95% confidence limits: 1.29, 2.32).
Statistical analysis
Person-years of follow-up were calculated from enrollment until time of clinical ascertainment of incident TMD, loss to follow-up, or the end of the follow-up period in May 2011. The main predictor was subjective sleep quality, measured at enrollment with the PSQI and thereafter with the Sleep Quality NRS. Adjusted means for sleep quality were calculated from a generalized estimating equation regression model in which the Sleep Quality NRS (range 0-10, higher scores denote worse sleep quality) was the dependent variable. Predictor variables were time of data collection (4 reporting periods), and TMD incident case classification (2 categories) and their 2-way interaction. Estimates were adjusted for study site, sex, age in years and race/ethnicity. In addition to computing mean values of it continuous measure, the Sleep Quality NRS was used dichotomized at its median value of 6, interpreted as ratings of 0-6 representing good sleep quality and ratings of >6 representing poor sleep quality.
Cox models with a time-varying covariate were used to evaluate the contribution of temporally-varying subjective sleep quality to risk of developing first-onset TMD. The time-varying Sleep Quality NRS variable was “lagged” by selecting the follow-up questionnaire completed in the quarter prior to the quarter used when calculating the partial likelihood. This lagged method avoided the problem of reverse causation by using Sleep Quality NRS reported in the questionnaire that preceded the concurrent quarter. Because all participants, including incident cases, were TMD-free in the lagged quarter, time-varying Sleep Quality NRS could not be influenced by TMD because TMD had not yet developed, even in incident cases.
An initial Cox model included the time-constant covariate of PSQI sleep quality reported at baseline, together with demographics and study site. A second model additionally adjusted for the other covariates.
The third model then determined the contribution of a time-varying sleep quality over time during follow-up, which we expected to be a more informative indicator of risk than a single baseline measurement.
The likelihood ratio test statistic measured overall fit for each model, and hazard ratios with corresponding 95% confidence intervals (95% CI) were estimated for each predictor variable. To address potential problems of residual confounding and information bias created by dichotomizing continuous sleep quality variables, an alternative to Model 3 used PSQI and NRS measures as continuous variables, each divided by its respective standard deviation. Resulting hazard ratios represent the effects of a 1-standard-deviation increase in each of the predictor variables.
To investigate potential mediation of the relationship between sleep quality and incident TMD by experimental pain sensitivity, we proceeded to decompose the total effect into the natural direct (effects not mediated through experimental pain sensitivity) and the natural indirect effect using the counterfactual based approach to mediation analysis that allows for non-linear dependencies. 43 Two potential mediators were considered: pressure pain threshold and average pinprick pain. Each of these potential mediators was then modeled separately in 2 ways, first as the corresponding baseline measure and second as change from baseline measure i.e. difference between follow-up and baseline score. To control for confounding, we created inverse probability of exposure (sleep quality) and mediator (experimental pain sensitivity and change from baseline pain sensitivity) weights and fitted an inverse probability weighted Cox proportional hazards regression using robust variance estimation to obtain hazard ratios and 95% CI for the intended effects. A detailed description of the mediation analysis methods is available in the online appendix.
RESULTS
A total of 2,453 OPPERA cohort participants had valid PSQI data at enrollment and completed at least two follow-up Quarterly Health Update questionnaires over the median 2.8-years of follow-up. TMD developed at a rate of 3.0% of people per annum (Table 1).
Table 1.
Description of subjective sleep quality,(a) and rate of TMD incidence (% per annum, 95% confidence limits (CL)) for selected characteristics measured at baseline, OPPERA Prospective Cohort Study, 2006–2011 (n=2,453).
| N (%) in cohort | PSQI mean (95%CL) | Site-adjusted TMD incidence rate % per annum (95%CL) | |
|---|---|---|---|
| All participants | 2,453 (100.0) | 4.6 (4.5, 4.7) | 3.0 (2.7, 3.5) |
| Sex | |||
| Male | 982 (40.0) | 4.6 (4.4, 4.8) | 2.3 (1.8, 3.0) |
| Female | 1,471 (60.0) | 4.6 (4.5, 4.8) | 3.1 (2.6, 3.7) |
| Age (y) | |||
