Abstract
Fertilization is a very dynamic period of comprehensive chromatin remodelling, from which two specialized cells result in a totipotent zygote. The formation of a totipotent cell requires extensive epigenetic remodeling that, while independent of modifications in the DNA sequence, still entails a profound cell fate change, supported by transcriptional profile modifications. As a result of finely-tuned interactions between numerous mechanisms, the goal of fertilization is to form a full healthy new individual. To avoid the persistence of alterations in epigenetic marks, the epigenetic information contained in each gamete is reset during early embryogenesis. Covalent modification of DNA by methylation, as well as post-translational modifications of histone proteins and non-coding RNAs, appear to be the main epigenetic mechanisms that control gene expression. These allow different cells in an organism to express different transcription profiles, despite each cell containing the same DNA sequence. In the context of replacement of spermatic protamine with histones from the oocyte, active cell division, and specification of different lineages, active and passive mechanisms of epigenetic remodeling have been revealed as critical for editing the epigenetic profile of the early embryo. Importantly, redundant factors and mechanisms are likely in place, and only a few have been reported as critical for fertilization or embryo survival by the use of knockout models. The aim of this review is to highlight the main mechanisms of epigenetic remodeling that ensue after fertilization in mammals.
Keywords: DNA methylation, Histone modifications, Embryo, Imprinting, Zygote, Reprogramming, Pronucleus
Introduction
Fertilization is among the most exciting events in nature, consisting of the union of two specialized cells (oocyte and sperm) to create a totipotent embryo with the potential to produce a complex organism. Throughout this process, various cell fate decisions take place, governed in part by the information contained within the DNA sequence, but also by epigenetic marks, which in parallel determine and establish the different cellular phenotypes. Genetic information contained within the DNA sequence is nearly identical for all cells within the body. In contrast, epigenetic information is different in every cell and is responsible for the maintenance of different cell types within the organism. The main epigenetic marks in mammals are covalent modifications of the DNA (e.g. methylation) and post-translational modifications of histone proteins (histone code). Together, these modifications affect transcription and readout of the DNA, but they do not alter the DNA sequence. Epigenetic marks reinforce cell-fate decisions and establish barriers against reversion to preceding cellular states; however, at two distinct stages of mammalian development, after fertilization in the pre-implantation embryo and during primordial germ cell specification, epigenetic information is erased to a basal state in a process referred to as epigenetic reprogramming.
In mammals, covalent modification of DNA occurs through methylation on position 5 of cytosine and is essential for embryonic development [1]. After fertilization, the parental genomes undergo a global asymmetric demethylation and subsequent lineage-specific reacquisition of methylation. In addition, a second wave of DNA demethylation takes place during germ line formation, when primordial germ cells (PGCs) undergo a more drastic demethylation, which is essential for erasure of genomic imprints and formation of mature sperm and oocytes with a unique and differential epigenome. The identification of different enzymes that can actively remove methyl groups from DNA, together with advances in methylation analysis, has allowed a greater understanding of this epigenetic mark. Histones are a family of positively charged proteins that associate with DNA (negatively charged due to phosphate groups) to package chromatin into nucleosomes. This packaging allows DNA condensation to a smaller volume, but also restricts access of regulatory proteins to the DNA strand, affecting transcriptional readout. Nucleosomes are composed of 146 base pairs of DNA wrapped around an octamer of canonical histones -two molecules of each histone variant H2A, H2B, H3 and H4- plus the H1 linker. In this configuration, the four histone tails are exposed on the nucleosome surface, allowing for modification at the N-terminus by different enzymes that alter the chromatin structure to be more or less accessible. Post-translational modifications of histone proteins configure the histone code and are implicated in DNA replication, recombination and repair. Additionally, these modifications influence gene transcriptional outcome by modulating chromatin structure, allowing transcription and replication when it is less compact, while transcriptionally silent regions are held less accessibly [2]. The major histone tail modifications are acetylation and methylation, but other possibilities include ubiquitination, SUMOylation and phosphorylation of specific histone amino acids (lysine can be acetylated, methylated or ubiquitinated; arginine can be methylated, and serine and threonine can be phosphorylated). The position and number of modifications that are added to histone amino acids (for example: mono-, di-, or trimethylation of lysine at position 4 in histone H3) correlates with different biological effects --transcriptional repression or activation- and illustrates the complexity of histone tail modification [3, 4]. Specific enzymes, known as histone modifiers, are responsible for catalyzing the addition and removal of epigenetic marks on histone tails and they participate in the rapid switch between gene expression programs that takes place during early embryo development [3, 4]. Histone modifiers also contribute to PGC specification, which involves comprehensive epigenetic remodeling, including reversion of the epigenetic state to a pluripotent state, promotion of the germ-cell program, and acquisition of unipotency. Aside from these epigenetic controls, a hidden layer of internal signals, the so-called non-coding RNAs (ncRNAs), appear to control various levels of gene expression. The relevance of the non-coding portion of the genome has progressed from being considered as “junk DNA” to being involved in the regulation of all recognized epigenetic mechanisms. De novo DNA methylation of transposable elements in fetal male germ cells, X-inactivation in female embryos, and gene imprinting exemplify the involvement of ncRNAs during epigenetic reprogramming [5]. Throughout this manuscript, we review known epigenetic control mechanisms and their involvement during fertilization and early embryo development.
DNA methylation
DNA methylation is the best studied epigenetic mark and participates in the majority of epigenetic mechanisms, including genomic imprinting, transposon silencing, X-inactivation and gene repression. Moreover, DNA methylation is involved in diverse key cellular processes such as early embryogenesis [6], stem cell differentiation, regulation of neuronal development and cancer development [7].
With some exceptions, the methylated state is the default state for CpG dinucleotides in the mammalian genome and is associated classically with repression of transcription initiation at CpG islands (CpGi) in the promoters, although this rule is not valid for promoters with low CpG density. Moreover, DNA methylation in the gene body has been associated with active transcription [8]. Recent studies have shown that DNA methylation can also result in activation of transcription; this uncoupling is evident in oocytes, germ cells and pluripotent cells [9–11]. Proposed mechanisms by which DNA methylation represses gene transcription include the prevention of binding of specific transcriptional activators or the recruitment of methyl binding proteins with repressor activity [12]. DNA methylation occurs by addition of a methyl group to the carbon-5 position of the pyrimidine ring of cytosine, producing 5-methyl cytosine (5mC). DNA methylation is found genome-wide, and methylome data have shown high methylation at CpG-poor DNA regions compared with CpGi, which remain mostly unmethylated [13, 14]. Some exceptions exist, however, and specific CpGi in somatic cells could appear methylated. For example, methylation at the CpGi in the promoters of key developmental genes, such as germline, pluripotency and Hox genes, restricts pluripotency and differentiation [15]. Dedifferentiation of somatic cells into iPSCs involves demethylation at pluripotency and germline-specific gene promoters, and the reprogramming efficiency can be increased by DNA demethylation [16, 17].
