Abstract
A multi-residue analytical method was developed for the determination in amniotic fluid (AF) of 13 illicit phenethylamines, including 12 compounds never investigated in this matrix before. Samples were subject to solid-phase extraction using; hydrophilic–lipophilic balance cartridges which gave good recoveries and low matrix effects on analysis of the extracts. The quantification was performed by liquid chromatography electrospray tandem mass spectrometry. The water–acetonitrile mobile phase containing 0.1% formic acid, used with a C18 reversed phase column, provided adequate separation, resolution and signal-to-noise ratio for the analytes and the internal standard. The final optimized method was validated according to international guidelines. A monitoring campaign to assess fetal exposure to these 13 substances of abuse has been performed on AF test samples obtained from pregnant women. All mothers (n = 194) reported no use of drugs of abuse during pregnancy, and this was confirmed by the analytical data.
Introduction
Prenatal substance abuse is a growing issue. In the last decade, a rapid escalation in the availability and misuse of psychotropic substances has been reported. Several neonatal problems related to health and development have been undoubtedly linked to fetal exposure to drugs and alcohol (1, 2): a fetus exposed to such substances during intrauterine development can be affected by reduced birth weight, small head circumference, premature birth, fetal distress, potential birth defects and perinatal complications (3).
Phenethylamines are among the most commonly used addictive drugs provoking elevation of mood and wakefulness, and increasing self-confidence, talkativeness and agitation. In addition, because of their adrenaline-like effects, phenethylamines cause hypertension, tachycardia, dry mouth and sweating.
The intake of phenethylamines during pregnancy could be dangerous to the fetus as these drugs of abuse are fat soluble and can readily pass through the placental barrier (4), reaching the fetal compartment and causing harmful side effects (5). Several investigations have indeed found links between prenatal methamphetamine (MA) and 3,4-methylenedioxymethamphetamine (MDMA) exposure and specific adverse outcomes (6, 7). Furthermore, recent studies have proved that one of the most important effects of prenatal exposure to phenethylamines is a persistent alteration of the metabolism of monoamines, especially dopamine and serotonin (5). Other significant effects are intrauterine growth retardation (8) and intellectual and behavioral problems in infants (9, 10). Equally important are the effects on maternal health, since the anorectic action of these drugs on the mother may in turn be dangerous for the developing fetus.
Consequently, due to the increasing diffusion and abuse of these drugs, and the appearance of new phenethylamine derivatives on the black market, there is a need for sensitive and specific bioanalytical methods that allow these substances to be detected and quantified, not only in traditional maternal matrices but also in fetal samples. This could make it possible to assess the exposure to these substances which, although illicit, are sometimes consumed by women, who are often not aware of their pregnancy.
It is important to highlight that the phenethylamine on the illicit market are constantly changing, as with small structural molecular changes it is possible to obtain new compounds temporarily lawful but equally dangerous. For this reason, it is essential to have updated analytical methods in order to detect these new molecules.
Fetal exposure to drugs of abuse can be diagnosed through a positive maternal history and detection of the investigated compounds themselves in maternal and/or neonatal matrices. In addition to traditional specimens (such as maternal blood and urine), there are many others available, such as meconium, umbilical cord tissue and amniotic fluid (AF). AF is essentially a filtrate of maternal blood. Drugs reach the AF by diffusing across the placenta. The fetus is exposed to xenobiotics dissolved in the AF through two main routes: oral ingestion and transdermal diffusion, particularly relevant in the early stage of pregnancy, because the skin is less developed. The only disadvantage of this matrix is that its collection is invasive. However, for drug testing purposes an aliquot can be taken of the AF sample collected during amniocentesis, a medical procedure performed to detect genetic disorders. Although the collection of an AF sample is more troublesome than other conventional matrices, one unique feature of this matrix is that it can measure second/third trimester drug exposure, compared with perinatal exposure in urine. Similarly, it gives a more direct measure of fetal exposure than maternal blood as it indicates actual drug concentrations than have passed the placental barrier. In another study, we previously investigated the intake of caffeine and acetaminophen during pregnancy (11).
