Abstract
The competitive equilibrium between deciduous and evergreen plant species to a large extent depends on the intensity of the reduction in carbon gain undergone by evergreen leaves, associated with the leaf traits that confer resistance to stressful conditions during the unfavourable part of the year. This study explores the effects of winter harshness on the resistance traits of evergreen leaves. Leaf mass per unit area (LMA), leaf thickness and the concentrations of fibre, nitrogen (N), phosphorus (P), soluble protein, chlorophyll and ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) were determined in three evergreen and two deciduous species along a winter temperature gradient. In the evergreen species, LMA, thickness, and P and structural carbohydrate concentrations increased with the decrease in winter temperatures. Nitrogen and lignin concentrations did not show definite patterns in this regard. Chlorophyll, soluble proteins and Rubisco decreased with the increase in winter harshness. Our results suggest that an increase in LMA and in the concentration of structural carbohydrates would be a requirement for the leaves to cope with low winter temperatures. The evergreen habit would be associated with higher costs at cooler sites, because the cold resistance traits imply additional maintenance costs and reduced N allocation to the photosynthetic machinery, associated with structural reinforcement at colder sites.
Keywords: leaf fibre content, leaf mass per unit area, leaf thickness, nutrient content, Rubisco, winter temperature gradient
Introduction
For many years, the study of leaf traits has been an important focus of research in ecology because leaves govern the patterns of gas exchange and many other processes that affect the properties of ecosystems. The fact that leaves are plastic organs with marked variability in their responses to environmental conditions has led the study of leaf traits to acquire great relevance in the investigation of climate change (Taub 2010, Guerin et al. 2012). In many tree species, significant correlations have been observed between intraspecific variations in leaf traits and different environmental factors (Valladares et al. 2002, Klein et al. 2013). Accordingly, study of phenotypic variation among populations within a given species has become a useful tool for predicting variations in the distribution and composition of forest species in a changing climatic scenario (Nicotra et al. 2010, Matesanz and Valladares 2014). However, even for the most frequently studied species, the number of observations available in the existing databases is limited, especially with respect to the coverage of the geographic range of a species (Niinemets 2015). Thus, more data are needed to gain insight into within-species variation in leaf traits.
Among the different leaf traits, leaf mass per unit area (LMA) has attracted the most attention, mainly because changes in LMA are accompanied by changes in other characteristics, such as leaf lifespan and fibre and nutrient contents (Wright et al. 2004), which leads to important trade-offs between productivity and persistence (Reich 2014). It has been reported that LMA varies widely at the single-species level in response to differences in the harshness of the habitat (He et al. 2006, Messier et al. 2010). Traditionally, these changes in LMA have been interpreted mainly as responses to changes in water or nutrient stress (Niinemets 2001, Wright et al. 2004, Poorter et al. 2009). Less attention has been focused on changes in LMA in response to low winter temperatures (Ogaya and Peñuelas 2007, Mediavilla et al. 2012). However, if lengthening leaf lifespan demands a structural reinforcement that will allow leaves to overcome climate harshness (Kikuzawa et al. 2013), we also could expect that the harsher the climate conditions in winter, the greater the demand for structural reinforcement in the leaves that must survive during winter. This means that keeping leaves alive during winter (the evergreen habit) should be associated with larger investments in structural tissues at colder sites. Accordingly, low winter temperatures may also be involved as an additional factor in the trade-offs between productivity and persistence.
The competitive equilibrium between deciduous and evergreen tree species depends strongly on leaf productivity along the different seasons of the year and on the morphological and chemical adaptations necessary for leaf survival during the different seasons. Prolonging leaf lifespan over more than one growth season is only advantageous if the lengthening of productive life compensates for both additional maintenance costs and reduced carbon gain, which are associated with tolerance to unfavourable circumstances (van Ommen Kloeke et al. 2012). A greater LMA requires more plant material to achieve a given leaf area for light interception and, hence, implies higher construction costs per unit leaf area. However, the costs associated with a greater LMA derive mainly from its negative relationship with instantaneous carbon assimilation (Reich et al. 1997, Niinemets and Sack 2006). The greater allocation of biomass and nitrogen (N) to structural components versus photosynthetic components has been proposed to be one of the factors responsible for the lower carbon assimilation rate in leaves with a larger LMA (Vitousek et al. 1990, Niinemets 1999). At the interspecific level, some authors have reported a reduction in the proportion of N allocated to ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) with an increase in LMA, suggesting that this would reflect the need for greater N investment in structural components (Ellsworth et al. 2004, Onoda et al. 2004, Takashima et al. 2004). For example, it is known that cell walls accumulate a significant amount of N compounds: up to 10% of the cell content (Reiter 1998). A greater LMA in the leaves produced at colder sites would be explained by their greater thickness (Mediavilla et al. 2012), which would be achieved via a thickening of the cell walls, a characteristic of leaves growing in cold climates (Kubacka-Zębalska and Kacperska 1999, Stefanowska et al. 1999). Accordingly, it can be expected that a greater amount of available N would be allocated to cell walls in environments with harsher winters, leading to a reduction in the amount available for allocation to chlorophyll (CF) or photosynthetic proteins (PTs), which would negatively affect CO2 assimilation rates. In addition, an increased thickness of cell walls contributes to increasing the photosynthetic limitations due to internal diffusion (Niinemets et al. 2011), which should exacerbate the disadvantages of the structural reinforcement under unfavourable climatic conditions.
In the present work, we analyse the effects of the differences in climate harshness during winter on the morphology (LMA and thickness) and chemical composition (content of N, P, fibres, CF, soluble PTs and Rubisco) of the leaves of three evergreen (Quercus ilex ssp. ballota (Desf.) Samp, Quercus suber L. and Pinus pinaster Aiton) and two deciduous (Quercus pyrenaica Willd. and Quercus faginea Lam.) species. These species are widely distributed across the Iberian Peninsula. Although some authors have addressed possible changes in LMA in response to decreases in temperature along altitudinal gradients, their results are so disparate that it is not possible to draw reliable conclusions. Thus, whereas an increase in LMA with altitude has been observed in several species (Vitousek et al. 1990, Bresson et al. 2011, Körner 2012), it has also been found that it remains constant (Birmann and Körner 2009, Vitasse et al. 2014) or even decreases in some cases (Schoettle and Rochelle 2000, Wright et al. 2004). A similar situation is seen in the case of nutrients. The levels of N and phosphorus (P) in plant tissues have been positively correlated with altitude and hence with decreases in temperature in several works (Weih and Karlsson 2001, Reich and Oleksyn 2004, Jian et al. 2009). In contrast, other authors have found a marked decrease in the N content per unit leaf area (Zhang et al. 2005, Li et al. 2006) or no trends with altitude (Hultine and Marshall 2000, Premoli and Brewer 2007) or with temperature (Niinemets 2015). With respect to the remaining leaf components included in the present study, as far as we are aware, no other authors have analysed the changes at the single-species level in response to gradients of winter harshness. Differences in the contents of CF, PTs and Rubisco in response to differences in light intensity (Miyazawa et al. 2004), drought stress (Haldimann et al. 2008), CO2 concentrations (Blaschke et al. 2001) and growth temperatures (Campbell et al. 2007) have been analysed, but only based on short-term responses in controlled environments. Possible differences in the photosynthetic machinery associated with a greater or lesser structural reinforcement of the leaves in different environments have not been addressed. Our general aim here is to check whether winter climate harshness (in particular, the intensity and frequency of frosts) contributes to intensifying the leaf traits that confer persistence, and hence to reducing the amount of N associated with photosynthetic components and photosynthetic capacity. We surmise that in temperate climates, the evergreen habit would involve costs of adaptation to freezing that are largely unknown (van Ommen Kloeke et al. 2012) and that must logically be stronger in colder environments. Our hypothesis is that the most important cost would be an unfavourable distribution of N, associated with a greater concentration of fibre in cold climates. Thus, our aim is to clarify the implications of cold for leaf traits and productivity with a view to unravelling the possible repercussions of climate change on the distribution of woody species.
Materials and methods
Study species and area
The set of species studied included three evergreens with leaf lifespans of more than 1 year, and accordingly with leaves that survive during at least one winter (P. pinaster Aiton, Q. suber L. and Q. ilex ssp. ballota (Desf.) Samp), and two deciduous species (Q. pyrenaica Willd. and Q. faginea Lam.) with leaf lifespans of about 5–7 months (data taken from Mediavilla and Escudero 2003).
