Abstract
The accumulation of epicuticular waxes over stomata in Quercus coccifera L. contributes to a severe reduction in maximum stomatal conductance (gs,max) under Mediterranean (MED) conditions. However, this phenomenon was not observed in this species under temperate (TEM) conditions, which could lead to differences in the ability to assimilate CO2 between the sites. We hypothesise that the overall importance of such a reduction in gs,max on photosynthesis is modulated by other factors affecting carbon gain, mainly mesophyll conductance to CO2 (gm), through a plastic response to changes in environmental conditions (i.e., vapour pressure deficit, VPD, and mean daily quantum flux density, Qint). The results reveal that leaves grown at the TEM site did not show an increased ability for net CO2 assimilation (AN), mainly due to an equal gm at both sites. This fact is explained by a trade-off between an increased conductance of the gas phase (gias) and a reduced conductance of the liquid phase (gliq) at the TEM site compared with the MED site. In spite of the reduction in gs,max at the MED site, transpiration (E) did not diminish during midsummer to the levels of the TEM site due to a higher VPD found at the MED site, yielding a higher water use efficiency (AN/E) at the TEM site. Moreover, photosynthetic nitrogen use efficiency was also higher at the TEM site, indicating these leaves can reach similar values of AN with lower nitrogen investment that those at the MED site. These results suggest that Q. coccifera does not always use the main resources (water and nutrients) at leaf level as efficiently as possible. Moreover, the different patterns of resource use (in particular N), together with the functional plasticity, cannot overcome the morpho-functional constraints that limit photosynthetic activity, even under potentially favourable conditions.
Keywords: epicuticular waxes, Mediterranean climate, mesophyll conductance, photosynthesis, PNUE, stomatal conductance, WUE
Introduction
Kermes oak (Quercus coccifera L.) is a sclerophyllous species that is considered one of the main components of the shrublands in the most arid areas of the Iberian Peninsula (Castro-Díez and Navarro 2007, Peguero-Pina et al. 2008). This species is able to withstand long and intense drought periods during summer, the driest season in the Mediterranean region (Vilagrosa 2002, Vilagrosa et al. 2003, Peguero-Pina et al. 2008). This high drought tolerance is associated with its ability to tolerate very low water potentials without signs of irreversible damage in either the apoplast or the symplast (Vilagrosa et al. 2010) and by the remarkable plasticity of its photo-protective systems in response to environmental challenges (García-Plazaola et al. 2003, 2008). Quercus coccifera has been considered a water-saving plant (Valladares et al. 2005) due to its capacity to regulate water loss and thereby withstand prolonged dry periods (Sakcali and Ozturk 2004, Ozturk et al. 2010). In addition, its constitutive drought tolerance is manifested in lower values of maximum stomatal conductance (gs,max) than those reported for other co-occurring species that are also considered to be genuine representative of the Mediterranean woody flora (Peguero-Pina et al. 2009, Vilagrosa et al. 2010).
Roth-Nebelsick et al. (2013) found that this low gs,max can be partly explained by particular morphological features of the stomata in this species. Specifically, they reported a c. sixfold reduction in stomatal pore area in mature leaves of Q. coccifera growing under Mediterranean conditions due to the accumulation of epicuticular waxes overarching the cuticular rims of the stomatal pore. Such reduction of stomatal pore area, which has a major influence on gs,max (Franks and Beerling 2009, Kaiser 2009, Dow et al. 2014), caused a strong reduction in transpiration under high vapour pressure deficit (VPD) conditions registered during the Mediterranean summer (Roth-Nebelsick et al. 2013).
Although Q. coccifera is well adapted to Mediterranean conditions, it covers a large climatic space similar to congeneric Quercus ilex L. (e.g., Peguero-Pina et al. 2014, Niinemets 2015), exceptionally even reaching the Atlantic coast of the Iberian Peninsula on south-facing limestone slopes under oceanic temperate climate conditions (Castro-Díez et al. 1997, Castro-Díez and Navarro 2007). The occurrence of Q. coccifera in these contrasting habitats is in part explained by its capacity to plastically respond to environmental variations at leaf and crown level (Rubio de Casas et al. 2007). Under these temperate conditions, with summer VPD values much lower than those registered under typical Mediterranean conditions, Roth-Nebelsick et al. (2013) reported a smaller degree of stomatal closure by cuticular wax cover, with stomatal pore area being c. 4 times higher than that found under Mediterranean conditions. This phenomenon was interpreted as a plastic response of gs,max in Q. coccifera to changes in environmental conditions during plant growth, i.e., air temperature and relative humidity, with further consequences for net CO2 assimilation (Roth-Nebelsick et al. 2013).
Species response to site climatic drivers is affected by both genetic and phenotypic (i.e., plastic) components of variation that cannot be separated in field studies (Niinemets 2015). In fact, several common garden studies have demonstrated important genetic sources of variation among Mediterranean evergreen oak ecotypes (Balaguer et al. 2001, Valladares et al. 2002a, Gimeno et al. 2009, Ramírez-Valiente et al. 2010). However, there is currently little information on adaptability of stomatal traits in Q. coccifera as driven by genetic and plastic sources of variation. Furthermore, provided that plastic components of variation dominate stomatal differentiation, the important question is when and how rapidly cuticular wax crypts can develop. There is evidence that cuticle thickness and wax deposition increase through leaf development (Pallardy and Kozlowski 1980, Hauke and Schreiber 1998), but to our knowledge there is little data on development of cuticular crypts around stomata.
Though it is well known that stomatal conductance plays a key role in determining maximum rates of carbon assimilation, other factors such as mesophyll conductance to CO2 (gm) can constrain photosynthesis and, under certain conditions, be the most significant photosynthetic limitation (Flexas et al. 2012, 2014), especially in evergreen species with inherently low gm (Niinemets et al. 2009, 2011). Recently, several studies have quantified the importance of several leaf anatomical traits (including leaf thickness, packing of mesophyll cells relative to the distance and position of stomata, chloroplast exposed surface area facing intercellular air spaces, thickness of mesophyll cell walls and chloroplast size) in determining the variability in gm and photosynthetic capacity among species (Tomás et al. 2013) or even within the same species growing under contrasting environmental conditions (Terashima et al. 2011, Peguero-Pina et al. 2015).
We hypothesise that the overall importance of cryptic stomata as a determinant of gs,max on foliage photosynthetic performance depends on how other foliage photosynthetic traits, including foliage photosynthetic capacity and gm, respond to environment, i.e., the extent to which plasticity in the amount of wax covering stomatal pores and gm and photosynthetic capacity of Q. coccifera are linked under contrasting environmental conditions. In order to test this hypothesis, the specific objectives of this study were: (i) to determine if the existence of differences in stomatal conductance has further consequences for net CO2 assimilation and other gas exchange parameters between Q. coccifera growing under contrasting environmental conditions, (ii) to determine if the modification of gs,max shown by Roth-Nebelsick et al. (2013) in Q. coccifera is mainly due to environmental effects (plastic component of variation) regardless of the genetic background, and (iii) to analyse the influences of site environmental conditions on several morphological and anatomical leaf traits of Q. coccifera that could modify gm and plant photosynthetic performance.
Materials and methods
Plant material and experimental conditions
In this study we used individuals from the same open-pollinated family in order to reduce the within-species genetic variability (Himrane et al. 2004). Seeds of Q. coccifera L. were collected from the same mother tree in a natural population of the Sistema Ibérico Meridional provenance of Spain. The seeds were sown in 2009 and cultivated in 0.5 l containers inside a greenhouse under the same conditions with a mixture of 80% compost (Neuhaus Humin Substrat N6; Klasman-Deilmann GmbH, Geeste, Germany) and 20% perlite. After the first growth cycle, seedlings were transplanted to 25 l containers filled with the same mixture of compost and perlite and cultivated outdoors at CITA de Aragón (41°39′N, 0°52′W, Zaragoza, Spain) under Mediterranean (MED) conditions (see Figure S1 available as Supplementary Data at Tree Physiology Online, mean annual temperature 15.4 °C, total annual precipitation 298 mm). Nutrients were supplied as slow-liberation fertilizer (Osmocote Plus, Sierra Chemical, Milpitas, CA, USA). The fertilizer (3 g l−1 growth substrate) was applied to the top 10-cm layer of substrate. At the end of 2011, half of these seedlings were randomly selected and moved to the Jardín Botánico de Iturrarán (43°13′N, 02°01′W, 70 m a.s.l., Gipuzkoa, Spain), which features temperate (TEM) conditions (see Figure S1 available as Supplementary Data at Tree Physiology Online, mean annual temperature 14.5 °C, total annual precipitation 1631 mm). Measurements were performed during the summer of 2012 on the current-year fully developed leaves of 4-year-old seedlings of Q. coccifera at both the MED and the TEM site.