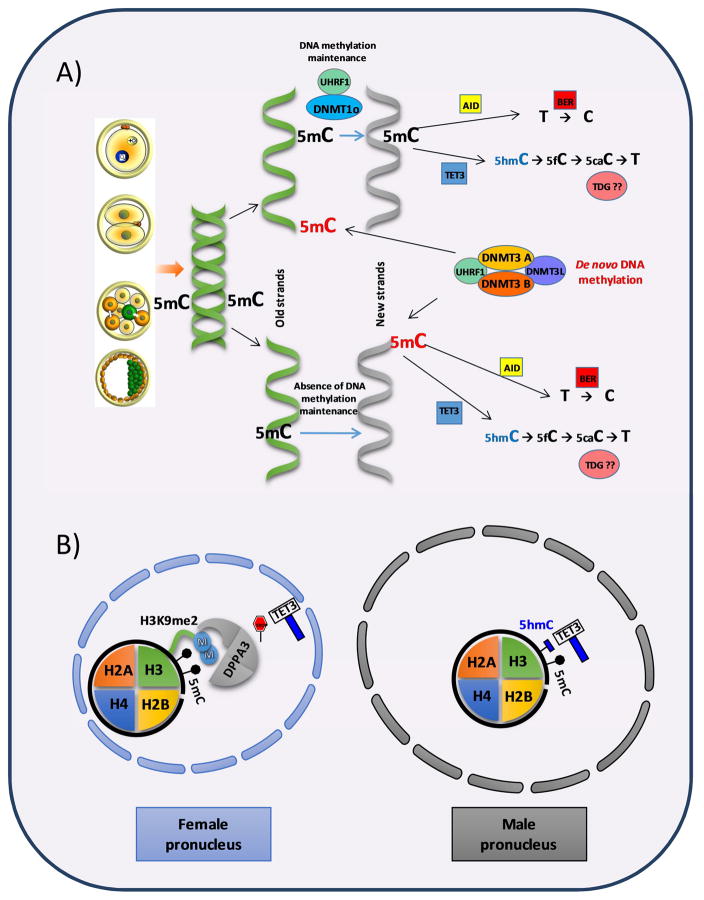
DNA methylation is relatively stable, and can be propagated through cell division by a system of DNA methylation maintenance coupled to DNA replication, which is responsible for “copying” the old DNA methylation pattern to the new DNA strand [18, 19]; however, DNA methylation marks undergo extensive reprogramming at two specific developmental stages: in the early embryo and in PGCs. Moreover, recent studies have reported DNA demethylation occurring in response to different stimuli; this process is also perturbed in different diseases, particularly in most types of cancer (reviewed in [20]). Methylation status is the result of the preservation of DNA methylation through cell divisions and specific de novo methylation: two actions executed by specific and differential enzymes (Figure 1.A). The methyltransferase DNMT1 and its crucial cofactor UHRF1 [18, 21] have been classically considered responsible for the maintenance of DNA methylation during cell divisions, whereas de novo DNA methylation (addition of methyl group at previously unmethylated cytosines) is mainly established by the methyltransferases DNMT3A and DNMT3B [1, 22]; however, an active role of DNMT1 for de novo methylation has also been documented [23]. In addition, an oocyte specific form of DNMT1 called DNMT1o, which has similar activity to DNMT1 [24], coexists with DNMT in oocytes and early embryos at different localizations: the nucleus and cytoplasm, respectively [25, 26]. A third member of the DNMT3 family, DNMT3-like (DNMT3L), which has no catalytic activity, functions as a regulator of DNMT3a and DNMT3b [27], and participates in de novo DNA methylation of imprinted control elements and retrotransposons after genome-wide epigenetic erasure during germ cell development [27, 28].
Figure 1. DNA methylation dynamics during chromatin replication in the early embryo.
A) During cell division in the early embryo two scenarios could happen: i) DNA methylation maintenance (top), where DNMTs recognize the hemimethylated strand and methylate it, ii) absence of DNA methylation maintenance (bottom), so new strand of DNA will be temporarily unmethylated and DNA double helix will result in hemimethylation. In both, de novo DNA methylation and demethylation by hydroximethylation (TET3) or deamination (AID) could take place. B) The activity of TET3 at the maternal PN is limited by the activity of DPPA3 interacting with H3K9me2 which is highly abundant in the maternal PN but not in the paternal PN.
DNMT1 is considered essential for life, as knockout results in embryonic lethality at day 8.5–9 [29] and deletion of its cofactor UHRF1 produces similar effect [30]. On the other hand, knockouts of DNMT3A or DNMT3B in mice both produce embryos that develop longer, but do not reach term, or die one month after birth, respectively [22]. Deletion of DNMT3L produces offspring which are infertile because of imprinting errors in the gametes [27].
Recently, different studies have contributed to identify potential pathways for the active removal of 5mC. Ten eleven translocation (TET) enzymes can quickly alter the methylation status of DNA [31] by catalyzing the oxidation of 5mC to 5-hydroxymethylcytosine (5hmC), which could represent an intermediate stage in the cytosine demethylation reaction. Furthermore, TET proteins can also catalyze the oxidation of 5hmC to 5-formylcytosine (5fC) and 5-carboxylcytosine (5caC) [32, 33]. The three TET proteins (TET1, TET2 and TET3) found in vertebrates show broad expression patterns in various tissues, with TET3 being the only form expressed in oocytes and preimplatation embryos [34, 35]. TET3 homozygous mutant mice die at birth [35], while TET1 and TET2 knockouts are compatible with life. Nevertheless, TET1 and TET2 knockouts result in reduced body and litter sizes, and deficiencies that promote chronic myelomonocytic leukemia, respectively [36, 37].
One possible scenario for the participation of TET proteins in the active erasure of DNA methylation is that the oxidation derivatives lead to passive demethylation because they are not properly recognized by the methylation maintenance machinery, as illustrated by the fact that DNMT1 is not active on hemi-hydroxymethylated DNA [38]. Another possibility is that 5hmC, 5caC or 5fC trigger erasure by DNA glycosylases such as thymine DNA glycosylase (TDG), followed by base excision repair (BER) (Figure 1). Other putative players that participate in demethylation are deaminases of the AID/APOBEC family, which could trigger demethylation by BER. These deaminases could directly deaminate 5mC, creating T:G mismatches that could be repaired by TDG followed by BER [39]. Although the role of these enzymes in vivo remains unclear, AID has been shown to be necessary for promoter demethylation and induction of OCT4 and NANOG gene expression during reprogramming toward pluripotency, and it participates actively in DNA demethylation during nuclear reprogramming toward pluripotency in human somatic cells [17]; however, there are reasonable doubts when considering TDG as an important factor in active erasure of DNA methylation, since oocyte-specific TDG conditional knockout resulted in normal offspring and no changes in zygotic 5-hmC levels. Indeed, conversion of 5-mC to 5-hmC was normal in the TDG knockout zygotes without accumulation of 5-fC or 5-caC, which would have been expected to be altered if TDG were involved in the removal of these marks [40].
Recent improvements in the techniques to analyze the methylome coupled to high-throughput DNA Sequencing have provided potent tools to decipher the methylome of specific cells in a wide range of developmental stages, while the ability to analyze all potential methylated variants still does not exist. Currently, three major approaches are used to study genome-wide DNA methylation: chemical modification (bisulfite, BS), affinity enrichment, and differential enzymatic digestion. Different variables, such as reproducibility, cost, input DNA amount or aim/nature of the studies, need to be considered to select the most suitable technique for DNA methylation studies. For example, the BS sequencing generates a quantitative measure of modified cytosine across the whole genome at single-base resolution, and can be performed on relatively low input DNA quantities. In addition, BS sequencing is also sensitive to relatively small changes in methylation and is thus considered the “gold standard”; however, this method does not distinguish 5mC from 5hmC and is therefore not appropriate for studies investigating dynamic DNA demethylation. An alternative, affinity-based, approach is genome-wide methylated DNA immunoprecipitation coupled with sequencing (meDIP-seq), which can distinguish 5mC and 5hmC with specific antibodies.
DNA methylation in early embryo development
After fertilization, parental genomes remain physically separated until syngamy takes place, and during this period (aprox. 24h) they show asymmetric epigenetic marks. At this time parental genomes undergo almost global asymmetrical demethylation which culminates with the establishment of two differentially methylated cell populations at the early blastocyst stage - inner cell mass (ICM) and trophoectoderm (TE). Then, cells quickly reacquire methylation, and only primordial germ cells will go through a second, even more dramatic, round of DNA demethylation.
Sperm and oocyte DNA methylation patterns are differentially established and follow distinct pathways during their epigenetic remodeling after fertilization. Sperm show higher (90%) genome-wide DNA methylation of non-CpGi than oocytes (40%). In addition, there are differentially methylated CpGs between male and female gametes, with oocytes showing a higher ratio of hypermethylated CpGs (10%) than somatic cells (3%) [10]. After gamete fusion, the spermatic genome undergoes dramatic remodeling, starting with removal of protamines and their replacement by acetylated histones [41, 42]. Then, paternal DNA undergoes a global and active demethylation before the first cell division, except for paternally imprinted genes, IAP retrotransposons and heterochromatin [6, 10, 43, 44]. Simultaneously to the loss of 5mC in the paternal pronucleus (PN), there is a strong increase in 5hMC, and moderate in 5fC and 5caC [35, 45–47]. Among the TET-protein family, TET3 is highly expressed in the zygote and binds specifically to paternal genome. Thus, TET-3 is considered responsible for these 5mC modifications, indicating that 5mC oxidation plays a key role during differential demethylation of the paternal genome [35, 47]. Classically, passive demethylation has been considered the mechanism responsible for maternal genome methylation erasure, by exclusion of the DNMT1 from the nucleus during preimplantation development [48, 49]; however, recent studies in mice using genome-wide methylation profiling and SNPs to distinguish between maternal and paternal genomes show active demethylation in the maternal genome, with presence of 5hmC in the female PN [40, 50]. The presence of this oxidative derivate in the female genome has been also reported in cows, pigs and rabbits [47, 51]. Indeed, two studies have also reported reduction of methylation in both PN using isolated male and female PN, and reduced representation bisulfate sequencing (RBBS). Using aphidicolin to block DNA replication, it was shown that DNA demethylation in the PN is mainly a consequence of DNA replication, and only partially dependent on TET3 activity, with a more major role in paternal than in maternal PN [40, 52]. This is also supported by the observed increase in hemimethylated DNA molecules after the first round of DNA replication in the zygote [53]. In humans, a 50% decrease in DNA methylation levels at the 2-cell stage compared to gametes has been reported, which suggests replication-dependent dilution of DNA methylation; however, 5hmC was also detected, mainly in male PN [54–56]. In human blastocysts, sperm-specific methylation are mostly erased while maternal marks persist [52, 55]. While the role of 5hmC is unclear, a recent study showed that round spermatid sperm injection (ROSI)-derived zygotes show higher 5mC and lower 5hmC levels than ICSI-derived zygotes at the PN3 stage, which could be related to the lower developmental efficiency of ROSI-derived embryos [57]. In conclusion, recent results show that maternal and paternal PN use the three different routes, here ordered by importance, to remove 5mC from the genome: replication-dependent, 5hmC replication-dependent, and active removal. Moreover, asymmetric removal between PN is present.