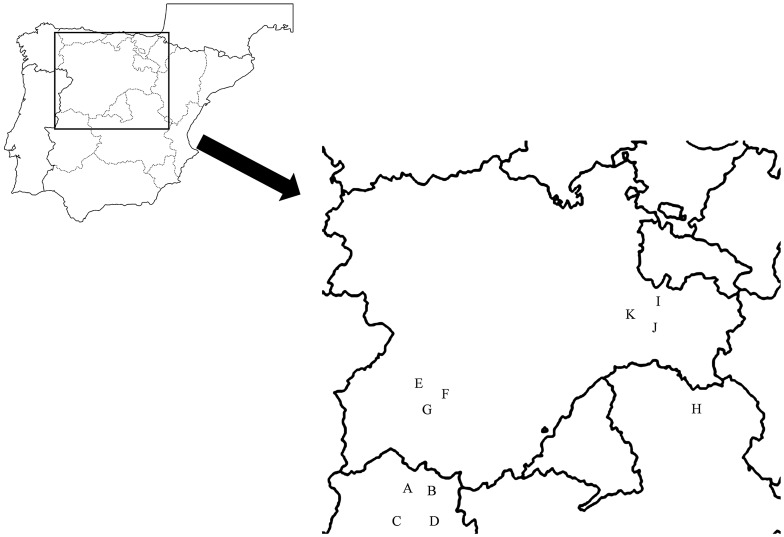
The species were distributed on 11 sites located in the regions of Castilla-Leon and Extremadura (central-western Spain) between latitudes 41°45′N and 40°01′N and between longitudes 6°22′W and 2°08′W (Figure 1). Owing to differences in altitude and to the effects of continentality, there were strong between-sites differences in temperature that were especially pronounced for the minimum winter temperatures and the number of frosts per year (Table 1). In contrast, the differences in summer temperatures were less intense. Accordingly, the annual temperature range was higher in colder sites, especially because of the effects of continentality (Ninyerola et al. 2000). The sites consisted of flat areas with sparse populations (between 50 and 100 specimens ha−1) of mature (>100 years old) individuals. Trunk diameter at 1.3 m height ranged from 20 to 60 cm and mean heights were 4–10 m. Each site was selected so as to include as many study species as possible and to cover a wide gradient in winter temperatures, although taking care that the rest of climate characteristics would be as homogeneous as possible. Nevertheless, there is a tendency for rainfall levels to be higher in the hottest and southernmost sites, which helps to reduce the differences in the intensity of drought stress between cold and hot site (Table 1). Owing to its lower tolerance to low temperatures, Q. suber was absent at the coldest sites. Rainfall and solar irradiance data were obtained from the digital climatic atlas of the Iberian Peninsula (Ninyerola et al. 2005): a set of digital climatic maps of mean air temperature, precipitation and solar radiation elaborated with 200 m resolution using data from climate stations and a combination of geographical variables (altitude, latitude, continentality, solar radiation and terrain curvature). Temperature data were obtained for each site by means of data loggers (Hobo Pendant temperature/light data logger, Part UA-002-08, Onset Computer Corporation, Pocasset, MA, USA). The data loggers were programmed to obtain temperature data every 10 min and they were kept at each site for 4 years (October 2008–October 2012).
Figure 1.
Distribution of the sample locations.
Table 1.
Site characteristics. Pp, Pinus pinaster; Qi, Quercus ilex; Qs, Quercus suber; Qp, Quercus pyrenaica; Qf, Quercus faginea.
| Stands |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | K | |
| Longitude (W) | 5°57′ | 5°48′ | 6°22′ | 5°02′ | 5°52′ | 5°47′ | 6°07′ | 2°08′ | 2°37′ | 2°43′ | 2°52′ |
| Latitude (N) | 40°13′ | 40°01′ | 39°49′ | 40°11′ | 41°14′ | 41°08′ | 40°55′ | 40°59′ | 41°45′ | 41°32′ | 41°43′ |
| Altitude (m above sea level) | 619 | 466 | 600 | 449 | 985 | 834 | 832 | 1246 | 1189 | 982 | 1045 |
| Mean temperature (°C) | |||||||||||
| Annual | 16.5 | 16.6 | 16.8 | 16.4 | 12.3 | 12.7 | 13.0 | 10.1 | 10.3 | 11.0 | 10.6 |
| Absolute maximum temperature | 40.4 | 43.6 | 43 | 44.1 | 39.6 | 38.9 | 43.9 | 39.2 | 41.1 | 39.5 | 40.4 |
| Absolute minimum temperature | −2.15 | −4.15 | −3.55 | −5.50 | −8.18 | −9.82 | −7.50 | −13.1 | −13.7 | −15.0 | −16.9 |
| Days with frost per year | 7 | 15 | 5 | 20 | 57 | 61 | 59 | 98 | 105 | 105 | 112 |
| Annual rainfall (mm) | 1109 | 986 | 672 | 1001 | 495 | 460 | 554 | 605 | 567 | 535 | 646 |
| Emberger's index | 72 | 105 | 60 | 60 | 52 | 46 | 48 | 58 | 79 | 69 | 78 |
| Daily mean radiation (W m−2) | 184 | 185 | 184 | 186 | 186 | 186 | 183 | 182 | 177 | 179 | 177 |
| Soil | |||||||||||
| Sand content (%) | 81.1 | 77.2 | 73.9 | 75.7 | 84.6 | 74.3 | 67.9 | 57.8 | 75.1 | 84.8 | 84.0 |
| Clay content (%) | 8.5 | 11.7 | 12.3 | 10.6 | 6.4 | 12.2 | 16.2 | 22.8 | 14.4 | 8.1 | 8.3 |
| Silt content (%) | 10.4 | 11.1 | 13.8 | 13.7 | 9.00 | 13.5 | 15.9 | 19.4 | 10.5 | 7.10 | 7.7 |
| Soil N content (%) | 0.082 | 0.143 | 0.104 | 0.126 | 0.072 | 0.021 | 0.078 | 0.112 | 0.115 | 0.040 | 0.048 |
| Available P (p.p.m.) | 31 | 38 | 5 | 6 | 10 | 4 | 20 | 2 | 7 | 9 | 6 |
| Organic matter (%) | 1.66 | 4.47 | 4.10 | 2.78 | 2.6 | 0.37 | 1.66 | 1.96 | 5.12 | 0.69 | 1.92 |
| pH | 5.7 | 4.5 | 4.7 | 5.1 | 4.6 | 4.5 | 4.4 | 7.6 | 4.8 | 7.0 | 6.1 |
| Species | Qi, Qs, Qp, Qf | Qs, Qp | Pp, Qi, Qs | Pp, Qi, Qs | Pp, Qi, <Qs, Qp | Pp, Qi, Qs, Qp, Qf | Qi, Qs, Qp, Qf | Qi, Qf | Pp, Qi, <Qp, Qf | Pp, Qi, Qp | Pp, Qi |
We used the Emberger's pluviothermic index (Emberger 1930) to analyse the effects of water stress on leaf traits:
where P is the annual precipitation (mm), TX is the average temperature of the hottest month (°C) and TN is the average temperature of the coldest month (°C). This index is commonly used in Mediterranean climates (Kunstler et al. 2007).
Soil samples were taken up to a depth of 20 cm (excluding the forest floor) from each stand. Determinations of soil granulometry, pH, and N and P concentrations were carried out at the Soils Laboratory of the Institute of Natural Resources and Agrobiology in Salamanca according to the methods described by Chapman and Pratt (1973) (Olsen analysis for available P) and Walkley and Black (1934).
Measurements of leaf morphology and chemistry
At each site, four or five mature specimens of each species were selected randomly during each sampling session. A composite sampling of sun-exposed branches with leaves from different crown positions in each canopy was undertaken for each individual selected. Samples were collected during three different periods of the year (autumn, winter and end of spring–beginning of summer) from October 2008 to October 2012, providing 4-year data for each sampling date. Some additional samplings during the same years were made to obtain estimates of leaf lifespan.
The samples were taken immediately to the laboratory and the branches were separated into annual segments (shoots) of different age classes. Only one flush of leaf growth was observed in all species. Accordingly, all the leaves sprouting in a given year were considered to belong to the same age class. All shoots bearing leaves of a given age were identified as belonging to the same age class. In the evergreen species, the number of leaves or needles per shoot was counted for each age class and the data were used to construct static life tables, which made it possible to estimate the mean leaf lifespan for each species according to standard methods.
For morphological analyses, 25 individual leaf samples for each species and leaf age class were finally selected at each site and sampling date. Leaf thickness was measured with a digital micrometer (Digimatic Micrometer, Mitutoyo, Japan) as a mean of three measurements taken at random positions on each leaf or needle, avoiding the main ribs on flat leaves. The total projected leaf and needle areas were determined by an image analysis system (Delta-T Devices Ltd, Cambridge, UK). In the case of Pinus, we also measured needle length with the digital micrometer. The leaf volume of flat leaves was calculated as the product of mean leaf thickness × leaf area. The transverse cross-sectional area of needles was measured with amplified scanning images, and needle volume was estimated as cross-sectional area × needle length. The samples were then oven-dried at 70 °C to constant weight and the total dry mass was determined. From the data thus obtained, we calculated the leaf dry mass per area (LMA) and leaf tissue density (dry mass/volume). Once all the data had been collected, a value for each species and age class at each site and sampling date were calculated as the average of 25 leaves taken in each case.
Once dried, the 25 individual leaves taken from each species and leaf age class selected at each site and sampling date were ground together to obtain a sufficient amount of sample for the chemical analyses. Leaf N concentrations were determined with a CE-Instruments NA-2100 autoanalyser (ThermoQuest, Milan, Italy). Phosphorus concentrations were measured colorimetrically as molybdo-vanado-phosphoric acid (Duque-Macias 1970).
After the N and P analyses, the remaining material was used to analyse the fibre content (hemicellulose, cellulose and lignin) with an Ankom Analyser (A220, ANKOM, Macedon, NY, USA), following the method of Goering and Van Soest (1970). The nutrient and fibre contents of leaves were expressed per unit dry mass (as milligrams of nutrient or fibre per gram of leaf dry mass), and nutrients also as per unit of leaf area, obtained as the nutrient concentration per unit dry mass multiplied by LMA.