In the work reported here, we developed and validated a liquid chromatography tandem mass spectrometry (LC–MS-MS) method for the determination and quantitation in AF of 13 phenethylamine derivatives—3,4-methylenedioxy-N-methylamphetamine (MDMA), 3,4-methylenedioxy-N-ethylamphetamine (MDE), p-methoxyamphetamine (PMA), p-methoxymethamphetamine (PMMA), 3,4,5-trimethoxyamphetamine (TMA), 2,5-dimethoxyamphetamine (DMA), 2,5-dimethoxy-4-methylamphetamine (DOM), 2,5-dimethoxy-4-ethylamphetamine (DOET), 2,5-dimethoxy-4-bromoamphetamine (DOB), 2,5-dimethoxy-4-bromophenethylamine (2C-B), 2,5-dimethoxy-4-iodophenethylamine (2C-I), 2,5-dimethoxy-4-ethylthiophenethylamine (2C-T-2) and 2,5-dimethoxy-4-n-propylthiophenethylamine (2C-T-7). All of these are officially recognized as illicit drugs by Italian Legislation (12, 13) and other European countries (14, 15). Twelve of these compounds have never been investigated in AF before. AF samples were subject to solid-phase extraction (SPE) using hydrophilic–lipophilic balance (HLB) cartridges to minimize matrix interference and to concentrate extracts prior to LC–MS-MS analysis. Finally, the method has been validated in order to monitor 194 test samples of human AF.
Experimental
Chemicals and reagents
The target analytes [3,4-methylenedioxy-N-methylamphetamine (MDMA), 3,4-methylenedioxy-N-ethylamphetamine (MDE), p-methoxyamphetamine (PMA), p-methoxymethamphetamine (PMMA), 3,4,5-trimethoxyamphetamine (TMA), 2,5-dimethoxyamphetamine (DMA), 2,5-dimethoxy-4-methylamphetamine (DOM), 2,5-dimethoxy-4-ethylamphetamine (DOET), 2,5-dimethoxy-4-bromoamphetamine (DOB), 2,5-dimethoxy-4-bromophenethylamine (2C-B), 2,5-dimethoxy-4-iodophenethylamine (2C-I), 2,5-dimethoxy-4-ethylthiophenethylamine (2C-T-2) and 2,5-dimethoxy-4-n-propylthiophenethylamine (2C-T-7)] were purchased from Lipomed AG (Arlesheim, Switzerland). The internal standard (2,3-dimethoxyphenethylamine-d3, IS) was synthesized in our laboratory and had a purity of >99%. Deionized and distilled water was filtered through a Milli Q water system (Millipore, Billerica, MA, USA). Other reagents and solvents were of the highest commercial quality and were obtained from Sigma Aldrich (Milano, Italy). Individual methanolic stock solutions (1.0 mg/mL of each analyte and the IS) were used to prepare the working solutions by appropriate dilution. All solutions were stored at −20°C in the dark.
Supel™-Select HLB SPE columns (30 mg/1 mL) were purchased from Supelco (Bellafonte, PA). SPE columns were mounted on a VacElut vacuum manifold (Sigma Aldrich, Milan, Italy).
Sample collection
A total of 194 women (15–21 weeks gestation) undergoing routine amniocentesis in our university hospital voluntarily participated in this study, giving signed consent. To perform the drug testing, an aliquot (1,000 µL) of the AF collected during amniocentesis was taken and stored at −80°C until analyzed. All experiments were conducted according to institutional guidelines and were approved by the Local Ethics Committee.
Sample preparation
The analytes were extracted as follows: the HLB cartridge (Supel™-Select, 30 mg/mL) was first activated and conditioned with 1 mL of methanol and 1 mL of ammonium acetate buffer (25 mM, pH 10). Then 0.5 mL of AF mixed with 0.5 mL of the same ammonium acetate buffer was applied to the cartridge. After application of the sample, the column was washed (1 mL of Milli-Q water and 1 mL of 5% methanol in water) and dried for 5 min under a stream of air. The analytes were then eluted with 1 mL of methanol/acetonitrile (1/1). Finally, the eluate, evaporated to dryness under a stream of nitrogen, was reconstituted in 0.2 mL of the initial mobile phase.
