Abstract
Sleep disturbance is common in children with autism, resulting in a great need for effective treatments. To evaluate treatments for sleep disturbance in this population, it is critical to understand the relationship between measures of sleep captured by parent report and objective measures. The Children’s Sleep Habits Questionnaire (CSHQ) and actigraphy-measured data from 80 children with autism and sleep onset delay were evaluated. Reported problems with sleep onset delay were concurrent with sleep duration problems in 66% of children, night wakings in 72% of children, and bedtime resistance in 66% of children; 38% of children were reported to have problems with all CSHQ insomnia domains. Actigraphy-measured sleep duration was correlated with estimates using CSHQ-reported bed and wake times.
Autism spectrum disorder (ASD) is characterized by impairments in social communication and social interaction as well as the presence of restricted and repetitive behavioral patterns. (American Psychiatric Association 2013) Within this definition, the severity of clinical presentation is quite variable and many individuals present with a number of comorbidities, endophenotypes, and biomarkers. (SCHAIN and FREEDMAN 1961; Fombonne et al. 1999; Gottesman and Gould 2003; Mulder et al. 2004; Tuchman and Cuccaro 2011) Sleep difficulties, in particular insomnia, are commonly observed comorbidities in patients with ASD and prevalence estimates range from 50–80%. (Couturier et al. 2005; Krakowiak et al. 2008; Goldman et al. 2009; Sivertsen et al. 2012) Children with ASD often have attention deficit hyperactivity disorder and irritability which are symptoms commonly seen in individuals with sleep deprivation (Gozal 1998; Maquet 2001). As such, sleep disturbances may further impede children with ASD, having detrimental effects on cognitive development and daily functioning. (Wiggs and Stores 2004; Souders et al. 2009) In addition, poor sleep in children with ASD has been shown to severely affect the entire family. (Wiggs and Stores 2004; Meltzer 2008; Souders et al. 2009)
Defining sleep status can be challenging in study populations such as children with ASD who have limited sleep-related data available. Epidemiological data on sleep behaviors and sleep quality in individuals with ASD have been largely derived from parent report. It is not clear how parent reports of different sleep behaviors relate to each other in children with ASD. The majority of sleep-related information available in the large, public ASD databases is limited to the Children’s Sleep Habits Questionnaire (CSHQ). (Owens et al. 2000) The CSHQ, a widely used and validated instrument, is based on parent report and may differ from objective measures of a sleep disturbance in a child with ASD.
Children with ASD may have difficulty tolerating polysomnography (PSG), considered the “gold standard” for objective sleep measurements, due to tactile sensitivities and the novel environment of a sleep laboratory. Actigraphy, on the other hand, provides a significant advantage to PSG in that it can easily be conducted at home, and allows measurement of night-to-night variability. (Mindell et al. 2006) To better understand how parent reporting of sleep problems relates to objective measures, we evaluated how sleep variables from the CSHQ related to actigraphy measurements of the corresponding sleep behavior.
As with many other behavioral phenotypes, the environment can substantially influence expression of sleep-related traits. (Brescianini et al. 2011; Moore et al. 2011; Sletten et al. 2013) Behavioral causes of insomnia are particularly important for understanding sleep difficulties in ASD as the behavioral deficits associated with ASD likely impede the establishment of good sleep habits (e.g., difficulty with transitions). (Malow et al. 2013) Previous studies have shown that implementation of behavioral therapies, such as teaching parents of children with ASD to practice strategies which promote sleep, are useful to improving insomnia in pediatric populations. (Reed et al. 2009; Kelly et al. 2013; Malow et al. 2013; Meltzer and Mindell 2014) However, there are limited data evaluating whether educating parents about sleep might also improve the accuracy of parent report.
The aim of the present study was to determine the relationship of parent-reported insomnia domains on the CSHQ to each other, and to actigraphy. Furthermore, we were interested in determining if parent sleep education could improve the relationship between parent report and actigraphy. Our goal was to understand the information provided by the CSHQ in large sleep and autism datasets that lacked objective data.
