Abstract
Smad and STAT proteins are critical signal transducers and transcription factors in controlling cell growth and tumorigenesis. Here we report that the STAT3 signaling pathway attenuates TGF-β-induced responses through a direct Smad3-STAT3 interplay. Activated STAT3 blunts TGF-β-mediated signaling. Depletion of STAT3 promotes TGF-β-mediated transcriptional and physiological responses, including cell cycle arrest, apoptosis and epithelial-to-mesenchymal transition. STAT3 directly interacts with Smad3 in vivo and in vitro, resulting in attenuation of the Smad3-Smad4 complex formation and suppression of DNA-binding ability of Smad3. The N-terminal region of DNA-binding domain of STAT3 is responsible for the STAT3-Smad3 interaction and also indispensable for STAT3-mediated inhibition of TGF-β signaling. Thus, our finding illustrates a direct crosstalk between the STAT3 and Smad3 signaling pathways that may contribute to tumor development and inflammation.
Keywords: Signal transduction, Transcription, Smad3 DNA binding, Tumor suppressor, TGF-β resistance
Introduction
TGF-β is a multifunctional cytokine that regulates diverse cellular responses, including apoptosis, cell growth inhibition, and immune surveillance (1–3). Due to its potent tumor-suppressive effects, the TGF-β signaling pathway is often inactivated in numerous cancers such as colon, pancreatic and gastric cancers, yet such somatic mutations are rare in other cancers such as breast, prostate and skin cancers (4–7). Because loss of TGF-β responses is common in all types of cancers, there are alternative mechanisms underlying TGF-β resistance in those cancer types without somatic mutations in the TGF-β pathway. Recent progress suggests that activation of oncogenes can suppress TGF-β growth inhibitory response (8–11).
TGF-β signals through a heteromeric complex of cell-surface serine-threonine kinase receptors, i.e. TβRI and TβRII, and intracellular signal transducers Smad2 and Smad3. In response to TGF-β ligands, TβRII transphosphorylates TβRI, which in turn mediates phosphorylation of Smad2 and Smad3. Phosphorylated (activated) Smad2/3 associate with Smad4, and translocate to the nucleus to regulate gene expression (3,12). Each step of this signal transduction pathway can be regulated by a variety of intracellular factors (13). For example, Erk MAP kinase-dependent phosphorylation of the linker region impairs Smad3 nuclear accumulation (14). In the nucleus, many transcription factors interact with R-Smads to modulate the final gene transcription output (15,16). The majority of these cooperative partners are also components of other signaling pathways. For example, FAST1/2 and c-Jun/c-Fos cooperate with Smad2-Smad4 (17,18) and Smad3-Smad4 complexes (19), respectively, to regulate transcription. The cooperative or antagonistic interactions of Smads with other transcriptional factors depend on the physiological contexts and therefore dictate the final physiological outputs.
STAT3 is a common downstream effector of some cytokines overwhelmingly expressed in tumor environment such as IL-6, IL-11 and VEGF (20). Moreover, STAT3 is also a critical mediator of EGFR signaling, which is aberrantly activated in various tumors (20). Therefore, STAT3 is a point of convergence for numerous oncogenic signaling pathways, and this makes it a potential candidate to modulate TGF-β signaling in tumor progression. Several earlier studies have demonstrated two-way interplays between STAT3 and TGF-β signaling pathways. For instance, STAT3 can promote TGF-β1 expression to enhance hepatic fibrosis in HCC development (21); Activated STAT3 also induces Smad7 expression to desensitize TGF-β signaling (22). TGF-β conversely inhibits IL-6-mediated STAT3 activation and affects its target gene expression (23, 24). Smads can also attenuate STAT3-mediated pathway by inhibiting its DNA binding ability and cooperation with p300 (25). However, many of these reports are conflicting and the underlying mechanisms behind these observations have not been elucidated.
In this study, we identified and characterized a direct interaction between STAT3 and Smad3. Cellular and molecular evidence lead to the conclusion that the STAT3-Smad3 interaction contributes to STAT3-mediated inhibition of TGF-β signaling. These findings elucidate a novel mechanism underlying the crosstalk between TGF-β antiproliferative signaling and STAT3-dependent growth promoting signaling pathways.
Results
STAT3 is essential for EGF/IL-6-mediated desensitization of the TGF-β responses
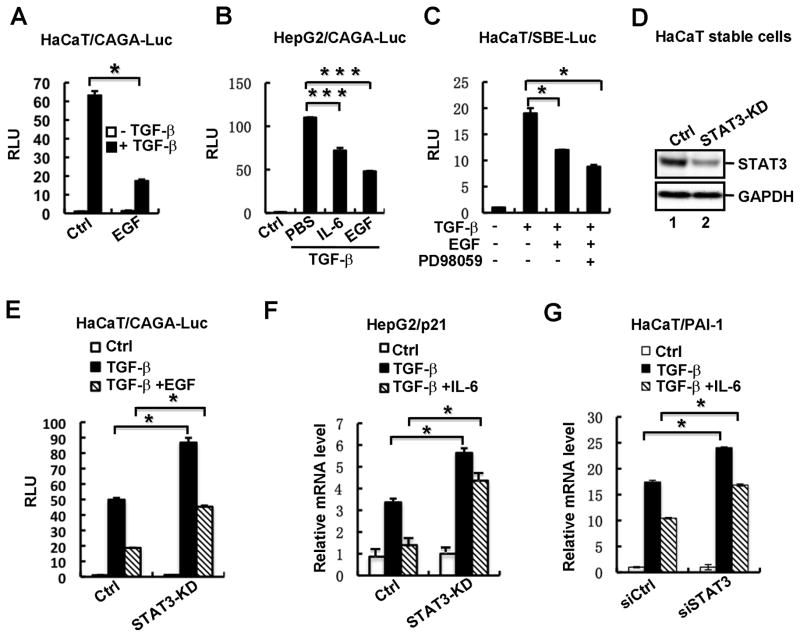
TGF-β and EGF signaling pathways are both independently implicated as key regulators in tumor formation and progression. Previous studies have shown that EGF may exert as an antagonist of TGF-β signaling in several cellular contexts (29,30), implying a potential crosstalk between these two signaling pathways. To elucidate mechanisms underlying EGF-mediated regulation of TGF-β signaling, we firstly investigated and confirmed the inhibitory effect of EGF in HaCaT cells. HaCaT is a human keratinocyte cell line that is highly responsive to TGF-β. A Smad3-dependent luciferase reporter CAGA-Luc containing 12 copies of Smad-binding elements (SBE) was transiently transfected into HaCaT cells to quantitatively determine TGF-β signaling sensitivity. Notably, TGF-β-activated stimulation of CAGA-Luc activity was significantly reduced in cells treated with EGF (Fig. 1A). This inhibitory effect of EGF on TGF-β signaling is not restricted to HaCaT cells, as EGF exerted a consistently inhibitory role in human hepatoma HepG2 cells (Fig. 1B). It was known that IL-6 and EGF stimulate similar downstream signaling pathways such as the JAK/STAT and Ras/MEK/ERK pathways. Aberrant IL-6/gp130 signaling also causes the desensitization of TGF-β signaling in the gastric epithelium (22). Thus, we also examined the effect of IL-6 on TGF-β signaling. Results showed that similarly to EGF, IL-6 decreased TGF-β-meditated CAGA-Luc expression (Fig. 1B).
Fig. 1. STAT3 is essential for EGF/IL-6-mediated desensitization of the TGF-β responses.
(A) EGF suppresses TGF-β-induced CAGA-Luc activity in HaCaT cells. HaCaT cells were transfected with the TGF-β-responsive reporter plasmid CAGA-Luc, and then treated with TGF-β (2 ng/ml) in the presence or absence of EGF (10 ng/ml). TGF-β/EGF treatment and luciferase assays were done as described under “Materials and Methods”.
(B) IL-6 and EGF suppresses TGF-β-induced CAGA-Luc activity in HepG2 cells. Experiment was carried out as described in Fig. 1A. IL-6 was used at a concentration of 10 ng/ml.
