Abstract
Muscle fascicle lengths are commonly measured in vivo using static 2D ultrasound. However, static ultrasound is best suited for muscles with shorter, pennate fascicles, in which entire fascicles can be viewed in one static image. An informal review of data from cadaver dissections suggests that over 60% of muscles in the upper and lower limbs have optimal lengths longer than the field-of-view of standard ultrasound transducers. Extended field of view ultrasound (EFOV) has been validated for measurement of fascicle lengths, but has yet to be implemented in the upper extremity in humans. In this study, EFOV ultrasound was used to measure the lengths of fascicles sampled from the anterior portion of the biceps brachii (long head) and the distal half of the triceps brachii (lateral head). Data were collected from both limbs of eleven healthy subjects in three elbow postures under passive conditions. Image analysis was completed via Image J. Fascicle length measurements were highly reliable, with intra-class correlations ranging from .92 to .95 for biceps and .81 – .92 for triceps (p<.001). Systematic, significant differences in measured lengths, consistent with muscle function, were observed between elbow positions. In vivo measurements for both muscles in this study were within the range of cadaver data. This work establishes the feasibility and reliability of EFOV ultrasound for measurement of the long fascicles of muscles in the upper limb.
Keywords: Muscle architecture, fascicle length, ultrasonography, upper extremity
Introduction
Muscle architecture, an important determinant of an individual muscle’s function, describes the number, length, and arrangement of a muscle’s fibers and their orientation with respect to the axis of force generation (Lieber and Friden, 2000; Lieber and Ward, 2011). Optimal fiber length (defined as the length at which the muscle generates its maximum isometric force (Zajac, 1989)), is a critical architectural parameter that defines a muscle’s excursion capacity and absolute shortening velocity (Lieber and Friden, 2000). To calculate optimal fiber length, researchers must first measure the lengths of a sample of its fascicles, the corresponding lengths of the sarcomeres within those fascicles, and then normalize the fascicle lengths (Lieber et al., 1990; Murray et al., 2000; Ward et al., 2009; 2006; Wickiewicz et al., 1983) to the optimal sarcomere length of human muscle (2.6–2.8 μm (Burkholder and Lieber, 2001; Lieber et al., 1994; Walker and Schrodt, 1974; Woledge et al., 1985)). Fascicle lengths are measured (vs. fiber lengths) due to challenges associated with isolating individual fibers from human muscle.
Traditionally, samples of fascicles for length measurements are obtained from cadaver dissections (Murray et al., 2000; Ward et al., 2009; Wickiewicz et al., 1983). However, with the rise of multiple imaging modalities, subsets of muscle architectural parameters are increasingly assessed in vivo (Bolsterlee et al., 2015; Kwah et al., 2013; Lansdown et al., 2007; Scott et al., 1993). The most common imaging modality used for the in vivo assessment of fascicle length is ultrasound, shown to be both valid and reliable for the in vivo measurement of fascicle length for a number of muscles (Kwah et al., 2013). The most problematic limitation of static ultrasound imaging for in vivo measurement of fascicle lengths is the inability to view entire fascicles that are longer than the probe field-of-view (generally 4–6 cm in standard linear transducers (Weng et al., 1997)). Thus, muscles with longer fascicles are only rarely assessed in living subjects. Importantly, recent work has established extended field-of-view ultrasound (EFOV US) as a reliable and accurate method for imaging fascicles that are longer than a standard US image (Ema et al., 2013; Fornage et al., 2000; Noorkoiv et al., 2010). Specifically, a gold standard study that compared fascicle lengths measured from the swine vastus lateralis using EFOV to direct measurements from dissected fascicles reported the accuracy of EFOV to be 2.4 ± 1.2%, with an intra-class correlation coefficient of .99 (Noorkoiv et al., 2010).
The aim of this study is to use extended field-of-view ultrasound to quantify in vivo lengths of samples of fascicles from the biceps and triceps brachii and to quantify the reliability of our methodology. To our knowledge, this study represents the first application of EFOV US in the upper extremity for these purposes.