Owing to the high cost in time and money involved in determining the Rubisco content, in this case, and for the CF and soluble PT content, we limited our analyses to current-year leaves of the two oak species (Q. ilex and Q. suber) and six sites (the two warmest, two intermediate and the two coldest in order to obtain replications of each of the temperature ranges). The leaf samples were taken from five mature specimens of each species at each site during winter and at the end of spring–beginning of summer of 2012. At the laboratory, the plant material was weighed and immediately plunged into liquid N and kept at −80 °C until analysis. For PT extraction and CF and PT determinations, we used the method of Agrisera (Sweden). Chlorophyll was measured according to Whatley and Arnon (1963) and total soluble PT was measured according to Bradford (1976). The dry mass and LMA of the leaves used for the analyses were also determined, and the CF and PT contents of leaves were expressed per unit dry mass and per unit leaf area. For western blotting and Rubisco analysis, we used the method of Agrisera, with minor modifications (see Vicente et al. 2011). The relative amount of the Rubisco large subunit was calculated by densitometric scanning of polyvinylidene difluoride membranes by image analysis using the Scion ImagePC software (Scion, Frederick, MD, USA) and expressed in arbitrary units.
Data analysis
The relationships between leaf traits and the different temperature measurements were described by means of linear regression analysis. To better explore site effects on leaf traits, for each site, we obtained a single value for the absolute annual maximum and minimum temperatures, the number of days with frost per year and the total annual rainfall.
The effects of climatic variables on leaf traits were initially explored by simple regression analyses. However, several environmental variables were correlated with each other. Accordingly, the data were also explored using multiple regression models with stepwise selection of variables based on Akaike's information criterion to determine the best model for leaf traits with environmental variables.
Temperature is known to exert a marked effect on the time of leaf emergence. Accordingly, the leaf traits data corresponding to the spring period for the current-year leaf cohort were excluded in order to avoid possible differences among the sites due to the different state of development of the recently emerged leaves. We did not include the leaves of the second age class of Q. suber either, since its maximum leaf lifespan is slightly longer than 1 year and we did not find old leaves in sufficient numbers to be able to perform the different chemical analyses. The test for significantly different slopes in analysis of covariance (ANCOVA) was used to determine whether the slopes of the change in each leaf trait with temperature change were significantly different for the different species. Between-sites differences in CF, soluble PT and Rubisco concentrations were explored using one-way analysis of variance. We performed the statistical tests using R ver. 3.0.3 software (R Development Core Team 2007).
Results
Within-species variability of leaf morphology <related to temperature gradients
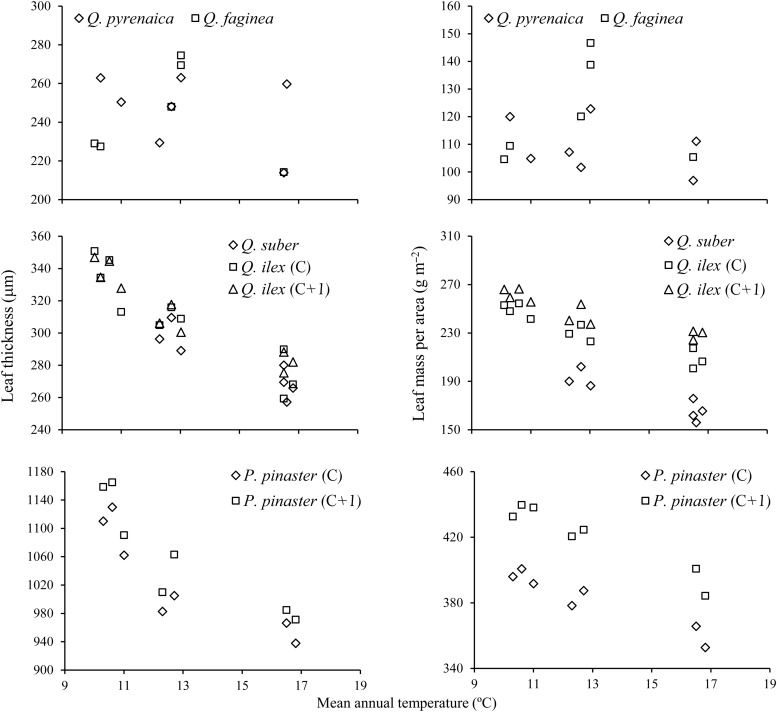
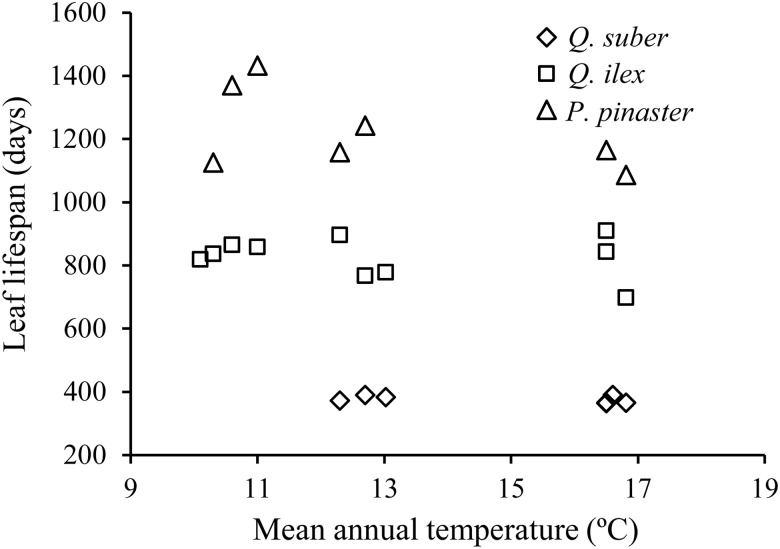
Leaves of evergreen species showed a definite trend to decreasing LMA and thickness with the increase in mean annual temperatures across the different sites (Figure 2). This trend was not apparent in the deciduous species, which also exhibited smaller between-sites differences in LMA and leaf thickness (Figure 2, upper panels). Both leaf traits presented higher values in the species with longer leaf lifespan, and the interspecific differences tended to be stronger at colder sites. Among the evergreens, leaf lifespan was independent from mean annual temperature for Q. suber and Q. ilex (Figure 3). However, P. pinaster needles tended to maintain longer duration in the coldest sites.
Figure 2.
Leaf thickness and LMA as a function of mean annual temperature at the different sites. C, current-year leaves; C + 1, 1-year-old leaves.
Figure 3.
Leaf lifespan of evergreen species as a function of mean annual temperature at the different sites.
Leaf responses of the evergreen species to temperature gradients were more pronounced when calculated only for winter conditions. In all leaf age classes, LMA and leaf thickness showed a pronounced response to the harshening of winter climatic variables, since the minimum temperatures and the number of days with frost were the two variables that best accounted for the highest percentage of variation observed among sites in thickness and LMA (Table 2). In contrast, in many cases, the relationships with the maximum temperatures disappeared or, if present, always reached a much lower significance level. Leaf tissue density was the only trait that did not show any definite trend along the thermal gradient for any type of leaf (data not shown). Therefore, the increase in LMA as the harshening of winter conditions progresses seems to occur only through leaf thickening, with no associated changes in density. Leaf lifespan of P. pinaster was marginally (P = 0.054) significantly correlated with absolute minimum temperatures. The two deciduous species showed no responses to any of the environmental factors studied. In some cases, the differences in leaf traits of evergreen species were also correlated with other climatic and soil variables, although always with relatively low percentages of variance explained. Since the warmest sites tended to have higher rainfall and solar radiation levels (Table 1), we also explored the combined effects of minimum winter temperatures and other factors by means of multiple regression analysis. In all cases, the minimum annual temperature was selected as the primary predictor for LMA and leaf thickness at each site. In a few cases, the best models included other independent variables, but always with lower significance levels than minimum temperatures (Table 3).
Table 2.
Linear regression parameters for different morphological leaf traits (LT, leaf thickness; LMA, leaf mass per unit area) depending on different climatic and soil variables (C, current-year leaves; C + 1, 1-year-old leaves; C + 2, 2-year-old leaves). Only significant (P < 0.05) relationships are shown. R, total annual rainfall; I, Emberger's pluviothermic index; SR, solar global radiation; min T, minimum winter temperature; max T, maximum summer temperature; F, number of days with frost per year.