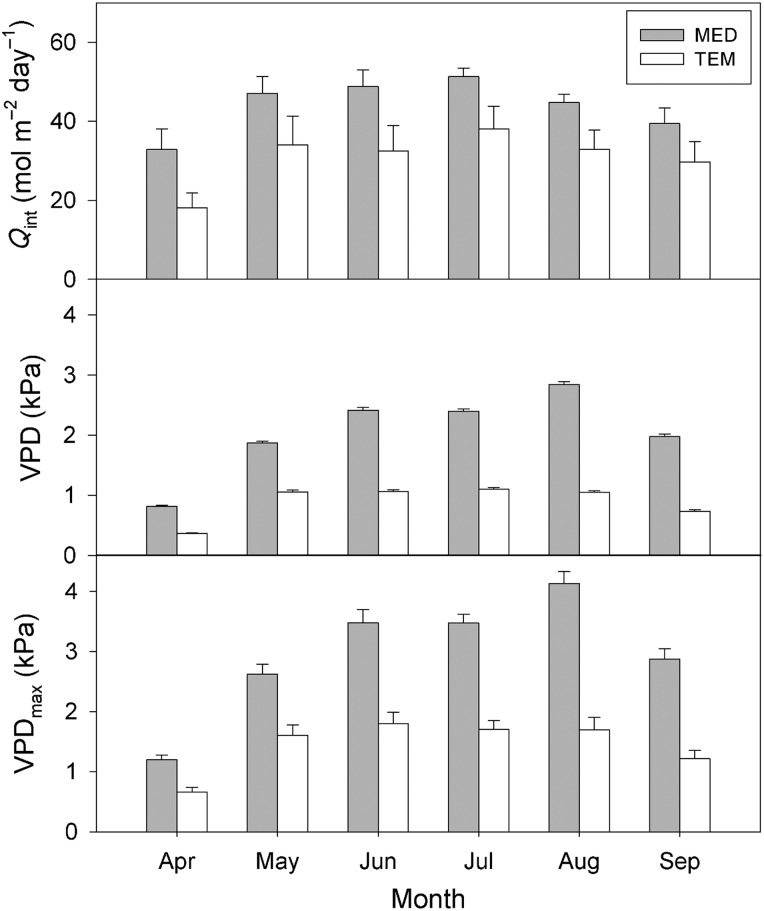
Air temperature (T, °C) and relative humidity (RH, %) were measured at both sites using three Hobo Pro temp/RH data loggers (Onset Computer, Bourne, MA, USA) located at 1.30 m above the soil surface. Measurements were recorded every 30 min during the growing season (April to September) of 2012. Vapour pressure deficit (VPD, kPa) was calculated for both sites from mean values of T and RH according to Rundel and Jarrell (1989). Mean daily quantum flux density (Qint, mol m−2 day−1) was calculated for both sites from the mean values of incident global solar radiation (Cescatti and Zorer 2003) obtained from the nearby meteorological stations for both sites. At the MED site, Qint and VPD were consistently higher than at the TEM site (P < 0.05) (Figure 1). Moreover, maximum diurnal vapour pressure deficit (VPDmax, kPa) was also higher at the MED site (P < 0.05), reaching values up to ∼4 kPa during summer (Figure 1).
Figure 1.
Mean daily quantum flux density (Qint, mol m−2 day−1), mean diurnal (from dawn to sunset) vapour pressure deficit (VPD, kPa) and maximum diurnal (from dawn to sunset) vapour pressure deficit (VPDmax, kPa) for the Mediterranean (MED) and temperate (TEM) sites during the growing season of 2012 (from April to September). Data are mean ± SE. All the values were statistically different (P < 0.05) between sites.
Stomatal pore area
The size of the orifice of the cuticular cover above stomata and the stomatal pore area of Q. coccifera leaves grown at the MED and TEM sites were measured on 20 fully mature leaves collected from four plants (five leaves from each plant) per site. To obtain the dimensions of the stomatal pore, which is usually occluded by the cuticular cover, it was necessary to remove epicuticular waxes by immersion of leaf samples twice for 30 s into chloroform at room temperature (Jetter et al. 2000). After this treatment, samples were viewed with scanning electron microscopy (VP-SEM S-3400N, Hitachi, Japan, low vacuum range 6–270 Pa) and stomatal characteristics were measured from micrographs with ImageJ software (http://rsb.info.nih.gov/nih-image/).
Analysis of changes in leaf area, leaf dry mass per area and stomatal pore area during the growing season at the MED site
At the MED site, changes in leaf area, leaf dry mass per unit area (LMA) and cuticular cover above stomata were periodically measured from 7 May 2012 (Day 1, 1 week after bud burst). Twenty leaves were collected from four plants (five leaves from each plant) at each sampling date. Leaf area was measured from scanned leaf images using ImageJ software. Leaves were then oven-dried at 70 °C for 72 h, and their dry mass was estimated. LMA was calculated as the ratio of foliage dry mass to foliage area, and was used as an estimator of sclerophylly (Castro-Díez et al. 1997, Corcuera et al. 2002, Niinemets 2015). Another set of 20 leaves was used to quantify changes in stomatal pore area due to cuticular cover. Stomatal characteristics were measured with ImageJ software from micrographs obtained with a scanning electron microscope (VP-SEM S-3400N, Hitachi, Japan, low vacuum range 6–270 Pa).
Leaf gas exchange and chlorophyll fluorescence measurements
Leaf gas exchange parameters were measured simultaneously with measurements of chlorophyll fluorescence using an open gas exchange system (CIRAS-2, PP-Systems, Amesbury, MA, USA) fitted with an automatic universal leaf cuvette (PLC6-U, PP-Systems) with an FMS II portable pulse amplitude modulated fluorometer (Hansatech Instruments Ltd, King's Lynn, UK). Six CO2 response curves were measured at both the MED and the TEM site using light-adapted mature leaves. The photosynthesis measurements started at a CO2 concentration surrounding the shoot (Ca) of 400 μmol mol−1, and a saturating photosynthetic photon flux density (PPFD) of 1500 μmol m−2 s−1. Leaf temperature and VPD were maintained at 25 °C and 1.25 kPa, respectively, during all the measurements. Once the steady state gas-exchange rate was reached under these conditions (usually in 30 min after clamping the leaf), net assimilation rate (AN), stomatal conductance (gs) and effective quantum yield of PSII (ΦPSII) were estimated. Thereafter, Ca was decreased stepwise down to 50 μmol mol−1. Upon completion of measurements at low Ca, Ca was increased again to 400 μmol mol−1 to restore the original value of AN. Ca was further increased stepwise to 1800 μmol mol−1 and all the steady-state photosynthetic characteristics were recorded at each Ca. Leakage of CO2 in and out of the cuvette was determined for the same range of CO2 concentrations with a photosynthetically inactive leaf enclosed (obtained by heating the leaf until no variable chlorophyll fluorescence was observed), and used to correct measured leaf fluxes (Flexas et al. 2007a).
For ΦPSII, steady-state fluorescence (FS) and maximum fluorescence during a light-saturating pulse of ∼8000 μmol m−2 s−1 were estimated, and ΦPSII was calculated as (Genty et al. 1989). Photosynthetic electron transport rate (Jflu) was then calculated according to Krall and Edwards (1992), multiplying ΦPSII by PPFD and by α (a term which includes the product of leaf absorbance and the partitioning of absorbed quanta between photosystems I and II); α was previously determined as the slope of the relationship between ΦPSII and (i.e., quantum efficiency of CO2 fixation) obtained by varying light intensity under non-photorespiratory conditions in an atmosphere containing <1% O2 (Valentini et al. 1995). Five photosynthesis vs PPFD response curves at both the MED and the TEM site were measured to determine α.
Estimation of mesophyll conductance by gas exchange and chlorophyll fluorescence
Mesophyll conductance (gm) was estimated according to the method of Harley et al. (1992), as follows:
| (1) |
where AN and substomatal CO2 concentration (Ci) were taken from gas exchange measurements at saturating light, whereas Γ* (chloroplastic CO2 compensation point in the absence of mitochondrial respiration) and RL (respiration rate in the light) were estimated for both sites following the methodology described in Flexas et al. (2007b). The values of gm obtained were used to convert AN–Ci into AN–Cc curves (where Cc is chloroplastic CO2 concentration) using the equation Cc = Ci − AN/gm. Maximum carboxylation and photosynthetic electron transport capacities (Vc,max and Jmax, respectively) were calculated from the AN–Cc curves, using the Rubisco kinetic constants and their temperature dependence described by Bernacchi et al. (2002). The Farquhar model was fitted to the data by applying iterative curve-fitting (minimum least-square difference) using the Solver tool of Microsoft Excel.
Morphological and anatomical measurements
After gas-exchange measurements, sections of 1 × 1 mm were cut between the main veins for anatomical measurements from the same leaves used for gas exchange. Leaf material was quickly fixed under vacuum with p-formaldehyde (2%) and glutaraldehyde (4%) in 0.1 M phosphate buffer (pH = 7.2) and post-fixed 1 h in 1% osmium tetroxide. Samples were dehydrated in (i) a graded ethanol series and (ii) propylene oxide and subsequently embedded in Embed-812 embedding medium (EMS, Hatfield, PA, USA). Semi-thin (0.8 μm) and ultrathin (90 nm) cross-sections were cut with an ultramicrotome (Reichert–Jung model Ultracut E). Semi-thin cross-sections were stained with 1% toluidine blue and viewed under a light microscope (Optika B-600TiFL, Optika Microscopes, Ponteranica, Italy). Ultrathin cross-sections were contrasted with uranyl acetate and lead citrate and viewed under a transmission electron microscope (H600, Hitachi, Japan). ImageJ software was further used to measure leaf anatomical characteristics from the micrographs. Light microscopy images were used to determine leaf thickness, mesophyll thickness between the two epidermal layers, number of palisade layers, fraction of the mesophyll tissue occupied by intercellular air spaces (fias), and mesophyll (Sm/S) and chloroplast (Sc/S) surface area facing intercellular air spaces per leaf area (Evans et al. 1994, Syvertsen et al. 1995, Tomás et al. 2013). All parameters were analysed in at least four different fields of view and in three different sections. Electron microscopy images were used to determine cell wall thickness (Tcw), cytoplasm thickness (Tcyt), chloroplast length (Lchl) and chloroplast thickness (Tchl) (Tomás et al. 2013). Three different sections and four to six different fields of view were used for measurements of each anatomical characteristic.