(C) EGF-mediated inhibition on the TGF-β SBE-luc response is MEK-independent. HaCaT cells were transfected with SBE-Luc reporter plasmid, pretreated with or without MEK inhibitor PD98059 (50 μM) for 4 h, and then treated with TGF-β (2 ng/ml) and/or EGF (10 ng/ml) for another 12 h. Luciferase assays were done as described in Fig. 1A.
(D) Stable knockdown of STAT3 in HaCaT cells. The protein level of STAT3 was detected by using Western blotting.
(E) EGF-mediated inhibition on the CAGA-Luc response is STAT3-dependent. Transfection, TGF-β/EGF treatment and luciferase assays in STAT3-depleted stable cells and control cells were done as described under “Materials and Methods”.
(F) IL-6-mediated inhibition of the TGF-β-induced p21 transcription is STAT3-dependent. HaCaT STAT3-depleted stable cells and control cells were treated with TGF-β (2 ng/ml) and/or IL-6 (10 ng/ml), and total RNAs were extracted for qRT-PCR analysis of p21 mRNA.
(G) IL-6-mediated inhibition of the TGF-β-induced PAI-1 transcription is STAT3-dependent. HaCaT cells were transfected with STAT3 siRNAs, treated with TGF-β (2 ng/ml) and/or IL-6 (10 ng/ml), and total RNAs were extracted for qRT-PCR analysis of PAI-1 mRNA.
The Ras-MAPK pathway, one of the most documented pathways activated by EGF, has been implicated in modulating Smad activation (14). To examine whether this pathway was solely involved in EGF-mediated desensitization of TGF-β signaling, we used a pharmacological inhibitor PD98059 to block MEK activity. PD98059 was not able to block EGF-mediated inhibition of TGF-β-induced SBE-Luc reporter activity (Fig. 1C), suggesting that the desensitization of TGF-β pathway by EGF is not through the MEK/ERK signaling.
Another important downstream signaling stimulated by EGF was the JAK/STAT pathway. It has been shown that sustained STAT3 activation results in the inhibition of TGF-β signaling (22). Therefore, we speculated whether STAT3 was involved in EGF-mediated desensitization of TGF-β responses. We established a HaCaT cell line with stable STAT3 knockdown (STAT3-KD) and investigated the effect of STAT3 depletion on TGF-β-induced responses. The protein levels of STAT3 were efficiently reduced in STAT3-KD cells (Fig. 1D). Stable knockdown of STAT3 increased TGF-β-activated CAGA-Luc activity and impaired the inhibitory effects of EGF stimulation (Fig. 1E). More apparent attenuation of TGF-β response by IL-6 was observed when the TGF-β-induced mRNA level of target p21 was analyzed, and furthermore this attenuation was lost when STAT3 was knocked down (Fig. 1F). Similar result was obtained by using transient tranfection. Two sequence-specific siRNAs against STAT3, which could effectively reduce STAT3 protein levels (data not shown), enhanced TGF-β-induced PAI-1 mRNA expression, and partially diminished IL-6-mediated inhibition (Fig. 1G). Taken together, these results demonstrate that EGF/IL-6-mediated suppression of TGF-β pathway is dependent of STAT3.
Activated STAT3 suppresses TGF-β-induced growth inhibitory responses
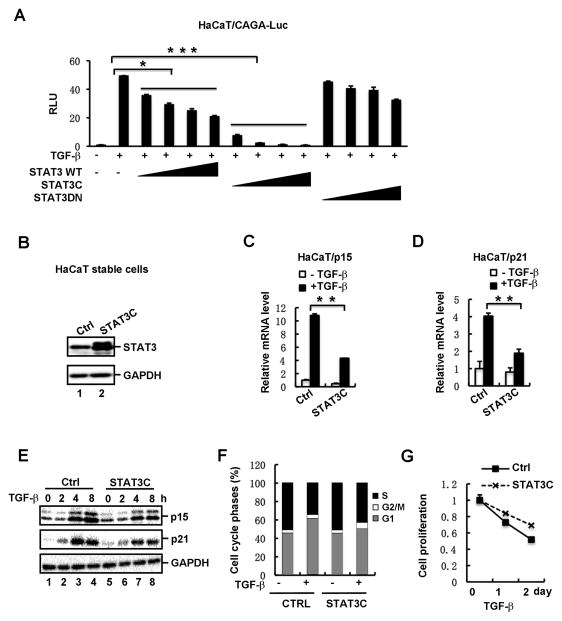
After having shown that STAT3 is involved in EGF/IL-6-mediatd inhibition of TGF-β signaling, we next set out to examine the effect of activated STAT3 on TGF-β-induced responses. STAT3 or its two variants was transiently transfected into TGF-β-responsive HaCaT cells. While STAT3C is a constitutively active mutant of STAT3 and can mimic the aberrant STAT3 activation in cancer cells (22), STAT3DN harbor a Y705F mutation that acts as a dominant negative mutant (22). As shown in Fig. 2A, STAT3C strongly decreased TGF-β-activated CAGA-Luc activity in a dose-dependent manner in HaCaT cells. In contrast, STAT3 wildtype (WT) had a very weak inhibition, and STATDN has no inhibition at all on TGF-β signaling. Because TGF-β is a potent inhibitor of cell proliferation by arresting epithelial cells in G1 phase, we reasoned that STAT3 would desensitize cells to TGF-β-mediated growth inhibition. We established a HaCaT cell line to stably express STAT3C, and then determined the expression level of STAT3C which was found to be comparable to that of endogenous STAT3 (Fig. 2B). As key effectors and indicators for TGF-β-mediated cell cycle arrest, induction of CDK inhibitors p15 and p21 was evaluated in HaCaT-STAT3C cells. While TGF-β induced 11-fold and 4-fold increase in p15 and p21 mRNA levels, respectively, STAT3C clearly attenuated this TGF-β-dependent transcriptional induction (Fig. 2C and 2D). Analysis of protein levels also supported the suppressing effect of STAT3C on TGF-β-dependent p15 and p21 induction (Fig. 2E). In accordance, expression of STAT3C caused a decrease in the G1 phase of the cell cycle, accompanied by an increase in the S phase, when the TGF-β response were compared in HaCaT-STAT3C cells vs. control cells (Fig. 2F). Consistently, STAT3C decreased the activity of TGF-β to suppress proliferation of HaCaT cells (Fig. 2G).
Fig. 2. Activated STAT3 suppresses TGF-β-induced growth inhibitory responses.
(A) STAT3C potently attenuates TGF-β-induced reporter expression in a dose-dependent manner. HaCaT cells were transfected with increasing amounts of STAT3 wildtype (WT) or variants (STAT3C or STAT3DN), together with CAGA-Luc, and then treated with or without TGF-β (2 ng/ml).
(B) Stable expression of STAT3C in HaCaT cells. The protein level of STAT3 was detected by using Western blotting. Note that the protein level of exogenous Flag-STAT3C is similar to that of endogenous STAT3.
(C) Stable expression of STAT3C decreases TGF-β-induced p15 mRNA expression in HaCaT cells. Cells were treated with or without TGF-β (2 ng/ml) for 2h, and total RNA was extracted for qRT-PCR analysis.
(D) STAT3C decreases TGF-β-induced p21 mRNA expression in HaCaT cells.
(E) Stable expression of STAT3C decreases TGF-β-induced p15 and p21 protein expression in HaCaT cells. Cells were treated with TGF-β for 0, 2, 4 and 8 h, respectively, and then the protein levels of p15 and p21 were detected by using Western blotting.
(F) STAT3C promotes G1-S progression in TGF-β-treated cells. STAT3C cells and control cells were treated with TGF-β (2 ng/ml) for 2 days. Cell cycle progression was determined by flow cytometry as described under “Materials and Methods”.
(G) STAT3C interferes with TGF-β-mediated inhibition on cell proliferation. STAT3C stable cells and control cells were treated with TGF-β, and cell growth rates were examined by actual cell numbers being counted at indicated time point (0, 1 and 2 d) of TGF-β treatment.