| Independent variables | R2 | F | Intercept | Slope | P | Independent variables | R2 | F | Intercept | Slope | P |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LT (μm) | LMA (g m−2) | ||||||||||
| Q. suber C (n = 49) | Q. suber C (n = 49) | ||||||||||
| R (mm) | 0.49 | 25.3 | 319 | −0.051 | <0.0001 | R (mm) | 0.51 | 27.0 | 215 | −0.051 | <0.0001 |
| I | 0.35 | 13.8 | 330 | −0.601 | <0.0001 | I | 0.39 | 16.5 | 228 | −0.623 | <0.0001 |
| SR (W m−2) | 0.15 | 4.7 | −702 | 5.32 | 0.0390 | F | 0.64 | 45.9 | 158 | 0.597 | <0.0001 |
| F | 0.69 | 59.0 | 261 | 0.636 | <0.0001 | Min T (°C) | 0.69 | 57.9 | 143 | −5.78 | <0.0001 |
| Min T (°C) | 0.73 | 71.4 | 245 | −6.08 | <0.0001 | Max T (°C) | 0.21 | 6.7 | 342 | −3.93 | 0.0155 |
| Max T (°C) | 0.31 | 11.8 | 489 | −4.96 | 0.0020 | Soil N (%) | 0.51 | 26.8 | 207 | −343 | <0.0001 |
| Soil N (%) | 0.63 | 44.4 | 316 | −390 | <0.0001 | Soil P (p.p.m.) | 0.32 | 12.3 | 190 | −0.797 | 0.0017 |
| Soil P (p.p.m.) | 0.32 | 12.1 | 294 | −0.808 | 0.0018 | ||||||
| Q. ilex C (n = 75) | Q. ilex C (n = 75) | ||||||||||
| R (mm) | 0.27 | 13.7 | 366 | −0.086 | <0.0001 | R (mm) | 0.31 | 16.7 | 268 | −0.056 | <0.0001 |
| SR (W m−2) | 0.25 | 12.6 | 1165 | −4.70 | 0.0011 | SR (W m−2) | 0.33 | 18.0 | 816 | −3.21 | <0.0001 |
| F | 0.64 | 66.8 | 266 | 0.679 | <0.0001 | F | 0.75 | 114 | 203 | 0.443 | <0.0001 |
| Min T (°C) | 0.62 | 59.7 | 257 | −5.47 | <0.0001 | Min T (°C) | 0.76 | 115 | 196 | −3.65 | <0.0001 |
| Max T (°C) | 0.12 | 5.1 | 571 | −6.40 | 0.0295 | Max T (°C) | 0.21 | 9.8 | 438 | −5.05 | 0.0034 |
| soil P (p.p.m.) | 0.25 | 12.3 | 329 | −1.95 | 0.0012 | Soil P (p.p.m.) | 0.31 | 16.5 | 245 | −1.31 | <0.0001 |
| Q. ilex C + 1 (n = 107) | Q. ilex C + 1 (n = 107) | ||||||||||
| R (mm) | 0.32 | 26.1 | 361 | −0.073 | <0.0001 | R (mm) | 0.25 | 19.1 | 276 | −0.045 | <0.0001 |
| SR (W m−2) | 0.42 | 39.8 | 1176 | −4.74 | <0.0001 | SR (W m−2) | 0.30 | 24.6 | 755 | −2.79 | <0.0001 |
| F | 0.83 | 266 | 275 | 0.599 | <0.0001 | F | 0.60 | 85.3 | 224 | 0.352 | <0.0001 |
| Min T (°C) | 0.83 | 265 | 265 | −4.94 | <0.0001 | Min T (°C) | 0.62 | 92.1 | 218 | −2.95 | <0.0001 |
| Max T (°C) | 0.27 | 20.8 | 616 | −7.40 | <0.0001 | Max T (°C) | 0.23 | 16.3 | 437 | −4.65 | <0.0001 |
| soil P (p.p.m.) | 0.30 | 23.9 | 330 | −1.68 | <0.0001 | soil P (p.p.m.) | 0.26 | 19.7 | 258 | −1.07 | <0.0001 |
| P. pinaster C (n = 56) | P. pinaster C (n = 56) | ||||||||||
| I | 0.16 | 5.0 | 808 | 2.74 | 0.0341 | SR (W m−2) | 0.25 | 8.6 | 844 | −2.53 | 0.0069 |
| SR (W m−2) | 0.39 | 16.5 | 3655 | −14.4 | <0.0001 | F | 0.53 | 29.2 | 356 | 0.390 | <0.0001 |
| F | 0.45 | 20.9 | 919 | 1.63 | <0.0001 | Min T (°C) | 0.52 | 27.8 | 347 | −3.31 | <0.0001 |
| Min T (°C) | 0.45 | 21.6 | 881 | −14.1 | <0.0001 | Max T (°C) | 0.27 | 9.7 | 635 | −6.19 | 0.0045 |
| R (mm) | 0.11 | 4.6 | 457 | −0.059 | 0.0382 | ||||||
| P. pinaster C + 1 (n = 78) | P. pinaster C + 1 (n = 78) | ||||||||||
| R (mm) | 0.10 | 4.2 | 1172 | −0.172 | 0.0481 | SR (W m−2) | 0.15 | 7.0 | 944 | −2.88 | 0.0118 |
| I | 0.14 | 6.2 | 868 | 2.44 | 0.0168 | F | 0.36 | 22.2 | 389 | 0.464 | <0.0001 |
| SR (W m−2) | 0.45 | 31.9 | 3791 | −15.0 | <0.0001 | Min T (°C) | 0.35 | 21.2 | 379 | −3.93 | <0.0001 |
| F | 0.55 | 47.1 | 950 | 1.72 | <0.0001 | Max T (°C) | 0.22 | 11.0 | 750 | −8.05 | 0.0020 |
| Min T (°C) | 0.55 | 46.9 | 910 | −14.8 | <0.0001 | R (mm) | 0.13 | 5.5 | 499 | −0.072 | 0.0239 |
| Max T (°C) | 0.16 | 7.2 | 1905 | −20.5 | 0.0106 | ||||||
| P. pinaster C + 2 (n = 78) | P. pinaster C + 2 (n = 78) | ||||||||||
| SR (W m−2) | 0.26 | 13.0 | 3512 | −13.3 | <0.0001 | I | 0.12 | 5.1 | 386 | 0.836 | 0.0298 |
| F | 0.36 | 21.4 | 974 | 1.70 | <0.0001 | SR (W m−2) | 0.45 | 31.0 | 1452 | −5.49 | <0.0001 |
| Min T (°C) | 0.36 | 21.1 | 936 | −14.5 | <0.0001 | F | 0.64 | 66.5 | 406 | 0.701 | <0.0001 |
| Max T (°C) | 0.13 | 5.8 | 2013 | −22.6 | 0.0213 | Min T (°C) | 0.62 | 62.2 | 391 | −5.94 | <0.0001 |
| Max T (°C) | 0.21 | 10.2 | 817 | −8.88 | 0.0028 | ||||||
Table 3.
Multiple regression for different morphological leaf traits against minimum annual temperature and other climatic and soil factors. Model selection was conducted based on Akaike's information criteria. Abbreviations as in Table 2.
| Independent variables | R2 | F | Intercept | Slope | P | Independent variables | R2 | F | Intercept | Slope | P |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LT (μm) | LMA (g m−2) | ||||||||||
| Q. suber C (n = 49) | |||||||||||
| Min T (°C) | 0.82 | 58 | 274 | −4.18 | <0.0001 | Min T (°C) | 0.62 | 62 | 391 | −5.94 | <0.0001 |
| Soil N (%) | −198 | 0.00142 | |||||||||
| Q. ilex C (n = 75) | |||||||||||
| Min T (°C) | 0.62 | 60 | 257 | −5.47 | <0.0001 | Min T (°C) | 0.80 | 46 | −146 | −4.77 | <0.0001 |
| SR (W m−2) | 1.78 | 0.02 | |||||||||
| Soil N (%) | 84 | 0.05 | |||||||||
| Q. ilex C + 1 (n = 107) | |||||||||||
| Min T (°C) | 0.85 | 100 | −31 | −5.93 | <0.0001 | Min T (°C) | 0.62 | 92 | 218 | −2.95 | <0.0001 |
| Soil N (%) | 92 | 0.02 | |||||||||
| SR (W m−2) | 1.520 | 0.03 | |||||||||
| P. pinaster C (n = 56) | |||||||||||
| Min T (°C) | 0.45 | 22 | 881 | −14.13 | <0.0001 | Min T (°C) | 0.52 | 28 | 347 | −3.31 | <0.0001 |
| P. pinaster C + 1 (n = 78) | |||||||||||
| Min T (°C) | 0.57 | 25 | 950 | −15.68 | <0.0001 | Min T (°C) | 0.35 | 21 | 379 | −3.93 | <0.0001 |
| Soil P (p.p.m.) | −7.44 | 0.18 | |||||||||
| P. pinaster C + 2 (n = 78) | |||||||||||
| Min T (°C) | 0.36 | 21 | 936 | −14.50 | <0.0001 | Min T (°C) | 0.62 | 62 | 391 | −5.94 | <0.0001 |
Patterns of leaf chemical composition with <respect to winter temperatures
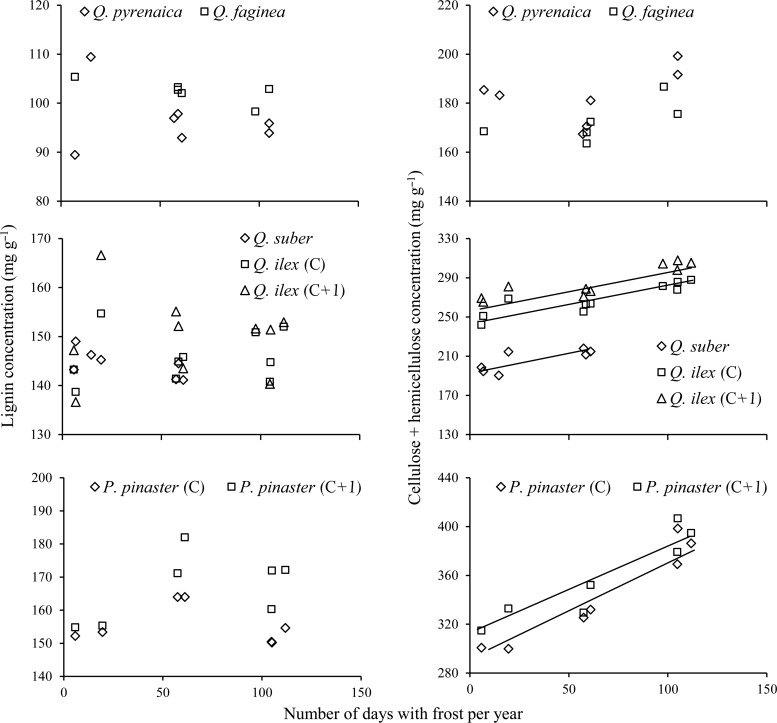
In all evergreen species, the structural carbohydrate concentration (cellulose + hemicellulose) increased between sites with the intensity of their winter harshness (mean number of days with frost per year along the study period) (Figure 4). The maximum temperatures either had no influence or the effect was less significant than that of winter harshness (not shown). Again, among the deciduous species, no trends with temperature were observed in fibre concentration (Figure 4, upper panels). No trend was observed also for the lignin concentration in response to any of the different temperature estimates in any of the species (Figure 4). Fibre concentration tended to be higher in the species with longer leaf lifespan. In addition, the ANCOVA results revealed that the slopes of the regression lines of cellulose + hemicellulose concentration against number of frosts did not differ for the two evergreen Quercus species, but were significantly higher in Pinus than in both Quercus species. This resulted in stronger between-species differences in structural carbohydrate concentration for cold sites (Figure 4).