Materials and Methods
Participants
Eleven subjects (6 female, 5 male, age range 23–71, 53.7 ± 13.4), with no history of significant shoulder or elbow injury, or any evidence of neurological disorder, volunteered to participate in the study. Northwestern University Institutional Review Board approved the human subject protocol, and all subjects provided informed consent prior to participating in the study.
Experimental Setup
Subjects were seated, trunk secured, with the arm in approximately the horizontal plane (shoulder abduction 85°, shoulder rotation 0°, wrist in neutral). Subjects were casted at the forearm and wrist and secured to a metal manipulandum (System 3, Biodex Medical Systems, Inc, Shirley, NY), positioned in different trials to enable measurements at different elbow postures. Surface EMG electrodes (Delsys Bagnoli™ 16-channel desktop EMG system, Delsys, Inc., Natick, MA) were placed on the biceps and triceps brachii to monitor muscle activity throughout the experiment.
Ultrasound image acquisition
In order to quantify muscle fascicle lengths in the upper extremity, B-mode extended field-of-view ultrasound images (Siemens Antares™ Siescape v.5 software, Siemens Medical Solutions USA, Inc., Mountain View, CA, (Weng et al., 1997)) were taken of the biceps brachii (long head) and triceps brachii (lateral head) in both arms. The EFOV algorithm used in the Siescape software has been described in detail previously (Weng et al., 1997), and validated for the measurement of muscle fascicle lengths (Noorkoiv et al., 2010). For both muscles, images were acquired using a 11.43-MHz linear array probe (45 mm width) in three elbow positions: extended (25°), neutral (55°), and flexed (80°) with each muscle relaxed, as verified with EMG.
In order to optimize image acquisition and repeatability of the imaging process, prior to image acquisition the line of action of the biceps brachii (long head) was established through palpation and the use of static ultrasound to establish fascicle orientation for each subject. After verifying fascicle orientation, a line was drawn on the skin tracking the probe path that visualized muscle fascicles using static ultrasound. A similar method was followed for establishing the orientation of the fascicles for the distal portion of the triceps brachii (lateral head). During dynamic image acquisition, the probe was oriented parallel to the fascicle orientation and perpendicular to the skin.
Data Analysis
For each position, the three best images (defined subjectively by the experimenter as images with the most continuously visible fascicles) were chosen by visual inspection for data analysis (Figure 1). Fascicles were measured by manually tracing a fascicle along its path using an open-source digitizing software (Image J 2.0.0, Wayne Rasband, National Institutes of Health, Bethesda, MD) to measure its length. For each image, four fascicles were measured, for a total of 12 measurements per position. For the biceps brachii, all fascicle measurements were made in the anterior portion of the muscle due to optimal image quality in that region (Figure 2). For the triceps brachii (lateral head), all measurements were made in the section of the image of the distal muscle for which fascicles were consistently viewable for their entire length, from origin to insertion (Figure 2).
Figure 1.
(Top) Extended field-of-view ultrasound images of biceps brachii long head (left) and triceps brachii lateral head (right) in the neutral position. The dashed line represents a single fascicle as outlined during the data analysis protocol. (Bottom) Schematic diagrams of biceps brachii (left) and triceps brachii (right) illustrating each muscle’s architecture.
Figure 2.
Anatomy of the biceps and triceps brachii. (A) The shaded region indicates the approximate plane of image acquisition for the long head of the biceps brachii. (B) The shaded region represents the approximate region of image acquisition in the distal portion of the triceps lateral head. Adapted from Surgical Exposures in Orthopaedics: The Anatomic Approach (Hoppenfeld et al., 2009).