Loss of STAT3 enhances TGF-β-induced physiological responses
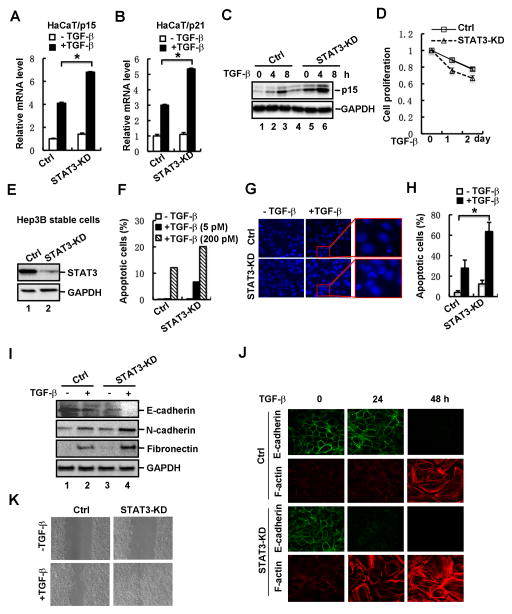
To elucidate the physiological functions of endogenous STAT3 in regulating TGF-β signaling, we examined the effect of STAT3 depletion on TGF-β responses in HaCaT cells. We found that shRNA-mediated stable knockdown of STAT3 expression resulted in a significant increase in the TGF-β-induced mRNA levels of p15 (Fig. 3A) and p21 (Fig. 3B). TGF-β-induced protein level of p15 was similarly enhanced by STAT3 depletion (Fig. 3C). In supporting these results, cell proliferation analysis showed that enhanced p15/p21 induction correlated to sensitization of cells to cell cycle arrest induced by a low dose of TGF-β (Fig. 3D).
Fig. 3. Loss of STAT3 enhances TGF-β-induced physiological responses.
(A) Depletion of STAT3 increases TGF-β-induced p15 mRNA expression in HaCaT cells. Cells were treated with or without TGF-β (2 ng/ml) for 2 h, and total RNA was extracted for qRT-PCR analysis.
(B) Depletion of STAT3 increases TGF-β-induced p21 mRNA expression in HaCaT cells.
(C) Depletion of STAT3 enhances TGF-β-induced p15 protein expression in HaCaT cells. Cells were treated with TGF-β for 0, 4 and 8 h, respectively, and then the protein level of p15 was detected by using Western blotting.
(D) Depletion of STAT3 in HaCaT cells enhances TGF-β-induced growth arrest. STAT3 knockdown stable cells and control cells were treated with TGF-β, and cell growth rates were examined by actual cell numbers being counted at indicated time point (0, 1 and 2 d) of TGF-β treatment (1 ng/ml).
(E) Stable knockdown of STAT3 in Hep3B cells. The protein level of STAT3 was detected by using Western blotting.
(F) Depletion of STAT3 enhances TGF-β-induced cell apoptosis. STAT3 knockdown stable cells and control cells were treated with TGF-β at indicated concentrations. Apoptotic cells were analyzed by flow cytometry. STAT3 knockdown stable cells and control cells were treated with or without TGF-β (2 ng/ml) for 48 h.
(G) Apoptotic nuclei and DNA fragments were visualized by nuclei staining.
(H) Quantification of apoptotic cells in panel G.
(I) Depletion of STAT3 enhances TGF-β-induced EMT marker expression. STAT3 knockdown stable cells and control cells were treated with or without TGF-β (2 ng/ml) for 36 h, and the protein levels of E-cadherin, N-cadherin and Fibronectin were detected by Western blotting.
(J) Depletion of STAT3 enhances TGF-β-mediated EMT. STAT3 knockdown stable cells and control cells were treated with TGF-β (2 ng/ml) for 0, 24 and 48 h, respectively. Cells were fixed and immuno-stained with anti-E-cadherin or anti-F-Actin antibody, and imaged by confocal microscope.
(K) Depletion of STAT3 promotes cell migration. STAT3 knockdown stable cells and control cells were grown to confluence, followed by a scratch on the cell patch. The status of cell gap closure was recorded at 24 hours post-wound in the presence or absence of TGF-β (2 ng/ml).
In addition to the cell cycle arrest, the tumor suppressing function of TGF-β also comes from its role in apoptosis. A good cell model is the human hepatoma Hep3B cells that undergo apoptosis when exposed to TGF-β. To this end, we established a stable cell line in Hep3B cells with STAT3 knockdown (STAT3-KD) (Fig. 3E) and investigated the effect of STAT3 depletion on TGF-β-induced apoptosis. In control cells, while TGF-β at a concentration of 200 pM induced apoptosis at a rate of 10%, the effect of a low concentration of TGF-β (5 pM) was not detectable (Fig. 3F). Notably, STAT3-KD cells underwent apoptosis with much increased sensitivity to TGF-β as 5 pM of TGF-β could elicit an apparent apoptosis response and 200 pM of TGF-β further increased apoptosis rate (Fig. 3F). We further examined condensed chromatin and nuclear fragmentation, two typical properties of apoptotic cell nuclei, by using Hoechest33258 staining. The staining result showed that STAT3 depletion markedly sensitized cells to TGF-β-mediated apoptosis (Fig. 3G and 3H).
As a multi-functional cytokine, TGF-β has a dual role in tumorigenesis. Whereas TGF-β induces cell cycle arrest and apoptosis in epithelial cells and early tumor cells, it promotes epithelial-to-mesenchymal transition (EMT), cell motility and tumor cell metastasis. Thus, we also sought to examine the role of STAT3 in TGF-β-induced EMT. The defined typical characteristics of cells undergoing EMT are downregulation of E-cadherin (an epithelial marker), upregulation of N-cadherin and Fibronectin (mesenchymal markers), and reorganization of actin to stress fiber. As shown in Figure 3I, whereas STAT3 depletion abolished expression of E-cadherin, it enhanced TGF-β-induced expression of EMT-associated proteins such as N-cadherin and Fibronectin in HaCaT cells. Loss of epithelial characteristics and acquisition of mesenchymal features were also analyzed by confocal microscopy. Compared to controls, STAT3 depletion strengthened EMT-associated changes in both unstimulated and TGF-β-stimulated cells. The effect of STAT3 was already apparent at an early time point of TGF-β treatment (24 h). At 24 h, wildtype HaCaT cells exhibited little changes in EMT markers and morphology (Fig. 3J). Depletion of STAT3 expression resulted in a dramatic loss of E-cadherin and gain of F-actin fiber (Fig. 3J). Consistent with the changes in cell morphology, STAT3-KD cells exhibited stronger migratory ability when treated with TGF-β in the wound healing analysis (Fig. 3K). These results support the notion that STAT3 also suppresses the EMT and migratory responses of TGF-β.
STAT3 selectively attenuates the formation of Smad3 signaling complexes
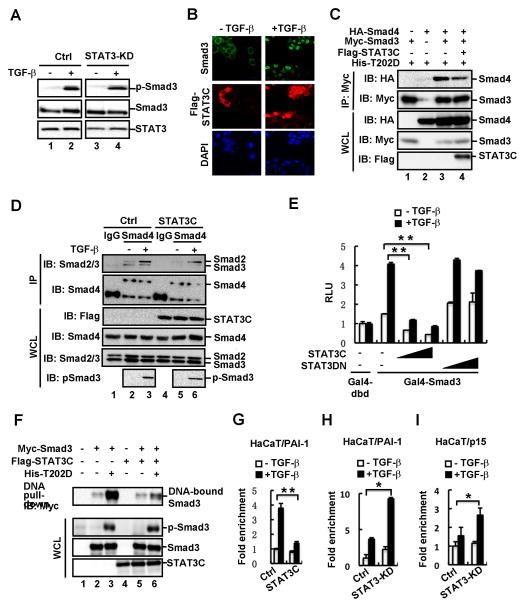
To elucidate the molecular mechanism underlying STAT3-mediated suppression of TGF-β signaling, we tested whether STAT3 could affect activation and signaling capacity of Smads, including Smad phosphorylation, nuclear localization, complex formation, and occupancy on the target gene promoters. By examining TGF-β-induced Smad3 phosphorylation, we found that stable knockdown of STAT3 did not obviously influence TGF-β-induced phosphorylation of endogenous Smad3 in HaCaT cells (Fig. 4A), suggesting that STAT3 probably does not regulate the expression or activity of TGF-β ligands or receptors in HaCaT cells. Moreover, STAT3 had no effect on TGF-β-induced Smad3 nuclear accumulation (Fig. 4B). Interestingly, TGF-β treatment stimulated not only the nuclear accumulation of Smad3, but also appeared to enhance that of STAT3 and thus co-localization of the two proteins in the nucleus (Fig. 4B).