Figure 4.
Relationships between the number of days with frost per year at each site and fibre concentration in the different leaf types. Each point is an average of eight sampling dates for current-year (C) leaves and 12 for 1-year-old (C + 1) leaves. Fitted equations for cellulose + hemicellulose concentrations: Q. suber (y = 0.34x + 195, R2 = 0.61, P = 0.04), Q. ilex C (y = 0.33x + 247, R2 = 0.78, P = 0.0008), Q. ilex C + 1 (y = 0.35x + 264, R2 = 0.79, P = 0.0005), P. pinaster C (y = 0.90x + 285, R2 = 0.92, P = 0.0007) and P. pinaster C + 1 (y = 0.77x + 308, R2 = 0.86, P = 0.0025). No significant relationships were observed for lignin concentration. Quercus pyrenaica and Q. faginea produced no significant relationships for any variable.
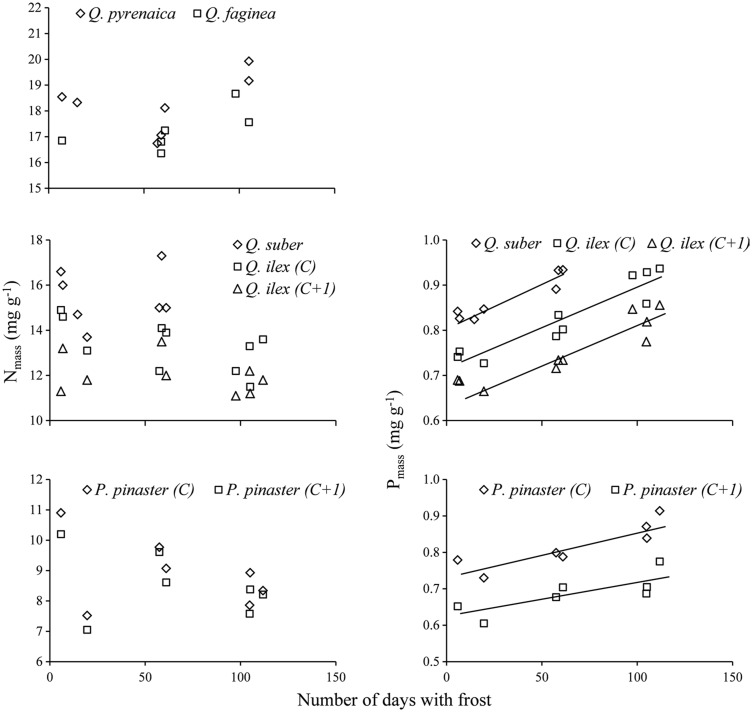
No significant relationships were observed between the leaf N concentration and the number of days with frost per year (Figure 5), despite a slight trend of the colder sites to exhibit lower concentrations. Owing to this non-significant trend, N contents per unit leaf area were also uncorrelated with winter temperatures, despite the increase in LMA observed in colder sites. Leaf N concentrations in the two deciduous species were also independent of temperature (Figure 5, upper panel). The differences in winter harshness were accompanied, however, by differences in the leaf P content in the evergreen species. The P concentrations per unit leaf mass responded to the changes in temperature, increasing among sites with the number of frosts per year along the study period (Figure 5). This was particularly patent when the P amounts were expressed per unit leaf area (not shown), because the increase in P concentration per unit mass was accompanied by the increase in LMA associated with the decrease in winter temperatures. Summer temperatures again either had no influence on P concentrations or the effect was less significant than that of winter harshness (data not shown). The same trends between sites were repeated in all the leaf age classes. In this case, the ANCOVA results revealed that there were no significant interspecific differences in the response slopes of P concentrations to the number of frosts. No relationship was observed between the concentration of N and P in the leaves at each site and the levels of both nutrients recorded in the soils of the same sites (see Table 1).
Figure 5.
Relationships between the number of days with frost per year in each site and nitrogen (Nmass) and phosphorus (Pmass) concentrations in the different leaf types. Each point is an average of eight sampling dates for current-year (C) leaves and 12 for 1-year-old (C + 1) leaves. Fitted equations for P concentrations: Q. suber (y = 0.0018x + 0.815, R2 = 0.89, P = 0.0015), Q. ilex C (y = 0.0018x + 0.716, R2 = 0.88, P < 0.0001), Q. ilex C + 1 (y = 0.0015x + 0.658, R2 = 0.84, P < 0.0001), P. pinaster C (y = 0.0013x + 0.733, R2 = 0.78, P = 0.0087) and P. pinaster C + 1 (y = 0.0010x + 0.622, R2 = 0.63, P = 0.0334). Phosphorus concentrations were not available for Q. pyrenaica and Q. faginea.
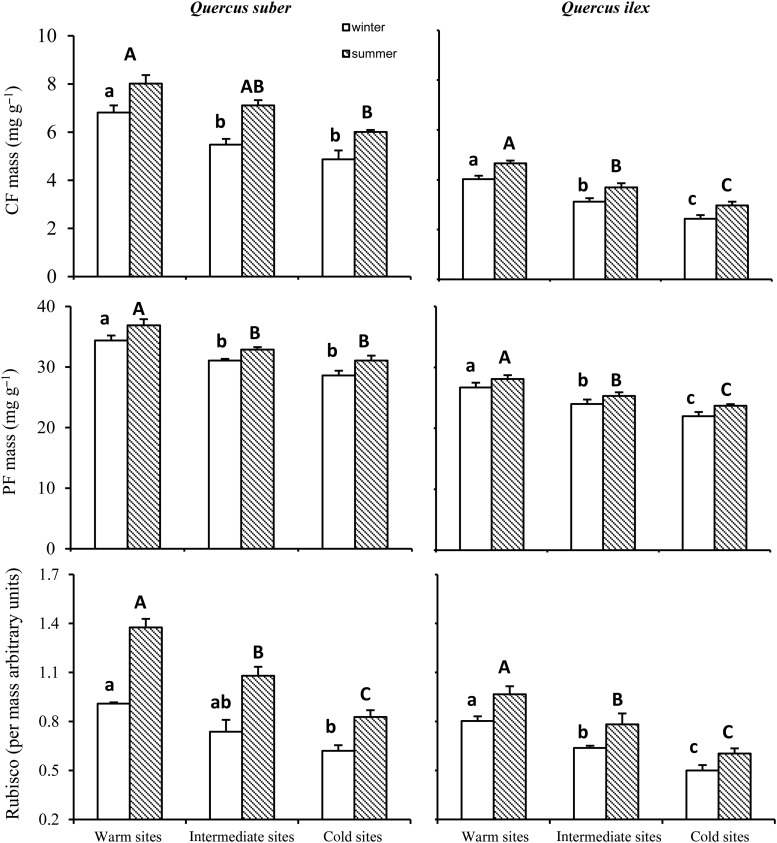
There were significant differences in the concentration of CF and soluble PT between sites with different temperatures. The data concerning the two sites selected for each climatic category as a function of the intensity of their winter harshness (two warmer sites, two intermediate sites and two sites with the coldest winters) were pooled after checking that there were no significant differences in the mean values obtained for each of them in any of the cases (data not shown). Expressed per unit leaf mass, the concentrations of CF and soluble PTs fell with the reduction in winter temperatures, with significantly higher values in the leaves produced in warmer environments with respect to the colder sites (Figure 6). The sites with intermediate conditions were not always significantly differentiated from the warmer or colder sites, although they consistently showed intermediate values between both. The same differences between environments persisted for the CF content but disappeared for that of soluble PTs when both were expressed per unit leaf area (not shown). In both species, it was also possible to note a decrease in the relative amounts of Rubisco per unit leaf mass with the increase in the intensity of winter harshness (Figure 6).
Figure 6.
Mean (±SE, n = 10) concentration of chlorophyll (CFmass), soluble protein (PTmass) and Rubisco for the different species. The significant differences among sites are marked with different letters (Fisher LSD test, P < 0.05).
Discussion
Most of the leaf traits analysed here showed significant differences between sites and such differences seemed to be related to differences in the intensity of winter harshness, with a similar response in all three evergreen species studied. Thus, the leaves from one species developed in environments with cooler winters had a greater LMA and a higher concentration of structural carbohydrates and P, but lower concentration of CF, soluble PTs and Rubisco than those produced at the sites with milder winters. A trend to decreasing Rubisco contents at high altitudes has also been reported for beech by Rajsnerová et al. (2015).