Leaf dry mass per area (LMA) was measured in 30 mature leaves sampled from 10 individuals per site (i.e., three leaves were randomly taken from each individual) as described above.
Mesophyll conductance modelled on the basis of anatomical characteristics
Leaf anatomical characteristics were used to estimate gm as a composite conductance for within-leaf gas and liquid components, according to the one-dimensional gas diffusion model of Niinemets and Reichstein (2003) as applied by Tosens et al. (2012):
| (2) |
where gias is the gas phase conductance inside the leaf from substomatal cavities to outer surface of cell walls, gliq is the conductance in liquid and lipid phases from outer surface of cell walls to chloroplasts, R is the gas constant (Pa m3 K−1 mol−1), Tk is the absolute temperature (K), and H is the Henry's law constant for CO2 (Pa m3 mol−1); gm is defined as a gas-phase conductance, and thus H/(RTk), the dimensionless form of the Henry's law constant, is needed to convert gliq to the corresponding gas-phase equivalent conductance (Niinemets and Reichstein 2003).
The intercellular gas-phase conductance (and the reciprocal term, rias) was obtained according to Niinemets and Reichstein (2003) as:
| (3) |
where ΔLias (m) is the average gas-phase thickness, τ is the diffusion path tortuosity (1.57 m m−1, Syvertsen et al. 1995), DA is the diffusivity of the CO2 in the air (1.51⋅10−5 m2 s−1 at 25 °C) and fias is the fraction of intercellular air spaces. ΔLias was taken as half the mesophyll thickness. Total liquid phase conductance (gliq) from the outer surface of cell walls to the carboxylation sites in chloroplasts is the sum of serial conductances in the cell wall, plasmalemma and inside the cell (Tomás et al. 2013):
| (4) |
The conductance of the cell wall was calculated as previously described in Peguero-Pina et al. (2012). For the conductance of plasma membrane we used an estimate of 0.0035 m s−1 as previously suggested (Tosens et al. 2012). The conductance inside the cell was calculated following the methodology described in Tomás et al. (2013), considering two different pathways of CO2 inside the cell: one for cell wall parts lined with chloroplasts and the other for interchloroplastial areas (Tholen et al. 2012).
Analysis of quantitative limitations of AN
To separate the relative controls on AN resulting from limited stomatal conductance (ls), mesophyll diffusion (lm) and limited photosynthetic capacity (lb), we used the quantitative limitation analysis of Grassi and Magnani (2005) as applied in Tomás et al. (2013). Different fractional limitations, ls, lm and lb (ls + lm + lb = 1) were calculated as:
| (5) |
| (6) |
| (7) |
where gs is the stomatal conductance to CO2, gm is the mesophyll conductance according to Harley et al. (1992, Eq. (1)), and gtot is the total conductance to CO2 from ambient air to chloroplasts (sum of the inverse CO2 serial conductances gs and gm). The value of ∂AN/∂Cc was calculated as the slope of AN–Cc response curves over a Cc range of 50–100 μmol mol−1. Quantitative limitations of photosynthesis were estimated for at least five different leaves of Q. coccifera at both the MED and the TEM site, and average estimates of the component photosynthetic limitations were calculated.
Leaf N, photosynthetic pigments and tocopherol analysis
Total leaf N concentration was determined in dried leaves from the MED and TEM sites using an Organic Elemental Analyzer (Flash EA 112, Thermo Fisher Scientific Inc., Waltham, MA, USA). Photosynthetic nitrogen use efficiency (PNUE) was calculated as the ratio between AN and N concentration per leaf area.
For pigment extraction six replicates per site of four leaf discs (6 mm ø) were frozen in liquid N2 and stored at −80 °C until use. Frozen samples were homogenised with a Tissue Tearor homogeniser (Model 395, Dremel, Mexico) in 1 ml of pure acetone solution buffered with CaCO3. The extracts were centrifuged at 16,100g for 20 min, and supernatants were filtered through 0.2-μm PTFE filters (Teknokroma, Barcelona, Spain). The pigments were separated by HPLC on a reversed-phase C18 column (Waters Spherisorb ODS1, 4.6 × 250 mm, Milford, MA, USA) and detected with a photodiode array detector, following the method of García-Plazaola and Becerril (1999, 2001). Tocopherol detection and quantification were performed with a Waters 474 Scanning Fluorescence Detector (SRD) operating in series with a photodiode array detector following García-Plazaola and Becerril (1999, 2001). The relative de-epoxidation state of the violaxanthin-cycle pigments was estimated by the ratio (A + Z)/(V + A + Z), abbreviated AZ/VAZ.
Statistical analyses and plasticity index
Data are expressed as mean ± standard error (SE). Student's t-test was used to compare the trait values between Q. coccifera leaves from the MED and TEM sites. One-way ANOVA was performed to compare the temporal changes in leaf area, LMA and stomatal pore area at different stages during the growing season at the MED site. Multiple comparisons were carried out among different stages for these variables using Tukey's post hoc honestly significant difference test. Furthermore, the plasticity index (PI) was calculated as the ratio between the range of variation for a parameter and its maximum value described (Valladares et al. 2002b, García-Plazaola et al. 2008). This index has the advantage that changes in variables expressed in different units can be compared. All statistical analyses were carried out using SAS version 8.0 (SAS Institute, Cary, NC, USA).
Results
Site differences in stomatal characteristics
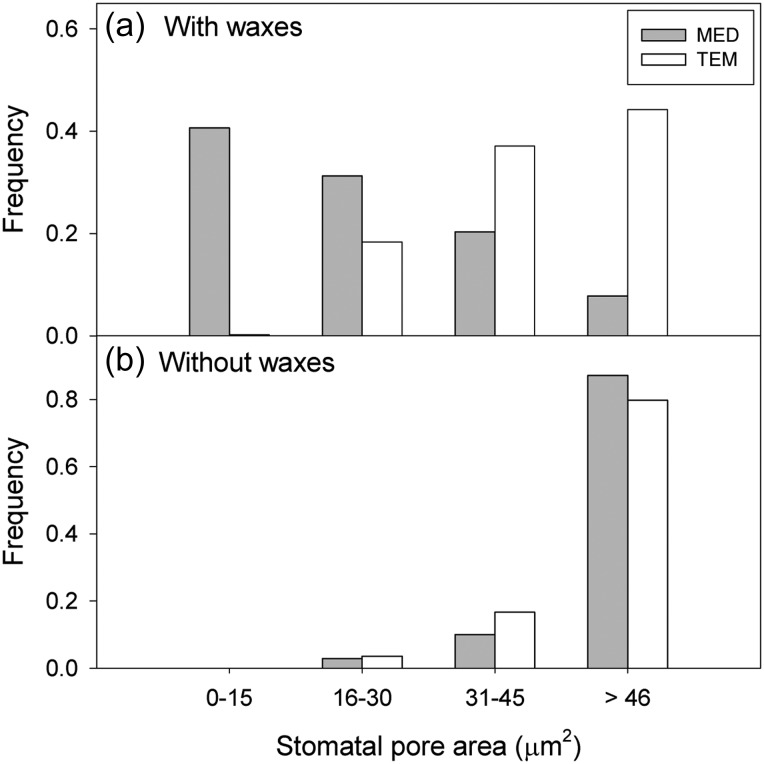
The aperture of the cuticular cup of Q. coccifera leaves from the MED site was significantly lower (P < 0.05) than that from the TEM site (Table 1). However, no significant differences (P > 0.05) were found among stomatal dimensions when epicuticular waxes were removed (Table 1). The distribution frequency of effective pore area in both sites revealed that, before wax removal, >40% of stomata at the MED site were smaller than 15 μm2 (Figure 2). By contrast, >40% of stomata were >45 μm2 for Q. coccifera leaves grown at the TEM site (Figure 2).
Table 1.
Area of the vent of the cuticular cups (stomatal pore area with waxes) and stomatal pore area without waxes of Q. coccifera leaves from the Mediterranean (MED) and temperate (TEM) sites. Data are mean ± SE. Different letters indicate statistically significant differences (P < 0.05) between sites.
| Characteristic | Stomatal pore area (μm2) |
|
|---|---|---|
| MED | TEM | |
| With waxes | 22 ± 2 b | 54 ± 4 a |
| Without waxes | 64 ± 2 a | 61 ± 2 a |
Figure 2.
Frequency distribution of stomatal pore area (μm2) for Q. coccifera leaves from the Mediterranean (MED) and temperate (TEM) sites with (a) and without (b) epicuticular waxes.