Methods
Dataset Demographics
The sample of 80 individuals was described in previously published work, and their demographics are included in Table 2 in the Results section of this previous work. (Malow et al. 2013) Briefly, the previous study was conducted among parents and their children with autism spectrum disorders who were participating in the Autism Speaks Autism Treatment Network (AS ATN). The study was funded by the Autism Intervention Research Network on Physical Health (AIR-P), and families were recruited at three AS ATN sites. Institutional Review Board approval was received at all three sites and all parents of children provided informed consent. Study inclusion criteria was as follows: age 2–10 years, DSM-IV-TR diagnosis of ASD (American Psychiatric Association 2000) study was conducted prior to DSM-5 (American Psychiatric Association 2013) by a clinician with expertise in ASD, confirmation of ASD diagnosis by the Autism Diagnostic Observation Schedule, (Lord et al. 1989) and sleep onset latency of ≥30 min on at least three nights per week based on parent report. Sleep onset latency of ≥30 min was confirmed by 14 days of actigraphy. Children with other sleep difficulties in addition to sleep onset delay (e.g., night wakings, short sleep duration, bedtime resistance) were included. Children were excluded if they had untreated medical and/or behavioral comorbidities that affect sleep (e.g., sleep apnea, epilepsy, gastrointestinal reflux disease, and depression). (Reynolds and Malow 2011; Malow et al. 2013) The final dataset was 80% male and 79% self-reported white ancestry. The parents of all children received sleep education, either in an individual or group format. (Malow et al. 2013) This sleep education included information about (1) Daytime and evening habits that contribute to good sleep patterns, (2) Appropriate timing of sleep, (3) Visual schedules to promote sleep, and (4) Healthy interactions when their children awakened during the night. The details of this sleep education program are available in Table 1 of that publication. (Malow et al. 2013)
Evaluation of Parent-Reported Insomnia Traits on the Children’s Sleep Habits Questionnaire
The CSHQ is a validated parentally-completed questionnaire that has been used to examine sleep behavior in toddlers, preschool and school-aged children with a variety of conditions, including ASD. (Goodlin-Jones et al. 2008; Malow et al. 2012) Subscales of the CSHQ measure insomnia-related dimensions such as bedtime resistance (BR), sleep anxiety, sleep onset delay (SOD), sleep duration (SD), and night wakings (NW), as well as other dimensions such as daytime sleepiness, sleep disordered breathing, and parasomnias. The CSHQ was included as a parent-reported measure due to its wide use in the ASD literature, and because we felt that some of the domains it measured, such as bedtime resistance, were important aspects of sleep behaviors not captured by actigraphy. In addition, we wanted to ensure that we also measured insomnia-related dimensions captured by actigraphy from the parent perspective. The CSHQ was scored by applying a scale of 1–3 (1 = rarely, 0–1 times a week; 2 = sometimes, 2–4 times a week; 3 = usually, 5–7 times a week) to each of the 33 questions on the CSHQ, following the recommendations of Dr. Owens (personal communication) and our prior work.
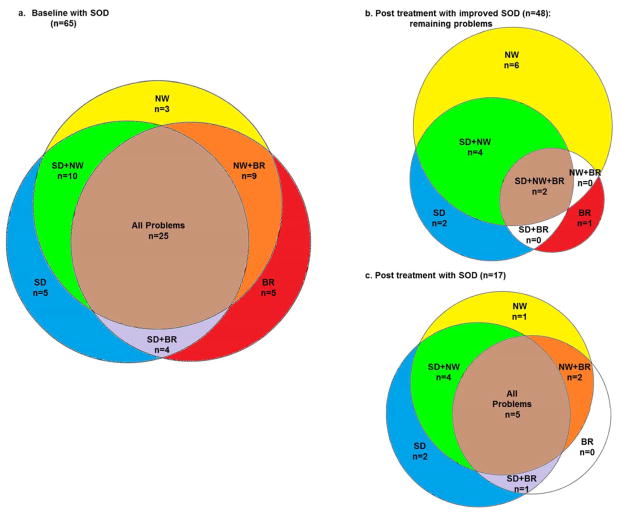
Parent responses on the CSHQ were evaluated with a focus on whether or not the child had problems with sleep duration, night wakings, and/or bedtime resistance, in relation to sleep onset delay. To visualize the overlap in CSHQ problems reported prior to sleep education, eulerAPE 3.0 (www.eulerdiagrams.com) (Micallef and Rodgers 2014) was used to plot Venn diagrams indicating the number of individuals with sleep onset delay also having problems reported in: 1) sleep duration, 2) night wakings, and 3) bedtime resistance.
Comparison of Parent-Reported and Actigraphy-Measured Sleep Traits
Complete details describing actigraphy and sleep diary data collection are provided in our previous work. (Malow et al. 2013) Briefly, all children wore AW Spectrum Actiwatches® (Philips Respironics, Bend, OR) for 14 days of data collection. Four weeks after the parents received sleep education, an additional 1–2 weeks of actigraphy data were collected (mean=12.2+/−3.1 days).