Fig. 4. STAT3 selectively attenuates the formation of Smad3 signaling complexes.
(A) Depletion of STAT3 does not affect TGF-β-induced phosphorylation of Smad3. STAT3 knockdown stable cells and control cells were treated with or without TGF-β (2 ng/ml) for 1 h. The protein levels of p-Smad3, Smad3 and STAT3 were detected by using Western blotting.
(B) STAT3C does not alter TGF-β-stimulated nuclear accumulation of Smad3. STAT3C stable cells were treated with or without TGF-β (2ng/ml) for 1h, fixed and immuno-stained with anti-Smad3 and anti-Flag antibodies. DNA was stained with DAPI.
(C) STAT3C attenuates the interaction between Smad3 and Smad4. HEK293T cells were transfected with indicated plasmids. Levels of these proteins in IP products and whole cell lysates were analyzed by Western blotting.
(D) STAT3C disrupts the endogenous Smad3-Smad4 complex formation. STAT3C stable cells and control cells were treated with or without TGF-β (2 ng/ml). Cell lysates were immunoprecipitated with anti-Smad4 antibody or control IgG antibody. The immuno-complexes and input were analyzed by using Western blotting with indicated antibodies.
(E) STAT3C, but not STAT3DN, inhibits the transactivation activity of Smad3. HaCaT cells were transfected with various amounts of STAT3C or STAT3DN together with pFR-luc, Gal4-dbd or Gal4-Smad3, and treated with TGF-β (2 ng/ml) for 8 h. Luciferase assay was performed as described under “Materials and Methods”.
(F) STAT3C decreases DNA-binding activity of Smad3. HEK293T cells were transfected with indicated plasmids. Cell lysates were immunoprecipitated with biotinylated SBE and streptavidin beads. DNA-bound Smad3 was then assessed by using Western blotting.
(G) STAT3 inhibits Smad3 binding to the PAI-1 promoter. Isolated chromatins from control and STAT3C cells (treated with or without TGF-β) were immunoprecipitated with IgG or anti-Smad3 antibody for ChIP assay. Specific fragments of the PAI-1 promoter DNA were determined by PCR.
(H) STAT3 depletion increases Smad3 binding to the PAI-1 promoter. The experiments were carried out as described in Fig. 4G.
(I) STAT3 depletion increases Smad3 binding to the p15 promoter. The experiments were carried out as described in Fig. 4G.
We next investigated the effect of STAT3 on the TGF-β-induced Smad complex formation. We found that STAT3C could compete with Smad4 for Smad3 binding as it inhibited TGF-β receptor-mediated Smad3-Smad4 interaction in HEK293T cells (Fig. 4C). Furthermore, stable expression of STAT3C abolished the endogenous Smad3-Smad4 complex in HaCaT cells (Fig. 4D). Notably, this STAT3C-induced disruption of the Smad complex was selective as it affected the Smad3-Smad4, but not Smad2-Smad4 association (Fig. 4D). Because the transcriptional activity of Smad3 depends on the activator role of Smad4 (31), we examined the effect of STAT3C on Smad3-mediated transcriptional activation using the heterologous Gal4 system. TGF-β stimulation could induce transcription activity of Gal4-fused Smad3, which was reflected by expression of the Gal4-driven luciferase reporter FR-Luc (Fig. 4E). STAT3C dramatically inhibited transcriptional activity of Gal4-Smad3 (Fig. 4E). In contrast, a dominant negative mutant of STAT3 (STAT3DN) did not exhibit any inhibitory effect (Fig. 4E).
A few transcription factors modulate the activity of Smad3 to bind to DNA in either synergistic or antagonistic manner (15). Therefore, we explored the possibility that STAT3 influences the DNA-binding activity of Smad3. In DNA pull-down assays, where a biotinylated DNA fragment consisting of SBEs were used to precipitate Smad3 in HEK293T cells, activated Smad3 (by TβRI-T202D) exhibited a high level of DNA binding (Fig 4F). In contrast, STAT3C markedly reduced the DNA-binding level of Smad3 (Fig. 4F). These results further prompted us to investigate the inhibitory effect of STAT3 on the natural promoters of two representative TGF-β target genes. Occupancy of Smad3 on the promoter regions of p15 and PAI-1 were examined by using chromatin immunoprecipitation. TGF-β induced the enrichment of Smad3 on the PAI-1 promoter, and this enrichment was abolished by STAT3C (Fig. 4G). Conversely, depletion of STAT3 profoundly increased TGF-β-induced accumulation of Smad3 on the promoters of PAI-1 (Fig. 4H) and p15 (Fig. 4I). Taken together, STAT3 impairs the Smad3-Smad4 complex formation and decreases Smad3 binding to chromatin, thereby resulting in inhibition of Smad3-mediated transcriptional activation.
STAT3 selectively and directly interacts with Smad3 under physiological conditions
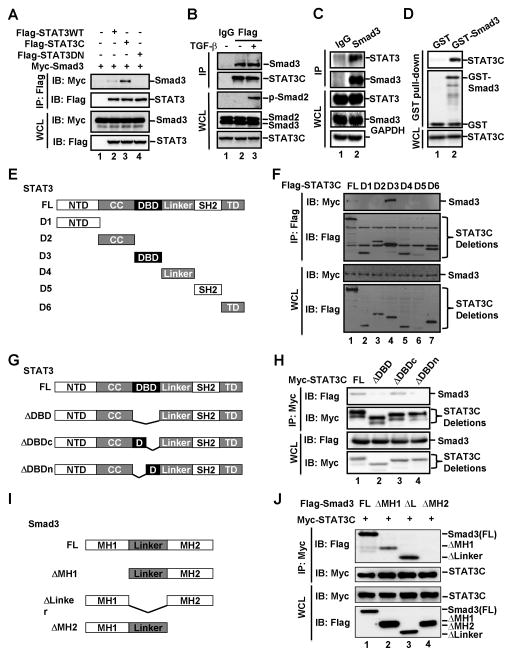
Based on the convincing evidence that STAT3 attenuates the transcriptional capacity of Smad3, we speculated that STAT3 might directly interact with Smad3. We analyzed the potential STAT3-Smad3 interactions by using co-immunoprecipitation (co-IP) and in vitro pull-down assays. In HEK293T cells transfected with expression plasmids for Flag-tagged Smad3 and Myc-tagged STAT3WT or STAT3C, we could detect both STAT3WT and STAT3C in the anti-Flag-Smad3 immunoprecipitates (Fig. 5A).
Fig. 5. STAT3 directly interacts with Smad3 under physiological conditions.
(A) STAT3 interacts with Smad3 in HEK293T cells. HEK293T cells were transfected with Myc-Smad3 and Flag-STAT3WT (wild-type STAT3), Flag-STAT3C (constitutively active STAT3) or Flag-STAT3DN (dominant negative mutant of STAT3). Levels of these proteins in IP products and whole cell lysates were analyzed by Western blotting.
(B) STAT3 interacts with endogenous Smad3. STAT3C expressing HaCaT stable cells were treated with or without TGF-β (2 ng/ml) for 1 h. Cell lysates were immunoprecipitated with anti-Flag antibody or control IgG antibody. The immune-complexes and input were analyzed by Western blotting with indicated antibodies.
(C) STAT3 interacts with Smad3 with both proteins at endogenous levels. HaCaT cell lysates were immunoprecipitated with anti-Smad3 antibody or control IgG antibody. The immune-complexes and input were analyzed by Western blotting with indicated antibodies.
(D) STAT3 directly binds to Smad3 in vitro. In vitro binding was carried out with purified GST or GST-Smad3 and in vitro translated STAT3C.
(E) Schematic diagram of STAT3 and its deletion mutants. Individual domains of STAT3 are shown.
(F) Smad3 binds with the DNA-binding domain of STAT3. Experiments were carried out as descried in Fig. 5A.
(G) Schematic diagram of deletion mutants of DNA binding domain of STAT3.