These temperature responses were not observed in the two deciduous species studied, which suggests that the between-sites differences in environmental conditions during the growth season were not responsible for the responses shown by evergreen leaves. Although other soil and climatic factors also exerted significant effects on leaf traits, these effects were mainly due to the existence of correlations among the different environmental variables. In multiple regression analysis, in most cases, only winter temperatures showed significant relationships with leaf traits, which suggests a direct effect of winter harshness on leaf characteristics. Differences in solar radiation between our sites were small. Possibly for this reason, despite the known effects of irradiance on LMA (Poorter et al. 2009), in the present study, we did not detect independent effects of this factor. In evergreen species, leaf lifespan tends to increase with decreasing temperatures (Wright et al. 2005), probably reflecting the longer payback time for construction costs in such conditions, due to the shorter growing season (Kikuzawa et al. 2013). These trends in leaf duration could have contributed to the responses in leaf traits observed in the present study. However, the increases in leaf lifespan with cold between our sites were undetectable for the two oak species and only marginally significant in P. pinaster, probably because the temperature gradient here studied was much smaller than those reported in global surveys, such as in Wright et al. (2005). Accordingly, differences in leaf lifespan seem to be too low to be responsible for the clear trends in leaf morphology and chemistry observed in our species.
A greater LMA has traditionally been interpreted as a trait aimed at guaranteeing leaf survival, acting as protection against different environmental factors such as drought or attack by herbivores (Turner 1994, Niinemets 2001). The highest mass per unit leaf area shown by the species studied here in the environments with the coldest winters and subjected to the most intense and continued frosts suggests that the harshest winter conditions also demand greater leaf reinforcement. The increase in LMA with winter harshness seems to be achieved exclusively through an increase in thickness, without changes in density. This increased thickness in colder sites could be a consequence of greater accumulation of photosynthetic biomass per unit leaf area (Niinemets 2015). In contrast, several authors have suggested that the greater thickness of leaves developing in colder climates would be achieved through a thickening of the leaf cell walls, characteristic of leaves growing under these conditions (Griffith and Brown 1982, Kubacka-Zębalska and Kacperska 1999). The amount of cell wall is related to the response capacity of plants to freezing, helping to increase tolerance to cold via a reduction in the freezing rate (Ball et al. 2002). Our results seem to confirm this higher amount of cell wall as being responsible for the greater leaf thickness in environments with harsh winters. All the evergreen species studied here showed a higher concentration per unit leaf mass of structural carbohydrates (cellulose + hemicellulose) in the colder environments. However, we did not note any trend in the lignin concentration associated with the differences in winter temperatures. Despite the traditional belief that lignin contributes to rigidity, and hence to leaf survival (Chabot and Hicks 1982, Cornelissen et al. 1999, Takashima et al. 2004), in recent years, many authors have reported the absence of a relationship between rigidity and leaf hardness and the lignin concentration (Kurokawa and Nakashizuka 2008, Kitajima et al. 2012) and hence between the amount of lignin and leaf duration (Mediavilla et al. 2008). The results of these authors suggest, as is the case here, that it is structural carbohydrates and not lignin that confer the leaves their hardness and thus increase their survival under adverse conditions, such as lower temperatures and greater frost intensity and duration in environments with harsher winters.
Although most leaf N is allocated to the components of the photosynthetic machinery, such as Rubisco and light-collecting complexes (Miyazawa et al. 2004), a significant amount of the N content is invested in other functions such as cell wall construction (Reiter 1998, Hikosaka and Shigeno 2009). If the leaves must reinforce themselves with greater amounts of cell wall material to be able to support lower winter temperatures, it is to be expected that a greater amount of available N must be allocated to the cell walls in environments with cold winters, leading to a reduction in the amount available for photosynthetic compounds. In our oak species, the mass-based concentration of CF, soluble PTs and Rubisco proved to be lower at the sites with the harshest winters. The concomitant variation in LMA tended to reduce to some extent the between-site differences in leaf chemical composition when the concentrations of CF, soluble PTs and Rubisco were expressed per unit area. However, if we assume that a main function of leaves is to deliver a profitable return on the investment that has been made in constructing the leaf (Westoby et al. 2013), mass-based differences may better reflect the costs associated with adaptation to cold. Unlike the increase in the N content of leaves with the decrease in temperatures reported by different authors (Weih and Karlsson 2001, Reich and Oleksyn 2004, Jian et al. 2009), in the present case, we failed to observe any significant trend in the amount of leaf N associated with differences in temperature. At the same sites as those studied in the present work, during the first 2 years of sampling, there was a significant trend towards higher N contents per unit leaf area at the colder sites (Mediavilla et al. 2012), which disappeared in the last 2 years. In fact, although plants grown in laboratory conditions typically contain greater leaf N when grown at low temperatures, field surveys tend to provide much more variable patterns (Reich and Oleksyn 2004, Niinemets 2015).
If the N concentration per unit leaf mass is independent of the differences in winter temperatures between sites, but the concentrations of CF, Rubisco and soluble PTs are lower at the colder sites, this suggests that there is a compromise in the distribution of N between photosynthesis and structural components. Our results, therefore, confirm those of other authors who have reported that the fraction of N allocated to Rubisco declines as LMA increases and the allocation of N to structural functions increases (Ellsworth et al. 2004, Onoda et al. 2004, Takashima et al. 2004). For example, the CF/N ratio in the leaves of the two oak species was significantly higher at the warmer sites (about 0.46 for Q. suber and 0.30 for Q. ilex) than at the colder sites (about 0.33 for Q. suber and 0.21 for Q. ilex). Accordingly, for the same amount of N, the amount of CF is higher in environments with milder winters, in parallel with the lower values of LMA, thickness and the concentration of structural carbohydrates. This suggests that the increase in the allocation of N to cell walls in colder environments occurs at the expense of reducing the allocation of N to the photosynthetic apparatus.
Regarding N, therefore, our results conflict with the hypothesis of a compensation of the reduction in the photosynthetic rate and other metabolic processes in colder environments by means of a greater allocation of N to leaves. In the literature on thermal acclimation, this idea is well established (Woods et al. 2003) and has been used to explain global patterns observed in plant leaf N in relation to temperature (Reich and Oleksyn 2004). Higher PT concentrations have been interpreted as an adaptation aimed at compensating the shorter length of the favourable period for growth at sites with lower temperatures. The discrepancy between our own results and this generally accepted idea could be explained in terms of the effect of drought stress during the summer in our Mediterranean environment. If drought stress were more intense at warmer sites, this would reverse the positive effects of higher temperatures and could shorten the growth season at the warmer sites when compared with the colder ones. However, in the present study, differences in summer temperatures were relatively low. At the same time, the warmer sites also received greater rainfall, which could reduce the differences in drought stress. In fact, the Emberger's pluviothermic index was uncorrelated with temperature (Table 1). Additionally, in a global survey, van Ommen Kloeke et al. (2012) found temperature to be the sole best predictor of the length of the growth season, while water availability (i.e., precipitation and evapotranspiration) had only marginal effects. We believe that the main factor responsible for the reduced allocation of N to the photosynthetic machinery at colder sites is the structural reinforcement detected by us, necessary for the evergreen leaves to cope with the winter conditions at the colder sites. This reinforcement allows leaf longevity to be extended beyond the first growing season and the lower instantaneous productivity to be compensated. As suggested by Kikuzawa et al. (2013), increasingly higher potential photosynthetic rates and nutrient concentrations as the length of the growing season decreases are important adaptations for deciduous leaves, but such an adjustment may not be required for evergreen plants, which can amortize construction costs over multiple seasons.
Contrary to leaf N, the P concentration responded to the changes in temperature in all three evergreen species, increasing among sites with the intensity of their winter harshness. Some authors have suggested changes in the leaf P content to be associated with latitudinal and temperature gradients, with a decrease in the P content with the increase in temperature and nearness to the equator. These authors proposed that this relationship would arise as a result of the differences in the age of the soil substrate, which has been shown to influence soil P availability and leaf P, with lower levels in older soils, closer to the equator, when compared with the younger and less leached soils farther away from the equator (Reich and Oleksyn 2004, Reich 2014). However, in our case, we did not observe any relationship between soil P contents and the P concentration in leaves, and neither did we note any trend in soil P levels associated with latitude or with the temperature at the study sites. In contrast, the highest levels of P found in the leaves of the populations occupying colder sites could respond to the limiting effect that this nutrient seems to exert in the study area. The N/P ratio in plant tissues has been proposed (Koerselman and Meuleman 1996) as a good predictor of limitations of these nutrients in the soil, with a leaf N/P ratio <14 indicating N limitation, a ratio >16 indicating P limitation and a ratio between 14 and 16 indicating that either N or P may limit plant growth or both elements are equally limiting. In our study, the N/P ratio was far above the limit value of 16 in all cases, reflecting P limitation at all our sites. A study conducted on Australian soils, which tend to be particularly P-deficient in comparison with soils from other continents (Atwell et al. 1999), found that leaf P had significance beyond leaf N in predicting the assimilation rate by unit leaf mass (Wright et al. 2001). Since in our case P was the element that supposedly acted as limiting, a higher concentration of this nutrient in the leaves of the trees growing in colder environments would compensate the unfavourable effects of low temperatures on CO2 assimilation.