| 18-24 | 1,300 (53.0) | 4.3 (4.2, 4.4) | 2.2 (1.8, 2.8) |
| 24-34 | 666 (27.2) | 4.7 (4.5, 4.9) | 3.3 (2.6, 4.2) |
| 45-44 | 487 (19.9) | 5.4 (5.1, 5.7) | 3.4 (2.6, 4.6) |
| Race/ethnicity | |||
| White | 1,351 (55.1) | 4.4 (4.3, 4.5) | 2.7 (2.2, 3.3) |
| African American | 625 (25.5) | 5.4 (5.2, 5.7) | 3.6 (2.7, 4.7) |
| Asian | 239 (9.7) | 4.2 (3.9, 4.5) | 1.2 (0.6, 2.3) |
| Hispanic | 163 (6.6) | 4.0 (3.6, 4.3) | 2.4 (1.5, 4.1) |
| Other | 75 (3.1) | 4.6 (3.9, 5.3) | 2.8 (1.3, 5.8) |
| Pittsburgh Sleep Quality Index global score(b) | |||
| 0-5 | 1,725 (70.3) | 2.1 (1.7, 2.5) | |
| >5 | 728 (29.7) | 4.6 (3.8, 5.7) | |
| Perceived Stress Scale | |||
| Low tertile (≤11) | 853 (34.9) | 3.5 (3.3, 3.6) | 2.0 (1.5, 2.6) |
| Mid tertile (>11 to <17) | 739 (30.3) | 4.4 (4.3, 4.6) | 2.4 (1.8, 3.1) |
| High tertile (≥17) | 850 (34.8) | 6.0 (5.8, 6.2) | 4.0 (3.3, 4.9) |
| Pennebaker Inventory of Limbic Languidness | |||
| Low tertile (≤78) | 857 (35.0) | 3.6 (3.4, 3.7) | 1.9 (1.5, 2.5) |
| Mid tertile (79 to 94) | 774 (31.6) | 4.4 (4.3, 4.6) | 2.0 (1.5, 2.7) |
| High tertile (≥95) | 820 (33.5) | 5.9 (5.7, 6.1) | 4.6 (3.8, 5.6) |
| Non-pain facial symptoms | |||
| None | 2,010 (82.1) | 4.4 (4.3, 4.5) | 2.3 (1.9, 1.9) |
| 1 or more | 437 (17.9) | 5.6 (5.3, 5.9) | 5.2 (4.0, 4.0) |
| Count of 20 comorbidities | |||
| None | 1,571 (64.8) | 4.1 (3.9, 4.2) | 1.9 (1.6, 2.4) |
| 1 or more | 854 (35.2) | 5.6 (5.4, 5.8) | 4.4 (3.6, 5.3) |
| Trapezius pressure pain threshold (kPa) | |||
| Low tertile (≤281.5) | 841 (34.6) | 4.7 (4.5, 4.9) | 3.1 (2.5, 4.0) |
| Mid tertile (>281.5 to 450.0) | 797 (32.8) | 4.7 (4.5, 4.9) | 2.8 (2.2, 3.6) |
| High tertile (>450.0) | 793 (32.6) | 4.5 (4.3, 4.7) | 2.2 (1.7, 2.9) |
| Mechanical cutaneous (pinprick) pain (0-100 rating) | |||
| Low tertile (≤5) | 762 (32.0) | 4.9 (4.6, 5.1) | 3.0 (2.3, 3.8) |
| Mid tertile (>5 to 20) | 840 (35.2) | 4.6 (4.4, 4.8) | 2.5 (2.0, 3.3) |
| High tertile (>20) | 782 (32.8) | 4.4 (4.2, 4.6) | 2.6 (2.0, 3.4) |
Subgroup numbers for some characteristics may sum to less than 2,453 due to missing data for that characteristic
Pittsburgh Sleep Quality Index score in which higher scores denote worse sleep quality
The rate of TMD incidence was twice as high in participants whose baseline subjective sleep quality was poor (4.6%, 95% CL: 3.8, 5.7) rather than good (2.1%, 95% CL: 1.7, 2.5). Mean baseline sleep quality (PSQI score) did not differ significantly between men and women. Mean PSQI scores exceeded 5—the threshold for poor sleep quality—for African Americans, participants with heightened perceived stress or somatic awareness, and participants with non-pain facial symptoms or comorbid conditions. Of note, baseline sleep quality was not associated with baseline measures of trapezius pressure pain threshold or ratings of pinprick pain (Table 1).
During the observation period, the mean level of impairment in sleep quality increased from the first quarter by 11% on average among those who became incident cases (p=0.001). By contrast, no significant change in sleep quality was observed for matched controls (3%, p=0.5) (Appendix Table 1). At the final quarter that precipitated the follow-up visit in which TMD incidence was determined, the magnitude of difference in sleep quality between incident cases and matched controls was 17%.
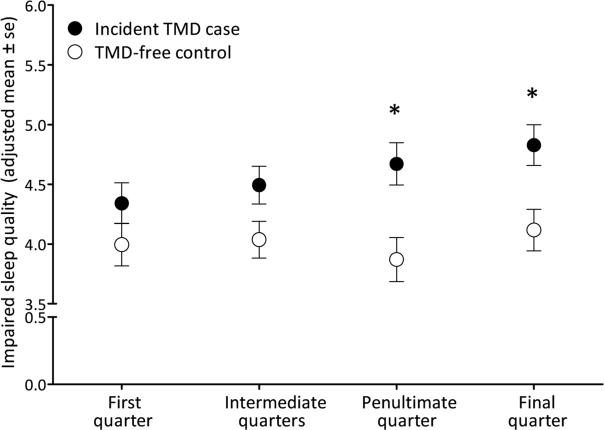
Depicted graphically (Figure 1), sleep quality measured at both the penultimate and final quarters was significantly worse than the baseline level for TMD incident cases while remaining unchanged for matched controls.
Fig 1.
In the nested case control study, sleep quality worsened over time in the lead-up to first-onset TMD (n=220), but remained stable for matched controls (n=193). The four time periods on the horizontal axis refer to: the first quarter (i.e. 3 months) after enrollment; intermediate quarters fall between the first and penultimate quarters; the penultimate quarter immediately preceded the final quarter; and the final quarter (i.e. the 3 months prior to the follow-up visit at which presence or absence of TMD was determined). Estimates are adjusted means calculated from a generalized estimating equation regression model in which the numeric rating scale of sleep quality (range 0–10, higher scores denote worse sleep quality) was the dependent variable. Predictor variables were follow-up period (4 categories), incident TMD case classification (2 categories) and their 2-way interaction. Error bars represent ± 1 standard error (se) of the adjusted mean. Estimates are adjusted for study site, sex, age in years and race/ethnicity.
The * symbol signifies a statistically significant difference (P <0.05) compared to the first quarter in the same study group.