CSHQ subscale item scores (i.e., SOD, SD, and NW) and questions of interest to insomnia that were measurable via actigraphy were compared to determine the consistency of parent report in relation to actigraphy. Subscale scores from the CSHQ were calculated based on the work of Owens et al (2000). The rationale for including these subscale scores, rather than focusing on total CSHQ scores, is to provide specific information regarding the types of sleep problems a child was having. The subscale item score for SOD is based on the question asking how often the child falls asleep within 20 minutes. The subscale item score for SD was calculated from questions asking how often the child sleeps too little, sleeps the right amount, and sleeps the same amount each day. The subscale item score for NW was calculated from questions asking how often the child moves to another person’s bed at night, awakes once during the night, and awakes more than once. The comparable actigraphy measurements were sleep latency (SL), sleep time during the night (Act ST), and wake time after sleep onset (WASO). In addition, the subscale item score for bedtime resistance (BR) was also included in correlations. This score is calculated from questions asking how often the child goes to bed at the same time, falls asleep in his/her own bed, falls asleep in someone else’s bed, needs a parent in the room to sleep, struggles at bedtime, and is afraid of sleeping alone. The correlation structure across all traits evaluated in the dataset prior to parent education was determined by calculating pairwise Spearman’s rank correlation coefficients (ρ) in STATA 11.2. (Statacorp 2009) To allow more commensurate measures across the evaluated CSHQ subscale item scores, the average score from all of the questions included in each specific subscale item score was used.
To fully evaluate sleep durations, in addition to the SD subscale item score from the CSHQ, we also included quantitative data related to the total amount of sleep reported and measured for each child. It is notable that the question on the CSHQ asking about the child’s usual amount of sleep each day includes any time spent napping. As such, if the child takes naps during the day, this estimate of sleep duration may not reflect night-time sleep as measured by actigraphy. Therefore, a total amount of time slept during the night was calculated using parent-reported weekday bedtimes and waketimes obtained from the CSHQ (Bed/Wake). Bedtimes and waketimes during the week were chosen rather than weekend times as weekday times should better represent the normal routine of the child. The specific questions asked in the original CSHQ were “What is the child’s usual bedtime?” and “What is the child’s usual wake time?”. In the version of the CSHQ which was used for this study (AS ATN version), the corresponding questions were worded slightly differently: “During the week what time does your child usually go to sleep?”, “During the week what time does your child usually wake up?”, “On the weekend what time does your child usually go to sleep?”, and “On the weekend what time does your child usually wake up?”. Given that the CSHQ question used to define ‘bedtime’ in the calculated sleep duration variable was worded “What time does your child usually go to sleep?”, it was important to better define whether parents were reporting on the CSHQ the time they put their child to bed, or the time the child actually fell asleep. To address these questions, paired t-tests were conducted comparing bedtimes reported on the CSHQ question to bedtimes recorded with sleep diaries/actigraphy event markers. Bedtimes reported on the CSHQ were similarly compared to the actigraphy-measured times of sleep onset.
While all children were reported by their parents to have a sleep latency of ≥30 min at least three nights per week, not all children were reported by their parents to have sleep onset delay as a problematic behavior on the CSHQ. Actigraphy measurements in children whose parent indicated the corresponding behavior (SOD, SD, and NW) was a problem on the CSHQ were compared to measurements in children who did not have a reported problem by performing Wilcoxon rank sum tests in STATA 11.2. To ensure that the child’s age had no effect on parent reports of sleep problems, ordered logistic regression was conducted regressing reports of problems on age in years.
Comparison of Parent-Reported and Actigraphy-Measured Sleep Traits Following Parent Education
To further evaluate changes in the relationship between parent report and actigraphy with regard to parent sleep education, correlations between CSHQ responses and actigraphy measurements taken after the sleep education intervention were also examined. In addition, Wilcoxon ranks sum tests were conducted as described above on data collected following parent education.
Results
Parent-Reported Sleep Problems Related to Sleep Onset Delay
Sleep onset delay was the most frequently reported sleep problem in the evaluated dataset (65 children; 81%) which focused on ascertaining individuals with this particular sleep disturbance. The reason that sleep onset delay was not reported to be a problem in 100% of children was because inclusion in the study was based on parent report of sleep onset delay of 30 minutes or greater for 3 or more nights in a week, even if sleep onset delay was not considered to be a problem. Problems with sleep onset delay were concurrent with problems with sleep duration in 44 out of 65 children, night wakings in 47 out of 65 children, and bedtime resistance in 43 out of 65 children. Many children (48 out of 65 children) were reported to have problems in more than one behavior concurrent with sleep onset delay. Twenty-five children were reported to have problems with all insomnia-related sleep variables (Figure 1a). With sleep education, reported problems with sleep onset delay improved for a majority of children (48 children; 74%). See Figure 1b for diagram of remaining problems. Only 17 children (26%) were reported to have problems with sleep onset delay following treatment. See Figure 1c for diagram of remaining problems.