Statistical Analysis
For each muscle, two-factor (arm, position) repeated measures ANOVA tested whether average fascicle length (mean of the 12 repeated measurements) differed between the three elbow postures or across arms. Intra-rater error associated with the manual digitization process was quantified via the typical error of measurement (TEM, (Bland and Altman, 1996)). Intra-rater measurement reliability was assessed using the coefficient of variation (Atkinson and Nevill, 1998; Noorkoiv et al., 2010) for repeated measurements of the same fascicle. To establish these metrics, the same rater measured one fascicle in one image per position for all 11 subjects (33 measurements), and repeated the same measurement 2 days later. TEM was calculated as the standard deviation (SD) of the difference between the fascicle measurements divided by √2. Coefficient of variation (CV%) was calculated using the following equation:
To evaluate the reliability of fascicle measurements, the intra-class correlation coefficient (ICC, two-way random effects model, absolute agreement) was calculated within each image (among the 4 measurements), and across images in a trial (12 total measurements), with an ICC of ≥.75 indicating excellent reliability (Andresen, 2000). Finally, we quantified differences in fascicle lengths between arms by calculating the percent difference in average fascicle length:
where positive indicates longer fascicles in the dominant limb. Significance for all tests was set at p<.05, and Bonferroni correction was used where appropriate to adjust for multiple comparisons.
Results
Measured biceps and triceps fascicle lengths were consistently longer than the field-of-view of the ultrasound probe (4.5 cm field-of-view). Across all 11 subjects and each of the 3 elbow positions evaluated, biceps fascicles ranged from 8.26 cm to 15.51 cm (Figure 3A). These lengths exceeded the field of view of the probe for static imaging by at least 1.8 times, with the largest value measured (15.51 cm) being almost 3.5 times longer than the probe field-of-view. Triceps fascicles sampled from the distal half of the lateral head were substantially shorter than the fascicles measured from the biceps brachii (Figure 3B); the longest average triceps fascicle length measured among our subjects (7.53 cm) was still shorter than the shortest biceps fascicle lengths. Despite this, triceps fascicle lengths for all subjects ranged from 1 to 1.7 times the field-of-view of the ultrasound probe in the neutral and flexed positions.
Figure 3.
Biceps brachii long head (A) and triceps brachii lateral head (B) average fascicle lengths measured in N = 11 healthy control subjects using extended field-of-view ultrasound imaging. The shaded bar to the left in each plot represents the range of non-normalized fascicle measurements seen in 5 male cadaver specimens (Murray et al., 2000).
Biceps fascicle lengths were substantially longer than the field of view available for the linear transducer used in our study (4.5 cm). For triceps brachii, only two subjects had average fascicle lengths that fell within the range of the transducer’s field of view. Even in these subjects, these lengths only occurred in the most extended elbow position, where triceps is the shortest. Fascicle lengths were significantly different between positions (p < .001) for both muscles.
Fascicle measurements for both muscles were highly reliable. The typical error of measurement (TEM) for the digitizing process was on average .84 ± .56 mm for biceps and .53 ± .39 mm for triceps. The CV% calculated for the intra-rater measurement process was on average 1.0 ± .7% for biceps brachii and 1.3 ± 1.0% for triceps brachii. ICC analysis revealed highly reliable fascicle measurements within single images (4 measurements per image), and across images, with ICCs for biceps across both arms ranging from .916–.949 (p<.001, Table 1), and triceps ICC values ranging from .812–.919 (p<.001).
Table 1.
ICC values with 95% confidence intervals for fascicle measurements within each image (4 measurements) and across all three images (12 total measurements) across all positions for both arms. ICC values ranged from .916–.949 for biceps brachii and from .812–.919 for triceps brachii, indicating excellent reliability of fascicle measurements. P-values for all ICC values were < .001.