In multivariable Cox proportional hazards analysis that adjusted for study site and demographic characteristics (Table 2, Model 1), baseline poor sleep quality (PSQI score >5) was associated with a significantly increased risk of TMD incidence (adjusted HR=2.04, 95% CL: 1.55, 2.70).
Table 2.
Association between subjective sleep quality and risk of first-onset TMDa among 2,410 OPPERA prospective cohort study participants adjusted for baseline predictors of TMD; hazard ratios (95% confidence limits) (n=2,410)
| Model 1: PSQI at baseline | Model 2: adds four covariates | Model 3: adds time-varying sleep quality | |
|---|---|---|---|
| Baseline PSQI score >5 [ref=0-5] | 2.04 (1.55, 2.70)* | 1.39 (1.02, 1.90)* | 1.28 (0.94, 1.75) |
| Any non-pain facial symptom at baseline [ref=None] | 1.67 (1.22, 2.27)* | 1.69 (1.23, 2.30)* | |
| Any comorbid health condition at baseline [ref=None] | 1.65 (1.22, 2.23)* | 1.63 (1.20, 2.19)* | |
| Baseline PSS score per 6.4 units | 1.14 (0.98, 1.33) | 1.14 (0.98, 1.32) | |
| Baseline PILL score per 21.0 units | 1.16 (1.01, 1.34)* | 1.14 (0.99, 1.32) | |
| Time-varying covariate of sleep quality rating >6 [ref=0-6]: | 1.73 (1.29, 2.32)* | ||
| Model fit: likelihood ratio statistic (df) | 78.1 (10) | 123.0 (14) | 137.8 (15) |
All models are adjusted for study site, age, sex and race/ethnicity
The symbol denotes a statistically significant predictor of first-onset TMD
Further adjustment for perceived stress, somatic awareness, comorbid conditions and non-pain facial symptoms attenuated the degree of association by 32% (HR=1.39, 95% CL: 1.02, 1.90), but baseline poor sleep quality remained a significant predictor of TMD (Table 2, model 2). In the presence of all of these factors, the dichotomized time-varying sleep quality rating was an independent risk factor (Table 2, model 3).
The risk of developing TMD was 73% higher (HR=1.73: 95% CL: 1.29, 2.32) in participants with poor sleep quality (numeric rating >6) during the observation period compared to participants with a sleep quality rating in the range of 0 to 6. Findings were analogous using continuous measures of sleep quality (Appendix Table 3). The likelihood ratio test statistic increased across successive models, indicating better model fit as variables were added.
At baseline, there was no statistically significant relationship between sleep quality and the QST measures, either in TMD cases or matched controls (Appendix Table 2). Neither did QST measures modify the relationship between baseline sleep quality and TMD status. Moreover the magnitude of change in QST measures from baseline to follow-up did not differ between TMD incident cases and matched controls.
The results of our mediation analysis suggest that the relationship between sleep quality and incident TMD is not mediated by pressure pain threshold (Table 3) or average pinprick pain (appendix table 3). While the estimates of the direct effect were stronger when baseline experimental pain measures were modeled as mediators as opposed to change from the baseline measures, this was not the case for the respective indirect effects, which were all estimated as null (Table 3 and appendix Table 2).
Table 3.
A mediation analysis showing hazard ratios (95%CI) for first-onset TMD comparing poor versus good baseline sleep quality, OPPERA prospective cohort study
| Nested case control (N=431) |
||||
|---|---|---|---|---|
| Model | Baseline sleep quality | Potential mediator | Natural direct effect | Natural indirect effect |
| 1((a), (c)) | PSQI ≤5 (ref) | Baseline pressure pain threshold | Ref. | Ref. |
| PSQI >5 (poor quality) | 1.91 (1.59, 2.29)* | 1.00 (0.83, 1.19) | ||
| 2(b) | PSQI ≤5 (ref) | Baseline pressure pain threshold | Ref. | Ref. |
| PSQI >5 | 1.41 (1.15, 1.74)* | 1.00 (0.83, 1.19) | ||
| 3(a) | PSQI ≤5 (ref) | Change in pressure pain threshold | Ref. | Ref. |
| PSQI >5 | 1.90 (1.59, 2.28)* | 1.00 (0.84, 1.20) | ||
| 4((b), (d)) | PSQI ≤5 (ref) | Change in pressure pain threshold | Ref. | Ref. |
| PSQI >5 | 1.42 (1.16, 1.74)* | 0.99 (0.83, 1.19) | ||
Models 1 and 3 are adjusted for study site, age, sex and race/ethnicity
Models 2 and 4 are adjusted for study site, age, sex, race/ethnicity, perceived stress (Perceived Stress Scale, continuous variable), somatic awareness (PILL score, continuous variable), comorbid conditions (≥1 vs. 0) and non-pain facial symptoms (≥1 vs. 0)
For models 1 and 2, the mediator was a binary measure of baseline pressure pain threshold (PPT), dichotomized at the lower tertile (≤273.25 vs. >273.35).
For models 3 and 4, the mediator was a binary measure of change from baseline PPT, dichotomized at the lower tertile (≤77.5 vs. >77.5).
The symbol denotes a statistically significant predictor of first-onset TMD
After taking account of the major risk factors for TMD, the risk of developing TMD remained elevated 73% in poor sleepers compared to good sleepers.
DISCUSSION
In this cohort of initially TMD-free adults, subjective sleep quality deteriorated progressively prior to the onset of pain symptoms in people who developed painful TMD. By contrast, there was no change in sleep quality during follow-up among those who remained TMD-free.