Temporal changes in foliage characteristics <at the MED site
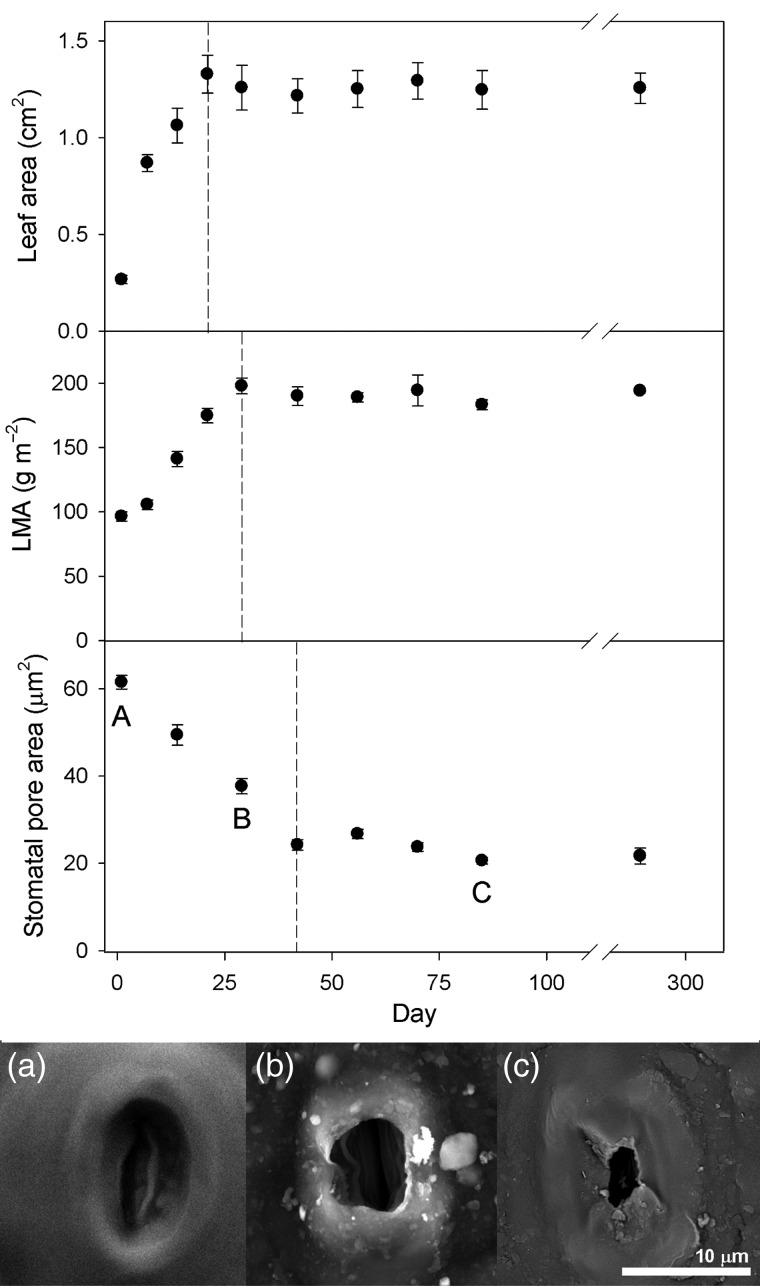
The cuticular wax deposition around stomatal pores found at the MED site was the consequence of a gradual process—in parallel with the increase in leaf area and LMA—since the onset of the growing season (Figure 3). The evolution of the different parameters indicates that leaf area reached the maximum value at Day 21 (c. 28 May), LMA at Day 29 (c. 5 June) and stomatal pore area at Day 42 (c. 18 June).
Figure 3.
Evolution of the leaf area (cm2), leaf mass area (LMA, g m−2) and stomatal pore area (μm2) for Q. coccifera leaves from the Mediterranean site (MED) since 7 May 2012 (Day 1, 1 week after bud bursting). Data are mean ± SE. Dashed lines indicate the end of the evolution of each measured parameter. Scanning electron micrographs correspond to different stages (a–c) of the evolution of the stomatal pore area due to cuticular wax covering during the growing season of 2012 at the MED site.
Site effects on foliage photosynthetic traits
Foliage photosynthetic measurements demonstrated that at 400 μmol CO2 mol−1 air and saturating light, gs was much higher at the TEM site (0.243 mol CO2 m−2 s−1) than at the MED site (0.143 mol CO2 m−2 s−1) (Table 2). Despite this, AN was very similar at both sites, and therefore, intrinsic water use efficiency (iWUE = AN/gs) was lower at the TEM site (Table 2). The mesophyll conductance to CO2 (gm) and the substomatal CO2 concentration (Ci) did not show statistically significant differences between the sites, whereas the chloroplastic CO2 concentration (Cc) was higher at the MED site (Table 2). Parameterisation of the Farquhar et al. (1980) model of photosynthesis yielded statistically significant higher values for Vc,max_Cc and Jmax_Cc at the TEM site, although the ratio Jmax_Cc:Vc,max_Cc did not show differences between sites (Table 2). The electron transport rate estimated by chlorophyll fluorescence (Jflu) was also higher at the TEM site (Table 2).
Table 2.
Mean values for the photosynthetic parameters of Q. coccifera leaves from the Mediterranean (MED) and temperate (TEM) sites. Data are mean ± SE. Different letters indicate statistically significant differences (P < 0.05) between sites. AN, net photosynthesis; gs, stomatal conductance; iWUE, intrinsic water use efficiency; gm, mesophyll conductance to CO2; Ci, substomatal CO2 concentration; Cc, chloroplastic CO2 concentration; Vc,max_Cc and Jmax_Cc, maximum velocity of Rubisco carboxylation and maximum capacity for electron transport; Jflu, electron transport rate estimated by chlorophyll fluorescence.
| Parameter | MED | TEM |
|---|---|---|
| AN (µmol CO2 m−2 s−1) | 10.2 ± 0.7 a | 12.4 ± 1.3 a |
| gs (mol CO2 m−2 s−1) | 0.143 ± 0.013 b | 0.243 ± 0.011 a |
| iWUE (µmol CO2 mol−1 H2O) | 46.8 ± 4.9 a | 31.6 ± 2.5 b |
| gm (mol CO2 m−2 s−1) | 0.050 ± 0.006 a | 0.050 ± 0.007 a |
| Ci (µmmol CO2 mol−1 air) | 304 ± 5 b | 312 ± 5 a |
| Cc (µmmol CO2 mol−1 air) | 120 ± 5 a | 74 ± 3 b |
| Vc,max_Cc (µmol m−2 s−1) | 178 ± 11 b | 258 ± 12 a |
| Jmax_Cc (µmol m−2 s−1) | 228 ± 21 b | 327 ± 17 a |
| Jflu (µmol m−2 s−1) | 216 ± 12 b | 363 ± 25 a |
| Jmax_Cc:Vc,max_Cc | 1.27 ± 0.06 a | 1.33 ± 0.07 a |
Differences in leaf morphological and anatomical characteristics between sites and implications for mesophyll conductance
Leaf thickness, LMA, total mesophyll thickness, spongy and palisade mesophyll thickness, number of palisade layers, Sm/S and Sc/S were higher at the MED site, whereas fias was higher at the TEM site (Table 3). However, no differences were found in Sc/Sm, Tcw, Tcyt, Lchl and Tchl between sites (Table 3).
Table 3.
Leaf mass area (LMA), leaf thickness, total mesophyll thickness, spongy and palisade mesophyll thickness, number of palisade layers, fraction of the mesophyll tissue occupied by the intercellular air spaces (fias), mesophyll surface area exposed to intercellular airspace (Sm/S), chloroplast surface area exposed to intercellular airspace (Sc/S), the ratio Sc/Sm, cell wall thickness (Tcw), cytoplasm thickness (Tcyt), chloroplast length (Lchl) and chloroplast thickness (Tchl) in Q. coccifera leaves from the Mediterranean (MED) and temperate (TEM) sites. Data are mean ± SE. Different letters indicate statistically significant differences (P < 0.05) between sites.
| Parameter | MED | TEM |
|---|---|---|
| LMA (g m−2) | 189.3 ± 6.5 a | 138.8 ± 6.3 b |
| Leaf thickness (μm) | 315 ± 8 a | 231 ± 3 b |
| Total mesophyll thickness (μm) | 278 ± 17 a | 195 ± 6 b |
| Spongy mesophyll thickness (μm) | 143 ± 5 a | 113 ± 3 b |
| Palisade mesophyll thickness (μm) | 135 ± 4 a | 83 ± 3 b |
| Number of palisade layers | 3 | 2 |
| fias | 0.08 ± 0.01 b | 0.14 ± 0.02 a |
| Sm/S (m2 m−2) | 17.3 ± 2.6 a | 13.3 ± 1.5 b |
| Sc/S (m2 m−2) | 9.9 ± 1.6 a | 6.1 ± 0.9 b |
| Sc/Sm | 0.57 ± 0.02 a | 0.47 ± 0.08 a |
| Tcw (μm) | 0.454 ± 0.026 a | 0.446 ± 0.016 a |
| Tcyt (μm) | 0.063 ± 0.026 a | 0.087 ± 0.028 a |
| Lchl (μm) | 5.13 ± 0.23 a | 5.10 ± 0.17 a |
| Tchl (μm) | 2.43 ± 0.13 a | 2.27 ± 0.09 a |
The anatomical characteristics were further used to estimate different components of the CO2 transfer conductances relative to total mesophyll conductance at both sites (see Materials and methods for details), resulting in a good correspondence between the estimates of gm from anatomy (Eqs (2–4)) and from gas exchange and chlorophyll fluorescence measurements (Eq. (1), Table 2). In the case of the gas phase, the results demonstrated that Q. coccifera leaves grown at the TEM site showed higher values of gias than those at the MED site (Table 4), which can be attributed to a higher fias and a lower mesophyll thickness found at the TEM site (Table 3). Regarding the liquid phase, Q. coccifera leaves grown at the MED site showed higher values of gliq than at the TEM site (Table 4), which can be attributed to a higher Sm/S value found at the MED site (Table 3). Consequently, the effects of gias and gliq on gm were opposite, explaining the absence of differences in the estimated value of gm between sites (Table 4).