LC–MS-MS analysis
A Shimadzu LC-20ADXR instrument (Shimadzu Italia, Milano, Italy) was employed for chromatographic separation using a Kinetex C18 column (50 × 2.1 mm i.d., particle size 2.6 μm; Phenomenex, Torrance, CA, USA) fitted with a 2.6-μm security guard cartridge (4 × 2.1 mm i.d., Phenomenex). The mobile phase was constituted by two solvents: 0.1% formic acid in water (solvent A) and 0.1% formic acid in acetonitrile (solvent B). Chromatography was undertaken at a flow rate of 0.3 mL/min using (i) a linear gradient from 10 to 90% B in 6 min followed by (ii) isocratic elution with 90% B for 1.5 min. Thereafter, the mobile phase was rapidly returned to 10% B in 0.1 min and the system re-equilibrated at the initial conditions for a further 4.9 min before each new sample injection. The total run time was thus 12.5 min. Each sample was injected three times.
Tandem mass spectrometric analysis was performed on an Applied Biosystem MDS Sciex API 2000 triple quadrupole (Concord, Ontario, Canada), equipped with an electrospray ionization (ESI) interface. Infusion experiments by the syringe-pump method were undertaken for each analyte and the IS at a concentration of 10 μg/mL in both negative and positive ion mode to optimize ESI parameters. It was observed that the signal intensities were greater in the positive ionization mode. The mass spectra revealed intense protonated molecular ion peaks (reported in Table I). These [M + 1]+ ions were selected as the precursors to optimize the fragmentation parameters in product ion experiments. For each target analyte, two product ions were selected; one transition was taken for confirmation of the identity of the analyte and the other for its quantitation. For the IS, only one transition was selected. The precursor/product ion transitions monitored are reported in Table I. The ion source temperature was set at 400°C. The ion spray voltage was set at 5,000 V, the curtain gas (CUR) was set at six arbitrary units and the collision gas (CAD) also at six arbitrary units. The optimized MS parameters are reported in Table I. Qualitative analysis was performed according to the selected multiple reaction monitoring (MRM) transitions and retention times. Positive analyte identification in samples required retention times to be within ±0.20 min of the average retention time of reference standards. Since two product ions were selected for each analyte, a further criterion of identification was to consider the ratio of the qualifier transition to the quantifier transition, which was required to be within ±30% of the average measured ratio from the calibration curve samples (16). Data acquisition was accomplished using the Applied Biosystem Analyst version 1.6 software. The Applied Biosystem MultiQuant version 2.1 software was used for data quantification, using the IS method.
Table I.
Tandem mass spectrometric conditions
| Compound | Precursor ion (m/z) | Target/qualifier ions (m/z) | DP (V) | FP (V) | EP (V) | CE (V) | CXP (V) |
|---|---|---|---|---|---|---|---|
| 2C-B | 260.0 | 243.0 228.0 |
15 | 300 | 4.0 | 18 30 |
10 4.6 |
| 2C-I | 308.1 | 291.1 276.1 |
15 | 300 | 5.0 | 21 35 |
6.5 5.4 |
| 2C-T-2 | 242.2 | 225.2 134.2 |
15 | 200 | 5.0 | 19 37 |
4.8 5.0 |
| 2C-T-7 | 256.2 | 239.2 197.2 |
15 | 300 | 5.0 | 19 30 |
5.4 8.0 |
| DMA | 196.2 | 151.2 179.2 |
15 | 200 | 8.0 | 16 24 |
3.2 6.0 |
| DOB | 274.0 | 257.0 229.0 |
15 | 300 | 5.0 | 22 33 |
5.9 4.3 |
| DOET | 224.2 | 207.2 179.2 |
11 | 300 | 4.0 | 20 27 |
4.7 7.4 |
| DOM | 210.2 | 193.2 178.2 |
10 | 200 | 6.0 | 18 27 |
4.3 3.5 |
| MDE | 209.1 | 164.1 78.1 |
21 | 200 | 7.0 | 19 63 |
10 12 |
| MDMA | 194.2 | 163.2 105.2 |
21 | 200 | 7.0 | 12 35 |
8 18 |
| PMA | 166.1 | 149.1 121.1 |
10 | 200 | 6.0 | 13 27 |
2.2 2.0 |
| PMMA | 180.1 | 121.1 149.1 |
15 | 200 | 6.0 | 18 25 |
2.2 4.6 |
| TMA | 226.2 | 209.2 181.2 |
15 | 200 | 4.0 | 16 29 |
4.6 3.0 |
| IS | 185.0 | 168.0 | 15 | 200 | 8.0 | 20 | 7 |
DP, declustering potential; FP, focusing potential; EP, entrance potential; CE, collision energy; CXP, collision cell exit potential; 2C-B, 2,5-dimethoxy-4-bromophenethylamine; 2C-I, 2,5-dimethoxy-4-iodophenethylamine; 2C-T-2, 2,5-dimethoxy-4-ethylthiophenethylamine; 2C-T-7, 2,5-dimethoxy-4-n-propylthiophenethylamine; DMA, 2,5-dimethoxyamphetamine; DOB, 2,5-dimethoxy-4-bromoamphetamine; DOET, 2,5-dimethoxy-4-ethylamphetamine; DOM, 2,5-dimethoxy-4-methylamphetamine; MDE, 3,4-methylenedioxy-N-ethylamphetamine; MDMA, 3,4-methylenedioxy-N-methylamphetamine; PMA, p-methoxyamphetamine; PMMA, p-methoxymethamphetamine; TMA, 3,4,5-trimethoxyamphetamine; IS, 2,3-dimethoxyphenethylamine-d3.
