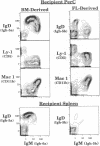
Abstract
Cell-transfer studies presented here distinguish three murine B cell lineages: conventional B cells, which develop late and are continually replenished from progenitors in adult bone marrow; Ly-1 B cells (B-1a), which develop early and maintain their numbers by self-replenishment; and Ly-1B "sister" (B-1b) cells, which share many of the properties of Ly-1 B cells, including self-replenishment and feedback regulation of development but can also readily develop from progenitors in adult bone marrow. The sequential emergence of these lineages, the time at which their progenitors function during ontogeny, and the distinctions among their repertoires and functions suggest that evolution has created a layered immune system in which the immune response potential of each successive lineage is adapted to its particular niche.
Full text
PDF


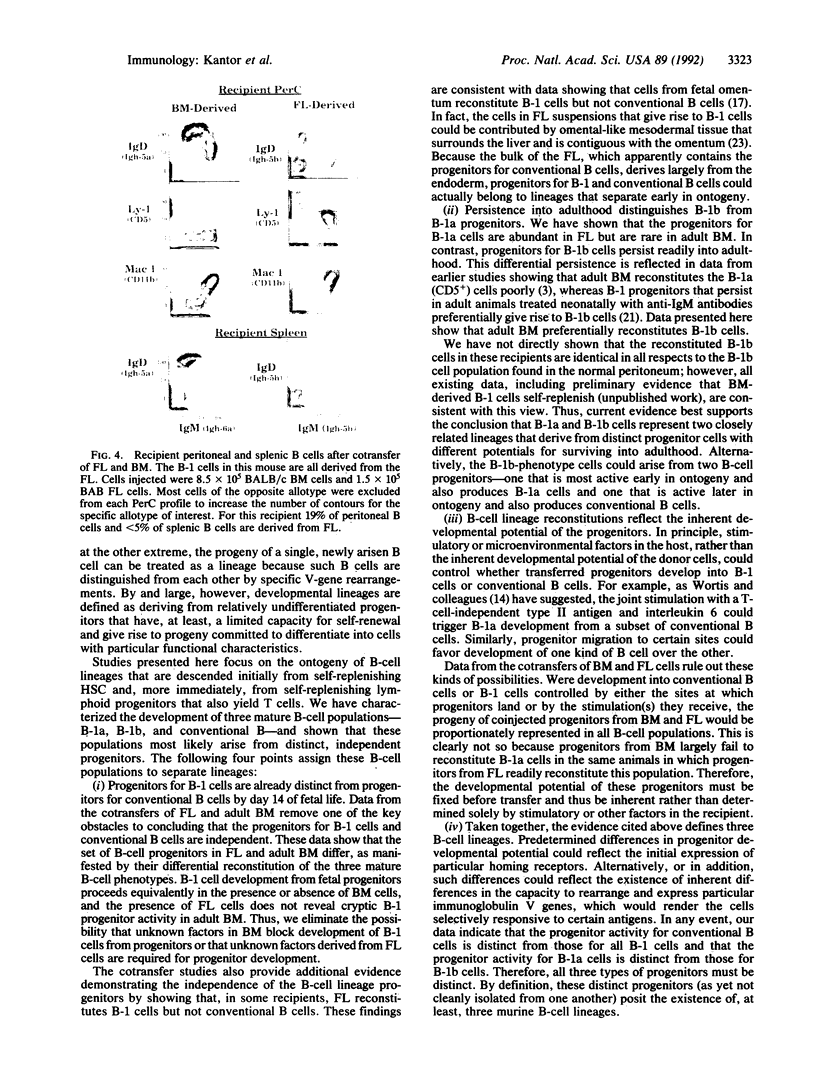

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carmack C. E., Shinton S. A., Hayakawa K., Hardy R. R. Rearrangement and selection of VH11 in the Ly-1 B cell lineage. J Exp Med. 1990 Jul 1;172(1):371–374. doi: 10.1084/jem.172.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L., Weissman I. L. B220: a B cell-specific member of th T200 glycoprotein family. Nature. 1981 Feb 19;289(5799):681–683. doi: 10.1038/289681a0. [DOI] [PubMed] [Google Scholar]
- Cong Y. Z., Rabin E., Wortis H. H. Treatment of murine CD5- B cells with anti-Ig, but not LPS, induces surface CD5: two B-cell activation pathways. Int Immunol. 1991 May;3(5):467–476. doi: 10.1093/intimm/3.5.467. [DOI] [PubMed] [Google Scholar]
- Cooper M. D., Kearney J. F., Gathings W. E., Lawton A. R. Effects of anti-Ig antibodies on the development and differentiation of B cells. Immunol Rev. 1980;52:29–53. doi: 10.1111/j.1600-065x.1980.tb00329.x. [DOI] [PubMed] [Google Scholar]
- Förster I., Gu H., Rajewsky K. Germline antibody V regions as determinants of clonal persistence and malignant growth in the B cell compartment. EMBO J. 1988 Dec 1;7(12):3693–3703. doi: 10.1002/j.1460-2075.1988.tb03251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Förster I., Rajewsky K. Sequence homologies, N sequence insertion and JH gene utilization in VHDJH joining: implications for the joining mechanism and the ontogenetic timing of Ly1 B cell and B-CLL progenitor generation. EMBO J. 1990 Jul;9(7):2133–2140. doi: 10.1002/j.1460-2075.1990.tb07382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Tarlinton D., Müller W., Rajewsky K., Förster I. Most peripheral B cells in mice are ligand selected. J Exp Med. 1991 Jun 1;173(6):1357–1371. doi: 10.1084/jem.173.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R. R., Carmack C. E., Shinton S. A., Kemp J. D., Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991 May 1;173(5):1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R. R., Carmack C. E., Shinton S. A., Riblet R. J., Hayakawa K. A single VH gene is utilized predominantly in anti-BrMRBC hybridomas derived from purified Ly-1 B cells. Definition of the VH11 family. J Immunol. 1989 May 15;142(10):3643–3651. [PubMed] [Google Scholar]