(H) Smad3 binds with the N-terminal region of STAT3 DNA-binding domain. Experiments were carried out as descried in Fig. 5A.
(I) Schematic diagram of Smad3 and its deletion mutants.
(J) STAT3 binds with the MH2 domain of Smad3. Experiments were carried out as descried in Fig. 5A.
To further investigate the specificity and physiological relevance of the STAT3-Smad3 interaction, we analyzed the STAT3-Smad3 interaction under physiological conditions. We first used co-IP experiments to examine the association between endogenous Smad2/3 and stably expressed Flag-STAT3C, the level of which is comparable to that of endogenous STAT3 (Fig. 2B). As shown in Figure 5B, anti-Flag immunoprecipitates (STAT3C) specifically retrieved Smad3, but not Smad2. This specificity was consistent with the result that STAT3 inhibited binding of Smad3, but not Smad2, to Smad4. We further examined the endogenous STAT3-Smad3 interaction in HaCaT cells, and found that STAT3 could be detected in the anti-Smad3 immunoprecipitates, but not in that of control IgG (Fig. 5C).
To evaluate whether the STAT3-Smad3 interaction is direct, we conducted an in vitro interaction assay where only recombinant proteins were used. Smad3 was expressed and purified from E. coli as a glutathione S-transferase (GST) fusion protein, whereas STAT3C was obtained from in vitro coupled transcription/translation in rabbit reticulocyte lysate. As shown in Fig. 5D, in vitro synthesized STAT3C was retrieved by GST-fused Smad3 protein, but not GST alone, indicating that STAT3 directly interacts with Smad3. Taken together, STAT3 directly interacts with Smad3 under physiological conditions.
To determine the structural features for STAT3-Smad3 interaction, we first mapped the region in STAT3 that mediates the STAT3-Smad3 interaction. STAT3 consists of several protein-protein interaction domains including coil-coil (CC), DNA-binding domain (DBD), and Src homology 2 (SH2) domains. Interaction of Smad3 with each of these individual domains of STAT3 was assessed by using co-IP assays (Fig. 5E). As shown in Figure 5F, DBD of STAT3 strongly bound to Smad3, whereas all other domains did not bind to Smad3. To further narrow down the interacting region in the DBD, three truncated mutants were created. While STAT3C-ΔDBD lacks the entire DBD, STAT3C-ΔDBDc and STAT3C-ΔDBDn lack the C-terminal and N-terminal regions of the STAT3 DBD, respectively (Fig. 5G). It is apparent that the N terminal half of DBD was critical for the STAT3-Smad3 interaction (Fig. 5H). We then determined the domains of Smad3 for STAT3 binding. Smads are structurally conserved proteins consisting of MH1 domain in the N terminus and MH2 domain in the C terminus, linked with a relatively less conserved linker region (Fig. 5I). Our co-IP binding assay found that Smad3 mutants with deletion of either the MH1 domain or the linker, but not the MH2 domain, retained the ability to bind with STAT3 (Fig. 5J, lane 2&3). Notably, deletion of the MH2 domain completely abolished Smad3 binding with STAT3. These results suggest that STAT3 binds to the MH2 domain of Smad3.
Binding to Smad3 is indispensable for STAT3 to inhibit TGF-β responses
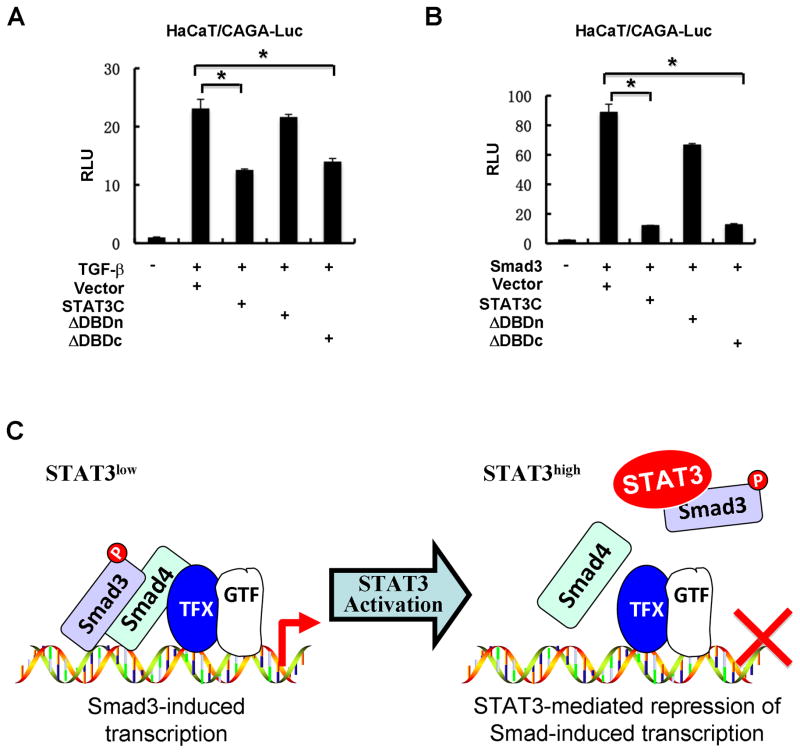
We further assessed the importance of the Smad3-interacting region of STAT3 in STAT3-mediated suppression of TGF-β signaling. In HaCaT cells, both the full-length STAT3C and STAT3C-ΔDBDc retained the inhibitory effect on TGF-β-induced CAGA-Luc activity. In contrast, STAT3C-ΔDBDn, which failed to interact with Smad3, was unable to suppress TGF-β signaling (Fig. 6A). Similarly, only the full-length STAT3C and STAT3C-ΔDBDc could obviously attenuate Smad3-induced transcriptional activation (Fig. 6B). Therefore, binding to Smad3 is essential for STAT3 to inhibit TGF-β signaling.
Fig. 6. STAT3 requires a direct interaction with Smad3 to antagonize TGF-β signaling.
(A) STAT3-ΔDBDn mutant fails to inhibit TGF-β-induced CAGA-Luc reporter activity. Experiments were carried out as descried in Fig. 1A.
(B) STAT3-ΔDBDn mutant fails to inhibit Smad3-activated CAGA-Luc reporter activity. Experiments were carried out as descried in Fig. 1A.
(C) A working model for STAT3-mediated repression of Smad-induced transcription. In normal cells, Smad3 mediates TGF-β growth inhibitory and transcriptional responses. In tumors where STAT3 is aberrantly activated, STAT3 sequesters Smad3 from the Smad nucleoprotein complex and thus suppresses TGF-β growth inhibitory and transcriptional responses.
Discussion
Cancer and inflammation are two reciprocally regulated events. Malignant cells can usually generate a local inflammatory environment, and conversely, inflammatory conditions will further promote oncogenic transformation. Increased expression of soluble cytokines and growth factors establish and maintain a tumor microenvironment where tumor cells, stroma cells and inflammatory cells are associated. TGF-β is recognized as a major mediator in either tumor-associated inflammation or inflammation-associated tumor progression. Crosstalks between TGF-β and other cytokines may play an important role in the diverse functions of TGF-β. There are several studies, albeit conflicting, reporting crosstalks between TGF-β and STAT3 signaling pathways. TGF-β reduces phosphorylation of STAT3, and then downregulates the expression of its downstream genes (23–25,32,33). Conversely, STAT3 induces the expression of TGF-β1 to promote fibrosis (21). It has also been previously reported that there are p300 coactivator-dependent interactions between STAT3 and Smad3 (34), between STAT1 and Smad3 (35) and between STAT3 and Smad1 (36). However, how STAT3 growth-promoting signaling antagonizes TGF-β growth inhibitory signaling remains elusive.