Method validation
Reliable qualitative and quantitative analysis constitutes the basis of a consistent toxicological assay in the field of clinical and forensic toxicology. For this reason, the method was validated according to the accepted guidelines (17–19) using drug-free AF samples obtained from different sources. The parameters determined were selectivity, linearity, limit of determination (LOD), limit of quantitation (LOQ), precision, accuracy, matrix effect and analyte stability.
Selectivity is defined as the ability of a bioanalytical method to measure unequivocally and to differentiate the analytes from other components which may be expected to be present, such as impurities and matrix components (20). This was assessed by analyzing 10 blank AF samples from different sources, extracted as described above. The presence of possible interference from endogenous or exogenous substances was verified by monitoring the MRM chromatograms specific for each investigated compound and the IS at their expected retention times. The absence of interference between the IS and the analytes was also determined by analyzing three blank AF samples fortified only with IS (zero standard), and three blank samples fortified only with the analytes, respectively.
Calibration curves were prepared by fortifying blank AF samples with IS (100 ng/mL) and the 13 target drugs at 6 concentrations (10, 25, 50, 100, 200 and 400 ng/mL), which were then extracted and analyzed as described above. The linearity of the compound-to-IS peak area ratio versus the theoretical concentration was verified in the AF using a 1/x weighted linear regression. The correlation coefficients (r) and the linearity were tested on a set of five calibration curves. The acceptance criterion for a calibration curve was an r of 0.990 or better (21).
The limits of determination (LODs) and the limits of quantitation (LOQs) were determined by the signal-to-noise ratio (S/N) method by analyzing blank samples extracted as described above. S/N values of greater than 3 and 10 were considered as the LOD and LOQ, respectively.
Accuracy was determined as % recovery. To assess this parameter, the standard mix of the analytes was used to spike AF blank samples from different sources in order to obtain final concentrations of 25, 200 and 400 ng/mL (n = 5). Recovery was calculated as the ratio of recovered analyte concentration to nominal concentration (calculated by analyzing standard samples in the initial mobile phase at the same concentration).
The precision of the method, evaluated at three concentrations (25, 200 and 400 ng/mL), was expressed as the percent relative standard deviation (RSD). The sample standard deviation (SD) was calculated for five replicates for each concentration for the intra-day repeatability and over five consecutive days for the inter-day repeatability.
The matrix effect was also determined for the method (22, 23). The sample matrix, co-eluting compounds and cross-talk can contribute to an alteration in analyte ionization and of the overall response. Accordingly,; the competition between analytes and co-eluting unknown matrix compounds with the primary ions formed in the MS interface may effectively decrease (ion suppression) or increase (ion enhancement) the efficiency of the protonation of the analytes of interest. The matrix effect was determined with the post-extraction addition method (24). Nine drug-free AF samples from different sources were first extracted as described above and subsequently fortified with analytes at three different concentrations (25, 200 and 400 ng/mL). For each analyte, the chromatographic peak areas obtained from the extracts were compared with the corresponding peak areas produced by the reference solutions prepared in the initial mobile phase at the same concentration. Each sample was analyzed three times. The matrix effect percentage was calculated according to the following equation:
The freeze-and-thaw stability of the analytes was determined after performing three freeze-and-thaw cycles on blank AF samples fortified at a low and high analyte concentration (25 and 400 ng/mL). AF samples (n = 3), fortified with the target compounds, were frozen for 24 h and then thawed for 2 h at room temperature. This cycle was performed three times. Fresh AF samples (n = 3) were prepared at the same two concentrations. The stability samples and the fresh samples were extracted as described above and subsequently analyzed.