Table 4.
CO2 transfer conductances across the intercellular air space (gias, m s−1), the liquid phase (gliq, m s−1), and the mesophyll conductance for CO2 (gm, mol m−2 s−1) calculated from anatomical measurements in Q. coccifera leaves from the Mediterranean (MED) and temperate (TEM) sites. Data are mean ± SE. Different letters indicate statistically significant differences (P < 0.05) between sites.
| Parameter | MED | TEM |
|---|---|---|
| gias (m s−1) | 0.0056 ± 0.0007 b | 0.0137 ± 0.0010 a |
| gliq (m s−1) | 0.0020 ± 0.0002 a | 0.0015 ± 0.0001 b |
| gm (mol m−2 s−1) | 0.057 ± 0.009 a | 0.055 ± 0.008 a |
Site effects on foliage chemistry
The concentration of leaf N was higher at the MED site, in terms of both dry mass and leaf area (Table 5). However, because AN values were similar between sites, TEM site plants showed higher photosynthetic nitrogen-use efficiency (PNUE) than MED site plants (Table 5). Pigment composition differed largely between leaves from the TEM and MED sites (Table 6). Thus, despite the higher LMA and thickness at the MED site, chlorophyll a content per unit of leaf area was higher at the TEM site. Chlorophyll a/b together with all the carotenoid to chlorophyll ratios also differed significantly, being higher for all parameters, except for the neoxanthin/chlorophyll ratio, at the MED site. According to the higher contents of protective carotenoids (violaxanthin-cycle total pool, lutein and β-carotene) at the MED site, the α-tocopherol to chlorophyll ratio and the de-epoxidation index of the xanthophyll cycle (AZ/VAZ) were also two- to threefold higher at the MED site.
Table 5.
Leaf N content per dry mass and per area and photosynthetic nitrogen use efficiency (PNUE) for Q. coccifera leaves from the Mediterranean (MED) and temperate (TEM) sites. Data are mean ± SE. Different letters indicate statistically significant differences (P < 0.05) between sites.
| Parameter | MED | TEM |
|---|---|---|
| N content per dry mass (%) | 1.37 ± 0.05 a | 1.21 ± 0.04 b |
| N content per area (mol m−2) | 0.18 ± 0.02 a | 0.12 ± 0.01 b |
| PNUE (µmol mol−1 s−1) | 55.3 ± 4.0 b | 103.3 ± 10.9 a |
Table 6.
Photosynthetic pigment and tocopherol contents for Q. coccifera leaves expressed per leaf area or per total chlorophyll content from the Mediterranean (MED) and temperate (TEM) sites. Data are mean ± SE. Different letters indicate statistically significant differences (P < 0.05) between sites. Chl, chlorophyll; Neo, neoxanthin; Lut, lutein; β-Car, β-carotene; α-Toc, α-tocopherol; VAZ, violaxanthin-cycle total pool; AZ/VAZ, de-epoxidation state of the violaxanthin-cycle pigments.
| Parameter | MED | TEM |
|---|---|---|
| Chl a (µmol m−2) | 318.2 ± 20.6 a | 393.1 ± 52.0 a |
| Chl b (µmol m−2) | 86.6 ± 6.8 a | 113.4 ± 13.8 a |
| Chl a/b | 3.7 ± 0.1 a | 3.4 ± 0.1 b |
| Neo/Chl (mmol mol−1) | 37.7 ± 0.6 b | 40.2 ± 0.7 a |
| Lut/Chl (mmol mol−1) | 126.8 ± 2.8 a | 106.6 ± 2.2 b |
| VAZ/Chl (mmol mol−1) | 88.1 ± 5.3 a | 64.5 ± 2.4 b |
| AZ/VAZ | 0.384 ± 0.048 a | 0.123 ± 0.008 b |
| β-Car/Chl (mmol mol−1) | 111.2 ± 0.9 a | 103.4 ± 1.5 b |
| α-Toc/Chl (mmol mol−1) | 1132.0 ± 71.3 a | 525.5 ± 57.2 b |
Discussion
Mediterranean climate constitutes a highly stressful environment for plants that has triggered a number of unique structural and physiological adaptations (Corcuera et al. 2002, Flexas et al. 2014, Niinemets and Keenan 2014). While such adaptation is highly beneficial in improving water use efficiency, constitutive expression of many of these traits, such as cryptic stomata, would reduce carbon gain when water becomes more available. The present study has demonstrated the existence of a plastic response in functional attributes of Q. coccifera leaves (see Table S1 available as Supplementary Data at Tree Physiology Online) to changes in environmental conditions (i.e., Qint and VPD) during growth, which agrees with previous studies on phenotypic responses of Q. coccifera saplings (Balaguer et al. 2001, Valladares et al. 2002a, 2005). The climatic conditions that demarcate the TEM and MED sites, both in terms of Qint and VPD (Figure 1), can be considered as the extremes of the Atlantic–Mediterranean gradient along which Q. coccifera is distributed (Castro-Díez et al. 1997).
The individual encryption of stomata by epicuticular waxes in Q. coccifera leaves grown at the MED site strongly reduced the area of the vent of the cuticular cup when compared with the TEM site (Table 1 and Figure 2). It should be noted that the process of stomatal wax covering at the MED site finished in late spring (Figure 3), while VPD values started to reach the maximum values registered during summer (Figure 1). Interestingly, both sites only differed in the degree of encryption, being identical in the dimensions of stomatal pore area after wax removal (Table 1 and Figure 2), as previously showed by Roth-Nebelsick et al. (2013) for this species. In this regard, the epicuticular wax deposition found at the MED site implied a sharp decrease in gs (c. 0.6 times) with respect to the TEM site (Table 2), which constitutes an effective mechanism for reducing water losses while keeping stomata open.
In spite of this, maximum water losses by transpiration (E) during midsummer would be c. 2 times higher here than at the TEM site (data not shown), due to the higher VPD found at the MED site (Figure 1). This fact reveals that this xeromorphic trait in Q. coccifera can only partially mitigate the extreme water vapour gradient between the mesophyll and the surrounding atmosphere found at the MED site. Consequently, water use efficiency (WUE)—expressed as the ratio between AN and E—is expected to be c. 2 times higher at the TEM site, but iWUE is instead larger at the MED site, as commonly found in plants of dry habitats (Field et al. 1983). These facts indicate that water losses per unit of carbon uptake for Q. coccifera living at the most arid extreme of its climatic gradient (the MED site) cannot be diminished to the level of the TEM site, even with such a large reduction in stomatal pore size by epicuticular wax deposition. In order to achieve WUE values similar to the TEM site, gs at the MED site should be ∼0.1 mol H2O m−2 s−1, which would imply a reduction in AN of c. threefold, as reported by Peguero-Pina et al. (2009) for this species growing under Mediterranean conditions.
Contrary to that suggested by Roth-Nebelsick et al. (2013) from modelled data, the absence of such extra resistance in stomata of Q. coccifera leaves grown at the TEM site did not imply an increased ability to take up carbon (Table 2). Therefore, the existence of adjustments in other traits influencing net CO2 assimilation should be considered. One of the key traits that determine the maximum photosynthetic rate is gm, which often is the most significant limitation on photosynthesis, especially in evergreens (Flexas et al. 2012, Galmés et al. 2014, Niinemets and Keenan 2014). In this way, the analysis of the quantitative limitations of photosynthesis revealed that AN in Q. coccifera was mainly limited by gm both at the MED (65%) and at the TEM site (76%) (data not shown). Moreover, contrary to gs, gm in Q. coccifera did not exhibit a plastic response to changes in growth environmental conditions (Table 2). Therefore, the lack of a plastic response in the most limiting factor to net CO2 assimilation in Q. coccifera—i.e., mesophyll conductance—may be one of the causes that explains the lack of differences in AN between the sites.