In the present study, we have identified a direct physical and functional interaction between STAT3 and Smad3. The interaction between STAT3 and Smad3 in hepatocytes was previously reported, yet it was indirect as it was bridged by p300 (34). We for the first time demonstrate that the STAT3-Smad3 interaction is direct by means of in vitro interaction assays. The interaction enables STAT3 to compete with Smad4 for Smad3, thereby disrupting the Smad3-Smad4 complex, and thus attenuate TGF-β-mediated Smad3-dependent growth inhibitory and transcriptional responses in epithelial cells (Fig. 6C), which is in contrast to the cooperative action of STAT3-Smad3 in STAT3-mediated gene expression in hepatocytes (34). The current finding is consistent with the oncogenic property of STAT3 and growth suppressor role of the TGF-β signaling. Loss of TGF-β tumor suppressing response, which is a hallmark in cancer (37), can be achieved through somatic mutations in the genes encoding components of the TGF-β tumor suppressing response pathway. Indeed, mutations are frequent in the Smad4 or TβRII gene in the gastrointestinal cancers such as pancreatic and colorectal cancers (4,6,38). In addition to genetic lesions in the genes encoding Smads and TGF-β receptors, accumulating evidence has demonstrated that the tumor suppressor functions of Smads are compromised by oncogene products such as c-Ski, Bcl6, c-Myc and Evi-1 through direct Smad-oncoprotein interactions (8–11). Overexpression or amplification of STAT3 is often observed in cancers (20). Thus, our study extends and expands the role of oncoproteins in suppressing the TGF-β tumor suppressing response.
It is previously reported that hyperactive STAT3 induces expression of inhibitory Smad7, and thus desensitizes TGF-β responses (22,30). However, there is a minimal induction of Smad7 mRNA by STAT3 overexpression in HaCaT cells, which cannot explain the profound suppressing effect of STAT3 on TGF-β responses (data not shown). STAT3 is one of the effectors mediating the growth-promoting effect of EGF, IL6 and related factors. Indeed, EGF and IL6 have suppressing effects on TGF-β-mediated transcriptional responses. We rule out the possibility that the effect of EGF may be mediated via an Erk-dependent manner. Although it is known that EGF-activated Erks mediate hyper-phosphorylation of Smad2/3 that can affect the nuclear translocation of Smad2/3 (14), MEK1 inhibitor PD98059 does not reverse the EGF-mediated attenuation of TGF-β signaling in our system. Neither does STAT3 alter nuclear accumulation of Smad3. Mechanistically, STAT3 competes with Smad4 for direct Smad3 binding and disables Smad3 to bind to DNA and to form the hetero-oligomeric complex with Smad4. As a consequence, STAT3 attenuates TGF-β-mediated growth inhibitory and transcriptional responses. Consistently, STAT3 depleted cells exhibit enhanced TGF-β-mediated responses.
Our current study has important implication in the development of more effective targeted cancer therapies, since TGF-β and JAK/STAT signaling have critical functions in tumor cell behaviors and tumor microenvironment. Our study provides compelling evidence demonstrating that STAT3 is a new oncogenic partner that interacts with Smads and suggests that STAT3 promotes tumorigenesis partly through disruption of the TGF-β pathway. Therefore, targeting of STAT3 with small molecules disrupting its canonical signaling in tumor cells may not be sufficient to halt tumor progression. Development of new STAT3 inhibitors targeting STAT3’s DBD, which confers both DNA-binding and Smad3-binding, may provide an advantage for not only inhibiting STAT3’s canonical growth promoting functions, but also restoring TGF-β tumor suppressing functions.
The observation that STAT3 selectively binds to Smad3, not Smad2, is very intriguing. Smad2 and Smad3 are the two closely related signal transducers for TGF-β/activin/myostatin in the TGF-β superfamily. Both are essential in most of TGF-β responses (15). However, while they share 92% sequence identity, Smad2 lacks the β-hairpin region that confers the DNA-binding function in Smad3. This DNA-binding difference may explain the differential roles of Smad2 and Smad3 in the regulation of certain TGF-β responses (39–43). Additionally, differential ability of Smad2 and Smad3 to bind to partner transcriptional factors can also contribute to differential TGF-β responses. Thus, the Smad3-STAT3 interaction may be involved in certain TGF-β responses. We previously reported that Smad2 is required for Th17 cell differentiation, where loss of Smad3 leads to enhanced Th17 differentiation (44,45). Although this study only describes the antagonistic activity of STAT3 on the Smad3 signaling, it is conceivable that there is mutual inhibition between STAT3 and Smad3. Lastly, given its oncogenic role, the ability of STAT3 to differentially block Smad3 signaling further implicates that Smad3 is a key player in the TGF-β tumor suppressor signaling. This is consistent with the fact that loss of the Smad3 expression occurs in human cancer (46,47).
Besides tumor and inflammation contexts, the interplay between TGF-β signaling and STAT3-dependent signaling exist in various physiological contexts, such as Th-17 cell differentiation where TGF-β and IL-6 collaborate (48), and murine embryonic stem cell self-renewal and differentiation where LIF and TGF-β/BMP signaling cooperatively control (49,50). How the direct interplay between STAT3 and Smad3 impacts these biological functions in vivo warrants more exciting investigation.
Materials and Methods
Plasmids
Expression plasmids for Myc- or Flag-tagged Smads and T202D (constitutively active TGF-β type I receptor) were described previously (26). Myc- or Flag-tagged STAT3WT, STAT3C (22) and STAT3DN (21) were generated by PCR and cloned into the EcoRI and SalI sites of pXF6F and pXF3HM. The pXF6F and pXF3HM were derived from pRK5 (Genentech).
Antibodies
Antibodies against Smad2 (#5339), Smad3 (#9523), Smad2/3 (#8685), p-Smad2 (#3108), p-Smad3 (#9520), STAT3 (#9139), p-STAT3 (#9145), HA tag C29F4 (#3724), p21 (#2947) and E-cadherin (#3195) were purchased from Cell Signaling Technology. Antibodies against Flag tag M2 (#F3165) and GAPDH (#G8795) were bought from Sigma. Antibodies against Myc tag 9E10 (#sc-40) and Fibronectin (#sc-8422) were from Santa Cruz. Antibodies against p15 (#C0287) were purchased from Assaybiotech. Antibody against N-cadherin (#610920) was from BD Biosciences.
Cell culture and transfection
HEK293T cells were grown in Dulbecco’s modified Eagle’s medium (DMEM, Corning) with 10% fetal bovine serum (FBS, GIBCO). HaCaT, Hep3B and HepG2 cells were cultured in MEM (Corning) with 10% FBS. HEK293T cells were transfected with PEI (Polyscience). HaCaT cells were transfected with X-treme GENE HP DNA transfection reagent (Roche). Hep3B and HepG2 cells were transfected with Lipofectamine2000 (Invitrogen). Stable cell lines were selected by puromycin with appropriate concentrations.
Luciferase reporter assays
Reporters CAGA-Luc, SBE-Luc and 3TP-Luc were used to measure TGF-β-induced transcription. Cells were co-transfected with indicated reporter plasmids and a Renilla luciferase plasmid to normalize transfection efficiency. Briefly, 24–36 h after transfection, cells were treated with TGF-β (2 ng/ml), IL-6 (10 ng/ml) and EGF (5 ng/ml) for 12 h. Cells were harvested and measured by Dual-Luciferase Reporter Assay System (Promega). All assays were carried out in duplicates and the activities of firefly were normalized against Renilla luciferase activities.
RNA interference and real-time PCR
siRNAs targeting STAT3 were transfected into cells with Lipofectamine RNAiMAX reagent (Invitrogen). siRNAs were synthesized by RIOBIO CO (#1 target sequence: 5-CCAACGACCUGCAGCAAUAUU; #2 target sequence: 5-CUCAGAGGAUCCCGGAAAUUU).
Total RNAs were isolated with TRIzol Reagent (Sigma) and corresponding cDNAs (complementary DNA) were obtained using PrimeScript RT reagent kit. Real-time PCRs were performed with SYBR Green Master Mix (Applied Biosystems) and specific primers. The primers for the following human genes were used: STAT3 (Forward: CATCCTGAAGCTGACCCAGG; Reverse: TATTGCTGCAGGTCGTTGGT); p15 (Forward: AAGCTGAGCCCAGGTCTCCTA; Reverse: CCACCGTTGGCCGTAAACT); p21 (Forward: ACCATGTGGACCTGTCACTGT; Reverse: TTAGGGCTTCCTCTTGGAGAA); PAI-1 (Forward: GTGTTTCAGCAGGTGGCGC; Reverse: CCGGAACAGCCTGAAGAAGTG); β-actin (Forward: CAAAGTTCACAATGTGGCCGAGGA; Reverse: GGGACTTCCTGTAACAACGCATCT).