In conclusion, our results suggest that an increase in LMA and in the concentration of structural carbohydrates would be an indispensable requirement for the trees to cope with the low winter temperatures and that evergreen species must reinforce their leaves to a greater extent at the coldest sites. This implies that the evergreen habit involves higher costs in these environments, which necessarily presupposes an additional disadvantage for the evergreen habit with respect to the deciduous one. A clear manifestation of these increased costs is that the interspecific differences in LMA and structural carbohydrate concentrations that are usually linked to differences in leaf lifespan are stronger in cold than in warm climates. For example, according to the results of the present article, current-year leaves of the evergreen Q. ilex have an average LMA 120% greater than that of the deciduous Q. pyrenaica at warm sites, but 135% greater at cold sites. Similarly, the concentration of structural carbohydrates per unit mass is 34% greater in Q. ilex with respect to Q. pyrenaica at warm sites, but the difference amounts to 45% at cold sites. These increased investments in structural reinforcement in evergreen species involve not only additional construction costs but also a reduced carbon gain (van Ommen Kloeke et al. 2012), and these costs seem to be stronger in colder environments.
Conflict of interest
None declared.
Funding
This article has received financial support from the Spanish Ministerio de Ciencia e Innovación—EU-FEDER (Projects No. CGL2006-04281 and CGL2010-21187), the Regional Government of Castilla-León (Project No. SA126A08) and the Miguel Casado S José Foundation.
References
- Atwell BJ, Kriedemann PE, Turnbull CGN (1999) Plants in action: adaptation in nature, performance in cultivation. MacMillan Education Australia, Melbourne, 664 pp. [Google Scholar]
- Ball MC, Wolfe J, Canny M, Hofmann M, Nicotra AB, Hughes D (2002) Space and time dependence of temperature and freezing in evergreen leaves. Funct Plant Biol 29:1259–1272. doi:10.1071/FP02037 [DOI] [PubMed] [Google Scholar]
- Birmann K, Körner C (2009) Nitrogen status of conifer needles at the alpine treeline. Plant Ecol Divers 2:233–241. doi:10.1080/17550870903473894 [Google Scholar]
- Blaschke L, Schulte M, Raschi A, Slee N, Rennenberg H, Polle A (2001) Photosynthesis, soluble and structural carbon compounds in two Mediterranean oak species (Quercus pubescens and Q. ilex) after lifetime growth at naturally elevated CO2 concentrations. Plant Biol 3:288–298. doi:10.1055/s-2001-15203 [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- Bresson CC, Vitasse Y, Kremer A, Delzon S (2011) To what extent is altitudinal variation of functional traits driven by genetic adaptation in European oak and beech? Tree Physiol 31:1164–1174. doi:10.1093/treephys/tpr084 [DOI] [PubMed] [Google Scholar]
- Campbell C, Atkinson L, Zaragoza-Castells J, Lundmark M, Atkin O, Hurry V (2007) Acclimation of photosynthesis and respiration is asynchronous in response to changes in temperature regardless of plant functional group. New Phytol 176:375–389. doi:10.1111/j.1469-8137.2007.02183.x [DOI] [PubMed] [Google Scholar]
- Chabot BF, Hicks DJ (1982) The ecology of leaf life spans. Annu Rev Ecol Syst 13:229–259. doi:10.1146/annurev.es.13.110182.001305 [Google Scholar]
- Chapman HD, Pratt PF (1973) Methods of analysis for soils, plants and water. University of California Press, Riverside, CA. [Google Scholar]
- Cornelissen JHC, Pérez-Harguindeguy N, Diaz S, Grime JP, Marzano B, Cabido M, Vendramini F, Cerabolini B (1999) Leaf structure and defence control litter decomposition rate across species and life forms in regional floras on two continents. New Phytol 143:191–200. doi:10.1046/j.1469-8137.1999.00430.x [Google Scholar]
- Duque-Macias F. (1970) Determinación conjunta de P, K, Ca, Mg, Fe, Mn, y Zn en plantas. Anal Edafol Agrobiol 30:207–229. [Google Scholar]
- Ellsworth DS, Reich PB, Naumburg ES, Koch GW, Kubiske ME, Smith SD (2004) Photosynthesis, carboxylation and leaf nitrogen responses of 16 species to elevated pCO2 across four free-air CO2 enrichment experiments in forest, grassland and desert. Glob Change Biol 10:2121–2138. doi:10.1111/j.1365-2486.2004.00867.x [Google Scholar]
- Emberger L. (1930) La végétation de la région méditerranéenne. Essai d'une classification des groupements végétaux. Rev Gén Bot 43:641–662, 705–729. [Google Scholar]
- Goering HK, Van Soest PJ (1970) Forage fibre analysis (apparatus, reagents, procedures and some applications). Agriculture Handbook no. 379 ARS-USDA, Washington, DC, 20 pp. [Google Scholar]
- Griffith M, Brown GN (1982) Cell wall deposits in winter rye Secale cereale L. ‘Puma’ during cold acclimation. Bot Gaz 143:486–490. doi:10.1086/337325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin GR, Wen H, Lowe AJ (2012) Leaf morphology shift linked to climate change. Biol Lett 8:882–886. doi:10.1098/rsbl.2012.0458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldimann P, Gallé A, Feller U (2008) Impact of an exceptionally hot dry summer on photosynthetic traits in oak (Quercus pubescens) leaves. Tree Physiol 28:785–795. doi:10.1093/treephys/28.5.785 [DOI] [PubMed] [Google Scholar]
- He JS, Wang ZH, Wang XP, Schmid B, Zuo WY, Zhou M, Zheng CY, Wang MF, Fang JY (2006) A test of the generality of leaf trait relationships on the Tibetan Plateau. New Phytol 170:835–848. doi:10.1111/j.1469-8137.2006.01704.x [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Shigeno A (2009) The role of Rubisco and cell walls in the interspecific variation in photosynthetic capacity. Oecologia 160:<443–451. doi:10.1007/s00442-009-1315-z [DOI] [PubMed] [Google Scholar]
- Hultine KR, Marshall JD (2000) Altitude trends in conifer leaf morphology and stable carbon isotope composition. Oecologia 123:32–40. doi:10.1007/s004420050986 [DOI] [PubMed] [Google Scholar]
- Jian Q, Keming M, Yuxin Z (2009) Leaf-trait relationships of Quercus liaotungensis along an altitudinal gradient in Dongling Mountain, Beijing. Ecol Res 24:1243–1250. doi:10.1007/s11284-009-0608-3 [Google Scholar]
- Kikuzawa K, Onoda Y, Wright IJ, Reich PB (2013) Mechanisms underlying global temperature-related patterns in leaf longevity. Global Ecol Biogeogr 22:982–993. doi:10.1111/geb.12042 [Google Scholar]
- Kitajima K, Llorens AM, Stefanescu C, Timchenko MV, Lucas PW, Wright SJ (2012) How cellulose-based leaf toughness and lamina density contribute to long leaf lifespans of shade-tolerant species. New Phytol 195:640–652. doi:10.1111/j.1469-8137.2012.04203.x [DOI] [PubMed] [Google Scholar]
- Klein T, Di Matteo G, Rotenberg E, Cohen S, Yakir D (2013) Differential ecophysiological response of a major Mediterranean pine species across a climatic gradient. Tree Physiol 33:26–36. doi:10.1093/treephys/tps116 [DOI] [PubMed] [Google Scholar]
- Koerselman W, Meuleman AFM (1996) The vegetation N : P ratio: a new tool to detect the nature of nutrient limitation. J Appl Ecol 33:1441–1450. doi:10.2307/2404783 [Google Scholar]
- Körner C. (2012) Alpine treelines: functional ecology of the global high elevation tree limits. Springer, Basel, 220 pp. [Google Scholar]
- Kubacka-Zębalska M, Kacperska A (1999) Low temperature-induced modifications of cell wall content and polysaccharide composition in leaves of winter oilseed rape (Brassica napus L. var. oleifera L.). Plant Sci 148:59–67. doi:10.1016/S0168-9452(99)00122-3 [Google Scholar]
- Kunstler G, Thuiller W, Curt T, Bouchaud M, Jouvie R, Deruette F, Lepart J (2007) Fagus sylvatica L. recruitment across a fragmented Mediterranean Landscape, importance of long distance effective dispersal, abiotic conditions and biotic interactions. Divers Distrib 13:799–807. doi:10.1111/j.1472-4642.2007.00404.x [Google Scholar]
- Kurokawa H, Nakashizuka T (2008) Leaf herbivory and decomposability in a Malaysian tropical rain forest. Ecology 89:2645–2656. doi:10.1890/07-1352.1 [DOI] [PubMed] [Google Scholar]
- Li CH, Zhang X, Liu X, Luukkanen O, Berninger F (2006) Leaf morphological and physiological responses of Quercus aquifolioides along an altitudinal gradient. Silva Fenn 40:5–13. [Google Scholar]
- Matesanz S, Valladares F (2014) Ecological and evolutionary responses of Mediterranean plants to global change. Environ Exp Bot 103:53–67. doi:10.1016/j.envexpbot.2013.09.004 [Google Scholar]
- Mediavilla S, Escudero A (2003) Photosynthetic capacity, integrated over the lifetime of a leaf, is predicted to be independent of leaf longevity in some tree species. New Phytol 159:203–211. doi:10.1046/j.1469-8137.2003.00798.x [DOI] [PubMed] [Google Scholar]