Figure 1. Overlap in Insomnia-related Problems Reported on CSHQ.
Shown are Venn diagrams plotting a) at baseline, the number of individuals in the dataset having problems with sleep onset delay for whom a parent reported other problems related to insomnia on the CSHQ (4 of the 65 children had no problems in addition to SOD), b) after sleep education, the number of individuals with other insomnia problems for whom a parent no longer reported problems with SOD (33 of the 48 children were no longer reported to have any problems), and c) after parent education, the number of individuals with other insomnia problems for whom a parent still reported problems with SOD (2 of the 17 children had no problems in addition to SOD). SOD=sleep onset delay, SD=sleep duration, NW=night wakings, BR=bedtime resistance.
Relationship Between Parent Report and Actigraphy
Sleep durations that were calculated using parent-reported weekday bedtimes and waketimes were correlated (ρ=0.30, p=0.008) at baseline with actigraphy-measured nighttime sleep durations (Table 1a). Following sleep education, the correlation was ρ=0.46, p<0.00001 (Table 1b).
Table 1. Sleep Data Correlation Structure.
Spearman’s rho correlations comparing parent report to actigraphy measurements. Significant correlations (p<0.05) are indicated in bold italics. Data were collected a) prior to or b) after implementation of parent education intervention. Post-education values were more strongly correlated than pre-education values for actigraphy-measured sleep latency/CSHQ sleep onset delay and actigraphy-measured sleep time (sleep duration)/CSHQ sleep times calculated from bedtime/waketime reports.
| a.
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Source | CSHQ | Actigraphy | ||||||
|
| ||||||||
| Variable | SOD | SD | NW | Bed/ Wake | Sleep Time | SOD | WASO | |
| CSHQ | SOD | 1.00 | ||||||
| SD | 0.19 | 1.00 | ||||||
| NW | 0.13 | 0.04 | 1.00 | |||||
| Bed/Wake | −0.25 | −0.40 | 0.05 | 1.00 | ||||
|
| ||||||||
| Actigraphy | Act ST | 0.06 | −0.13 | −0.06 | 0.30 | 1.00 | ||
| SL | 0.16 | 0.15 | −0.05 | −0.20 | −0.35 | 1.00 | ||
| WASO | −0.28 | −0.07 | 0.15 | 0.22 | −0.41 | 0.09 | 1.00 | |
| b.
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | CSHQ | Actigraphy | |||||||
|
| |||||||||
| Variable | SOD | SD | NW | Bed/ Wake | Sleep Time | SOD | WASO | ||
| CSHQ | SOD | 1.00 | |||||||
| SD | 0.41 | 1.00 | |||||||
| NW | 0.16 | 0.31 | 1.00 | ||||||
| Bed/Wake | −0.09 | −0.23 | −0.10 | 1.00 | |||||
|
| |||||||||
| Actigraphy | Act ST | 0.04 | −0.14 | −0.08 | 0.46 | 1.00 | |||
| SL | 0.43 | 0.23 | −0.06 | −0.14 | −0.21 | 1.00 | |||
| WASO | −0.20 | 0.02 | 0.11 | 0.24 | −0.28 | −0.22 | 1.00 | ||
SOD=CSHQ sleep onset delay subscale, SD=CSHQ sleep duration subscale, NW=CSHQ night wakings subscale, Bed/Wake=sleep duration calculated from reported weekday bedtimes and wake times, Act ST=actigraphy- measured sleep time at night, SL=actigraphy-measured sleep latency, WASO=actigraphy-measured wake time after sleep onset.
CSHQ-reported bedtimes were not different from bedtimes that were derived using sleep diaries and event markers accompanying actigraphy records. In contrast, CSHQ-reported bedtimes were different from actigraphy-measured times of sleep onset (p 0.001). CSHQ subscale item scores for sleep onset delay were not correlated with actigraphy-measured sleep latency prior to parent education but were following treatment (ρ=0.43, p=0.0001) (Table 1).
Age was negatively correlated with bedtime resistance both before (ρ=−0.37, p=0.0008) and after parent education intervention (ρ=−0.45, p<0.00001) with the younger children having more bedtime resistance. After treatment, age was also negatively correlated with CSHQ subscale item scores for night wakings (ρ=−0.23, p=0.04), and actigraphy-measured nighttime sleep durations (ρ=−0.26, p=0.02).
The child’s age was not associated with the parent reporting a problem on any of the CSHQ questions evaluated (p≥0.08). Parent reports on the CSHQ of problems in any evaluated sleep trait were not associated with differences in the actigraphy measurements of the comparable behaviors at baseline. Following treatment, problems reported on the CSHQ related to sleep onset delay and night wakings were indicative of differences in actigraphy-measured sleep latency and wake time after sleep onset (Table 2).