Immunofluorescence
HaCaT cells grown on coverslips were fixed with 4% formaldehyde for 15 min at 4°C, permeabilized with 0.5% Triton X-100 for 15 min, followed by 5% BSA blocking for 1 h. Cells were subsequently probed with indicated primary antibodies, and then with Alexa Fluor 488-conjugated or Alexa Fluor 546-secondary antibodies (Invitrogen). Fluoresence images were captured by Zeiss LSM710 confocal microscope (Carl Zeiss).
DNA pull-down assay
DNA pull-down assay was carried out as previously described (27). HEK293T cells were lysated in buffer (10 mM HEPES [pH 7.5], 150 mM NaCl, 1 mM MgCl2, 0.5 mM EDTA, 0.5 mM DTT, and 0.1% NP-40, 10% glycerol), then incubated with 0.5 μmol biotinylated SBE oligonucleotides together with 10 μg poly (dI-dC). DNA-protein complexes were then collected by precipitation on streptavidin beads (GE Healthcare) for 15 min, washed extensively with binding buffer, and detected by Western blotting.
Chromatin immunoprecipitation (ChIP) assay
HaCaT cells were treated with TGF-β (2 ng/ml) for 2 h. Cells were cross-linked with 1% formaldehyde for 10 min at 37°C, and then quenched with 0.125 M glycine for 5 min at room temperature. Subsequently, cell pellets were suspended in SDS lysis buffer and sonicated to generate DNA fragments. A Smad3 antibody was used to immunoprecipitated Smad3 and IgG was used as a control. Smad3-bound DNAs were determined by RT-PCR. ChIP primers used in the experiment are as follows: p15 Forward CTGCCTGGGGATGAATTTAAC; p15 Reverse GGTTTCACTGTGGAGACGTTG; PAI-1 Forward GCAGGACATCCGGGAGAGA; PAI-1 Reverse: CCAATAGCCTTGGCCTGAGA.
Immunoprecipitation and Western blotting
Immunoprecipitation-coupled Western blotting was performed as previously described (28). Briefly, Cells were transfected with indicated plasmids and harvested 24–48 h after transfection. Co-immunoprecipitation was carried out with appropriate antibody and protein A Sepharose (GE Healthcare), followed by extensive washes. Precipitated proteins were eluted in SDS loading buffer and separated by SDS-PAGE and detected in Western blotting using appropriate antibodies.
In vitro protein binding assay
Recombinant glutathione S-transferase (GST) fusion protein of Smad3 was prepared from E. coli strain BL21 (DE3). In vitro translation of STAT3 was carried out using Quick Coupled Transcription/Translation System (Promega). GST-Smad3 was incubated with STAT3 in the in vitro binding buffer (0.5% NP-40, 150 mM NaCl, 50 mM Tris-HCl, 5 mM EDTA) for 2 h. GST-Smad3 was retrieved using glutathione sepharose beads and examined by Western blotting.
Cell cycle analysis
Cell cycle distribution was quantitatively evaluated by flow cytometry analysis. HaCaT cells were cultured in the absence or presence of TGF-β (2 ng/ml) for 2 days. Cells were detached and collected in 15 mL tube, and then fixed in 70% ice-cold ethanol for 24 h at 20 °C. Fixed cells were washed once with 1×PBS and stained with 1 mg/mL of Propidium Iodide (PI) at room temperature for 30 min. DNA content was then analyzed using Flow Cytometer (Beckman Coulter).
Apoptosis Assay
Hep3B cells were cultured in the absence or presence of TGF-β at indicated concentrations for 2 days. Cells were fixed as cell cycle analysis and then stained as manufacturer’s instruction (Annexin V/PI apoptosis kit, Multisciences). The stained cells were analyzed by Flow Cytometer (Beckman Coulter).
Acknowledgments
We thank David Luskutoff for p800 (PAI-1)-luc, Peter ten Dijke for CAGA-luc, Bert Vogelstein for WWP1 (p21)-luc and SBE-luc, and Xiao-Fan Wang for p15-luc. We are grateful to colleagues in our laboratories for helpful discussion and technical assistance. This research was partly supported by grants from MOST (2012CB966600) and NSFC (31571447; 31090360), NIH (R01GM63773, R01AR053591, R01CA108454, and R01DK073932), Project 111, Project 985, and the Fundamental Research Funds for the Central Universities.
References
- 1.Derynck R, Miyazono K. The TGF-β Family. 2007. pp. 1–1114. [Google Scholar]
- 2.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limón P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10:554–67. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu MY, Hill CS. TGF-beta superfamily signaling in embryonic development and homeostasis. Dev Cell. 2009;16:329–43. doi: 10.1016/j.devcel.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Massagué J. TGFβ in Cancer. Cell. 2008;134:215–30. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikushima H, Miyazono K. TGFβ signalling: a complex web in cancer progression. Nat Rev Cancer. 2010;10:415–424. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- 6.Rik Derynck Rosemary J, Akhurst, Balmain Allan. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 7.Drabsch Y, ten Dijke P. TGF-β signalling and its role in cancer progression and metastasis. Cancer Metastasis Rev Springer US. 2012;31:553–68. doi: 10.1007/s10555-012-9375-7. [DOI] [PubMed] [Google Scholar]
- 8.Deheuninck J, Luo K. Ski and SnoN, potent negative regulators of TGF-beta signaling. Cell Res. 2009;19:47–57. doi: 10.1038/cr.2008.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D, Long J, Dai F, Liang M, Feng X-H, Lin X. BCL6 represses Smad signaling in transforming growth factor-beta resistance. Cell Res. 2008;68:783–9. doi: 10.1158/0008-5472.CAN-07-0008. [DOI] [PubMed] [Google Scholar]
- 10.Hirai H, Izutsu K, Kurokawa M, Mitani K. Oncogenic mechanisms of Evi-1 protein. Cancer Chemother and Pharmacol. 2001;48:S35–S40. doi: 10.1007/s002800100303. [DOI] [PubMed] [Google Scholar]
- 11.Feng X-H, Liang Y-Y, Liang M, Zhai W, Lin X. Direct interaction of c-Myc with Smad2 and Smad3 to inhibit TGF-beta-mediated induction of the CDK inhibitor p15(Ink4B) Mol Cell. 2002;9:133–43. doi: 10.1016/s1097-2765(01)00430-0. [DOI] [PubMed] [Google Scholar]
- 12.Massagué J, Gomis RR. The logic of TGFbeta signaling. FEBS Lett. 2006;580:2811–20. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 13.Wrighton KH, Lin X, Feng X-H. Phospho-control of TGF-β superfamily signaling. Cell Res. 2009;19:8–20. doi: 10.1038/cr.2008.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kretzschmar M, Doody J, Timokhina I, Massagué J. A mechanism of repression of TGFbeta/ Smad signaling by oncogenic Ras. Genes Dev. 1999;13:804–16. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng X-H, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–93. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 16.Massagué J, Seoane J, Wotton D. Smad transcription factors. Genes Dev Genes Dev. 2005;19:2783–810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 17.Labbé E, Silvestri C, Hoodless PA, Wrana JL, Attisano L. Smad2 and Smad3 positively and negatively regulate TGF beta-dependent transcription through the forkhead DNA-binding protein FAST2. Mol Cell. 1998;2:109–20. doi: 10.1016/s1097-2765(00)80119-7. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Weisberg E, Fridmacher V, Watanabe M, Naco G, Whitman M. Smad4 and FAST-1 in the assembly of activin-responsive factor. Nature. 1997;389:85–9. doi: 10.1038/38008. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Feng XH, Derynck R. Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-beta-induced transcription. Nature. 1998;394:909–13. doi: 10.1038/29814. [DOI] [PubMed] [Google Scholar]