Although the mechanism that controls differential demethylation in male and female PN is not fully understood, some factors involved in this process have been identified. DPPA3 (STELLA/PGC7) protects maternal PN 5mC from Tet3-mediated conversion to 5hmC through its binding to dimehylated lysine 9 on histone H3 (H3K9me2)-containing chromatin (Figure 1.B) although this histone modification (H3K9me2) also participates in the protection of imprinted loci in mature sperm that avoid demethylation [58–60]. TET2 and TET3 activity is inhibited by human DPPA3, which localizes to specific loci and is controlled by H3K9me2 marks and DNA sequence, and could specifically protect the female PN from DNA demethylation as well as specific regions such as imprinted genes [61]. In mice, DPPA3-null embryos show impaired DNA replication, ectopic micronuclei, abnormal chromosome segregation of maternal chromosomes, and aberrant H2AX phosphorylation, which inhibits DNA replication [62]. These results demonstrate that DPPA3 protects maternal DNA from aberrant epigenetic modifications to ensure early embryogenesis.
As a result of the demethylation process, differentially methylated CpGi in sperm and oocyte are reset at the pre-implantation stage, potentially preventing epigenetic marks acquired during life from being passed on to the progeny. Nonetheless, a significant number persist, including imprinted genes and retrotransposons [10], which could constitute a basis for epigenetic inheritance.
Histone acetylation
Histone acetylation consists of the reversible addition of acetyl groups to lysine in histones H3 and H4. Acetylation neutralizes the positive charge on the histones, thereby decreasing their interaction with the negatively charged DNA backbone and allowing an open chromatin structure that favors the accessibility of transcription factors and proteins to the DNA sequence [63]. In addition, acetylated lysines can be recognized by bromodomains, creating binding points to recruit regulatory proteins [64]. Histone acetylation has been most often analyzed at gene promoters; however, low levels of acetylation are also found throughout transcribed genes, although little is known about the function of global acetylation.
The level of histone acetylation is regulated by two opposing actions: i) histone acetylation by histone acetyltransferases (HATs) and ii) removal of acetyl groups (deacetylation) by histone deacetylases (HDACs) [65]. A large number of transcription-related proteins are known to have HAT activity, which could be classified in four families: GNAT, MYST, p300/CBP and HAT1. HATs are highly diverse and are often made up of multisubunit complexes. In this respect, the functions of the catalytic subunit depend largely on the context of the other subunits in the complex [66]. Regarding HDAC, thus far eighteen mammalian HDACs have been identified with the capacity to remove histone acetyl groups, and they are grouped into four classes according to phylogenetic analysis [67]. The class I HDAC, HDAC1, has been found to play an important role during mouse embryo development [68]. Histone deacetylation leads to a tighter wrapping of the DNA around the histone core, which results in reduced gene transcription. Aberrantly high HDAC activity leads to transcriptional silencing of a subset of genes, including those involved in tumor suppression (p53, RUNX3) and apoptosis or control of proliferation (p21, p63, p16). Hence, some cancer therapies use HDAC inhibitors as cytostatic agents to induce cell cycle arrest, differentiation and/or apoptosis [69].
Histone methylation
In contrast to acetylation, histone methylation can correlate with either silencing (H3K9me3 and H3K27me3) or activation (H3K4me3, H3K36me3) of transcription, depending on the position and number of methyl groups that are added to histone tail amino acids (7). Histone methylation consists of the addition of one or two methyl groups to arginine, while lysine on histones H3 and H4 can be mono-, di- or tri-methylated. Histone lysine methylation does not neutralize charges, but instead functions to recruit silencing or regulatory proteins to methylated histones and plays a key role during lineage specification and cell differentiation.
Trimethylation of histone 3 lysine 27 (H3K27me3) is a typical repressive mark of many key developmental genes in ES cells, and is considered a temporary signal with an essential role in stem cell maintenance [70, 71] and regulation of pluripotency genes [72]. Di- and trimethylation of H3K27 is mediated by polycomb repressive complex 2 (PRC2), which has three essential components: EZH2, EED and SUZ12 [73], while demethylation of this specific residue is regulated by UTX, JMJD3 and KIA1718 [74–76].
H3K9me3 is an additional repressive mark, which functions differently with respect to H3K27me3. H3K9me3 is considered to be a permanent repression signal and is preferentially detected in gene-poor regions (G-banding regions) and in retrotransposons, whereas H3K27me3 marks are temporary signals and are associated with CpG-rich sequences (R-banding regions) [77, 78]. H3K9me3 is catalyzed by several methyltransferases: i) SUV39H1 and SUV39H2 are responsible for marking H3K9me3 at constitutive heterochromatin [79], ii) EHMT1 (GLP), and EHMT2 (G9A) target H3K9 in euchromatic regions [80], and iii) SETDB1 is considered a key regulator for maintaining the pluripotency and self-renewal properties of ES cells [81, 82].
For many years, histone methylation was considered an irreversible process until lysine-specific demethylase (LSD1), with demethylation activity on mono- and dimethylated H3K4 and H3K9, was discovered in 2004 [83]. Later, JumonjiC domain-containing proteins JMJD1A, 2A, 2B, 2C, 2D and KIAA1718, PHF2, PHF8 with demethylase activity at H3K9 residues were reported (for references, see [84]).
H3K4me3 is one of the most studied histone tail modifications, which is catalyzed by mammalian homologues of the trithorax group (trxG) of methyl transferases and activates transcription through the recruitment of nucleosome remodeling complexes and histone-modifying enzymes [85–87]. H3K4me3 plays a key role in mammalian gene expression and is critical for animal embryonic development. H3K4me3 is present genome-wide and occupies almost 75% of all gene promoters in human ES (hES) cells [72]. In mammals, over 10 different H3K4 histone methyl transferases –HMTs- have been discovered (MLL1—5, SET1A/B, SET7/9, SET and ASH1L, SETMAR, PRDM9); among these, six are close homologs of yeast genes (see [88] and references therein). Demethylation of H3K4me3 is carried out by LSD1 and LSD2, which remove methyl groups from mono- and dimethylated H3K4 [83, 89], and the JARID1 family of histone demethylases (JARID1A—D), which erase H3K4me3 and H3K4me2 [88, 90]. Although H3K4me3 is associated with actively transcribed genes, it is also localized in the promoters of numerous silenced genes in ES cells. H3K4me3 frequently co-localizes with the H3K27me3 repressive mark in the promoters of critical differentiation genes (Hox gene clusters) that are transcriptionally inactive in ES cells [72, 91, 92]. These structures, containing opposite marks in chromatin regions, so-called “bivalent domains”, function to maintain differentiation-specific genes in a repressive state in pluripotent stem cells, indicating that repressive H3K27me3 modifications generally overrule the activating effect of H3K4me3 [92]. The role of bivalent domains is to provide a flexible mechanism for gene activation or repression during early development, when transcriptional circuits are most dynamic. H3K4me3 also protects genes from permanent silencing by countering transcriptional repressors or blocking DNA methylation [93].