Results and discussion
Sample preparation
Sample clean-up is a critical step, especially when a complex matrix such as AF is analyzed. To the best of our knowledge, the extraction of these illicit drugs in AF has never been reported in the literature before. The extraction was performed with HLB SPE cartridges, which have superior reversed phase capacity, created by a balanced ratio of two monomers, hydrophilic N-vinylpyrrolidone and lipophilic divinylbenzene, that enhance the retention of polar analytes. These cartridges gave good recoveries and low matrix effects on analysis of extracted samples.
Method validation
The optimization of the sample preparation and analytical parameters provided adequate separation, resolution and signal-to-noise ratio for the analytes and the IS. All analytes detected in a positive ion mode were well separated on the reversed phase HPLC column used. Each molecule in the concentration range 10–400 ng/mL exhibited a single chromatographic peak that was easily discernible from the baseline noise. Qualitative analysis was performed on the basis of analyte retention times and two MRM transitions for each compound (Tables I and II). Selection of the quantifier and qualifier transitions was based on transitions from the molecular ion to the most and second-most predominant fragment ions, respectively. For positive identification of each analyte, the ratio of the qualifier transition to the quantifier transition was required to be within ±30% of the average measured ratio from standards (16). The method was validated according to accepted protocols (17–19).
Table II.
Validation parameters
| Compound | tR (min) | r ± SD (n = 5) | y = ax + b (n = 5) | LOQ (ng/mL) | LOD (ng/mL) |
|---|---|---|---|---|---|
| 2C-B | 3.03 | 0.998 ± 0.015 | y = 2.2895x + 0.1515 | 19 | 6 |
| 2C-I | 3.39 | 0.999 ± 0.011 | y = 1.6691x + 0.1811 | 12 | 4 |
| 2C-T-2 | 3.27 | 0.999 ± 0.016 | y = 1.2307x + 0.1785 | 13 | 4 |
| 2C-T-7 | 3.87 | 0.998 ± 0.013 | y = 0.9736x + 0.2057 | 14 | 5 |
| DMA | 2.59 | 0.999 ± 0.024 | y = 0.9591x − 0.0271 | 20 | 3 |
| DOB | 3.52 | 0.999 ± 0.013 | y = 1.922x + 0.1807 | 19 | 6 |
| DOET | 3.73 | 0.999 ± 0.008 | y = 0.9164x + 0.1573 | 15 | 5 |
| DOM | 3.15 | 0.998 ± 0.011 | y = 1.108x + 0.0493 | 19 | 6 |
| MDE | 2.85 | 0.999 ± 0.021 | y = 0.9819x − 0.2373 | 9 | 3 |
| MDMA | 2.49 | 0.999 ± 0.009 | y = 1.0967x − 0.2097 | 10 | 3 |
| PMA | 2.30 | 0.999 ± 0.014 | y = 4.9197x − 0.0695 | 19 | 6 |
| PMMA | 2.57 | 0.998 ± 0.017 | y = 1.7031x − 0.1245 | 18 | 6 |
| TMA | 2.17 | 0.997 ± 0.012 | y = 1.6812x + 0.2365 | 13 | 4 |
tR, retention time; SD, standard deviation; LOQ, limit of quantitation; LOD, limit of detection.
Ten drug-free AF samples were extracted and analyzed in order to evaluate selectivity, by verifying the absence of potential endogenous interference. Selectivity was satisfactory, as no interfering peaks were observed at the same retention times as the target analytes and the IS. Working calibration curves presented an acceptable linearity range and correlation coefficients (r), higher than 0.997 for all target compounds (Table II). LODs and LOQs ranged from 3 to 6 ng/mL and from 9 to 20 ng/mL, respectively (Table II). It is important to emphasize that these values refer to the whole analytical procedure, incorporating mutual ionization suppression and recovery losses, while the detection limits estimated from solvent calibration curves incorrectly estimate these values.