- Hardy R. R., Hayakawa K. A developmental switch in B lymphopoiesis. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11550–11554. doi: 10.1073/pnas.88.24.11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havran W. L., Allison J. P. Developmentally ordered appearance of thymocytes expressing different T-cell antigen receptors. Nature. 1988 Sep 29;335(6189):443–445. doi: 10.1038/335443a0. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Herzenberg L. A., Herzenberg L. A. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985 Jun 1;161(6):1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Honda M., Herzenberg L. A., Steinberg A. D., Herzenberg L. A. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2494–2498. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R. Normal, autoimmune, and malignant CD5+ B cells: the Ly-1 B lineage? Annu Rev Immunol. 1988;6:197–218. doi: 10.1146/annurev.iy.06.040188.001213. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Parks D. R., Herzenberg L. A. The "Ly-1 B" cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983 Jan 1;157(1):202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Stall A. M., Herzenberg L. A., Herzenberg L. A. Immunoglobulin-bearing B cells reconstitute and maintain the murine Ly-1 B cell lineage. Eur J Immunol. 1986 Oct;16(10):1313–1316. doi: 10.1002/eji.1830161021. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Herzenberg L. A. Toward a layered immune system. Cell. 1989 Dec 22;59(6):953–954. doi: 10.1016/0092-8674(89)90748-4. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Stall A. M., Lalor P. A., Sidman C., Moore W. A., Parks D. R., Herzenberg L. A. The Ly-1 B cell lineage. Immunol Rev. 1986 Oct;93:81–102. doi: 10.1111/j.1600-065x.1986.tb01503.x. [DOI] [PubMed] [Google Scholar]
- Ikuta K., Kina T., MacNeil I., Uchida N., Peault B., Chien Y. H., Weissman I. L. A developmental switch in thymic lymphocyte maturation potential occurs at the level of hematopoietic stem cells. Cell. 1990 Sep 7;62(5):863–874. doi: 10.1016/0092-8674(90)90262-d. [DOI] [PubMed] [Google Scholar]
- Ito K., Bonneville M., Takagaki Y., Nakanishi N., Kanagawa O., Krecko E. G., Tonegawa S. Different gamma delta T-cell receptors are expressed on thymocytes at different stages of development. Proc Natl Acad Sci U S A. 1989 Jan;86(2):631–635. doi: 10.1073/pnas.86.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinfield R., Hardy R. R., Tarlinton D., Dangl J., Herzenberg L. A., Weigert M. Recombination between an expressed immunoglobulin heavy-chain gene and a germline variable gene segment in a Ly 1+ B-cell lymphoma. 1986 Aug 28-Sep 3Nature. 322(6082):843–846. doi: 10.1038/322843a0. [DOI] [PubMed] [Google Scholar]
- Kroese F. G., Butcher E. C., Stall A. M., Lalor P. A., Adams S., Herzenberg L. A. Many of the IgA producing plasma cells in murine gut are derived from self-replenishing precursors in the peritoneal cavity. Int Immunol. 1989;1(1):75–84. doi: 10.1093/intimm/1.1.75. [DOI] [PubMed] [Google Scholar]