| ARM | BICEPS (within images) | BICEPS (across 3 images) | TRICEPS (within images) | TRICEPS (across 3 images) |
|---|---|---|---|---|
| Dominant | .949 | .929 | .919 | .897 |
| 95% CI: (.931 – .963) | 95% CI: (.892 – .959) | 95% CI: (.890 – .942) | 95% CI: (.841 – .943) | |
| Non-Dominant | .933 | .916 | .889 | .812 |
| 95% CI: (.911 – .952) | 95% CI: (.872 – .951) | 95% CI: (.852 – .920) | 95% CI: (.722 – .892) |
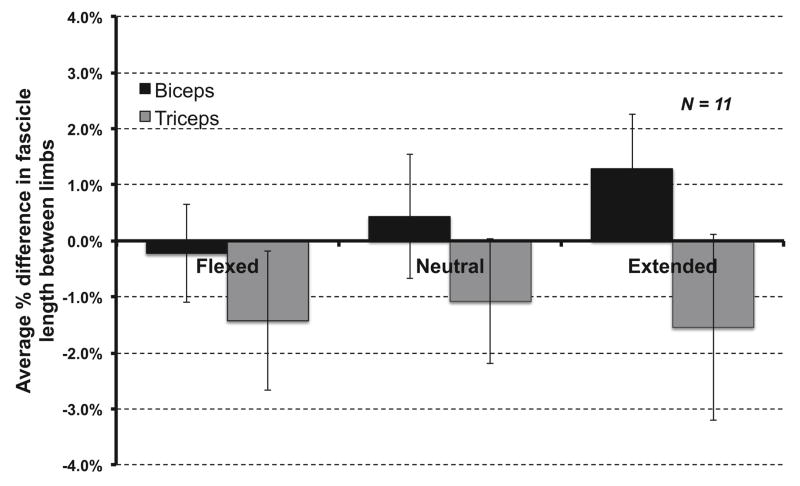
Systematic, significant differences in measured fascicle lengths were observed between elbow positions for both the biceps and triceps. Across all subjects, biceps fascicles increased an average of 2.50 cm (SE .23) as the elbow was extended, while triceps fascicles decreased an average of 1.53 cm (SE .19) as the elbow was extended (Figure 3). For both biceps and triceps, there was a significant main effect for position (p<.001), as well as significant pairwise comparisons between individual positions (p<.001, Bonferroni correction for multiple comparisons). There were no significant main effects for the factor of arm (p=.455 biceps, p=.375, triceps). Across all positions, the largest average biceps percent difference between limbs was 1.3% (Figure 4). Similarly, the largest average percent difference in triceps fascicle lengths between limbs was −1.5% (Figure 4).
Figure 4.
Average interlimb % differences in average fascicle length for biceps brachii (long head) and triceps brachii (lateral head) for different elbow postures, shown with ± 1 standard error. Percent differences were normalized to the dominant arm.
Discussion
This study provides the first known application of extended field-of-view ultrasound technology for measurement of muscle fascicle lengths in the upper extremity. We demonstrate excellent reliability in repeated fascicle measurements, showing high reliability in measurements made both within an image and across different images within a session. We also demonstrate that this methodology characterizes differences in fascicle lengths that are expected from anatomy as a muscle is lengthened (biceps during elbow extension) or shortened (triceps during elbow extension) over a joint’s range of motion.
The range of biceps and triceps fascicle lengths measured using EFOV US were within the range of non-normalized fascicle length measurements quantified in a previous cadaveric study (Murray et al., 2000). The observed variation in average fascicle lengths across all 11 subjects was large; previous cadaver research has shown similar interspecimen variability and a significant correlation between subject size and optimal fiber length (Murray et al., 2000). Although we did not quantify anthropometric data from our subjects, the similar variability observed in cadavers for the same muscles suggests different sizes among our various participants as a reasonable explanation. We have also shown that fascicle lengths are significantly different between various elbow positions, and the magnitude of change is greater than the typical error of measurement (TEM) for the digitizing process we report for both muscles.
We were able to demonstrate high reliability in our fascicle measurements as evidenced by ICC values greater than .8 for both biceps and triceps fascicle lengths. Although ultrasound has been shown to be a reliable and valid method for measurement of muscle fascicle length, previous research has also shown that some error in fascicle length can occur when the probe orientation is tilted away from the true fascicular plane (Bénard et al., 2009; Klimstra et al., 2007, Weng et al., 1997). Therefore, any variation in ultrasound probe orientation or position between images in this study could contribute to small differences in measured fascicle measurements. In the present study, multiple practice scans were used to maximize image quality and visualization of complete fascicles. The use of a visual cue for line of action of the muscle also assisted the experimenter to achieve consistent scans within a trial. Despite slightly lower ICC values for fascicle measurements across images, ICC values were still in a range considered excellent, which would indicate that any difference in measurements across images due to probe orientation was minimal. Finally, the image reconstruction algorithm employed in this study takes into account small tissue motion artifacts (Weng et al., 1997) that could be present due to variable transducer pressure during the length of the scan. Therefore, any measurement error resulting from variation in transducer pressure during the scan is expected to be minimal.