Table 2.
Comparison Between CSHQ and Actigraphy.Results testing the null hypothesis that there is no difference between actigraphy measurements for children whose parents did not report a problem in the corresponding behavior compared to those whose parents did report a problem. Measurements that were significantly different when the parent reported the behavior as a problem are indicated in bold italics. Following parent education, actigraphy measurements of SL and WASO were significantly different based on whether or not the parent reported a problem with sleep onset delay and night wakings (i.e. awaking more than once during the night).
| CSHQ Question Reported as a Problem (subscale) | Actigraphy Measure min (sd) | p-value | |
|---|---|---|---|
| Before Parent Education (Baseline) | |||
| ‘falls asleep in 20 min.’ (sleep onset delay) | Yes | Act SL=59.91 (27.27) | 0.0618 |
| No | Act SL=50.77 (35.94) | ||
| ‘sleeps too little’ (sleep duration) | Yes | Act ST=483.29 (54.95) | 0.4364 |
| No | Act ST=490.00 (45.43) | ||
| ‘sleeps the right amount’ (sleep duration) | Yes | Act ST=486.52 (51.09) | 0.8707 |
| No | Act ST=485.58 (51.81) | ||
| ‘sleeps same amount each day’ (sleep duration) | Yes | Act ST=480.21 (48.03) | 0.3791 |
| No | Act ST=488.42 (52.78) | ||
| ‘moves to other’s bed in night’ (night wakings) | Yes | WASO=64.42 (23.29) | 0.3247 |
| No | WASO=60.32 (27.14) | ||
| ‘awakes once during night’ (night wakings) | Yes | WASO=63.43 (24.76) | 0.5066 |
| No | WASO=59.59 (27.19) | ||
| ‘awakes more than once’ (night wakings) | Yes | WASO=63.80 (26.63) | 0.6377 |
| No | WASO=60.09 (24.95) | ||
| After Parent Education (Treatment) | |||
| ‘falls asleep in 20 min.’ (sleep onset delay) | Yes | SL=53.87 (23.77) | 0.0003 |
| No | SL=35.65 (18.06) | ||
| ‘sleeps too little’ (sleep duration) | Yes | Act ST=481.20 (51.93) | 0.5236 |
| No | Act ST=486.13 (48.79) | ||
| ‘sleeps the right amount’ (sleep duration) | Yes | Act ST=476.71 (33.37) | 0.4955 |
| No | Act ST=485.68 (50.90) | ||
| ‘sleeps same amount each day’ (sleep duration) | Yes | Act ST=466.50 (24.85) | 0.0858 |
| No | Act ST=487.55 (51.70) | ||
| ‘moves to other’s bed in night’ (night wakings) | Yes | WASO=61.21 (32.86) | 0.9303 |
| No | WASO=58.15 (24.20) | ||
| ‘awakes once during night’ (night wakings) | Yes | WASO=63.49 (22.79) | 0.3155 |
| No | WASO=57.23 (26.75) | ||
| ‘awakes more than once’ (night wakings) | Yes | WASO=60.09 (25.67) | 0.0407 |
| No | WASO=55.22 (25.17) | ||
sd=standard deviation, Act SL=sleep latency, Act ST=actigraphy sleep time, WASO=wake after sleep onset.
Discussion
In this study, we observed that sleep durations estimated using parent-reported bedtimes and waketimes were related to the corresponding actigraphy measurements. This finding suggests that the amount of sleep calculated from parent report can be used to estimate sleep duration in samples where objective measurements are not available. Furthermore, following sleep education, this parent report of sleep duration showed an even stronger correlation with actigraphy measurements. In addition, reports of sleep onset delay on the CSHQ were more closely related to the objective measurements of this behavior following treatment.
Parents no longer reporting problems with SOD more frequently reported improvements in bedtime resistance compared to reports of improvements in either sleep duration, or night wakings. This suggests that when a child is less likely to struggle with the bedtime routine, there is less delay in sleep onset. Furthermore, in general, when sleep onset latency was no longer reported to be a problem, problems with sleep duration and night wakings were also no longer reported.
Estimating sleep durations using parent-reported weekday bedtimes and waketimes from the CSHQ appears to be more similar to the corresponding actigraphic measurements than either parent reports of specific problems or CSHQ subscale items scores for SD, SOD and NW. Given that the parent has received sleep education, this CSHQ-derived measure of total sleep time in children with ASD appears to be even more consistent with actigraphy measurements. This calculated variable from the CSHQ should be useful for future research studies of sleep duration in ASD.