Participants with baseline poor sleep quality developed first-onset TMD at twice the rate as participants with good sleep quality (demographically adjusted HR 2.04 95% CI: 1.55, 2.70). The magnitude of this effect size has considerable importance to clinical practice, given that sleep quality is amenable to intervention. The implication is that, if poor sleep quality were to be mitigated in these individuals, their rate of TMD incidence would be halved. After taking account of the major risk factors for TMD, the risk of developing TMD remained elevated 73%, in poor sleepers compared to good sleepers.
The effect of deteriorating sleep quality on TMD onset was independent of baseline sleep quality and other baseline measures that were among the strongest predictors of TMD in OPPERA. These were somatic awareness, perceived stress, comorbid health conditions and non-painful facial symptoms. We found no evidence that poor sleep quality was associated with sensitivity to experimental pain stimuli. Moreover, we found no evidence that the effect of poor sleep quality on risk of TMD was mediated via sensitivity to experimental pain.
These findings build upon our earlier OPPERA finding that poor sleep quality at baseline predicted increased risk of developing TMD. 35 First we monitored sleep quality longitudinally over a median 2.8 year follow-up and found a downward trajectory before the onset and clinical confirmation of TMD. Second we contrasted this dynamic trajectory to the stable trajectory in a comparison group that remained TMD-free. Third we showed that the effect of poor sleep quality on TMD onset was not mediated by pain sensitivity. Our analytic Cox models, including the mediation models, controlled for the potential confounding of perceived stress. This was necessary as stress is associated with primary insomnia17 and experimentally-evoked pain sensitivity 23 and predicts the subsequent development of TMD.15
Our findings permit interpretation that poor sleep quality is not merely a surrogate marker of psychological stress. A large number of studies have shown that baseline sleep problems increase risk for development of widespread musculoskeletal pain. However we believe this to be the first population-based cohort to have monitored sleep quality every few months as a risk factor for pain onset.
Our a priori expectation that worsening subjective sleep quality would predict increased sensitivity to experimental pain was based on knowledge that disruptive sleep enhances pain perception,20 predicts the number of tender points26, and disturbs pain inhibiting processing 38 including among adults with chronic TMD.13 However, inconsistent findings are reported. For example, interruption in delta wave sleep over three consecutive nights predicted widespread pain, but did not predict reduced pain thresholds. 28 One possibility is that sleep deprivation modulates nociception differently at different stages of sleep. The effects on pain of chronic sleep restriction and sleep fragmentation (such as the arousals induced by obstructive sleep apnea) have received considerably less research attention than total sleep deprivation which is a deprivation of all sleep stages, including rapid eye movement sleep.
It is important to note that OPPERA's longitudinal analysis only weakly supported the premise that elevated sensitivity to pain predicted TMD onset. The best prediction was observed when change in pressure pain thresholds was measured from enrollment to onset TMD,37 which is why we used change scores in our mediation analysis, not simply baseline scores of QST. Yet even the change scores did not mediate the effect of sleep quality on TMD.
For the mediation analysis, we made untestable assumptions that the variables used in creating the inverse probability weights were sufficient to adjust for confounding between: sleep quality and TMD onset; sleep quality and pain sensitivity; and pain sensitivity and TMD onset. We also assumed that there were no confounders of pain sensitivity and TMD onset affected by sleep quality. We also point out that mediation was explored in only a subset of the OPPERA cohort—the nested case control participants—so our power to detect an association between sleep quality and TMD onset was lowered somewhat, although importantly the number of incident cases in the nested case control study was no less than in the prospective cohort study.
At no time during the study was sleep assessed clinically. Neither was objective information collected on sleep disorders such as insomnia, periodic limb movement, and sleep-disordered breathing. In this study sleep quality was assessed differently at baseline (PSQI) than during follow-up (Sleep Quality NRS), which precluded evaluation of change in sleep quality in the first post-enrollment quarter. This probably attenuated the magnitude of sleep quality deterioration in people who later developed TMD, and hence our estimates are conservative.
Use of the sleep quality NRS is mostly restricted to clinical trials treating fibromyalgia patients with pregabalin, and its psychometric properties have been validated in those settings.8, 25 It has also been applied to explore the impact of sleep disorders on postural stability1, 2 but few other studies are reported. Despite its limited application to sleep, the NRS is a widely used methodology that has long been well accepted in as a measure of pain intensity. It is simple to use and requires no training. For these reasons, there is no reason to think that the NRS is not a reliable and responsive measure of other subjective phenomenon, such as sleep quality.
Self-reported sleep quality is only modestly correlated with objective assessment obtained by polysomnogram (PSG). Hence discordant findings from the two modalities are common. For example, menopausal women in the Wisconsin Sleep Cohort Study reported worse sleep quality than their premenopausal peers, yet PSG found that postmenopausal women had more deep sleep and significantly longer total sleep time than the premenopausal women.44 In the Sleep Heart Health Study, older women had good subjective sleep quality, yet poor PSG-determined sleep quality, while the reverse was true for older men.41 Therefore we point out that subjective and objective sleep quality should not be viewed as equivalent constructs.
This leaves open the possibility that objectively-measured aspects of sleep might likewise affect risk of TMD, and through pathways that are mediated via experimental pain sensitivity. Objectively evaluated sleep quality parameters would offer physiological validity to ratings of habitual sleep quality and enhance our understanding of this complex relationship.
A major strength of this study was the repeated prospective ascertainment of sleep quality before the onset of TMD, the measurement of individual change in sleep quality, and the modeling of these correlated time-varying covariates in Cox models. Another strength was the measurement of QST measures at enrollment and when incident TMD was confirmed. A third strength was the utilization of a nested case control design, which capitalized on the longitudinal design of the cohort and provided a matched control. Finally, the formal mediation analysis is a powerful technique with longitudinal data to understand causal pathways between an exposure and an outcome.