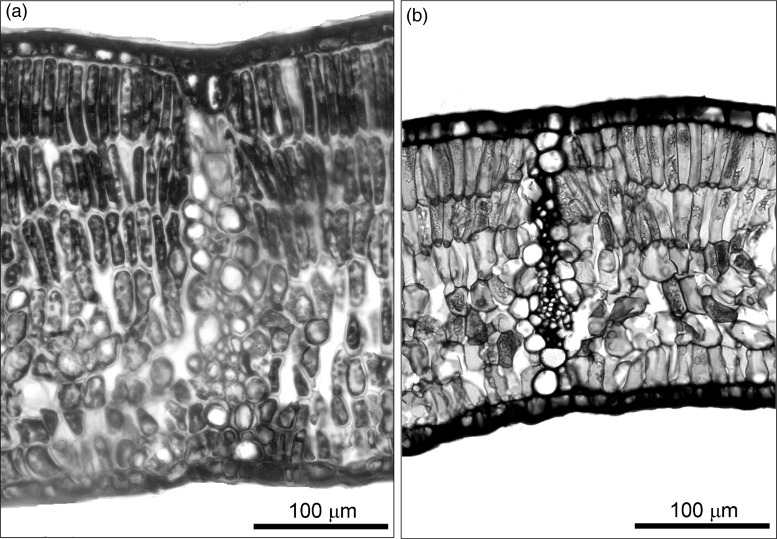
Besides the sharp increase in gs, several anatomical leaf traits in Q. coccifera also showed a plastic response to changes in growth environmental conditions, especially mesophyll thickness, fias, Sm/S and Sc/S (Table 3 and Figure 4). Recently, several studies have quantitatively determined the importance of these leaf anatomical traits in determining the variability in gm and photosynthetic capacity among species (see Tomás et al. 2013 and references therein). According to the one-dimensional gas diffusion model used to estimate gm from leaf anatomical characteristics (Niinemets and Reichstein 2003, Tosens et al. 2012), the higher gias (due to higher fias and lower mesophyll thickness) counteracted the lower gliq (due to lower Sm/S) in Q. coccifera leaves grown at the TEM site when compared with the MED site (Table 4). Consequently, this trade-off resulted in the same estimated value of gm for both sites (Table 4) which, as stated above, may be one of the causal factors explaining the lack of differences in net CO2 assimilation. On the other hand, no differences were found in ultrastructural anatomical traits influencing gm, such as cell wall and chloroplast thickness (Table 3). Recently, Peguero-Pina et al. (2015) have found changes in these anatomical traits in Abies pinsapo in response to changes in light environment that strongly determined changes in gm. However, it should be noted that the light gradients found by Peguero-Pina et al. (2015) were much higher than the differences in Qint found between the MED and TEM sites of Q. coccifera.
Figure 4.
Transverse section of the mesophyll of Q. coccifera from the Mediterranean (MED; a) and temperate (TEM; b) sites.
The changes in morphological and anatomical leaf traits found in the present study agree with those previously found in other studies in response to growth irradiance in this (Balaguer et al. 2001, Valladares et al. 2002a) and other species (Oguchi et al. 2003, Terashima et al. 2011). These previous studies also found changes at biochemical level in response to changes in growth irradiance that point in the same direction as those found in the present study (Table 5). In this regard, the lower N at the TEM site could constitute an additional limiting factor for net CO2 assimilation. On the other hand, the chlorophyll a/b ratio was lower at the TEM site (Table 6), suggesting a larger antenna size (Esteban et al. 2015). This conclusion was also supported by the higher content of neoxanthin, a carotenoid that is mostly bound to the outer trimeric antenna proteins (Morosinotto and Bassi 2012). These observations are indicative of a larger allocation of N to light harvesting proteins in the less stressful TEM site.
The combination of lower gs and higher growth irradiance at the MED site can be potentially harmful for the maintenance of photosynthetic function. In fact, comparison of pigment composition between both sites showed that at the MED site the structure of photosynthetic apparatus had a greater capacity for dissipating the excess of light energy than at the TEM site, being characterised by a higher content of protective carotenoids (β-carotene, lutein and violaxanthin-cycle total pool) (Esteban et al. 2015). Furthermore, this plastic photo-protective response was maximised for the content of lipophilic antioxidants (α-tocopherol) and AZ/VAZ. The exceptional relevance of these lipophilic photo-protective mechanisms has been observed in this and other Mediterranean evergreens in response to climatic extremes (García-Plazaola et al. 2003, 2008, Camarero et al. 2012).
In agreement with pigment data, photosynthesis on a mass basis was clearly higher at the TEM site (0.089 μmol CO2 g−1 s−1) than at MED site (0.054 μmol CO2 g−1 s−1). This is a direct consequence of the reduction in LMA found at the TEM site (Table 3) and is in line with predictions of leaf economics spectrum (Wright et al. 2004). Previously, such a within-species shift within the economics spectrum has been developed for congeneric Q. ilex across its bioclimatic range (Niinemets 2015). Moreover, PNUE was also higher at the TEM site (Table 5), indicating that leaves developed under the environmental conditions of the TEM site can reach similar values of AN with lower nitrogen investment that those leaves grown at the most arid extreme of its climatic gradient (MED). In this regard, it can be discussed how long the higher nitrogen content on an area basis associated with a higher thickness at the MED site has a direct counterpart in terms of AN. So-called ‘resource substitution’ postulates the existence of a trade-off between stomatal conductance and foliar N that has been linked to changes in photosynthetic rate (Buckley and Roberts 2006, Taylor and Eamus 2008). According to this, similar AN values might be reached at the expense of a higher N investment, compensating for the lower gs at the MED site, as leaf nitrogen concentration is often correlated with leaf photosynthetic rates (see Meziane and Shipley 2001 and references therein). On the other hand, as stated above, the same values of AN for different gs in both sites may be the consequence of a similar gm, which can be explained by the higher Sm/S associated with the higher mesophyll thickness found at the MED site. Previous studies have already related the increase in leaf thickness in Q. coccifera to xeric habitats (Balaguer et al. 2001, Valladares et al. 2002a). Another factor directly related to leaf construction cost is the higher amount of epicuticular wax production associated with stomatal covering at the MED site (Villar and Merino 2001), which has been associated with a conservative leaf economics spectrum strategy (Mason and Donovan 2015).
Conclusions
From an ecological perspective, Q. coccifera has been considered the main component of the climax vegetation under climatic conditions that correspond to the MED site, while its presence is assumed to be merely marginal in areas under climates similar to that defining the TEM site (Castro-Díez et al. 1997). This seems to be contradictory to the better physiological performance in terms of WUE, PNUE and photosynthesis on a mass basis of Q. coccifera found at the TEM site. A lower investment in N and dry matter for a given leaf area at the TEM site would constitute an advantage in terms of leaf construction costs (Merino et al. 1982) A possible explanation for this apparent paradox is that Q. coccifera can compete successfully in stressful environments such as the MED site due to its ability to withstand long and intense drought periods (i) without signs of irreversible damage in either the apoplast or the symplast (Vilagrosa et al. 2010), (ii) through a high resistance to drought-induced cavitation (Vilagrosa et al. 2003), and (iii) through the exceptional capacity for activation of drought-mediated photo-protective mechanisms (García-Plazaola et al. 2008, Peguero-Pina et al. 2009). The results found in the present study suggest that Q. coccifera, a Mediterranean evergreen oak species, does not always use the main resources (water and nutrients) at leaf level as efficiently as possible. In this, it would be interesting to consider the existence of this adaptation process at the whole-plant level, which is a matter that deserves further investigation.
Overall, in the case study reported here, the different patterns of resource use (in particular N) together with functional plasticity are not able to overcome the morpho-functional constraints that limit photosynthetic activity, even under potentially favourable conditions. This limitation in the expression of phenotypic plasticity probably represents an example of genetic canalisation (Valladares et al. 2002a), a process frequent in unstable and unpredictable environments such as the Mediterranean ecosystems.
Supplementary data
Supplementary data for this article are available at Tree Physiology Online.
Conflict of interest
None declared.
Funding
Financial support is acknowledged from Gobierno de Aragón (H38 research group) and by the Spanish Ministry of Economy and Competitiveness (MINECO) (Research grant BFU 2010-15021 co-funded by the ERDF-FEDER). The work of D.S.-K. is supported by a DOC INIA contract co-funded by the Spanish National Institute for Agriculture and Food Research and Technology (INIA) and the European Social Fund (ESF). Financial support to B.F.-M. is acknowledged from the Research Vicerrectorate (UPV/EHU) and the EU (Marie Curie Action FP7-PEOPLE-2012-IEF 328370 ‘MELISSA’). J.F. acknowledges support from Plan Nacional, Spain (project CTM2014-53902-C2-1-P).
Supplementary Material
Acknowledgments
The authors are grateful to José Almandoz and the people of Jardín Botánico de Iturrarán for their help in the maintenance of plant material.