Further, parent responses to the CSHQ question “During the week what time does your child usually go to sleep?” were more comparable to sleep diary and actigraphy event markers indicating bedtime than to actigraphy-measured times of sleep onset. This suggests that parents were reporting the child’s bedtime in response to this CSHQ question rather than trying to estimate the time the child actually fell asleep.
Prior to treatment with sleep education, parents reporting problems (vs. no problems) related to CSHQ-measured sleep traits were not associated with differences in the actigraphy measurements of the comparable behaviors. However, after sleep education, parent report of problems with sleep onset delay on the CSHQ were associated with differences in actigraphy-measured sleep latency when compared to children whose parent did not report a problem with the child falling asleep. This further suggests that sleep education improves the reliability of parent report of sleep onset delay in relation to actigraphy.
Of note, prior to sleep education, age was not correlated with CSHQ night wakings or actigraphy-measured sleep duration but following treatment age was negatively correlated with both. It is possible that prior to treatment there was more statistical variability in the data related to these traits and that implementing sleep education helped reveal a true relationship between age and these sleep traits, with younger children having more night wakings and longer sleep durations. This finding is consistent with a prior report. (Goldman et al. 2012)
Surprisingly, parents reporting problems with children sleeping too little showed comparable sleep durations, as measured by actigraphy, to children whose parents did not report a problem with sleeping too little. Sleep education did not affect these findings. This indicates that parents of children with ASD may vary in expectations-- some may feel that shorter sleep duration is a symptom of ASD that cannot be altered while others may expect their child with ASD to meet the sleep duration norms established for children of typical development. More study will be needed to better understand parent expectations regarding sleep duration. Our findings also indicate that parent reports of problems with sleep duration (as opposed to their reports of specific sleep and bed/waketimes) may not be useful for accurate definitions of short sleep in this population.
Implementing sleep education strengthened the correlations between parent-reported and actigraphy-measured sleep duration and sleep onset delay. It is likely the parents became more facile at recording these behaviors following treatment because they were asked to keep diaries as part of sleep education. In addition, sleep education may have helped the child sleep more consistently with less night-to-night variability in measurements leading to greater accuracy on the part of the parents. Deciphering the effects of environmental risk factors from that of other risk factors (e.g., genetic, biological) will lead to an understanding of how insomnia relates to ASD and help identify individuals of interest to treatment studies. In the current study, sleep education may have helped identify children having underlying biological issues affecting sleep patterns and, as a result, more consistent expression of insomnia-related traits compared to children whose sleep patterns are largely impacted by external factors and have more variable expression of insomnia. It is also possible that lack of response to behavioral interventions potentially identifies unique subsets of individuals with ASD that harbor a greater load of underlying risk factors and may require a pharmacological intervention.
While there are no finite amounts of sleep that are considered ideal for individuals with ASD, there are guidelines that have been established for children with typical development. Several studies have used a definition of short sleep that was based on sleep durations of less than the 10th percentile for reported norms by age. (Nixon et al. 2008; Owens et al. 2008; Paavonen et al. 2009; Pesonen et al. 2010) Based on this definition, short sleepers with typical development have previously been defined using cutoffs of <10 hours for children age 3 to 5.9 years, and <9 hours for children age 6 to 12.9 years. (Owens et al. 2008; Pesonen et al. 2010) The majority of children with ASD evaluated in the current study meet these criteria for short sleep duration, at least according to the standards for children of typical development. The average sleep duration measured by actigraphy in this report was 8.2hrs ± 1hr for children age 2 to 5.9 years, and 7.9 hours ±0.5 hr for children age 6 to 12 years.
Parent report provides information regarding sleep duration that is related to sleep duration measured with actigraphy. This indicates that, in the absence of objectively-measured sleep duration data, parent-reported sleep durations derived from bedtimes and waketimes collected via the CSHQ provide estimates of total sleep time that are consistent with actigraphy measurements. Furthermore, the relationship between parent report and objective measures is strengthened after parents receive sleep education. To fully understand the usefulness of parent report, it will be necessary to further evaluate this relationship using objective measurements taken in larger ASD datasets, focusing on children with comorbid insomnia.