Our findings point to directions for future research. Psychometricians will continue to test the properties of the sleep quality NRS. The OPPERA investigators are interested in determining whether sleep quality may itself be a mediator of the relationship between psychological stress and TMD incidence. Another question of particular interest to clinicians is whether interventions that improve sleep quality prevent the onset of pain in high-risk groups, and mitigate pain in people with existing pain disorders.
Supplementary Material
PERSPECTIVE.
Subjective sleep quality deteriorates progressively prior to the onset of painful temporomandibular disorder (TMD), but sensitivity to experimental pain does not mediate this relationship. Furthermore, the relationship is independent of potential confounders such as psychological stress, somatic awareness, comorbid conditions, non-pain facial symptoms and various demographic factors.
Highlights.
■ Subjective sleep quality worsened progressively prior to the onset of painful TMD.
■ Sleep quality was stable over time in participants who remained free of TMD.
■ Sleep quality was a stronger predictor of TMD than well-established TMD risk factors.
■ Worsening sleep quality did not predict an increase in pain sensitivity.
■ Pain sensitivity did not mediate the relationship between sleep quality and TMD.
ACKNOWLEDGEMENT
This work was supported by the National Institutes of Health (NIH) and National Institutes of Dental and Cranial Research (NIDCR) (grant numbers U01-DE17018 and R03-DE022595). Roger Fillingim, Shad Smith and Gary Slade are consultants and equity stock holders, and William Maixner is a founder and equity stock holder in Algynomics, Inc., a company providing research services in personalized pain medication and diagnostics.
Appendix
Statistical Methods-Mediation analysis
To investigate potential mediation of the sleep quality-incident TMD association by experimental pain sensitivity, we proceeded to decompose the total effect into the direct (effects not mediated through experimental pain sensitivity) and indirect effects. To accomplish this, we used the counterfactual based approach to mediation analysis as described by Lange and colleagues (Lange et al., 2012). First the exposure (PSQI score) was dichotomized and individuals with score >5 were considered as having poor sleep quality. We chose pressure pain threshold of the trapezius muscle and average pinprick pain as our potential mediators. These potential mediators were modeled separately as baseline pressure pain threshold, change from baseline pressure pain threshold, baseline average pinprick pain and change from baseline average pinprick pain. And similar to the exposure, we dichotomized these variables at the lower tertile of their distribution. Next, we proceeded to identify a sufficient set of confounding variables C (study site, age, sex, race/ethnicity, the Perceived Stress Scale score, the Pennebaker Inventory of Limbic Languidness (PILL) score, a count of 20 comorbidities, and a count of non-pain facial symptoms) of the exposure-outcome, exposure mediator and mediator outcome relationships. We then created inverse probability (IP) weights separately for the exposure and mediators. The inverse probability of exposure weight is the inverse of the predicted probability of the exposure conditional on observed covariates C. The purpose of weighting is to create a pseudo-population consisting of wi copies of each subject i (Robins et al., 2000), such that a given individual with for instance a weight of 6 contributes 6 copies of themselves to the pseudo-population. Thus, in this pseudo-population, the exposure is no longer associated with confounders C. In other words, the IP of exposure weight controls for confounding by the set of covariates C used in constructing it.
In small to moderate sized samples, the inverse probability weights tend to be unstable because certain individuals with large weights tend to dominate the estimation (Robins et al., 2000). Thus we stabilized this weight by substituting in the numerator of the weight, the marginal probability of the exposure for the exposed and 1 minus this value for the unexposed. The stabilized inverse probability of exposure weight is thus:
Where xi and ci are the actual values of the exposure and covariates for individual i. Next we created mediator weights as detailed in Lange et al (Lange et al., 2012) by first constructing a new dataset with replicates of each observation in the original dataset twice and creating a new variable (xstar) that captures the 2 possible values of the exposure relative to the indirect path. For the first replication of each observation, xstar was set to the actual value of the exposure (x) and set to the opposite of the actual exposure for the second replicate. We then proceeded to create mediator weights that correspond to the direct and indirect paths. Specifically, we estimated the following weights:
The numerator of the weight corresponds to covariate and exposure conditional predicted probability relative to the indirect path and the denominator is the same but for the direct path.
To obtain the final weight, we multiplied the exposure weight by the mediator weight and fitted an inverse probability weighted cox proportional hazards model with robust variance estimation to obtain Hazard ratios and 95% CI.
The coefficient β1 for x corresponds to the log hazard estimate of the natural direct effect while the coefficient β0 for xstar corresponds to the log hazard estimate of the natural indirect effect. A final caveat is that the validity of this method depends on correct specification of both the exposure and mediator weight models. We also assumed that the set of confounders used in creating these weights controlled for the exposure-outcome, exposure-mediator and mediator-outcome confounding and that there were no exposure induced mediator-outcome confounders.
Appendix Table 1.