References
- Balaguer L, Martínez-Ferri E, Valladares F, Pérez-Corona ME, Baquedano FJ, Castillo FJ, Manrique E (2001) Population divergence in the plasticity of the response of Quercus coccifera to the light environment. Funct Ecol 15:124–135. doi:10.1046/j.1365-2435.2001.00505.x [Google Scholar]
- Bernacchi CJ, Portis AR, Nakano H, von Caemmerer S, Long SP (2002) Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiol 130:1992–1998. doi:10.1104/pp.008250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley TN, Roberts DW (2006) How should leaf area, sapwood area and stomatal conductance vary with tree height to maximize growth? Tree Physiol 26:145–157. doi:10.1093/treephys/26.2.145 [DOI] [PubMed] [Google Scholar]
- Camarero JJ, Olano JM, Arroyo SJ, Fernández-Marín B, Becerril JM, García-Plazaola JI (2012) Photoprotection mechanisms in Quercus ilex under contrasting climatic conditions. Flora 207:557–564. doi:10.1016/j.flora.2012.06.003 [Google Scholar]
- Castro-Díez P, Navarro J (2007) Water relations of seedlings of three Quercus species: variations across and within species grown in contrasting light and water regimes. Tree Physiol 27:1011–1018. doi:10.1093/treephys/27.7.1011 [DOI] [PubMed] [Google Scholar]
- Castro-Díez P, Villar-Salvador P, Pérez-Rontomé C, Maestro-Martínez M, Montserrat-Martí G (1997) Leaf morphology and leaf chemical composition in three Quercus (Fagaceae) species along a rainfall gradient in NE Spain. Trees 11:127–134. [Google Scholar]
- Cescatti A, Zorer R (2003) Structural acclimation and radiation regime of silver fir (Abies alba Mill.) shoots along a light gradient. Plant Cell Environ 26:429–442. doi:10.1046/j.1365-3040.2003.00974.x [Google Scholar]
- Corcuera L, Camarero JJ, Gil-Pelegrín E (2002) Functional groups in Quercus species derived from the analysis of pressure–volume curves. Trees 16:465–472. doi:10.1007/s00468-002-0187-1 [Google Scholar]
- Dow GJ, Bergmann DC, Berry JA (2014) An integrated model of stomatal development and leaf physiology. New Phytol 201:<1218–1226. doi:10.1111/nph.12608 [DOI] [PubMed] [Google Scholar]
- Esteban R, Barrutia O, Artetxe U, Fernández-Marín B, Hernández A, García-Plazaola JI (2015) Internal and external factors affecting photosynthetic pigment composition in plants: a meta-analytical approach. New Phytol 206:268–280. doi:10.1111/nph.13186 [DOI] [PubMed] [Google Scholar]
- Evans JR, von Caemmerer S, Setchell BA, Hudson GS (1994) The relationship between CO2 transfer conductance and leaf anatomy in transgenic tobacco with a reduced content of Rubisco. Aust J Plant Physiol 21:475–495. doi:10.1071/PP9940475 [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90. doi:10.1007/BF00386231 [DOI] [PubMed] [Google Scholar]
- Field C, Merino J, Mooney HA (1983) Compromises between water-use efficiency and nitrogen-use efficiency in five species of California evergreens. Oecologia 60:384–389. doi:10.1007/BF00376856 [DOI] [PubMed] [Google Scholar]
- Flexas J, Díaz-Espejo A, Berry JA, Galmés J, Cifre J, Kaldenhoff R, Medrano H, Ribas-Carbó M (2007a) Analysis of leakage in IRGA's leaf chambers of open gas exchange systems: quantification and its effects in photosynthesis parameterization. J Exp Bot 58:1533–1543. doi:10.1093/jxb/erm027 [DOI] [PubMed] [Google Scholar]
- Flexas J, Ortuño MF, Ribas-Carbó M, Díaz-Espejo A, Flórez-Sarasa ID, Medrano H (2007b) Mesophyll conductance to CO2 in Arabidopsis thaliana. New Phytol 175:501–511. doi:10.1111/j.1469-8137.2007.02111.x [DOI] [PubMed] [Google Scholar]
- Flexas J, Barbour MM, Brendel O et al. (2012) Mesophyll diffusion conductance to CO2: an unappreciated central player in photosynthesis. Plant Sci 193–194:70–84. doi:10.1016/j.plantsci.2012.05.009 [DOI] [PubMed] [Google Scholar]
- Flexas J, Díaz-Espejo A, Gago J, Gallé A, Galmés J, Gulías J, Medrano H (2014) Photosynthetic limitations in Mediterranean plants: a review. Environ Exp Bot 103:12–23. doi:10.1016/j.envexpbot.2013.09.002 [Google Scholar]
- Franks PJ, Beerling DJ (2009) Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc Natl Acad Sci USA 106:10343–10347. doi:10.1073/pnas.0904209106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galmés J, Andralojc PJ, Kapralov MV, Flexas J, Keys AJ, Molins A, Parry MAJ, Conesa MÀ (2014) Environmentally driven evolution of Rubisco and improved photosynthesis and growth within the C3 genus Limonium (Plumbaginaceae). New Phytol 203:989–999. doi:10.1111/nph.12858 [DOI] [PubMed] [Google Scholar]
- García-Plazaola JI, Becerril JM (1999) A rapid high-performance liquid chromatography method to measure lipophilic antioxidants in stressed plants: simultaneous determination of carotenoids and tocopherols. Phytochem Anal 10:307–313. [Google Scholar]
- García-Plazaola JI, Becerril JM (2001) Seasonal changes in photosynthetic pigments and antioxidants in beech (Fagus sylvatica) in a Mediterranean climate: implications for tree decline diagnosis. Aust J Plant Physiol 28:225–232. [Google Scholar]
- García-Plazaola JI, Olano JM, Hernández A, Becerril JM (2003) Photoprotection in evergreen Mediterranean plants during sudden periods of intense cold weather. Trees 17:285–291. [Google Scholar]
- García-Plazaola JI, Esteban R, Hormaetxe K, Fernández-Marín B, Becerril JM (2008) Photoprotective responses of Mediterranean and Atlantic trees to the extreme heat-wave of summer 2003 in Southwestern Europe. Trees 22:385–392. doi:10.1007/s00468-007-0199-y [Google Scholar]
- Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92. doi:10.1016/S0304-4165(89)80016-9 [Google Scholar]
- Gimeno TE, Pias B, Lemos-Filho JP, Valladares F (2009) Plasticity and stress tolerance override local adaptation in the responses of Mediterranean holm oak seedlings to drought and cold. Tree Physiol 29:87–98. doi:10.1093/treephys/tpn007 [DOI] [PubMed] [Google Scholar]
- Grassi G, Magnani F (2005) Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ 28:834–849. doi:10.1111/j.1365-3040.2005.01333.x [Google Scholar]
- Harley PC, Loreto F, Di Marco G, Sharkey TD (1992) Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiol 98:1429–1436. doi:10.1104/pp.98.4.1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauke V, Schreiber L (1998) Ontogenetic and seasonal development of wax composition and cuticular transpiration of ivy (Hedera helix L.) sun and shade leaves. Planta 207:67–75. doi:10.1007/s004250050456 [Google Scholar]
- Himrane H, Camarero JJ, Gil-Pelegrín E (2004) Morphological and ecophysiological variation of the hybrid oak Quercus subpyrenaica (Q. faginea × Q. pubescens). Trees 18:566–575. doi:10.1007/s00468-004-0340-0 [Google Scholar]
- Jetter R, Schäffer S, Riederer M (2000) Leaf cuticular waxes are arranged in chemically and mechanically distinct layers: evidence from Prunus laurocerasus L. Plant Cell Environ 23:619–628. doi:10.1046/j.1365-3040.2000.00581.x [Google Scholar]
- Kaiser H. (2009) The relation between stomatal aperture and gas exchange under consideration of pore geometry and diffusional resistance in the mesophyll. Plant Cell Environ 32:1091–1098. doi:10.1111/j.1365-3040.2009.01990.x [DOI] [PubMed] [Google Scholar]
- Krall JP, Edwards GE (1992) Relationship between photosystem II activity and CO2 fixation in leaves. Physiol Plant 86:180–187. doi:10.1111/j.1399-3054.1992.tb01328.x [Google Scholar]
- Mason CM, Donovan LA (2015) Does investment in leaf defenses drive changes in leaf economic strategy? A focus on whole-plant ontogeny. Oecologia 177:1053–1066. doi:10.1007/s00442-014-3177-2 [DOI] [PubMed] [Google Scholar]
- Merino J, Field C, Mooney HA (1982) Construction and maintenance costs of Mediterranean-climate evergreen and deciduous leaves. I. Growth and CO2 exchange analysis. Oecologia 53:208–213. doi:10.1007/BF00545665 [DOI] [PubMed] [Google Scholar]
- Meziane D, Shipley B (2001) Direct and indirect relationships between specific leaf area, leaf nitrogen and leaf gas exchange. Effects of irradiance and nutrient supply. Ann Bot 88:915–927. doi:10.1006/anbo.2001.1536 [Google Scholar]
- Morosinotto T, Bassi R (2012) Assembly of light harvesting pigment-protein complexes in photosynthetic eukaryotes. In: Eaton-Rye JJ, Tripathy BC, Sharkey TD (eds) Photosynthesis: plastid biology, energy conversion and carbon assimilation. Advances in Photosynthesis and Respiration 34. Springer, Dordrecht, pp 113–126. [Google Scholar]
- Niinemets Ü. (2015) Is there a species spectrum within the world-wide leaf economics spectrum? Major variations in leaf functional traits in the Mediterranean sclerophyll Quercus ilex. New Phytol 205:79–96. doi:10.1111/nph.13001 [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Keenan T (2014) Photosynthetic responses to stress in Mediterranean evergreens: mechanisms and models. Environ Exp Bot 103:24–41. doi:10.1016/j.envexpbot.2013.11.008 [Google Scholar]