Acknowledgments
We acknowledge the efforts of our study coordinators including Deborah Wofford, Amanda Wyatt, Harriet Austin, Bonnie Chan, Ester Hsueh, and Pam Green, educators Kim Frank, Kay Artibee, and Cathy Petta, and the families who generously participated in this project. Special thanks to Diane Fawkes, CCRC and Suzanne Goldman, PhD for collection and scoring of actigraphy data. This research was conducted as part of the Autism Speaks Autism Treatment Network and the Autism Intervention Research Network on Physical Health. Main support came from a cooperative agreement (UA3 MC 11054) from the U.S. Department of Health and Human Services, Health Resources and Services Administration, Maternal and Child Health Research Program, to the Massachusetts General Hospital. The views expressed in this publication do not necessarily reflect the views of Autism Speaks, Inc. or The Maternal and Child Health Bureau. Additional support was provided by CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Reference List
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington DC: American Psychiatric Association; 2000. Text Revision. [Google Scholar]
- 2.American Psychiatric Association. Report of DSM-5 Proposed Criteria for Autism Spectrum Disorder Designed to Provide More Accurate Diagnosis and Treatment. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 3.Brescianini S, Volzone A, Fagnani C, Patriarca V, Grimaldi V, Lanni R, et al. Genetic and environmental factors shape infant sleep patterns: a study of 18-month-old twins. Pediatrics. 2011;127:e1296–e1302. doi: 10.1542/peds.2010-0858. [DOI] [PubMed] [Google Scholar]
- 4.Couturier JL, Speechley KN, Steele M, Norman R, Stringer B, Nicolson R. Parental perception of sleep problems in children of normal intelligence with pervasive developmental disorders: prevalence, severity, and pattern. J Am Acad Child Adolesc Psychiatry. 2005;44:815–822. doi: 10.1097/01.chi.0000166377.22651.87. [DOI] [PubMed] [Google Scholar]
- 5.Fombonne E, Roge B, Claverie J, Courty S, Fremolle J. Microcephaly and macrocephaly in autism. J Autism Dev Disord. 1999;29:113–119. doi: 10.1023/a:1023036509476. [DOI] [PubMed] [Google Scholar]
- 6.Goldman SE, Richdale AL, Clemons T, Malow BA. Parental sleep concerns in autism spectrum disorders: variations from childhood to adolescence. J Autism Dev Disord. 2012;42:531–538. doi: 10.1007/s10803-011-1270-5. [DOI] [PubMed] [Google Scholar]
- 7.Goldman SE, Surdyka K, Cuevas R, Adkins K, Wang L, Malow BA. Defining the sleep phenotype in children with autism. Dev Neuropsychol. 2009;34:560–573. doi: 10.1080/87565640903133509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodlin-Jones BL, Sitnick SL, Tang K, Liu J, Anders TF. The Children’s Sleep Habits Questionnaire in toddlers and preschool children. J Dev Behav Pediatr. 2008;29:82–88. doi: 10.1097/dbp.0b013e318163c39a. [DOI] [PubMed] [Google Scholar]
- 9.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 10.Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics. 1998;102:616–620. doi: 10.1542/peds.102.3.616. [DOI] [PubMed] [Google Scholar]
- 11.Kelly Y, Kelly J, Sacker A. Changes in bedtime schedules and behavioral difficulties in 7 year old children. Pediatrics. 2013;132:e1184–e1193. doi: 10.1542/peds.2013-1906. [DOI] [PubMed] [Google Scholar]
- 12.Krakowiak P, Goodlin-Jones B, Hertz-Picciotto I, Croen LA, Hansen RL. Sleep problems in children with autism spectrum disorders, developmental delays, and typical development: a population-based study. J Sleep Res. 2008;17:197–206. doi: 10.1111/j.1365-2869.2008.00650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, et al. Autism diagnostic observation schedule: a standardized observation of communicative and social behavior. J Autism Dev Disord. 1989;19:185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- 14.Malow B, Adkins KW, McGrew SG, Wang L, Goldman SE, Fawkes D, et al. Melatonin for sleep in children with autism: a controlled trial examining dose, tolerability, and outcomes. J Autism Dev Disord. 2012;42:1729–1737. doi: 10.1007/s10803-011-1418-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malow BA, Adkins KW, Reynolds A, Weiss SK, Loh A, Fawkes D, et al. Parent-Based Sleep Education for Children with Autism Spectrum Disorders. J Autism Dev Disord. 2013 doi: 10.1007/s10803-013-1866-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–1052. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- 17.Meltzer LJ. Brief report: sleep in parents of children with autism spectrum disorders. J Pediatr Psychol. 2008;33:380–386. doi: 10.1093/jpepsy/jsn005. [DOI] [PubMed] [Google Scholar]