Adjusted(a) Mean Sleep Quality Numeric Rating Scale Scores(b) at Four Time Points in the OPPERA Nested Case-Control Study of TMD (N=413)(c)
| First quarter | Intermediate quarters | Penultimate quarter | Final quarter | |
|---|---|---|---|---|
| TMD incident cases (n= 220) | ||||
| Sleep quality score, mean (SE) | 4.34 (0.17) | 4.49 (0.16) | 4.67 (0.18) | 4.83 (0.17) |
| % Change relative to first quarter | - | 3% | 8% | 11% |
| P value for change from first quarter | - | 0.244 | 0.032 | 0.001 |
| Matched controls (n= 193) | ||||
| Sleep quality score, mean (SE) | 4.00 (0.18) | 4.04 (0.15) | 3.87 (0.18) | 4.12 (0.17) |
| % Change relative to first quarter | - | 1% | −3% | 3% |
| P value for change from first quarter | - | 0.761 | 0.464 | 0.451 |
| Contrast cases versus matched controls | ||||
| % Difference | 9% | 11% | 21% | 17% |
| P value for contrast | 0.084 | 0.006 | 0.000 | 0.000 |
Adjusted for study site, sex age in years and race/ethnicity
Higher mean scores denote worse sleep quality
Selection of TMD cases and matched controls is limited to participants in the nested case-control study who completed at least two Quarterly Health Update questionnaires during follow-up
Appendix Table 2.
Baseline estimates (mean (standard error (SE))(a) and change from baseline in quantitative sensory testing (QST) measures according to sleep quality for incident TMD cases and match controls in the OPPERA nested case-control study (n=431)
| Baseline sleep quality | P | ||||
|---|---|---|---|---|---|
| Good (PSQI 0-3) | Moderate (PSQI >3-5) | Poor (PSQI >5) | |||
| Baseline QST measures | Mean (SE) | Mean (SE) | Mean (SE) | ||
| Trapezius pressure pain threshold (kPa) | Case | 316 (17) | 370 (17) | 349 (13) | 0.3(d) |
| Matched control | 356 (15) | 370 (18) | 337 (20) | ||
| P=0.5(b) | P=0.1(c) | ||||
| Mean pinprick pain rating (0-100) (N=378) | Case | 18.0 (2.6) | 14.4 (2.7) | 17.8 (2.0) | 0.9(d) |
| Matched control | 22.4 (2.3) | 16.7 (2.7) | 20.8 (3.3) | ||
| P=0.1(b) | P=0.2(c) | ||||
| Pinprick post-stimulus rating (0-100) (N=377) | Case | 2.35 (1.0) | 2.71 (1.1) | 2.91 (0.8) | 0.4(d) |
| Matched control | 3.78 (1.0) | 1.97 (1.1) | 5.11 (1.3) | ||
| P=0.3(b) | P=0.3(c) | ||||
| Change from baseline QST measures | |||||
| Delta: trapezius pressure pain threshold (kPa) | Case | −41.4 (17) | −81.0 (17) | −49.9 (13) | 0.1(d) |
| Matched control | 13.1 (15) | 35.6 (18) | 60.2 (21) | ||
| P<0.0001(b) | P=0.3(c) | ||||
| Delta: Mean pinprick pain rating (0-100) (N=362) | Case | 6.60 (2.9) | 6.23 (3.0) | 3.05 (2.2) | 0.6(d) |
| Matched control | −1.98 (2.6) | −1.47 (2.9) | −0.06 (3.6) | ||
| P=0.005(b) | P=0.9(c) | ||||
| Delta: Pinprick post-stimulus rating (0-100) (N=362) | Case | 4.34 (1.2) | 1.24 (1.3) | 1.45 (0.9) | 0.3(d) |
| Matched control | −0.32 (1.1) | 0.14 (1.3) | −1.59 (1.5) | ||
| P=0.003(b) | P=0.2(c) | ||||
All mean estimates are adjusted for study site
P-value testing the null hypothesis of no statistically significant difference in baseline mean sleep quality between incident cases and matched controls (i.e. main effect)
P-value for testing the null hypothesis of no statistical significant difference between good, moderate and poor sleep quality (i.e. main effect)
P-value from the likelihood ratio test that assesses the statistical significance of the effect modification of the QST measures on the relationship between sleep quality and TMD case status (i.e. interaction)
Appendix Table 3.
Association between subjective sleep quality and risk of first-onset TMD(a) among 2,410 OPPERA prospective cohort study participants adjusted for baseline predictors of TMD; hazard ratios (95% confidence limits) (n=2,410)
| Hazard Ratio (95% confidence limits) |
|
|---|---|
| Baseline PSQI score per 2.8 units | 1.04 (0.90, 1.20) |
| Any non-pain facial symptom at baseline [ref=None] | 1.74 (1.28, 2.38)* |
| Any comorbid health condition at baseline [ref=None] | 1.64 (1.21, 2.21)* |
| Baseline PSS score per 6.4 units | 1.16 (1.00, 1.35) |
| Baseline PILL score per 21.0 units | 1.16 (1.01, 1.35)* |
| Time-varying covariate per 2.0 units | 1.23 (1.07, 1.42)* |
| Model fit: likelihood ratio statistic (df) | 135.2 (15) |
The model adjusts for study site, age, sex and race/ethnicity
The symbol denotes a statistically significant predictor of first-onset TMD
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Anne E. Sanders, Department of Dental Ecology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Aderonke A. Akinkugbe, Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Eric Bair, Department of Biostatistics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.; Department of Endodontics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA., USA. Center for Pain Research and Innovation, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Roger B. Fillingim, University of Florida, College of Dentistry, Gainesville, Florida, USA.
Joel D. Greenspan, Department of Neural and Pain Sciences, University of Maryland School of Dentistry, Baltimore, Maryland, USA. Brotman Facial Pain Clinic, University of Maryland School of Dentistry, Baltimore, Maryland, USA.
Richard Ohrbach, Department of Oral Diagnostic Sciences, University at Buffalo, Buffalo, New York, USA..
Ronald Dubner, Department of Neural and Pain Sciences, University of Maryland Dental School, Baltimore, Maryland, USA..