Method precision has been evaluated by three replicate injections of standards prepared in blank AF samples fortified at three concentrations on five consecutive days. The intraday and interday repeatabilities were under 15% (Table III).
Table III.
Matrix effect, recovery and repeatability
| Compound | Concentration (ng/mL) | Matrix effect (%) | Recovery (%) | Repeatability (RSD %) |
|
|---|---|---|---|---|---|
| Intraday | Interday | ||||
| PMA | 25 | −3.44 | 96.1 | 9.3 | 11.1 |
| 200 | −6.79 | 79.2 | 11.2 | 11.3 | |
| 400 | −5.55 | 73.0 | 10.5 | 9.8 | |
| PMMA | 25 | −6.25 | 82.9 | 8.6 | 10.0 |
| 200 | −6.77 | 88.9 | 7.7 | 9.9 | |
| 400 | −9.06 | 73.5 | 8.4 | 8.5 | |
| TMA | 25 | −8.65 | 72.3 | 9.8 | 13.0 |
| 200 | −4.53 | 88.0 | 6.9 | 12.3 | |
| 400 | −2.96 | 90.5 | 8.2 | 11.2 | |
| DMA | 25 | −0.76 | 96.0 | 11.4 | 15.0 |
| 200 | −3.05 | 92.9 | 12.5 | 14.5 | |
| 400 | −3.78 | 96.1 | 13.0 | 13.5 | |
| DOM | 25 | −10.7 | 77.5 | 10.0 | 9.9 |
| 200 | −6.97 | 88.9 | 9.2 | 10.5 | |
| 400 | −4.99 | 95.0 | 9.3 | 10.8 | |
| DOET | 25 | −9.30 | 84.6 | 13.1 | 14.7 |
| 200 | −11.5 | 89.9 | 14.0 | 14.1 | |
| 400 | −8.90 | 86.9 | 12.5 | 13.5 | |
| DOB | 25 | −7.87 | 85.4 | 8.8 | 10.2 |
| 200 | −6.97 | 95.5 | 8.6 | 10.8 | |
| 400 | −7.02 | 85.2 | 10.2 | 12.0 | |
| 2C-B | 25 | −8.82 | 91.9 | 13.3 | 13.8 |
| 200 | −5.45 | 94.4 | 12.0 | 13.1 | |
| 400 | −10.2 | 92.8 | 11.9 | 12.7 | |
| 2C-I | 25 | −7.36 | 75.2 | 7.8 | 10.9 |
| 200 | −8.21 | 91.1 | 8.7 | 11.5 | |
| 400 | −6.60 | 90.3 | 9.6 | 11.8 | |
| 2C-T-2 | 25 | −10.6 | 85.5 | 11.4 | 14.3 |
| 200 | −9.45 | 85.4 | 11.6 | 13.7 | |
| 400 | −7.31 | 81.3 | 12.0 | 13.5 | |
| 2C-T-7 | 25 | −4.09 | 94.3 | 14.0 | 13.2 |
| 200 | −9.34 | 75.7 | 12.1 | 12.6 | |
| 400 | −2.29 | 85.0 | 13.0 | 14.1 | |
| MDMA | 25 | −4.50 | 88.6 | 11.1 | 13.2 |
| 200 | −6.24 | 85.4 | 9.8 | 10.2 | |
| 400 | −6.33 | 89.0 | 8.6 | 11.0 | |
| MDE | 25 | −2.87 | 77.1 | 10.1 | 11.3 |
| 200 | −8.53 | 78.2 | 12.1 | 10.8 | |
| 400 | −5.12 | 79.3 | 9.7 | 10.7 | |
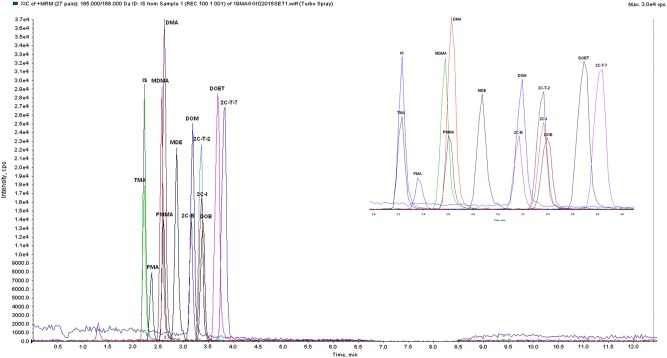
Accuracy is expressed as recoveries, determined by analyzing fortified AF samples since no certified reference materials were available for phenethylamines in this matrix. The recoveries were between 72.3 and 96.1% (Table III). The chromatogram of a drug-free AF sample fortified with the target analytes and IS are given in Figure 1. The chromatographic separation of all compounds was achieved in 4 min. In order to evaluate the carryover from previous injections, an extract of a drug-free AF sample has been analyzed immediately after the highest concentrations used in the calibration range. No quantifiable carryover was observed for any analyte. For all compounds, the loss observed after three freeze-and-thaw cycles was lower than 20% (data not shown).