Similar to lysine methylation, arginine methylation can be either an active or repressive mark for transcription. Several arginine residues on histones H2A, H3 and H4 can be monomethylated or dimethylated, asymmetrically (H3R2me2a, H3R26me2a, H3R17me2a, H4R3me2a and H2AR3me2a), or symmetrically (H4R3me2, H2AR3me2 and H3R8me2) by protein arginine methyltransferases (PRMT). In this respect, type I PRMTs (PRMT1, 2, 3, 4, 6, and 8) are responsible for asymmetric dimethylation, while type II PRMTs (PRMT5 and 7) catalyze the formation of symmetric dimethylation [94].
Different authors have proposed that there is a wide-spread cross-talk between arginine and lysine methylation, termed the “arginine/lysine-methyl/methyl switch”, and between arginine methylation and DNA methylation; however, the consequences of histone tail arginine methylation remain unclear. Dimethylation of H2AR3 and H4R3 has been classically associated with gene repression in mammals [95, 96]. Nevertheless, a recent study [97] revealed the unexpected result that no general correlation exists between gene expression and H4R3me2 enrichment. This study demonstrated a highly similar pattern of H4R3me2 in gene promoters of differentiated cells (embryonic fibroblasts) and ES cells, including those actively transcribed. Curiously, H4R3me2 marks are present at imprinting control regions (ICRs) and at intracisternal A particles (IAPs) in mouse embryos [96, 98]. H4R3me2 is mono-allelic at ICRs, and it marks the same parental allele as H3K9me3, H4K20me3 and DNA methylation [97].
Although histone methylation is very stable, there is no consensus about whether the modification can be enzymatically reversed, although a member of Jumonji family protein with arginine demethylase activity has been identified (JMJD6) [99].
Histone marks in embryo development
Immunostaining studies using antibodies specific for different histone modifications have revealed an asymmetric distribution of histone marks between the parental genomes (review by [42, 100, 101]). The functional relevance of these differences is not fully understood. Characterizing the global state of different epigenetic marks has progressed our understanding of epigenetic regulation during embryo development. In spite of the great value of these results, they only represent a general overview of chromatin status, and detailed analysis of epigenetic marks at specific genomic regions are in progress to further elucidate details in their regulation during early mammalian embryogenesis.
In general, the maternal genome shows a pattern of histone marks similar to somatic cells, with high abundance of H3K4me3 [102], H3K9me2/3 [103], H3K27me2/3 [104], H3K36me3 [105], H3K64me3 [106] and H4K20me3 [107]. On the other hand, the paternal chromatin presents these marks at low abundance (H3K4me1/3, H3K9me2, H3K27me2) or undetectable levels (trimethylation marks and constitutive heterochromatin features) (Figure 2).
Figure 2.
Dynamics of histone modification remodeling in the zygote.
In contrast to oocytes, DNA in sperm is mainly packaged by protamines, although between 2–15% of chromatin retains histones, which are non-randomly distributed, occupying developmentally important genes [108, 109]. Just after fertilization, the spermatic chromatin is decondensed and protamines are exchanged for newly synthesized histones derived from the ooplasm [44]. In addition, a small fraction of histones directly inherited from the sperm contain a high ratio of hyperacetylation at H4K8 and K12 [44, 108, 109], and could transmit epigenetic information and drive the incorporation of newly synthesized histones [110]. Until this time, oocyte chromatin remains relatively stable, and the female PN contains a higher amount of H3K9 methylation [58], which participates in the protection of 5mC from Tet3-mediated conversion to 5hmC [59]. Also, H3K4me3 and H3K27me3 are higher in the oocyte compared with sperm chromatin, which acquires methylation at these marks progressively during development [58]. Monomethylation is functionally uncoupled from dimethylation and trimethylation, and generally is established by different enzymes, so the observed delay in the appearance of trimethylated marks makes sense as a consequence of the new histone incorporation in the parental genome. For example, in mouse there is a preferential incorporation of the histone variant H3.3 to the male PN, which is associated with active transcription, and which is later trimethylated at K27 [111]. This allows a first phase of active transcription from the paternal chromatin, follow by a transcriptionally silent stage by acquisition of H3K27me3 [112].
Another relevant feature of histone post-translational modification during embryo development is the co-existence of active (H3K4m3) and repressive histone marks (H3K27me3/H3K9me3) at specific chromatin regions (bivalent domains), which enables the fine-tuning and precise control of gene expression. This was first reported in ES cells, and later demonstrated in pluripotent epiblast cells of early post-implantation embryos in mice [113, 114].
Histone modifications have been also proposed as an important epigenetic component in the segregation between ICM and TE, with a higher levels of H3K27me3 and lower levels of histone H2A and/or H4 phosphorylation in ICM; however, the relevance/role of this asymmetry is not fully understood, because these epigenetic differences could not be directly correlated with gene expression [42].
H3R26me3 represents a clear example of how epigenetic asymmetry could drive cell fate. In mouse 4-cell embryos, one of the four blastomeres shows lower levels of methylated H3H3R26; while high levels of this mark are observed in the remaining cells which are induced by CARM1. Low levels of H3R26me3 result in upregulation of Nanog and Sox2, promoting ICM fate in those cells [115].
In mouse embryos, an asymmetric distribution of H3K27me3 and H3K9me3 in ICM and TE has been also related with the lineage specification to embryonic and extraembryonic tissues [116]. H3K9me3 represses typical ICM genes in the TE [117] and this mark is also important to suppress expression of TE-specific factors during ICM formation [81, 82]. Different reports in pigs [118, 119], and in cows have indicated modifications in the H3K27me3 levels during early embryo development [120] and the role of JMJD3, a specific H3K27me3 demethylase, during blastocyst development [121]. These results show that H3K27me3 plays an important role during reprogramming after fertilization, allowing embryonic genome activation.
Imprinting
Genomic imprinting is an epigenetic regulatory mechanism present in mammals, whereby a small proportion of genes (imprinted genes) are differentially marked according to their parental (maternal or paternal) origin. As a result of this phenomenon, either the paternal or maternal allele of the gene is expressed. This mechanism entails that both maternal and paternal contributions are required for normal development (for review see [122]). Differential DNA methylation between the two parental chromosomes is the main mark present in imprinted genes; however, histone marks, long non-coding RNAs and other factors are also involved in imprinting [96, 123].
DNA methylation marks at imprinted loci survive the extensive DNA methylation erasure that takes place after fertilization in the zygote. Several maternal factors are involved in the maintenance of these DNA methylation marks [124], including DNMT1, DNMT3A, DNMT3B, DPPA3, ZFP57 and TRIM28. DPPA3 plays a role in imprinting maintenance by protecting the DNA methylation state at imprinted loci. Specifically, DPPA3 protects 5mC against Tet3 activity (conversion to 5hmC) in maternal chromatin which contains H3K9me2 [59, 60]. In addition, imprinting gene protection goes further: methylation marks at in these loci are propagated in the embryo during cleavage division, mainly by the oocyte-specific form of DNMT1 (DNMT1o) and some residual activity of the somatic form of DNMT1 [48, 125]. In spite of the fact that DNMT1 is mainly excluded from the nucleus (allowing passive demethylation of maternal genome) TRIM28/ZP57 participate in a noncanonical DNA methylation maintenance by mediating targeting of DNMT1 to imprinted gene regions [126]. Absence of TRIM28 causes highly asynchronous aberrant demethylation, creating complex chimeras. Pronuclear transfer into healthy enucleated zygotes can ameliorate epimutations caused by the absence of maternal TRIM28, which demonstrates the importance of inherited factors from oocyte and their role in early epigenetic reprogramming [127]. ZFP57 mutations in mouse produce maternal and zygotic lethality, and result in aberrant DNA methylation and expression patterns of imprinted genes [128]. Although protection of imprinted genes by ZFP57 is conserved between mice and humans [129], ZPF57 is not expressed in human oocytes, which suggests that ZP57 might not be essential in imprinting maintenance in humans [55].
Histone repressive marks such as H3K27me3, H3K9me3 and H4K20me3 are also associated with imprinting repression but they do not substitute for DNA methylation in all inactive allele when DMR is not present [130]. Most imprinted genes are grouped by clusters, which also contain an imprinting control region (ICR) and a noncoding RNA that mediates chromatin repression [131].