- Niinemets Ü, Reichstein M (2003) Controls on the emission of plant volatiles through stomata: a sensitivity analysis. J Geophys Res 108:4211 doi:10.1029/2002JD002626 [Google Scholar]
- Niinemets Ü, Díaz-Espejo A, Flexas J, Galmés J, Warren CR (2009) Role of mesophyll diffusion conductance in constraining potential photosynthetic productivity in the field. J Exp Bot 60:2249–2270. doi:10.1093/jxb/erp036 [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Flexas J, Peñuelas J (2011) Evergreens favored by higher responsiveness to increased CO2. Trends Ecol Evol 26:136–142. doi:10.1016/j.tree.2010.12.012 [DOI] [PubMed] [Google Scholar]
- Oguchi R, Hikosaka K, Hirose T (2003) Does the photosynthetic light-acclimation need change in leaf anatomy? Plant Cell Environ 26:505–512. doi:10.1046/j.1365-3040.2003.00981.x [Google Scholar]
- Ozturk M, Dogan Y, Sakcali MS, Doulis A, Karam F (2010) Ecophysiological responses of some maquis (Ceratonia siliqua L., Olea oleaster Hoffm. & Link, Pistacia lentiscus and Quercus coccifera L.) plant species to drought in the east Mediterranean ecosystem. J Environ Biol 31:233–45. [PubMed] [Google Scholar]
- Pallardy SG, Kozlowski TT (1980) Cuticle development in the stomatal region of Populus clones. New Phytol 85:363–368. doi:10.1111/j.1469-8137.1980.tb03174.x [Google Scholar]
- Peguero-Pina JJ, Morales F, Flexas J, Gil-Pelegrín E, Moya I (2008) Photochemistry, remotely sensed physiological reflectance index and de-epoxidation state of the xanthophyll cycle in Quercus coccifera under intense drought. Oecologia 156:1–11. doi:10.1007/s00442-007-0957-y [DOI] [PubMed] [Google Scholar]
- Peguero-Pina JJ, Sancho-Knapik D, Morales F, Flexas J, Gil-Pelegrín E (2009) Differential photosynthetic performance and photoprotection mechanisms of three Mediterranean evergreen oaks under severe drought stress. Funct Plant Biol 36:453–462. doi:10.1071/FP08297 [DOI] [PubMed] [Google Scholar]
- Peguero-Pina JJ, Flexas J, Galmés J, Niinemets Ü, Sancho-Knapik D, Barredo G, Villarroya D, Gil-Pelegrín E (2012) Leaf anatomical properties in relation to differences in mesophyll conductance to CO2 and photosynthesis in two related Mediterranean Abies species. Plant Cell Environ 35:2121–2129. doi:10.1111/j.1365-3040.2012.02540.x [DOI] [PubMed] [Google Scholar]
- Peguero-Pina JJ, Sancho-Knapik D, Barrón E, Camarero JJ, Vilagrosa A, Gil-Pelegrín E (2014) Morphological and physiological divergences within Quercus ilex support the existence of different ecotypes depending on climatic dryness. Ann Bot 114:301–313. doi:10.1093/aob/mcu108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peguero-Pina JJ, Sancho-Knapik D, Flexas J, Galmés J, Niinemets Ü, Gil-Pelegrín E (2015) Light acclimation of photosynthesis in two closely related firs (Abies pinsapo Boiss. and Abies alba Mill.): the role of leaf anatomy and mesophyll conductance to CO2. Tree Physiol (in press) doi:10.1093/treephys/tpv114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Valiente JA, Sánchez-Gómez D, Aranda I, Valladares F (2010) Phenotypic plasticity and local adaptation in leaf ecophysiological traits of 13 contrasting cork oak populations under different water availabilities. Tree Physiol 30:618–627. doi:10.1093/treephys/tpq013 [DOI] [PubMed] [Google Scholar]
- Roth-Nebelsick A, Fernández V, Peguero-Pina JJ, Sancho-Knapik D, Gil-Pelegrín E (2013) Stomatal encryption by epicuticular waxes as a plastic trait modifying gas exchange in a Mediterranean evergreen species (Quercus coccifera L.). Plant Cell Environ 36:579–589. doi:10.1111/j.1365-3040.2012.02597.x [DOI] [PubMed] [Google Scholar]
- Rubio de Casas R, Vargas P, Pérez-Corona E, Manrique E, Quintana JR, García-Verdugo C, Balaguer L (2007) Field patterns of leaf plasticity in adults of the long-lived evergreen Quercus coccifera. Ann Bot 100:325–334. doi:10.1093/aob/mcm112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundel PW, Jarrell WM (1989) Water in the environment. In: Pearcy RW, Ehleringer J, Mooney HA, Rundel PW (eds) Plant physiological ecology: field methods and instrumentation. Chapman and Hall, London, pp 29–56. [Google Scholar]
- Sakcali MS, Ozturk M (2004) Eco-physiological behaviour of some Mediterranean plants as suitable candidates for reclamation of degraded areas. J Arid Environ 57:141–153. doi:10.1016/S0140-1963(03)00099-5 [Google Scholar]
- Syvertsen JP, Lloyd J, McConchie C, Kriedemann PE, Farquhar GD (1995) On the relationship between leaf anatomy and CO2 diffusion through the mesophyll of hypostomatous leaves. Plant Cell Environ 18:149–157. doi:10.1111/j.1365-3040.1995.tb00348.x [Google Scholar]
- Taylor D, Eamus D (2008) Coordinating leaf functional traits with branch hydraulic conductivity: resource substitution and implications for carbon gain. Tree Physiol 28:1169–1177. doi:10.1093/treephys/28.8.1169 [DOI] [PubMed] [Google Scholar]
- Terashima I, Hanba YT, Tholen D, Niinemets U (2011) Leaf functional anatomy in relation to photosynthesis. Plant Physiol 155:108–116. doi:10.1104/pp.110.165472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholen D, Ethier G, Genty B, Pepin S, Zhu XG (2012) Variable mesophyll conductance revisited: theoretical background and experimental implications. Plant Cell Environ 35:2087–2103. doi:10.1111/j.1365-3040.2012.02538.x [DOI] [PubMed] [Google Scholar]
- Tomás M, Flexas J, Copolovici L et al. (2013) Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: quantitative limitations and scaling up by models. J Exp Bot 64:2269–2281. doi:10.1093/jxb/ert086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosens T, Niinemets Ü, Vislap V, Eichelmann H, Castro-Díez P (2012) Developmental changes in mesophyll diffusion conductance and photosynthetic capacity under different light and water availabilities in Populus tremula: how structure constrains function. Plant Cell Environ 35:839–856. doi:10.1111/j.1365-3040.2011.02457.x [DOI] [PubMed] [Google Scholar]
- Valentini R, Epron D, De Angelis P, Matteucci G, Dreyer E (1995) In situ estimation of net CO2 assimilation, photosynthetic electron flow and photorespiration in Turkey oak (Q. cerris L.) leaves: diurnal cycles under different levels of water supply. Plant Cell Environ 18:631–640. doi:10.1111/j.1365-3040.1995.tb00564.x [Google Scholar]
- Valladares F, Balaguer L, Martínez-Ferri E, Pérez-Corona E, Manrique E (2002a) Plasticity, instability and canalization: is the phenotypic variation in seedlings of sclerophyll oaks consistent with the environmental unpredictability of Mediterranean ecosystems? New Phytol 156:457–467. doi:10.1046/j.1469-8137.2002.00525.x [DOI] [PubMed] [Google Scholar]
- Valladares F, Chico JM, Aranda I, Balaguer L, Dizengremel P, Manrique E, Dreyer E (2002b) The greater seedling high-light tolerance of Quercus robur over Fagus sylvatica is linked to a greater physiological plasticity. Trees 16:395–403. [Google Scholar]
- Valladares F, Dobarro I, Sánchez-Gómez D, Pearcy RW (2005) Photoinhibition and drought in Mediterranean woody saplings: scaling effects and interactions in sun and shade phenotypes. J Exp Bot 56:483–494. doi:10.1093/jxb/eri037 [DOI] [PubMed] [Google Scholar]
- Vilagrosa A. (2002) Estrategias de resistencia al déficit hídrico en Pistacia lentiscus L. y Quercus coccifera L. Implicaciones en la repoblación forestal. PhD thesis University of Alicante, Spain. [Google Scholar]
- Vilagrosa A, Bellot J, Vallejo VR, Gil-Pelegrín E (2003) Cavitation, stomatal conductance, and leaf dieback in seedlings of two co-occurring Mediterranean shrubs during an intense drought. J Exp Bot 54:2015–2024. doi:10.1093/jxb/erg221 [DOI] [PubMed] [Google Scholar]
- Vilagrosa A, Morales F, Abadía A, Bellot J, Cochard H, Gil-Pelegrín E (2010) Are symplast tolerance to intense drought conditions and xylem vulnerability to cavitation coordinated? An integrated analysis of photosynthetic, hydraulic and leaf level processes in two Mediterranean drought-resistant species. Environ Exp Bot 69:233–242. doi:10.1016/j.envexpbot.2010.04.013 [Google Scholar]
- Villar R, Merino J (2001) Comparison of leaf construction costs in woody species with differing leaf life-spans in contrasting ecosystems. New Phytol 151:213–226. doi:10.1046/j.1469-8137.2001.00147.x [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Westoby M et al. (2004) The worldwide leaf economics spectrum. Nature 428:821–827. doi:10.1038/nature02403n [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.