- 20.Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer. 2014;14:736–46. doi: 10.1038/nrc3818. [DOI] [PubMed] [Google Scholar]
- 21.Ogata H, Chinen T, Yoshida T, Kinjyo I, Takaesu G, Shiraishi H, et al. Loss of SOCS3 in the liver promotes fibrosis by enhancing STAT3-mediated TGF-beta1 production. Oncogene. 2006;25:2520–30. doi: 10.1038/sj.onc.1209281. [DOI] [PubMed] [Google Scholar]
- 22.Jenkins BJ, Grail D, Nheu T, Najdovska M, Wang B, Waring P, et al. Hyperactivation of Stat3 in gp130 mutant mice promotes gastric hyperproliferation and desensitizes TGF-β signaling. Nat Med. 2005;11:845–52. doi: 10.1038/nm1282. [DOI] [PubMed] [Google Scholar]
- 23.Gunaje JJ, Bhat GJ. Distinct mechanisms of inhibition of interleukin-6-induced Stat3 signaling by TGF-beta and alpha-thrombin in CCL39 cells. Mol Cell Biol Res Commun. 2000;4:151–7. doi: 10.1006/mcbr.2001.0272. [DOI] [PubMed] [Google Scholar]
- 24.Walia B, Wang L, Merlin D, Sitaraman SV. TGF-beta down-regulates IL-6 signaling in intestinal epithelial cells: Critical role of SMAD-2. FASEB J. 2003;17:2130–2132. doi: 10.1096/fj.02-1211fje. [DOI] [PubMed] [Google Scholar]
- 25.Zauberman A, Lapter S, Zipori D. Smad proteins suppress CCAAT/enhancer-binding protein (C/EBP) beta- and STAT3-mediated transcriptional activation of the haptoglobin promoter. J Biol Chem. 2001;276:24719–25. doi: 10.1074/jbc.M005813200. [DOI] [PubMed] [Google Scholar]
- 26.Lin X, Duan X, Liang Y-Y, Su Y, Wrighton KH, Long J, et al. PPM1A Functions as a Smad Phosphatase to Terminate TGFβ Signaling. Cell. 2006;125:915–28. doi: 10.1016/j.cell.2006.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai F, Lin X, Chang C, Feng X-H. Nuclear export of Smad2 and Smad3 by RanBP3 facilitates termination of TGF-beta signaling. Dev Cell. 2009;16:345–57. doi: 10.1016/j.devcel.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin X, Liang Y-Y, Sun B, Liang M, Shi Y, Brunicardi FC, et al. Smad6 recruits transcription corepressor CtBP to repress bone morphogenetic protein-induced transcription. Mol Cell Biol. 2003;23:9081–93. doi: 10.1128/MCB.23.24.9081-9093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunfield LD, Nachtigal MW. Inhibition of the antiproliferative effect of TGFbeta by EGF in primary human ovarian cancer cells. Oncogene. 2003;22:4745–51. doi: 10.1038/sj.onc.1206617. [DOI] [PubMed] [Google Scholar]
- 30.Luwor RB, Baradaran B, Taylor LE, Iaria J, Nheu TV, Amiry N, et al. Targeting Stat3 and Smad7 to restore TGF-β cytostatic regulation of tumor cells in vitro and in vivo. Oncogene. 2012;32:2433–41. doi: 10.1038/onc.2012.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng X-H, Zhang Y, Wu R-Y, Derynck R. The tumor suppressor Smad4/DPC4 and transcriptional adaptor CBP/p300 are coactivators for Smad3 in TGF-beta. Genes Dev. 1998;12:2153–63. doi: 10.1101/gad.12.14.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wierenga ATJ, Schuringa JJ, Eggen BJL, Kruijer W, Vellenga E. Downregulation of IL-6-induced STAT3 tyrosine phosphorylation by TGF-β1 is mediated by caspase-dependent and -independent processes. Leukemia. 2002;16:675–82. doi: 10.1038/sj.leu.2402425. [DOI] [PubMed] [Google Scholar]
- 33.Campbell JD, Cook G, Robertson SE, Fraser A, Boyd KS, Gracie JA, et al. Suppression of IL-2-induced T cell proliferation and phosphorylation of STAT3 and STAT5 by tumor-derived TGF beta is reversed by IL-15. J Immunol. 2001;167:553–61. doi: 10.4049/jimmunol.167.1.553. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto T, Matsuda T, Muraguchi A, Miyazono K, Kawabata M. Cross-talk between IL-6 and TGF-beta signaling in hepatoma cells. FEBS Lett. 2001;492:247–53. doi: 10.1016/s0014-5793(01)02258-x. [DOI] [PubMed] [Google Scholar]
- 35.Ghosh AK, Yuan W, Mori Y, Chen Sj, Varga J. Antagonistic regulation of type I collagen gene expression by interferon-gamma and transforming growth factor-beta. Integration at the level of p300/CBP transcriptional coactivators. J Biol Chem. 2001;276:11041–8. doi: 10.1074/jbc.M004709200. [DOI] [PubMed] [Google Scholar]
- 36.Nakashima K, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Kawabata M, et al. Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science. 1999;284:479–82. doi: 10.1126/science.284.5413.479. [DOI] [PubMed] [Google Scholar]
- 37.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Miyazono K, Suzuki H, Imamura T. Regulation of TGF-beta signaling and its roles in progression of tumors. Cancer Sci. 2003;94:230–4. doi: 10.1111/j.1349-7006.2003.tb01425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ungefroren H, Groth S, Sebens S, Lehnert H, Gieseler F, Fändrich F. Differential roles of Smad2 and Smad3 in the regulation of TGF-β1-mediated growth inhibition and cell migration in pancreatic ductal adenocarcinoma cells: control by Rac1. Mol Cancer. 2011;10:67. doi: 10.1186/1476-4598-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng R, Xiong Q, Zuo B, Jiang S, Li F, Lei M, et al. Using RNA interference to identify the different roles of SMAD2 and SMAD3 in NIH/3T3 fibroblast cells. Cell Biochem Funct. 2008;26:548–56. doi: 10.1002/cbf.1464. [DOI] [PubMed] [Google Scholar]
- 41.Huang D, Liu Y, Huang Y, Xie Y, Shen K, Zhang D, et al. Mechanical compression upregulates MMP9 through SMAD3 but not SMAD2 modulation in hypertrophic scar fibroblasts. Connect Tissue Res. 2014;55:391–6. doi: 10.3109/03008207.2014.959118. [DOI] [PubMed] [Google Scholar]
- 42.Kim SG, Kim H-A, Jong H-S, Park J-H, Kim NK, Hong SH, et al. The endogenous ratio of Smad2 and Smad3 influences the cytostatic function of Smad3. Mol Biol Cell. 2005;16:4672–83. doi: 10.1091/mbc.E05-01-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmierer B, Schuster MK, Shkumatava A, Kuchler K. Activin a signaling induces Smad2, but not Smad3, requiring protein kinase a activity in granulosa cells from the avian ovary. J Biol Chem. 2003;278:21197–203. doi: 10.1074/jbc.M212425200. [DOI] [PubMed] [Google Scholar]
- 44.Martinez GJ, Zhang Z, Chung Y, Reynolds JM, Lin X, Jetten AM, et al. Smad3 differentially regulates the induction of regulatory and inflammatory T cell differentiation. J Biol Chem. 2009;284:35283–6. doi: 10.1074/jbc.C109.078238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martinez GJ, Zhang Z, Reynolds JM, Tanaka S, Chung Y, Liu T, et al. Smad2 positively regulates the generation of Th17 cells. J Biol Chem. 2010;285:29039–43. doi: 10.1074/jbc.C110.155820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han S-U, Kim H-T, Seong DH, Kim Y-S, Park Y-S, Bang Y-J, et al. Loss of the Smad3 expression increases susceptibility to tumorigenicity in human gastric cancer. Oncogene. 2004;23:1333–41. doi: 10.1038/sj.onc.1207259. [DOI] [PubMed] [Google Scholar]
- 47.Wolfraim LA, Fernandez TM, Mamura M, Fuller WL, Kumar R, Cole DE, et al. Loss of Smad3 in acute T-cell lymphoblastic leukemia. N Engl J Med. 2004;351:552–9. doi: 10.1056/NEJMoa031197. [DOI] [PubMed] [Google Scholar]
- 48.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 49.Okita K, Yamanaka S. Intracellular Signaling Pathways Regulating Pluripotency of Embryonic StemCells. Current Stem Cell Research Therapy. 2006;1:103–11. doi: 10.2174/157488806775269061. [DOI] [PubMed] [Google Scholar]
- 50.Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–92. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]