- 18.Meltzer LJ, Mindell JA. Systematic Review and Meta-Analysis of Behavioral Interventions for Pediatric Insomnia. J Pediatr Psychol. 2014 doi: 10.1093/jpepsy/jsu041. [DOI] [PubMed] [Google Scholar]
- 19.Micallef L, Rodgers P. eulerAPE: Drawing Area-Proportional 3-Venn Diagrams Using Ellipses. PLoS One. 2014;9:e101717. doi: 10.1371/journal.pone.0101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mindell JA, Emslie G, Blumer J, Genel M, Glaze D, Ivanenko A, et al. Pharmacologic management of insomnia in children and adolescents: consensus statement. Pediatrics. 2006;117:e1223–e1232. doi: 10.1542/peds.2005-1693. [DOI] [PubMed] [Google Scholar]
- 21.Moore M, Slane J, Mindell JA, Burt SA, Klump KL. Genetic and environmental influences on sleep problems: a study of preadolescent and adolescent twins. Child Care Health Dev. 2011;37:638–641. doi: 10.1111/j.1365-2214.2011.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulder EJ, Anderson GM, Kema IP, de BA, van Lang ND, den Boer JA, et al. Platelet serotonin levels in pervasive developmental disorders and mental retardation: diagnostic group differences, within-group distribution, and behavioral correlates. J Am Acad Child Adolesc Psychiatry. 2004;43:491–499. doi: 10.1097/00004583-200404000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Nixon GM, Thompson JM, Han DY, Becroft DM, Clark PM, Robinson E, et al. Short sleep duration in middle childhood: risk factors and consequences. Sleep. 2008;31:71–78. doi: 10.1093/sleep/31.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owens JA, Mehlenbeck R, Lee J, King MM. Effect of weight, sleep duration, and comorbid sleep disorders on behavioral outcomes in children with sleep-disordered breathing. Arch Pediatr Adolesc Med. 2008;162:313–321. doi: 10.1001/archpedi.162.4.313. [DOI] [PubMed] [Google Scholar]
- 25.Owens JA, Spirito A, McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23:1043–1051. [PubMed] [Google Scholar]
- 26.Paavonen EJ, Raikkonen K, Lahti J, Komsi N, Heinonen K, Pesonen AK, et al. Short sleep duration and behavioral symptoms of attention-deficit/hyperactivity disorder in healthy 7- to 8-year-old children. Pediatrics. 2009;123:e857–e864. doi: 10.1542/peds.2008-2164. [DOI] [PubMed] [Google Scholar]
- 27.Pesonen AK, Raikkonen K, Paavonen EJ, Heinonen K, Komsi N, Lahti J, et al. Sleep duration and regularity are associated with behavioral problems in 8-year-old children. Int J Behav Med. 2010;17:298–305. doi: 10.1007/s12529-009-9065-1. [DOI] [PubMed] [Google Scholar]
- 28.Reed HE, McGrew SG, Artibee K, Surdkya K, Goldman SE, Frank K, et al. Parent-based sleep education workshops in autism. J Child Neurol. 2009;24:936–945. doi: 10.1177/0883073808331348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reynolds AM, Malow BA. Sleep and autism spectrum disorders. Pediatr Clin North Am. 2011;58:685–698. doi: 10.1016/j.pcl.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 30.SCHAIN RJ, FREEDMAN DX. Studies on 5-hydroxyindole metabolism in autistic and other mentally retarded children. J Pediatr. 1961;58:315–320. doi: 10.1016/s0022-3476(61)80261-8. [DOI] [PubMed] [Google Scholar]
- 31.Sivertsen B, Posserud MB, Gillberg C, Lundervold AJ, Hysing M. Sleep problems in children with autism spectrum problems: a longitudinal population-based study. Autism. 2012;16:139–150. doi: 10.1177/1362361311404255. [DOI] [PubMed] [Google Scholar]
- 32.Sletten TL, Rajaratnam SM, Wright MJ, Zhu G, Naismith S, Martin NG, et al. Genetic and environmental contributions to sleep-wake behavior in 12-year-old twins. Sleep. 2013;36:1715–1722. doi: 10.5665/sleep.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Souders MC, Mason TB, Valladares O, Bucan M, Levy SE, Mandell DS, et al. Sleep behaviors and sleep quality in children with autism spectrum disorders. Sleep. 2009;32:1566–1578. doi: 10.1093/sleep/32.12.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Statacorp. Stata Statistical Software. College Station, TX: Statacorp LP; 2009. [Google Scholar]
- 35.Tuchman R, Cuccaro M. Epilepsy and autism: neurodevelopmental perspective. Curr Neurol Neurosci Rep. 2011;11:428–434. doi: 10.1007/s11910-011-0195-x. [DOI] [PubMed] [Google Scholar]
- 36.Wiggs L, Stores G. Sleep patterns and sleep disorders in children with autistic spectrum disorders: insights using parent report and actigraphy. Dev Med Child Neurol. 2004;46:372–380. doi: 10.1017/s0012162204000611. [DOI] [PubMed] [Google Scholar]