Conclusions
We demonstrate application of extended field-of-view ultrasound to measurement of the long fascicles of muscles in the upper limb. We establish that these methods produced reliable length measurements for samples of fascicles from the long head biceps and the lateral head of triceps brachii, and were sensitive enough to quantify changes in fascicle lengths resulting from changes in joint position. This study initiates the evaluation of in vivo biceps and triceps brachii fascicle architecture, previously ignored due to the limitations of traditional static US methods.
Acknowledgments
We would like to acknowledge funding from the American Heart Association (#14PRE20240022), Northwestern University terminal year fellowship, NIH-NIBIB (#T32EB009406), NIH R01 to Drs. Dewald and Murray (#1R01HD084009-01A1), Northwestern University Department of Physical Therapy and Human Movement Sciences, and the Searle Funds of the Chicago Community Trust. We would also like to acknowledge Ana Maria Acosta for her help with development of the experimental GUI, and Paul Krueger and Vikram Darbhe for their assistance during experiments.
Footnotes
Conflict of Interest Statement: I declare that none of the authors of this work have had any financial or personal relationships with other individuals or organizations that could inappropriately influence or bias this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature
- Andresen EM. Criteria for assessing the tools of disability outcomes research. Arch Phys Med Rehabil. 2000;81:S15–S20. doi: 10.1053/apmr.2000.20619. [DOI] [PubMed] [Google Scholar]
- Atkinson G, Nevill AM. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med. 1998;26:217–238. doi: 10.2165/00007256-199826040-00002. [DOI] [PubMed] [Google Scholar]
- Bénard MR, Becher JG, Harlaar J, Huijing PA, Jaspers RT. Anatomical information is needed in ultrasound imaging of muscle to avoid potentially substantial errors in measurement of muscle geometry. Muscle Nerve. 2009;39:652–665. doi: 10.1002/mus.21287. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistics notes: measurement error. Brit Med J. 1996;313:744. doi: 10.1136/bmj.313.7059.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolsterlee B, Veeger H, van der Helm F. Comparison of measurements of medial gastrocnemius architectural parameters from ultrasound and diffusion tensor images. J Biomech. 2015;48:1133–1140. doi: 10.1016/j.jbiomech.2015.01.012. [DOI] [PubMed] [Google Scholar]
- Burkholder TJ, Lieber RL. Sarcomere length operating range of vertebrate muscles during movement. J Exp Biol. 2001;204:1529–1536. doi: 10.1242/jeb.204.9.1529. [DOI] [PubMed] [Google Scholar]
- Ema R, Wakahara T, Mogi Y, Miyamoto N, Komatsu T, Kanehisa H, Kawakami Y. In vivo measurement of human rectus femoris architecture by ultrasonography- validity and applicability. Clin Physiol Funct Imaging. 2013;33:267–273. doi: 10.1111/cpf.12023. [DOI] [PubMed] [Google Scholar]
- Fornage BD, Atkinson EN, Nock LF, Jones PH. US with extended field of view: Phantom-tested accuracy of distance measurements. Radiology. 2000;214:579–584. doi: 10.1148/radiology.214.2.r00fe20579. [DOI] [PubMed] [Google Scholar]
- Hoppenfeld S, DeBoer P, Buckley RE. Surgical exposures in orthopaedics: the anatomic approach. 4. Wolters Kluwer/Lippincott Williams & Wilkins Health; 2009. [Google Scholar]