William Maixner, Department of Endodontics, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.; Center for Pain Research and Innovation, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
Gary D. Slade, Department of Dental Ecology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA. Center for Pain Research and Innovation, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
References
- 1.Akkaya N, Akkaya S, Atalay NS, Acar M, Catalbas N, Sahin F. Assessment of the relationship between postural stability and sleep quality in patients with fibromyalgia. Clin Rheumatol. 2013;32:325–331. doi: 10.1007/s10067-012-2117-y. [DOI] [PubMed] [Google Scholar]
- 2.Akkaya N, Doganlar N, Celik E, Aysse SE, Akkaya S, Gungor HR, Sahin F. Test-Retest Reliability of Tetrax(R) Static Posturography System in Young Adults with Low Physical Activity Level. Int J Sports Phys Ther. 2015;10:893–900. [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold LM, Emir B, Pauer L, Resnick M, Clair A. Time to improvement of pain and sleep quality in clinical trials of pregabalin for the treatment of fibromyalgia. Pain Med. 2015;16:176–185. doi: 10.1111/pme.12636. [DOI] [PubMed] [Google Scholar]
- 4.Bair E, Brownstein NC, Ohrbach R, Greenspan JD, Dubner R, Fillingim RB, Maixner W, Smith SB, Diatchenko L, Gonzalez Y, Gordon SM, Lim PF, Ribeiro-Dasilva M, Dampier D, Knott C, Slade GD. Study protocol, sample characteristics, and loss to follow-up: the OPPERA prospective cohort study. J Pain. 2013;14:T2–19. doi: 10.1016/j.jpain.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bair E, Ohrbach R, Fillingim RB, Greenspan JD, Dubner R, Diatchenko L, Helgeson E, Knott C, Maixner W, Slade GD. Multivariable modeling of phenotypic risk factors for first-onset TMD: the OPPERA prospective cohort study. J Pain. 2013;14:T102–115. doi: 10.1016/j.jpain.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bastuji H, Perchet C, Legrain V, Montes C, Garcia-Larrea L. Laser evoked responses to painful stimulation persist during sleep and predict subsequent arousals. Pain. 2008;137:589–599. doi: 10.1016/j.pain.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 7.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 8.Cappelleri JC, Bushmakin AG, McDermott AM, Sadosky AB, Petrie CD, Martin S. Psychometric properties of a single-item scale to assess sleep quality among individuals with fibromyalgia. Health Qual Life Outcomes. 2009;7:54. doi: 10.1186/1477-7525-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen S, Williamson G. In: The social psychology of health. Spacapan S, Oskamp S, editors. Sage; Newbury Park, CA: 1988. pp. 31–67. [Google Scholar]
- 10.Cooperman NR, Mullin FJ, Kleitman N. Studies on the physiology of sleep. XI. Further observations on the effects of prolonged sleeplessness. Am J Physiol. 1934;107:589–593. [Google Scholar]
- 11.Dubrovsky B, Raphael KG, Lavigne GJ, Janal MN, Sirois DA, Wigren PE, Nemelivsky LV, Klausner JJ, Krieger AC. Polysomnographic investigation of sleep and respiratory parameters in women with temporomandibular pain disorders. J Clin Sleep Med. 2014;10:195–201. doi: 10.5664/jcsm.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord. 1992;6:301–355. [PubMed] [Google Scholar]
- 13.Edwards RR, Grace E, Peterson S, Klick B, Haythornthwaite JA, Smith MT. Sleep continuity and architecture: associations with pain-inhibitory processes in patients with temporomandibular joint disorder. Eur J Pain. 2009;13:1043–1047. doi: 10.1016/j.ejpain.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faraut B, Leger D, Medkour T, Dubois A, Bayon V, Chennaoui M, Perrot S. Napping reverses increased pain sensitivity due to sleep restriction. PLoS One. 2015;10:e0117425. doi: 10.1371/journal.pone.0117425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Diatchenko L, Dubner R, Bair E, Baraian C, Mack N, Slade GD, Maixner W. Psychological factors associated with development of TMD: the OPPERA prospective cohort study. J Pain. 2013;14:T75–90. doi: 10.1016/j.jpain.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14:1539–1552. doi: 10.1016/j.jpain.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldman-Mellor S, Gregory AM, Caspi A, Harrington H, Parsons M, Poulton R, Moffitt TE. Mental health antecedents of early midlife insomnia: evidence from a four-decade longitudinal study. Sleep. 2014;37:1767–1775. doi: 10.5665/sleep.4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenspan JD, Slade GD, Bair E, Dubner R, Fillingim RB, Ohrbach R, Knott C, Mulkey F, Rothwell R, Maixner W. Pain sensitivity risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case control study. J Pain. 2011;12:T61–74. doi: 10.1016/j.jpain.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson L. Psychological and physiological changes following total sleep deprivation. In: Kales A, editor. Sleep Physiology and Pathology. Lippincott; Philadelphia: 1969. [Google Scholar]
- 20.Kundermann B, Spernal J, Huber MT, Krieg JC, Lautenbacher S. Sleep deprivation affects thermal pain thresholds but not somatosensory thresholds in healthy volunteers. Psychosom Med. 2004;66:932–937. doi: 10.1097/01.psy.0000145912.24553.c0. [DOI] [PubMed] [Google Scholar]
- 21.Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10:357–369. doi: 10.1016/j.smrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Lavigne G, Zucconi M, Castronovo C, Manzini C, Marchettini P, Smirne S. Sleep arousal response to experimental thermal stimulation during sleep in human subjects free of pain and sleep problems. Pain. 2000;84:283–290. doi: 10.1016/s0304-3959(99)00213-4. [DOI] [PubMed] [Google Scholar]
- 23.Maixner W, Fillingim R, Booker D, Sigurdsson A. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain. Pain. 1995;63:341–351. doi: 10.1016/0304-3959(95)00068-2. [DOI] [PubMed] [Google Scholar]
- 24.Maixner W, Fillingim R, Sigurdsson A, Kincaid S, Silva S. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain: evidence for altered temporal summation of pain. Pain. 1998;76:71–81. doi: 10.1016/s0304-3959(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 25.Martin S, Chandran A, Zografos L, Zlateva G. Evaluation of the impact of fibromyalgia on patients' sleep and the content validity of two sleep scales. Health Qual Life Outcomes. 2009;7:64. doi: 10.1186/1477-7525-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moldofsky H, Scarisbrick P, England R, Smythe H. Musculosketal symptoms and non-REM sleep disturbance in patients with “fibrositis syndrome” and healthy subjects. Psychosom Med. 1975;37:341–351. doi: 10.1097/00006842-197507000-00008. [DOI] [PubMed] [Google Scholar]
- 27.National Sleep Foundation [September 17 2015];Pain and Sleep. Available at: https://sleepfoundation.org/sleep-polls-data/2015-sleep-and-pain.