Concluding remarks
Until recently, our understanding of biological processes was largely sustained by DNA sequence-based knowledge. It is now recognized that epigenetic marks play a major role in the control of gene expression and participate in development, cell differentiation and human pathology. Epigenetic marks are complex, interrelated, and highly orchestrated. For didactic reasons, we discussed epigenetic mechanisms independently, but a great deal of interrelationship exists. Epigenetic alterations are increasingly recognized as causes of human disease. The International Human Epigenetic Consortium (IHEC), which studies a wide spectrum of human cell types, does not include early embryo and PGCs in their list of selected cells, likely because of ethical limitations and technical constraints. Methodological advances, such as the recently reported single cell DNA-methylation analysis [127, 132], are needed to overcome the inaccessibility of early-stage embryos and to analyze the impact of reproductive technologies at the epigenetic level. In this respect, ES cells and iPS cells represent an attractive alternative to extend our knowledge about epigenetic marks during this critical period, but they also come with limitations.
The process of epigenetic remodeling during preimplantation development is complex and dynamic, including changes in DNA methylation and histone modifications that occur both on a global scale but also differentially at specific loci. Uncovering the bases of these mechanisms will improve our understanding of early developmental processes, promising great potential for improving animal fertility and diagnosing and treating infertility problems. Also, as epigenetic mechanisms can be affected by environmental factors, understanding the role that assisted reproductive technologies and in utero exposure to different factors have on altering the embryonic epigenome, and potentially the individual’s epigenome, will continue to be an important area of research, especially as we understand how the epigenome influences short-and long-term health. Also, better a understanding the mechanisms and role of epigenetic states during early embryogenesis will likely lead to potential interventions aimed at improving animal health and productivity. Finally, the possibility of transgenerational epigenetic inheritance will depend in part on the epigenetic marks evading the reprogramming that ensues during preimplantation development.
Acknowledgments
Work in the Ross laboratory related to this manuscript is supported by NIH/NICHD RO1 HD070044 and USDA/NIFA Hatch projects W-3171 and W-2112. We thank Michelle Halstead for her careful and critical reading of our manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sebastian Canovas, Email: sebastian.canovas@juntadeandalucia.es.
Pablo Juan Ross, Email: pross@ucdavis.edu.
References
- 1.Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69:915–26. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 2.Cantone I, Fisher AG. Epigenetic programming and reprogramming during development. Nat Struct Mol Biol. 2013;20:282–9. doi: 10.1038/nsmb.2489. [DOI] [PubMed] [Google Scholar]
- 3.Peterson CL, Laniel MA. Histones and histone modifications. Curr Biol. 2004;14:546–51. doi: 10.1016/j.cub.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Kouzarides T. SnapShot: Histone-modifying enzymes. Cell. 2007;131:822. doi: 10.1016/j.cell.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Qureshi IA, Mehler MF. Emerging roles of non-coding RNAs in brain evolution, development, plasticity and disease. Nat Rev Neurosci. 2012;13:528–41. doi: 10.1038/nrn3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayer W, Niveleau A, Walter J, Fundele R, Haaf T. Demethylation of the zygotic paternal genome. Nature. 2000;403:501–2. doi: 10.1038/35000656. [DOI] [PubMed] [Google Scholar]
- 7.Dulac C. Brain function and chromatin plasticity. Nature. 2010;465:728–35. doi: 10.1038/nature09231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hellman A, Chess A. Gene body-specific methylation on the active X chromosome. Science (New York, NY) 2007;315:1141–3. doi: 10.1126/science.1136352. [DOI] [PubMed] [Google Scholar]
- 9.Spruijt CG, Vermeulen M. DNA methylation: old dog, new tricks? Nat Struct Mol Biol United States. 2014:949–54. doi: 10.1038/nsmb.2910. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi H, Sakurai T, Imai M, Takahashi N, Fukuda A, Yayoi O, et al. Contribution of intragenic DNA methylation in mouse gametic DNA methylomes to establish oocyte-specific heritable marks. PLoS genetics. 2012;8:e1002440. doi: 10.1371/journal.pgen.1002440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schroeder DL, Jayashankar K, Douglas KC, hirkill TL, York D, Dikinson PJ, et al. Early developmental and evolutionary origins of gene body DNA methylation patterns in mammalian placentas. PLoS genetics. 2015 doi: 10.1371/journal.pgen.1005442. IN PRESS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones PL, Veenstra GJ, Wade PA, Vermaak D, Kass SU, Landsberger N, et al. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet. 1998;19:187–91. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- 13.Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J, et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462:315–22. doi: 10.1038/nature08514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meissner A, Mikkelsen TS, Gu H, Wernig M, Hanna J, Sivachenko A, et al. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature. 2008;454:766–70. doi: 10.1038/nature07107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borgel J, Guibert S, Li Y, Chiba H, Schubeler D, Sasaki H, et al. Targets and dynamics of promoter DNA methylation during early mouse development. Nat Genet. 2010;42:1093–100. doi: 10.1038/ng.708. [DOI] [PubMed] [Google Scholar]
- 16.Mikkelsen TS. Dissecting direct reprogramming through integrative genomic analysis. Nature. 2008;454:49–55. doi: 10.1038/nature07056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhutani N, Brady JJ, Damian M, Sacco A, Corbel SY, Blau HM. Reprogramming towards pluripotency requires AID-dependent DNA demethylation. Nature England. 2010:1042–7. doi: 10.1038/nature08752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bostick M, Kim JK, Esteve PO, Clark A, Pradhan S, Jacobsen SE. UHRF1 plays a role in maintaining DNA methylation in mammalian cells. Science (New York, NY) 2007;317:1760–4. doi: 10.1126/science.1147939. [DOI] [PubMed] [Google Scholar]
- 19.Song J, Teplova M, Ishibe-Murakami S, Patel DJ. Structure-based mechanistic insights into DNMT1-mediated maintenance DNA methylation. Science. 2012;335:709–12. doi: 10.1126/science.1214453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Auclair G, Weber M. Mechanisms of DNA methylation and demethylation in mammals. Biochimie. 2012;94:2202–11. doi: 10.1016/j.biochi.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 21.Arita K, Ariyoshi M, Tochio H, Nakamura Y, Shirakawa M. Recognition of hemi-methylated DNA by the SRA protein UHRF1 by a base-flipping mechanism. Nature England. 2008:818–21. doi: 10.1038/nature07249. [DOI] [PubMed] [Google Scholar]
- 22.Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–57. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 23.Athanasiadou R, de Sousa D, Myant K, Merusi C, Stancheva I, Bird A. Targeting of de novo DNA methylation throughout the Oct-4 gene regulatory region in differentiating embryonic stem cells. PloS one. 2010;5:e9937. doi: 10.1371/journal.pone.0009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cirio MC, Ratnam S, Ding F, Reinhart B, Navara C, Chaillet JR. Preimplantation expression of the somatic form of Dnmt1 suggests a role in the inheritance of genomic imprints. BMC developmental biology. 2008;8:9. doi: 10.1186/1471-213X-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mertineit C, Yoder JA, Taketo T, Laird DW, Trasler JM, Bestor TH. Sex-specific exons control DNA methyltransferase in mammalian germ cells. Development. 1998;125:889–97. doi: 10.1242/dev.125.5.889. [DOI] [PubMed] [Google Scholar]