- Klimstra M, Dowling J, Durkin JL, MacDonald M. The effect of ultrasound probe orientation on muscle architecture measurement. J Electromyogr Kines. 2007;17:504–514. doi: 10.1016/j.jelekin.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Kwah LK, Pinto RZ, Diong J, Herbert RD. Reliability and validity of ultrasound measurements of muscle fascicle length and pennation in humans: a systematic review. J Appl Physiol. 2013;114:761–769. doi: 10.1152/japplphysiol.01430.2011. [DOI] [PubMed] [Google Scholar]
- Lansdown DA, Ding Z, Wadington M, Hornberger JL, Damon BM. Quantitative diffusion tensor MRI-based fiber tracking of human skeletal muscle. J Appl Physiol. 2007;103:673–681. doi: 10.1152/japplphysiol.00290.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber RL, Fazeli BM, Botte MJ. Architecture of selected wrist flexor and extensor muscles. J Hand Surg. 1990;15A:244–250. doi: 10.1016/0363-5023(90)90103-x. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Friden J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve. 2000;23:1647–1666. doi: 10.1002/1097-4598(200011)23:11<1647::aid-mus1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Loren GJ, Friden J. In-Vivo Measurement of Human Wrist Extensor Muscle Sarcomere-Length Changes. J Neurophysiol. 1994;71:874–881. doi: 10.1152/jn.1994.71.3.874. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Ward SR. Skeletal muscle design to meet functional demands. Philos T Roy Soc B. 2011;366:1466–1476. doi: 10.1098/rstb.2010.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray WM, Buchanan TS, Delp SL. The isometric functional capacity of muscles that cross the elbow. J Biomech. 2000;33:943–952. doi: 10.1016/s0021-9290(00)00051-8. [DOI] [PubMed] [Google Scholar]
- Noorkoiv M, Stavnsbo A, Aagaard P, Blazevich AJ. In vivo assessment of muscle fascicle length by extended field-of-view ultrasonography. J Appl Physiol. 2010;109:1974–1979. doi: 10.1152/japplphysiol.00657.2010. [DOI] [PubMed] [Google Scholar]
- Scott SH, Engstrom CM, Loeb GE. Morphometry of human thigh muscles. Determination of fascicle architecture by magnetic resonance imaging. J Anat. 1993;182:249–257. [PMC free article] [PubMed] [Google Scholar]
- Walker SM, Schrodt GR. I segment lengths and thin filament periods in skeletal muscle fibers of the Rhesus monkey and the human. Anat Record. 1974;178:63–81. doi: 10.1002/ar.1091780107. [DOI] [PubMed] [Google Scholar]
- Ward SR, Eng CM, Smallwood LH, Lieber RL. Are current measurements of lower extremity muscle architecture accurate? Clin Orthop Relat Res. 2009;467:1074–1082. doi: 10.1007/s11999-008-0594-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SR, Hentzen ER, Smallwood LH, Eastlack RK, Burns KA, Fithian DC, Friden J, Lieber RL. Rotator cuff muscle architecture. Clin Orthop Relat Res. 2006;448:157–163. doi: 10.1097/01.blo.0000194680.94882.d3. [DOI] [PubMed] [Google Scholar]
- Weng L, Tirumalai AP, Lowery CM, Nock LF, Gustafson DE, VonBehren PL, Kim JH. US extended-field-of-view imaging technology. Radiology. 1997;203:877–880. doi: 10.1148/radiology.203.3.9169720. [DOI] [PubMed] [Google Scholar]
- Wickiewicz TL, Roy RR, Powell PL, Edgerton VR. Muscle architecture of the human lower limb. Clin Orthop Relat Res. 1983;179:275–283. [PubMed] [Google Scholar]
- Woledge RC, Curtin NA, Homsher E. Energetic aspects of muscle contraction. Monographs of the Physiological Society. 1985:1–357. [PubMed] [Google Scholar]
- Zajac FE. Muscle and Tendon - Properties, Models, Scaling, and Application to Biomechanics and Motor Control. Crit Rev Biomed Eng. 1989;17:359–411. [PubMed] [Google Scholar]