- Kubai L., Auerbach R. A new source of embryonic lymphocytes in the mouse. Nature. 1983 Jan 13;301(5896):154–156. doi: 10.1038/301154a0. [DOI] [PubMed] [Google Scholar]
- Lafaille J. J., DeCloux A., Bonneville M., Takagaki Y., Tonegawa S. Junctional sequences of T cell receptor gamma delta genes: implications for gamma delta T cell lineages and for a novel intermediate of V-(D)-J joining. Cell. 1989 Dec 1;59(5):859–870. doi: 10.1016/0092-8674(89)90609-0. [DOI] [PubMed] [Google Scholar]
- Lalor P. A., Herzenberg L. A., Adams S., Stall A. M. Feedback regulation of murine Ly-1 B cell development. Eur J Immunol. 1989 Mar;19(3):507–513. doi: 10.1002/eji.1830190315. [DOI] [PubMed] [Google Scholar]
- Lalor P. A., Stall A. M., Adams S., Herzenberg L. A. Permanent alteration of the murine Ly-1 B repertoire due to selective depletion of Ly-1 B cells in neonatal animals. Eur J Immunol. 1989 Mar;19(3):501–506. doi: 10.1002/eji.1830190314. [DOI] [PubMed] [Google Scholar]
- Micklem H. S., Lennon J. E., Ansell J. D., Gray R. A. Numbers and dispersion of repopulating hematopoietic cell clones in radiation chimeras as functions of injected cell dose. Exp Hematol. 1987 Mar;15(3):251–257. [PubMed] [Google Scholar]
- Pennell C. A., Mercolino T. J., Grdina T. A., Arnold L. W., Haughton G., Clarke S. H. Biased immunoglobulin variable region gene expression by Ly-1 B cells due to clonal selection. Eur J Immunol. 1989 Jul;19(7):1289–1295. doi: 10.1002/eji.1830190721. [DOI] [PubMed] [Google Scholar]
- Pennell C. A., Sheehan K. M., Brodeur P. H., Clarke S. H. Organization and expression of VH gene families preferentially expressed by Ly-1+ (CD5) B cells. Eur J Immunol. 1989 Nov;19(11):2115–2121. doi: 10.1002/eji.1830191122. [DOI] [PubMed] [Google Scholar]
- Sims P. J., Wiedmer T. The response of human platelets to activated components of the complement system. Immunol Today. 1991 Sep;12(9):338–342. doi: 10.1016/0167-5699(91)90012-I. [DOI] [PubMed] [Google Scholar]
- Solvason N., Lehuen A., Kearney J. F. An embryonic source of Ly1 but not conventional B cells. Int Immunol. 1991 Jun;3(6):543–550. doi: 10.1093/intimm/3.6.543. [DOI] [PubMed] [Google Scholar]
- Stall A. M., Fariñas M. C., Tarlinton D. M., Lalor P. A., Herzenberg L. A., Strober S., Herzenberg L. A. Ly-1 B-cell clones similar to human chronic lymphocytic leukemias routinely develop in older normal mice and young autoimmune (New Zealand Black-related) animals. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7312–7316. doi: 10.1073/pnas.85.19.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlinton D., Stall A. M., Herzenberg L. A. Repetitive usage of immunoglobulin VH and D gene segments in CD5+ Ly-1 B clones of (NZB x NZW)F1 mice. EMBO J. 1988 Dec 1;7(12):3705–3710. doi: 10.1002/j.1460-2075.1988.tb03253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S., Berns A., Bonneville M., Farr A., Ishida I., Ito K., Itohara S., Janeway C. A., Jr, Kanagawa O., Katsuki M. Diversity, development, ligands, and probable functions of gamma delta T cells. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):31–44. doi: 10.1101/sqb.1989.054.01.005. [DOI] [PubMed] [Google Scholar]