- 26.Hayward BE, De Vos M, Judson H, Hodge D, Huntriss J, Picton HM, et al. Lack of involvement of known DNA methyltransferases in familial hydatidiform mole implies the involvement of other factors in establishment of imprinting in the human female germline. BMC Genet. 2003;4:2. doi: 10.1186/1471-2156-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bourc’his D, Xu GL, Lin CS, Bollman B, Bestor TH. Dnmt3L and the establishment of maternal genomic imprints. Science (New York, NY) 2001;294:2536–9. doi: 10.1126/science.1065848. [DOI] [PubMed] [Google Scholar]
- 28.Aravin AA, Sachidanandam R, Bourc’his D, Schaefer C, Pezic D, Toth KF, et al. A piRNA pathway primed by individual transposons is linked to de novo DNA methylation in mice. Mol Cell. 2008;31:785–99. doi: 10.1016/j.molcel.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lei H, Oh SP, Okano M, Juttermann R, Goss KA, Jaenisch R, et al. De novo DNA cytosine methyltransferase activities in mouse embryonic stem cells. Development (Cambridge, England) 1996;122:3195–205. doi: 10.1242/dev.122.10.3195. [DOI] [PubMed] [Google Scholar]
- 30.Sharif J, Muto M, Takebayashi S, Suetake I, Iwamatsu A, Endo TA, et al. The SRA protein Np95 mediates epigenetic inheritance by recruiting Dnmt1 to methylated DNA. Nature. 2007;450:908–12. doi: 10.1038/nature06397. [DOI] [PubMed] [Google Scholar]
- 31.Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–5. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ito S, D’Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466:1129–33. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koh KP, Yabuuchi A, Rao S, Huang Y, Cunniff K, Nardone J, et al. Tet1 and Tet2 regulate 5-hydroxymethylcytosine production and cell lineage specification in mouse embryonic stem cells. Cell Stem Cell. 2011;8:200–13. doi: 10.1016/j.stem.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Branco MR, Ficz G, Reik W. Uncovering the role of 5-hydroxymethylcytosine in the epigenome. Nat Rev Genet England. 2012:7–13. doi: 10.1038/nrg3080. [DOI] [PubMed] [Google Scholar]
- 35.Gu TP, Guo F, Yang H, Wu HP, Xu GF, Liu W, et al. The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes. Nature. 2011;477:606–10. doi: 10.1038/nature10443. [DOI] [PubMed] [Google Scholar]
- 36.Dawlaty MM, Ganz K, Powell BE, Hu YC, Markoulaki S, Cheng AW, et al. Tet1 is dispensable for maintaining pluripotency and its loss is compatible with embryonic and postnatal development. Cell stem cell. 2011;9:166–75. doi: 10.1016/j.stem.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moran-Crusio K, Reavie L, Shih A, Abdel-Wahab O, Ndiaye-Lobry D, Lobry C, et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell. 2011;20:11–24. doi: 10.1016/j.ccr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valinluck V, Sowers LC. Endogenous cytosine damage products alter the site selectivity of human DNA maintenance methyltransferase DNMT1. Cancer Res. 2007;67:946–50. doi: 10.1158/0008-5472.CAN-06-3123. [DOI] [PubMed] [Google Scholar]
- 39.Cortellino S, Xu J, Sannai M, Moore R, Caretti E, Cigliano A, et al. Thymine DNA glycosylase is essential for active DNA demethylation by linked deamination-base excision repair. Cell. 2011;146:67–79. doi: 10.1016/j.cell.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo F, Li X, Liang D, Li T, Zhu P, Guo H, et al. Active and passive demethylation of male and female pronuclear DNA in the mammalian zygote. Cell stem cell. 2014;15:447–58. doi: 10.1016/j.stem.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science (New York, NY) 2001;293:1089–93. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- 42.Burton A, Torres-Padilla ME. Chromatin dynamics in the regulation of cell fate allocation during early embryogenesis. Nat Rev Mol Cell Biol. 2014;15:723–34. doi: 10.1038/nrm3885. [DOI] [PubMed] [Google Scholar]
- 43.Oswald J, Engemann S, Lane N, Mayer W, Olek A, Fundele R, et al. Active demethylation of the paternal genome in the mouse zygote. Curr Biol. 2000;10:475–8. doi: 10.1016/s0960-9822(00)00448-6. [DOI] [PubMed] [Google Scholar]
- 44.Santos F, Hendrich B, Reik W, Dean W. Dynamic reprogramming of DNA methylation in the early mouse embryo. Dev Biol. 2002;241:172–82. doi: 10.1006/dbio.2001.0501. [DOI] [PubMed] [Google Scholar]
- 45.Inoue A, Zhang Y. Replication-dependent loss of 5-hydroxymethylcytosine in mouse preimplantation embryos. Science (New York, NY) 2011;334:194. doi: 10.1126/science.1212483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iqbal K, Jin SG, Pfeifer GP, Szabo PE. Reprogramming of the paternal genome upon fertilization involves genome-wide oxidation of 5-methylcytosine. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3642–7. doi: 10.1073/pnas.1014033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wossidlo M, Nakamura T, Lepikhov K, Marques CJ, Zakhartchenko V, Boiani M, et al. 5-Hydroxymethylcytosine in the mammalian zygote is linked with epigenetic reprogramming. Nat Commun. 2011;2:241. doi: 10.1038/ncomms1240. [DOI] [PubMed] [Google Scholar]
- 48.Howell CY, Bestor TH, Ding F, Latham KE, Mertineit C, Trasler JM, et al. Genomic imprinting disrupted by a maternal effect mutation in the Dnmt1 gene. Cell. 2001;104:829–38. doi: 10.1016/s0092-8674(01)00280-x. [DOI] [PubMed] [Google Scholar]
- 49.Branco MR, Oda M, Reik W. Safeguarding parental identity: Dnmt1 maintains imprints during epigenetic reprogramming in early embryogenesis. Genes Dev. 2008;22:1567–71. doi: 10.1101/gad.1690508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang L, Zhang J, Duan J, Gao X, Zhu W, Lu X, et al. Programming and inheritance of parental DNA methylomes in mammals. Cell. 2014;157:979–91. doi: 10.1016/j.cell.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bakhtari A, Ross PJ. DPPA3 prevents cytosine hydroxymethylation of the maternal pronucleus and is required for normal development in bovine embryos. Epigenetics. 2014;9:1271–9. doi: 10.4161/epi.32087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen L, Inoue A, He J, Liu Y, Lu F, Zhang Y. Tet3 and DNA replication mediate demethylation of both the maternal and paternal genomes in mouse zygotes. Cell stem cell. 2014;15:459–70. doi: 10.1016/j.stem.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arand J, Wossidlo M, Lepikhov K, Peat JR, Reik W, Walter J. Selective impairment of methylation maintenance is the major cause of DNA methylation reprogramming in the early embryo. Epigenetics & chromatin. 2015;8:1. doi: 10.1186/1756-8935-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo H, Zhu P, Yan L, Li R, Hu B, Lian Y, et al. The DNA methylation landscape of human early embryos. Nature. 2014;511:606–10. doi: 10.1038/nature13544. [DOI] [PubMed] [Google Scholar]
- 55.Okae H, Chiba H, Hiura H, Hamada H, Sato A, Utsunomiya T, et al. Genome-wide analysis of DNA methylation dynamics during early human development. PLoS genetics. 2014;10:e1004868. doi: 10.1371/journal.pgen.1004868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith ZD, Chan MM, Humm KC, Karnik R, Mekhoubad S, Regev A, et al. DNA methylation dynamics of the human preimplantation embryo. Nature. 2014;511:611–5. doi: 10.1038/nature13581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kurotaki YK, Hatanaka Y, Kamimura S, Oikawa M, Inoue H, Ogonuki N, et al. Impaired active DNA demethylation in zygotes generated by round spermatid injection. Hum Reprod. 2015;30:1178–87. doi: 10.1093/humrep/dev039. [DOI] [PubMed] [Google Scholar]
- 58.Santos F, Peters AH, Otte AP, Reik W, Dean W. Dynamic chromatin modifications characterise the first cell cycle in mouse embryos. Dev Biol. 2005;280:225–36. doi: 10.1016/j.ydbio.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 59.Nakamura T, Arai Y, Umehara H, Masuhara M, Kimura T, Taniguchi H, et al. PGC7/Stella protects against DNA demethylation in early embryogenesis. Nat Cell Biol. 2007;9:64–71. doi: 10.1038/ncb1519. [DOI] [PubMed] [Google Scholar]
- 60.Nakamura T, Liu YJ, Nakashima H, Umehara H, Inoue K, Matoba S, et al. PGC7 binds histone H3K9me2 to protect against conversion of 5mC to 5hmC in early embryos. Nature. 2012;486:415–9. doi: 10.1038/nature11093. [DOI] [PubMed] [Google Scholar]
- 61.Bian C, Yu X. PGC7 suppresses TET3 for protecting DNA methylation. Nucleic Acids Res. 2014;42:2893–905. doi: 10.1093/nar/gkt1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arakawa T, Nakatani T, Oda M, Kimura Y, Sekita Y, Kimura T, et al. Stella controls chromocenter formation through regulation of Daxx expression in 2-cell embryos. Biochem Biophys Res Commun. 2015;466:60–5. doi: 10.1016/j.bbrc.2015.08.106. [DOI] [PubMed] [Google Scholar]