Figure 1.
Chromatogram of a drug-free AF sample fortified with the target analytes (100 ng/mL) and IS (100 ng/mL). This figure is available in black and white in print and in color at JAT online.
In vivo application
The validated method was applied to the analysis of 194 test samples of human AF collected during amniocentesis. The obtained results were correlated with the information collected during interviews with the mothers. No mothers (n = 194) reported any intake of drug of abuse during pregnancy, and this was confirmed by the analytical results which revealed no detectable concentrations of the 13 phenethylamines in their AF samples.
Conclusion
The present method for determination of 13 illicit phenethylamines in AF samples was validated according to international guidelines. It may prove a useful method in hospitals to identify the intake of these substances during pregnancy, in order to take prompt medical action with respect to the mother and the child. Although the collection of an AF sample is more troublesome than other conventional matrices, it gives a more direct measure of fetal exposure than maternal blood, as it indicates actual drug concentrations that have passed the placental barrier. The literature concerning these drugs in pregnancy and their measurement is limited and to the best of our knowledge, no other method in AF has been developed.
References
- 1.Broening H.W., Morford L.L., Inman-Wood S.L., Fukumura M., Vorhees C.V. (2001) 3,4-methylenedioxymethamphetamine (ecstasy)-induced learning and memory impairments depend on the age of exposure during early development. Journal of Neuroscience, 21, 3228–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguirre N., Barrionuevo M., Lasheras B., Del Rio J. (1998) The role of dopaminergic systems in the perinatal sensitivity to 3,4-methylenedioxymethamphetamine-induced neurotoxicity in rats. Journal of Pharmacology and Experimental Therapy, 286, 1159–1165. [PubMed] [Google Scholar]
- 3.Huestis M.A., Choo R.E. (2002) Drug abuse's smallest victims: in utero drug exposure. Forensic Science International, 128, 20–30. [DOI] [PubMed] [Google Scholar]
- 4.Ramamoorthy J.D., Ramamoorthy S., Leibach F.H., Ganapathy V. (1995) Human placental monoamine transporters as targets for amphetamines. American Journal of Obstetrics & Gynecology, 173, 1782–1787. [DOI] [PubMed] [Google Scholar]
- 5.Campbell N.G., Koprich J.B., Kanaan N.M., Lipton J.W. (2006) MDMA administration to pregnant Sprague–Dawley rats results in its passage to the fetal compartment. Neurotoxicology and Teratology, 28, 459–465. [DOI] [PubMed] [Google Scholar]
- 6.LaGasse L.L., Wouldes T., Newman E., Smith L.M., Shah R.Z., Derauf C. et al. (2011) Prenatal methamphetamine exposure and neonatal neurobehavioral outcome in the USA and New Zealand. Neurotoxicology and Teratology, 33, 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singer L.T., Moore D.G., Fulton S., Goodwin J., Turner J.J.D., Min M.O. et al. (2012) Neurobehavioral outcomes of infants exposed to MDMA (Ecstasy) and other recreational drugs during pregnancy. Neurotoxicology and Teratology, 34, 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McFarlane J., Parker B., Soeken K. (1996) Physical abuse, smoking, and substance use during pregnancy: prevalence, interrelationships, and effects on birth weight. Journal of Obstetric, Gynecologic & Neonatal Nursering, 25, 313–320. [DOI] [PubMed] [Google Scholar]
- 9.Billing L., Eriksson M., Jonsson B., Steneroth G., Zetterstrom R. (1994) The influence of environmental factors on behavioural problems in 8-year-old children exposed to amphetamine during fetal life. Child Abuse Negligence, 18, 3–9. [DOI] [PubMed] [Google Scholar]
- 10.Cernerud L., Eriksson M., Jonsson B., Steneroth G., Zetterstrom R. (1996) Amphetamine addiction during pregnancy: 14-year follow-up of growth and school performance. Acta Paediatrica, 85, 204–208. [DOI] [PubMed] [Google Scholar]
- 11.Burrai L., Nieddu M., Trignano C., Carta A., Boatto G. (2015) LC-MS/MS analysis of acetaminophen and caffeine in amniotic fluid. Analytical Methods, 7, 405–410. [Google Scholar]
- 12.Decree of the Italian Republic President, n. 309/1990. “Testo unico delle leggi in materia di disciplina degli stupefacenti e sostanze psicotrope, prevenzione, cura e riabilitazione dei relativi stati di tossicodipendenza”. “Law for the discipline of abuse drugs and psychotropic substances, prevention, treatment and rehabilitation of drug addiction”. Italian Official Gazette No. 25/1990, Suppl.Ord. No. 67.