- 28.Older SA, Battafarano DF, Danning CL, Ward JA, Grady EP, Derman S, Russell IJ. The effects of delta wave sleep interruption on pain thresholds and fibromyalgia-like symptoms in healthy subjects; correlations with insulin-like growth factor I. J Rheumatol. 1998;25:1180–1186. [PubMed] [Google Scholar]
- 29.Onen SH, Alloui A, Gross A, Eschallier A, Dubray C. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. J Sleep Res. 2001;10:35–42. doi: 10.1046/j.1365-2869.2001.00240.x. [DOI] [PubMed] [Google Scholar]
- 30.Pennebaker JW. The psychology of physical symptoms. Springer-Verlag; New York: 1982. [Google Scholar]
- 31.Quartana PJ, Wickwire EM, Klick B, Grace E, Smith MT. Naturalistic changes in insomnia symptoms and pain in temporomandibular joint disorder: a cross-lagged panel analysis. Pain. 2010;149:325–331. doi: 10.1016/j.pain.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 32.Rener-Sitar K, John MT, Bandyopadhyay D, Howell MJ, Schiffman EL. Exploration of dimensionality and psychometric properties of the Pittsburgh Sleep Quality Index in cases with temporomandibular disorders. Health Qual Life Outcomes. 2014;12:10. doi: 10.1186/1477-7525-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roehrs TA, Harris E, Randall S, Roth T. Pain sensitivity and recovery from mild chronic sleep loss. Sleep. 2012;35:1667–1672. doi: 10.5665/sleep.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanders AE, Essick GK, Fillingim R, Knott C, Ohrbach R, Greenspan JD, Diatchenko L, Maixner W, Dubner R, Bair E, Miller VE, Slade GD. Sleep apnea symptoms and risk of temporomandibular disorder: OPPERA cohort. J Dent Res. 2013;92:70S–77S. doi: 10.1177/0022034513488140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanders AE, Slade GD, Bair E, Fillingim RB, Knott C, Dubner R, Greenspan JD, Maixner W, Ohrbach R. General health status and incidence of first-onset temporomandibular disorder: the OPPERA prospective cohort study. J Pain. 2013;14:T51–62. doi: 10.1016/j.jpain.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitter M, Kares-Vrincianu A, Kares H, Bermejo JL, Schindler HJ. Sleep-associated aspects of myofascial pain in the orofacial area among Temporomandibular Disorder patients and controls. Sleep Med. 2015;16:1056–1061. doi: 10.1016/j.sleep.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 37.Slade GD, Sanders AE, Ohrbach R, Fillingim RB, Dubner R, Gracely RH, Bair E, Maixner W, Greenspan JD. Pressure pain thresholds fluctuate with, but do not usefully predict, the clinical course of painful temporomandibular disorder. Pain. 2014;155:2134–2143. doi: 10.1016/j.pain.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30:494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
- 39.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8:119–132. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 40.Smith MT, Wickwire EM, Grace EG, Edwards RR, Buenaver LF, Peterson S, Klick B, Haythornthwaite JA. Sleep disorders and their association with laboratory pain sensitivity in temporomandibular joint disorder. Sleep. 2009;32:779–790. doi: 10.1093/sleep/32.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Unruh ML, Redline S, An MW, Buysse DJ, Nieto FJ, Yeh JL, Newman AB. Subjective and objective sleep quality and aging in the sleep heart health study. J Am Geriatr Soc. 2008;56:1218–1227. doi: 10.1111/j.1532-5415.2008.01755.x. [DOI] [PubMed] [Google Scholar]
- 42.Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology. 2007;18:805–835. doi: 10.1097/EDE.0b013e3181577511. [DOI] [PubMed] [Google Scholar]
- 43.VanderWeele TJ. Marginal structural models for the estimation of direct and indirect effects. Epidemiology. 2009;20:18–26. doi: 10.1097/EDE.0b013e31818f69ce. [DOI] [PubMed] [Google Scholar]
- 44.Young T, Rabago D, Zgierska A, Austin D, Laurel F. Objective and subjective sleep quality in premenopausal, perimenopausal, and postmenopausal women in the Wisconsin Sleep Cohort Study. Sleep. 2003;26:667–672. doi: 10.1093/sleep/26.6.667. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.