- 63.Clayton AL, Hazzalin CA, Mahadevan LC. Enhanced histone acetylation and transcription: a dynamic perspective. Mol Cell. 2006;23:289–96. doi: 10.1016/j.molcel.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 64.Zeng L, Zhou MM. Bromodomain: an acetyl-lysine binding domain. FEBS Lett. 2002;513:124–8. doi: 10.1016/s0014-5793(01)03309-9. [DOI] [PubMed] [Google Scholar]
- 65.Kuo MH, Allis CD. Roles of histone acetyltransferases and deacetylases in gene regulation. Bioessays. 1998;20:615–26. doi: 10.1002/(SICI)1521-1878(199808)20:8<615::AID-BIES4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 66.Lee KK, Workman JL. Histone acetyltransferase complexes: one size doesn’t fit all. Nature reviews Molecular cell biology. 2007;8:284–95. doi: 10.1038/nrm2145. [DOI] [PubMed] [Google Scholar]
- 67.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–84. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 68.Ma P, Schultz RM. Histone deacetylase 1 (HDAC1) regulates histone acetylation, development, and gene expression in preimplantation mouse embryos. Dev Biol. 2008;319:110–20. doi: 10.1016/j.ydbio.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gabrielli B, Brown M. Histone deacetylase inhibitors disrupt the mitotic spindle assembly checkpoint by targeting histone and nonhistone proteins. Adv Cancer Res. 2012;116:1–37. doi: 10.1016/B978-0-12-394387-3.00001-X. [DOI] [PubMed] [Google Scholar]
- 70.Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell United States2006. :315–26. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 71.Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–53. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- 72.Pan G, Tian S, Nie J, Yang C, Ruotti V, Wei H, et al. Whole-genome analysis of histone H3 lysine 4 and lysine 27 methylation in human embryonic stem cells. Cell Stem Cell. 2007;1:299–312. doi: 10.1016/j.stem.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 73.Simon JA, Kingston RE. Mechanisms of polycomb gene silencing: knowns and unknowns. Nat Rev Mol Cell Biol. 2009;10:697–708. doi: 10.1038/nrm2763. [DOI] [PubMed] [Google Scholar]
- 74.Agger K, Cloos PA, Christensen J, Pasini D, Rose S, Rappsilber J, et al. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature. 2007;449:731–4. doi: 10.1038/nature06145. [DOI] [PubMed] [Google Scholar]
- 75.Lan F, Bayliss PE, Rinn JL, Whetstine JR, Wang JK, Chen S, et al. A histone H3 lysine 27 demethylase regulates animal posterior development. Nature. 2007;449:689–94. doi: 10.1038/nature06192. [DOI] [PubMed] [Google Scholar]
- 76.Hong S, Cho Y, Yu L, Yu H, Veenstra T, Ge K. Identification of JmjC domain-containing UTX and JMJD3 as histone H3 lysine 27 demethylases. Proc Natl Acad Sci USA. 2007;104:18439–44. doi: 10.1073/pnas.0707292104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pauler FM, Sloane MA, Huang R, Regha K, Koerner MV, Tamir I, et al. H3K27me3 forms BLOCs over silent genes and intergenic regions and specifies a histone banding pattern on a mouse autosomal chromosome. Genome Res. 2009;19:221–33. doi: 10.1101/gr.080861.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G, et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–60. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peters AH, O’Carroll D, Scherthan H, Mechtler K, Sauer S, Schofer C, et al. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell. 2001;107:323–37. doi: 10.1016/s0092-8674(01)00542-6. [DOI] [PubMed] [Google Scholar]
- 80.Tachibana M, Sugimoto K, Nozaki M, Ueda J, Ohta T, Ohki M, et al. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev. 2002;16:1779–91. doi: 10.1101/gad.989402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yeap LS, Hayashi K, Surani MA. ERG-associated protein with SET domain (ESET)-Oct4 interaction regulates pluripotency and represses the trophectoderm lineage. Epigenetics Chromatin. 2009;2:12. doi: 10.1186/1756-8935-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yuan P, Han J, Guo G, Orlov YL, Huss M, Loh YH, et al. Eset partners with Oct4 to restrict extraembryonic trophoblast lineage potential in embryonic stem cells. Genes Dev. 2009;23:2507–20. doi: 10.1101/gad.1831909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–53. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 84.Kooistra SM, Helin K. Molecular mechanisms and potential functions of histone demethylases. Nat Rev Mol Cell Biol England. 2012:297–311. doi: 10.1038/nrm3327. [DOI] [PubMed] [Google Scholar]
- 85.Santos-Rosa H, Schneider R, Bannister AJ, Sherriff J, Bernstein BE, Emre NC, et al. Active genes are tri-methylated at K4 of histone H3. Nature. 2002;419:407–11. doi: 10.1038/nature01080. [DOI] [PubMed] [Google Scholar]
- 86.Bernstein BE, Kamal M, Lindblad-Toh K, Bekiranov S, Bailey DK, Huebert DJ, et al. Genomic maps and comparative analysis of histone modifications in human and mouse. Cell. 2005;120:169–81. doi: 10.1016/j.cell.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 87.Wysocka J, Allis CD, Coonrod S. Histone arginine methylation and its dynamic regulation. Front Biosci. 2006;11:344–55. doi: 10.2741/1802. [DOI] [PubMed] [Google Scholar]
- 88.Gu B, Lee MG. Histone H3 lysine 4 methyltransferases and demethylases in self-renewal and differentiation of stem cells. Cell Biosci. 2013;3:39. doi: 10.1186/2045-3701-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fang R, Barbera AJ, Xu Y, Rutenberg M, Leonor T, Bi Q, et al. Human LSD2/KDM1b/AOF1 regulates gene transcription by modulating intragenic H3K4me2 methylation. Mol Cell. 2010;39:222–33. doi: 10.1016/j.molcel.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iwase S, Lan F, Bayliss P, de la Torre-Ubieta L, Huarte M, Qi HH, et al. The X-linked mental retardation gene SMCX/JARID1C defines a family of histone H3 lysine 4 demethylases. Cell. 2007;128:1077–88. doi: 10.1016/j.cell.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 91.Zhao XD, Han X, Chew JL, Liu J, Chiu KP, Choo A, et al. Whole-genome mapping of histone H3 Lys4 and 27 trimethylations reveals distinct genomic compartments in human embryonic stem cells. Cell Stem Cell United States. 2007:286–98. doi: 10.1016/j.stem.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 92.Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–26. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 93.Lee S, Roeder RG, Lee JW. Prog Mol Biol Transl Sci. Netherlands: Elsevier Inc; 2009. Roles of histone H3-lysine 4 methyltransferase complexes in NR-mediated gene transcription; pp. 343–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Di Lorenzo A, Bedford MT. Histone arginine methylation. FEBS Lett. 2011;585:2024–31. doi: 10.1016/j.febslet.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Majumder S, Alinari L, Roy S, Miller T, Datta J, Sif S, et al. Methylation of histone H3 and H4 by PRMT5 regulates ribosomal RNA gene transcription. J Cell Biochem. 2010;109:553–63. doi: 10.1002/jcb.22432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Henckel A, Nakabayashi K, Sanz LA, Feil R, Hata K, Arnaud P. Histone methylation is mechanistically linked to DNA methylation at imprinting control regions in mammals. Hum Mol Genet. 2009;18:3375–83. doi: 10.1093/hmg/ddp277. [DOI] [PubMed] [Google Scholar]
- 97.Girardot M, Hirasawa R, Kacem S, Fritsch L, Pontis J, Kota SK, et al. PRMT5-mediated histone H4 arginine-3 symmetrical dimethylation marks chromatin at G + C-rich regions of the mouse genome. Nucleic Acids Res. 2014;42:235–48. doi: 10.1093/nar/gkt884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Singh P, Cho J, Tsai SY, Rivas GE, Larson GP, Szabo PE. Coordinated allele-specific histone acetylation at the differentially methylated regions of imprinted genes. Nucleic Acids Res. 2010;38:7974–90. doi: 10.1093/nar/gkq680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chang B, Chen Y, Zhao Y, Bruick RK. JMJD6 is a histone arginine demethylase. Science. 2007;318:444–7. doi: 10.1126/science.1145801. [DOI] [PubMed] [Google Scholar]
- 100.Burton A, Torres-Padilla ME. Epigenetic reprogramming and development: a unique heterochromatin organization in the preimplantation mouse embryo. Brief Funct Genomics. 2010;9:444–54. doi: 10.1093/bfgp/elq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Beaujean N. Histone post-translational modifications in preimplantation mouse embryos and their role in nuclear architecture. Molecular reproduction and development. 2014;81:100–12. doi: 10.1002/mrd.22268. [DOI] [PubMed] [Google Scholar]