- 13.Italian Decree “Provvedimento No. 99/cu del 30/10/2007, Intesa, ai sensi dell'articolo 8, comma 6, della legge n. 131/2003, in materia di accertamento di assenza di tossicodipendenza”. “Measure No. 99/cu del 30/10/2007, Agreement for the assessment of a drug addiction status, according to the article 8, comma 6, of the law n. 131/2003”. Italian Official Gazette No. 266/2007.
- 14.Council Decision 2003/847/JHA of 27 November 2003 concerning control measures and criminal sanctions in respect of the new synthetic drugs 2C-I, 2C-T-2, 2C-T-7 and TMA-2.
- 15.Council decision 2002/188/JHA of 28 February 2002 concerning control measures and criminal sanctions in respect of the new synthetic drug PMMA.
- 16.Kacinko S.L., Xu A., Homan J.W., McMullin M.M., Warrington D.M., Logan B.K. (2011) Development and validation of a liquid chromatography–tandem mass spectrometry method for the identification and quantification of JWH-018, JWH-073, JWH-019, and JWH-250 in human whole blood. Journal of Analytical Toxicology, 35, 386–393. [DOI] [PubMed] [Google Scholar]
- 17.Peters F.T., Maurer H.H. (2002) Bioanalytical method validation and its implications for forensic and clinical toxicology. Accreditation and Quality Assurance, 7, 441–444. [Google Scholar]
- 18.Taverniers I., de Loose M., Van Bockstaele E. (2004) Analytical method validation and quality assurance. Trends in Analytical Chemistry, 23, 533–536. [Google Scholar]
- 19.Peters F.T., Drummer O.H., Musshoff F. (2007) Validation of new methods. Forensic Science International, 165, 216–224. [DOI] [PubMed] [Google Scholar]
- 20.Shah V.P., Midha K.K., Hill H.M., Hulse J.D., McGilveray I.J., McKay G. et al. (2000) Bioanalytical method validation—a revisit with a decade of progress. Pharmaceutical Research, 17, 1551–1557. [DOI] [PubMed] [Google Scholar]
- 21.Cao Z., Kaleta E., Wang P. (2015) Simultaneous Quantitation of 78 Drugs and Metabolites in Urine with a Dilute-And-Shoot LC-MS-MS Assay. Journal of Analytical Toxicology, 39, 335–346. [DOI] [PubMed] [Google Scholar]
- 22.Souverain S., Rudaz S., Veuthey J.L. (2004) Matrix effect in LC-ESI-MS and LC-APCI-MS with off-line and on-line extraction procedures. Journal of Chromatography A, 1058, 61–66. [PubMed] [Google Scholar]
- 23.Annesley T. (2003) Ion suppression in mass spectrometry. Clinical Chemistry, 49, 1041–1044. [DOI] [PubMed] [Google Scholar]
- 24.Matuszewski B.K., Constanzer M.L., Chavez-Eng C.M. (2003) Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC−MS/MS. Analytical Chemistry, 75, 3019–3030. [DOI] [PubMed] [Google Scholar]