- Mediavilla S, Garcia-Criado B, Garcia-Ciudad A, Escudero A (2008) Testing the correlations between leaf life span and leaf structural reinforcement in 13 species of European Mediterranean woody plants. Funct Ecol 22:787–793. doi:10.1111/j.1365-2435.2008.01453.x [Google Scholar]
- Mediavilla S, Gallardo-López V, González-Zurdo P, Escudero A (2012) Patterns of leaf morphology and leaf N content in relation to winter temperatures in three evergreen tree species. Int J Biometeorol 56:915–926. doi:10.1007/s00484-011-0498-2 [DOI] [PubMed] [Google Scholar]
- Messier J, McGill BJ, Lechowicz MJ (2010) How do traits vary across ecological scales? A case for trait-based ecology. Ecol Lett 13:838–848. doi:10.1111/j.1461-0248.2010.01476.x [DOI] [PubMed] [Google Scholar]
- Miyazawa SI, Suzuki AA, Sone K, Terashima I (2004) Relationships between light, leaf nitrogen and nitrogen remobilization in the crowns of mature evergreen Quercus glauca trees. Tree Physiol 24:1157–1164. doi:10.1093/treephys/24.10.1157 [DOI] [PubMed] [Google Scholar]
- Nicotra AB, Atkin OK, Bonser SP et al. (2010) Plant phenotypic plasticity in a changing climate. Trends Plant Sci 15:684–692. doi:10.1016/j.tplants.2010.09.008 [DOI] [PubMed] [Google Scholar]
- Niinemets U. (1999) Components of leaf dry mass per area—thickness and density—alter leaf photosynthetic capacity in reverse directions in woody plants. New Phytol 144:35–47. doi:10.1046/j.1469-8137.1999.00466.x [Google Scholar]
- Niinemets U. (2001) Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology 82:453–469. doi:10.1890/0012-9658(2001)082[0453:GSCCOL]2.0.CO;2 [Google Scholar]
- Niinemets Ü. (2015) Is there a species spectrum within the world-wide leaf economics spectrum? Major variations in leaf functional traits in the Mediterranean sclerophyll Quercus ilex. New Phytol 205:79–96. doi:10.1111/nph.13001 [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Sack L (2006) Structural determinants of leaf light-harvesting capacity and photosynthetic potentials. Prog Bot 67:385–419. doi:10.1007/3-540-27998-9_17 [Google Scholar]
- Niinemets Ü, Flexas J, Peñuelas J (2011) Evergreens favored by higher responsiveness to increased CO2. Trends Ecol Evol 26:136–142. doi:10.1016/j.tree.2010.12.012 [DOI] [PubMed] [Google Scholar]
- Ninyerola M, Pons X, Roure JM (2000) A methodological approach of climatological modelling of air temperature and precipitation through GIS techniques. Int J Climatol 20:1823–1841. doi:10.1002/1097-0088(20001130)20:14<1823::AID-JOC566>3.0.CO;2-B [Google Scholar]
- Ninyerola M, Pons X, Roure JM (2005) Atlas Climático Digital de la Península Ibérica. Metodología y aplicaciones en bioclimatología y geobotánica. Universidad Autónoma de Barcelona, Bellaterra: http://opengis.uab.es/wms/iberia/espanol/es_model.htm (24 December 2015, date last accessed). [Google Scholar]
- Ogaya R, Peñuelas J (2007) Leaf mass per area ratio in Quercus ilex leaves under a wide range of climatic conditions. The importance of low temperatures. Acta Oecol 31:168–173. doi:10.1016/j.actao.2006.07.004 [Google Scholar]
- Onoda Y, Hikosaka K, Hirose T (2004) Allocation of nitrogen to cell walls decreases photosynthetic nitrogen-use efficiency. Funct Ecol 18:419–425. doi:10.1111/j.0269-8463.2004.00847.x [Google Scholar]
- Poorter H, Niinemets U, Poorter L, Wright IJ, Villar R (2009) Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol 182:565–588. doi:10.1111/j.1469-8137.2009.02830.x [DOI] [PubMed] [Google Scholar]
- Premoli AC, Brewer CA (2007) Environmental v. genetically driven variation in ecophysiological traits of Nothofagus pumilio from contrasting elevations. Aust J Bot 55:585–591. doi:10.1071/BT06026 [Google Scholar]
- Rajsnerová P, Klem K, Holub P et al. (2015) Morphological, biochemical and physiological traits of upper and lower canopy leaves of European beech tend to converge with increasing altitude. Tree Physiol 35:47–60. doi:10.1093/treephys/tpu104 [DOI] [PubMed] [Google Scholar]
- R Development Core Team (2007) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Reich PB. (2014) The world-wide ‘fast–slow’ plant economics spectrum: a traits manifesto. J Ecol 102:275–301. doi:10.1111/1365-2745.12211 [Google Scholar]
- Reich PB, Oleksyn J (2004) Global patterns of plant leaf N and P in relation to temperature and latitude. Proc Natl Acad Sci USA 101:11001–11006. doi:10.1073/pnas.0403588101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS (1997) From tropics to tundra: global convergence in plant functioning. Proc Natl Acad Scie USA 94:13730–13734. doi:10.1073/pnas.94.25.13730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter WD. (1998) The molecular analysis of cell wall components. Trends Plant Sci 3:27–32. [Google Scholar]
- Schoettle AW, Rochelle SG (2000) Morphological variation of Pinus flexilis (Pinaceae) a bird-dispersed pine, across a range of elevations. Am J Bot 87:1797–1806. doi:10.2307/2656832 [PubMed] [Google Scholar]
- Stefanowska M, Kuras M, Kubacka-Zebalska M, Kacperska A (1999) Low temperature affects pattern of leaf growth and structure of cell walls in winter Oilseed rape (Brassica napus L., var. oleifera L.). Ann Bot 84:313–319. doi:10.1006/anbo.1999.0924 [Google Scholar]
- Takashima T, Hikosaka K, Hirose T (2004) Photosynthesis or persistence: nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant Cell Environ 27:1047–1054. doi:10.1111/j.1365-3040.2004.01209.x [Google Scholar]
- Taub D. (2010) Effects of rising atmospheric concentrations of carbon dioxide on plants. Nat Educ Knowl 3:21. [Google Scholar]
- Turner IM. (1994) Sclerophylly: primarily protective? Funct Ecol 8: 669–675. doi:10.2307/2390225 [Google Scholar]
- Valladares F, Balaguer L, Martínez-Ferri E, Perez-Corona E, Manrique E (2002) Plasticity, instability and canalization: is the phenotypic variation in seedlings of sclerophyll oaks consistent with the environmental unpredictability of Mediterranean ecosystems? New Phytol 156:457–467. doi:10.1046/j.1469-8137.2002.00525.x [DOI] [PubMed] [Google Scholar]
- van Ommen Kloeke AEE, Douma JC, Ordoñez JC, Reich PB, van Bodegom PM (2012) Global quantification of contrasting leaf life span strategies for deciduous and evergreen species in response to environmental conditions. Global Ecol Biogeogr 21:224–235. doi:10.1111/j.1466-8238.2011.00667.x [Google Scholar]
- Vicente R, Morcuende R, Babiano J (2011) Differences in rubisco and chlorophyll content among tissues and growth stages in two tomato (Lycopersicon esculentum Mill.) varieties. Agron Res 9:501–507. [Google Scholar]
- Vitasse Y, Lenz A, Kollas C, Randin CF, Hoch G, Körner C (2014) Genetic vs. non-genetic responses of leaf morphology and growth to elevation in temperate tree species. Funct Ecol 28:243–252. doi:10.1111/1365-2435.12161 [Google Scholar]
- Vitousek PM, Field CB, Matson PA (1990) Variation in foliar δ13C in Hawaiian Metrosideros polymorpha: a case of internal resistance? Oecologia 84:362–370. doi:10.1007/BF00329760 [DOI] [PubMed] [Google Scholar]
- Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci 37:29–38. doi:10.1097/00010694-193401000-00003 [Google Scholar]
- Weih M, Karlsson PS (2001) Growth response of Mountain birch to air and soil temperature: is increasing leaf-nitrogen content an acclimation to lower air temperature? New Phytol 150:147–155. doi:10.1046/j.1469-8137.2001.00078.x [Google Scholar]
- Westoby M, Reich PB, Wright IJ (2013) Understanding ecological variation across species: area-based vs mass-based expression of leaf traits. New Phytol 199:322–323. doi:10.1111/nph.12345 [DOI] [PubMed] [Google Scholar]
- Whatley FR, Arnon DI (1963) Photosynthetic phosphorylation in plants. In: Colowick SP, Kaplan NO (eds) Methods in enzymology VI. Academic Press, New York, pp 308–313. [Google Scholar]
- Woods HA, Makino W, Cotner JB, Hobbie SE, Harrison JF, Acharya K, Elser JJ (2003) Temperature and the chemical composition of poikilothermic organisms. Funct Ecol 17:237–245. doi:10.1046/j.1365-2435.2003.00724.x [Google Scholar]
- Wright IJ, Reich PB, Westoby M (2001) Strategy shifts in leaf physiology, structure and nutrient content between species of high- and low-rainfall and high- and low-nutrient habitats. Funct Ecol 15: 423–434. doi:10.1046/j.0269-8463.2001.00542.x [Google Scholar]
- Wright IJ, Reich PB, Westoby M et al. (2004) The worldwide leaf economics spectrum. Nature 428:821–827. doi:10.1038/nature02403 [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Cornelissen JH et al. (2005) Modulation of leaf economic traits and trait relationships by climate. Global Ecol Biogeogr 14:411–421. doi:10.1111/j.1466-822x.2005.00172.x [Google Scholar]
- Zhang SB, Zhou ZK, Hu H, Xu K, Yan N, Li SY (2005) Photosynthetic performances of Quercus pannosa vary with altitude in the Hengduan Mountains, southwest China. For Ecol Manag 212:291–301. doi:10.1016/j.foreco.2005.03.031 [Google Scholar]