- 102.Lepikhov K, Walter J. Differential dynamics of histone H3 methylation at positions K4 and K9 in the mouse zygote. BMC Dev Biol. 2004;4:12. doi: 10.1186/1471-213X-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu H, Kim JM, Aoki F. Regulation of histone H3 lysine 9 methylation in oocytes and early pre-implantation embryos. Development. 2004;131:2269–80. doi: 10.1242/dev.01116. [DOI] [PubMed] [Google Scholar]
- 104.Erhardt S, Su IH, Schneider R, Barton S, Bannister AJ, Perez-Burgos L, et al. Consequences of the depletion of zygotic and embryonic enhancer of zeste 2 during preimplantation mouse development. Development. 2003;130:4235–48. doi: 10.1242/dev.00625. [DOI] [PubMed] [Google Scholar]
- 105.Boskovic A, Bender A, Gall L, Ziegler-Birling C, Beaujean N, Torres-Padilla ME. Analysis of active chromatin modifications in early mammalian embryos reveals uncoupling of H2A.Z acetylation and H3K36 trimethylation from embryonic genome activation. Epigenetics. 2012;7:747–57. doi: 10.4161/epi.20584. [DOI] [PubMed] [Google Scholar]
- 106.Daujat S, Weiss T, Mohn F, Lange UC, Ziegler-Birling C, Zeissler U, et al. H3K64 trimethylation marks heterochromatin and is dynamically remodeled during developmental reprogramming. Nat Struct Mol Biol. 2009;16:777–81. doi: 10.1038/nsmb.1629. [DOI] [PubMed] [Google Scholar]
- 107.Kourmouli N, Jeppesen P, Mahadevhaiah S, Burgoyne P, Wu R, Gilbert DM, et al. Heterochromatin and tri-methylated lysine 20 of histone H4 in animals. J Cell Sci. 2004;117:2491–501. doi: 10.1242/jcs.01238. [DOI] [PubMed] [Google Scholar]
- 108.Adenot PG, Mercier Y, Renard JP, Thompson EM. Differential H4 acetylation of paternal and maternal chromatin precedes DNA replication and differential transcriptional activity in pronuclei of 1-cell mouse embryos. Development (Cambridge, England) 1997;124:4615–25. doi: 10.1242/dev.124.22.4615. [DOI] [PubMed] [Google Scholar]
- 109.Hammoud SS, Nix DA, Zhang H, Purwar J, Carrell DT, Cairns BR. Distinctive chromatin in human sperm packages genes for embryo development. Nature. 2009;460:473–8. doi: 10.1038/nature08162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.van de Werken C, van der Heijden GW, Eleveld C, Teeuwssen M, Albert M, Baarends WM, et al. Paternal heterochromatin formation in human embryos is H3K9/HP1 directed and primed by sperm-derived histone modifications. Nat Commun. 2014;5:5868. doi: 10.1038/ncomms6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Torres-Padilla ME, Bannister AJ, Hurd PJ, Kouzarides T, Zernicka-Goetz M. Dynamic distribution of the replacement histone variant H3.3 in the mouse oocyte and preimplantation embryos. Int J Dev Biol. 2006;50:455–61. doi: 10.1387/ijdb.052073mt. [DOI] [PubMed] [Google Scholar]
- 112.Santenard A, Ziegler-Birling C, Koch M, Tora L, Bannister AJ, Torres-Padilla ME. Heterochromatin formation in the mouse embryo requires critical residues of the histone variant H3.3. Nature cell biology. 2010;12:853–62. doi: 10.1038/ncb2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alder O, Lavial F, Helness A, Brookes E, Pinho S, Chandrashekran A, et al. Ring1B and Suv39h1 delineate distinct chromatin states at bivalent genes during early mouse lineage commitment. Development (Cambridge, England) 2010;137:2483–92. doi: 10.1242/dev.048363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dahl JA, Reiner AH, Klungland A, Wakayama T, Collas P. Histone H3 lysine 27 methylation asymmetry on developmentally-regulated promoters distinguish the first two lineages in mouse preimplantation embryos. PloS one. 2010;5:e9150. doi: 10.1371/journal.pone.0009150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Torres-Padilla ME, Parfitt DE, Kouzarides T, Zernicka-Goetz M. Histone arginine methylation regulates pluripotency in the early mouse embryo. Nature. 2007;445:214–8. doi: 10.1038/nature05458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dahl JA, Reiner AH, Klungland A, Wakayama T, Collas P. Histone H3 lysine 27 methylation asymmetry on developmentally-regulated promoters distinguish the first two lineages in mouse preimplantation embryos. PLoS One. 2010;5:e9150. doi: 10.1371/journal.pone.0009150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rugg-Gunn PJ, Cox BJ, Ralston A, Rossant J. Distinct histone modifications in stem cell lines and tissue lineages from the early mouse embryo. Proc Natl Acad Sci USA. 2010;107:10783–90. doi: 10.1073/pnas.0914507107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gao Y, Hyttel P, Hall VJ. Regulation of H3K27me3 and H3K4me3 during early porcine embryonic development. Molecular reproduction and development. 2010;77:540–9. doi: 10.1002/mrd.21180. [DOI] [PubMed] [Google Scholar]
- 119.Park KE, Magnani L, Cabot RA. Differential remodeling of mono- and trimethylated H3K27 during porcine embryo development. Mol Reprod Dev. 2009;76:1033–42. doi: 10.1002/mrd.21061. [DOI] [PubMed] [Google Scholar]
- 120.Ross PJ, Ragina NP, Rodriguez RM, Iager AE, Siripattarapravat K, Lopez-Corrales N, et al. Polycomb gene expression and histone H3 lysine 27 trimethylation changes during bovine preimplantation development. Reproduction. 2008;136:777–85. doi: 10.1530/REP-08-0045. [DOI] [PubMed] [Google Scholar]
- 121.Canovas S, Cibelli JB, Ross PJ. Jumonji domain-containing protein 3 regulates histone 3 lysine 27 methylation during bovine preimplantation development. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:2400–5. doi: 10.1073/pnas.1119112109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bartolomei MS, Ferguson-Smith AC. Mammalian genomic imprinting. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a002592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mercer TR, Mattick JS. Structure and function of long noncoding RNAs in epigenetic regulation. Nat Struct Mol Biol. 2013;20:300–7. doi: 10.1038/nsmb.2480. [DOI] [PubMed] [Google Scholar]
- 124.Kelsey G, Feil R. New insights into establishment and maintenance of DNA methylation imprints in mammals. Philos Trans R Soc Lond B Biol Sci. 2013;368:20110336. doi: 10.1098/rstb.2011.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hirasawa R, Chiba H, Kaneda M, Tajima S, Li E, Jaenisch R, et al. Maternal and zygotic Dnmt1 are necessary and sufficient for the maintenance of DNA methylation imprints during preimplantation development. Genes Dev. 2008;22:1607–16. doi: 10.1101/gad.1667008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Messerschmidt DM, de Vries W, Ito M, Solter D, Ferguson-Smith A, Knowles BB. Trim28 is required for epigenetic stability during mouse oocyte to embryo transition. Science (New York, NY) 2012;335:1499–502. doi: 10.1126/science.1216154. [DOI] [PubMed] [Google Scholar]
- 127.Lorthongpanich C, Cheow LF, Balu S, Quake SR, Knowles BB, Burkholder WF, et al. Single-cell DNA-methylation analysis reveals epigenetic chimerism in preimplantation embryos. Science. 2013;341:1110–2. doi: 10.1126/science.1240617. [DOI] [PubMed] [Google Scholar]
- 128.Li X, Ito M, Zhou F, Youngson N, Zuo X, Leder P, et al. A maternal-zygotic effect gene, Zfp57, maintains both maternal and paternal imprints. Dev Cell. 2008;15:547–57. doi: 10.1016/j.devcel.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mackay DJ, Callaway JL, Marks SM, White HE, Acerini CL, Boonen SE, et al. Hypomethylation of multiple imprinted loci in individuals with transient neonatal diabetes is associated with mutations in ZFP57. Nat Genet. 2008;40:949–51. doi: 10.1038/ng.187. [DOI] [PubMed] [Google Scholar]
- 130.McEwen KR, Ferguson-Smith AC. Distinguishing epigenetic marks of developmental and imprinting regulation. Epigenetics Chromatin. 2010;3:2. doi: 10.1186/1756-8935-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bartolomei MS. Genomic imprinting: employing and avoiding epigenetic processes. Genes Dev. 2009;23:2124–33. doi: 10.1101/gad.1841409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Smallwood SA, Tomizawa S, Krueger F, Ruf N, Carli N, Segonds-Pichon A, et al. Dynamic CpG island methylation landscape in oocytes and preimplantation embryos. Nat Genet. 2011;43:811–4. doi: 10.1038/ng.864. [DOI] [PMC free article] [PubMed] [Google Scholar]