Leaf structure under frost: getting thicker in preparation for winter
Winter conditions in seasonal environments constitute a highly stressful environment for plant life due to low temperatures and other interacting stresses. Even chilling temperatures between 0 and 10 °C strongly reduce foliage photosynthetic activity (e.g., Huner et al. 1998, Venema et al. 2000, Allen and Ort 2001), especially on bright days that can lead to temporal and chronic photoinhibition (Öquist and Huner 1993, Huner et al. 1998, Ivanov et al. 2001). Frost stress further exacerbates reduction in leaf physiological activity due to freezing of plant tissues with potential cellular damage (e.g., Gray et al. 1997, Lamontagne et al. 1998). Furthermore, freezing of water in soil and xylem, and xylem embolism stop water delivery to foliage and can lead to major desiccation stress once the leaves thaw during warmer periods or upon exposure to bright sunlight. In addition, abrasive damage due to wind, snow and ice can strongly enhance foliage cuticular conductance, thereby further enhancing potential desiccation stress (Larcher 1985, Herrick and Friedland 1991, Koppel and Heinsoo 1994).
A plethora of structural and physiological adjustments occur in plants growing under low temperatures. Classical studies have demonstrated increases in leaf thickness in species growing at higher elevations and exposed to lower temperatures compared with lowland species (Woodward 1979, Körner et al. 1986). The temperature-dependent changes in thickness have been associated with a greater number of cell layers and increased thickness of individual cell layers, overall resulting in greater water content per unit leaf area (Woodward 1979, Körner et al. 1989, Atkin et al. 1996). Due to the high heat capacitance of water, such increases in leaf thickness and water content reduce the rate of leaf freezing (Ball et al. 2002), contributing to the reduction of physiological damage due to ice formation under relatively short and moderate freezing conditions alternating with periods of warmer temperatures, e.g., early morning freezing followed by leaf warm-up upon exposure to solar radiation (Ball et al. 2002, Poorter et al. 2009). Although a greater heat capacitance provides extra time until the leaves freeze, once fully frozen, the apparent benefits of being thick are less clear. In frozen leaves, greater water content per leaf area could contribute to reduced rate of thaw upon leaf exposure to sunlight and thereby result in reduced cellular damage (Steffen et al. 1989, Nilsen 1990, Fall et al. 2001). Thus, having a greater heat capacity could be especially beneficial for leaves exposed to multiple freeze–thaw cycles during the winter.
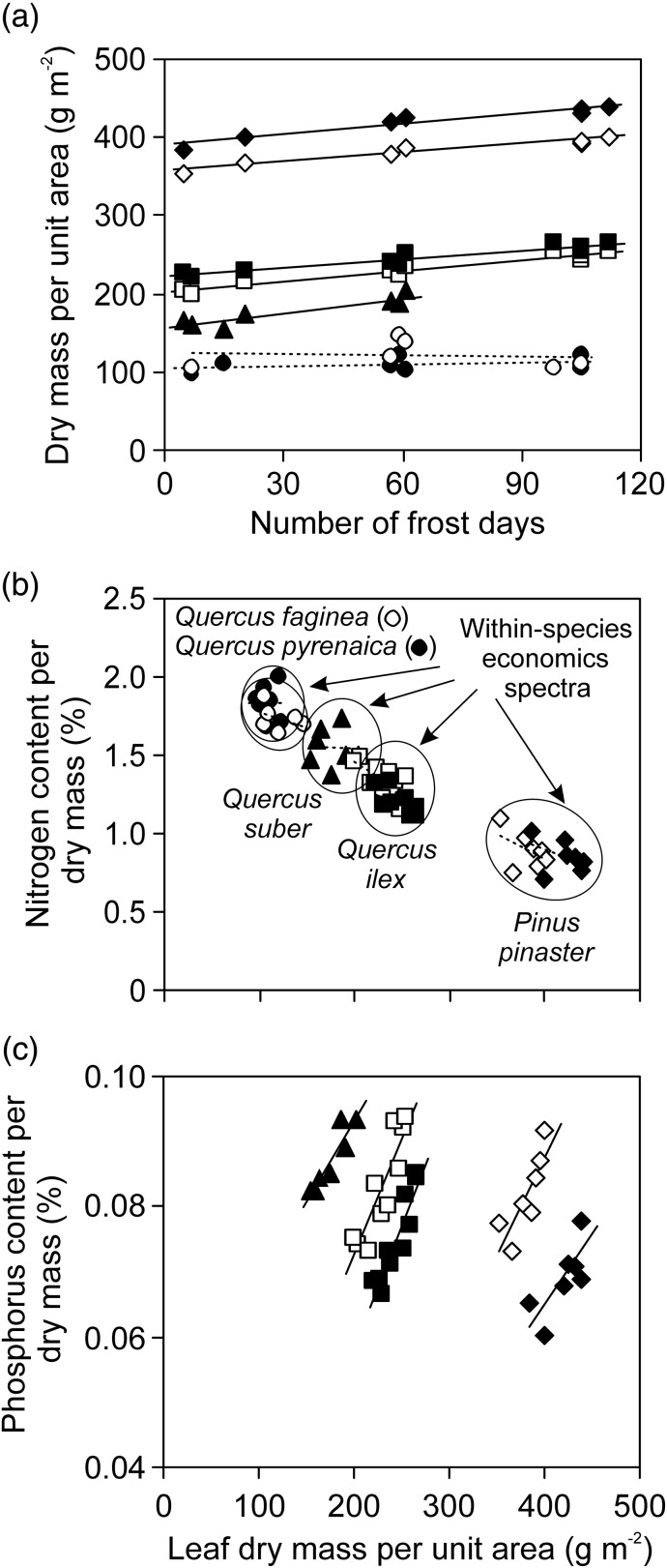
So far, much fewer data are available for within-species structural modifications as driven by variation in minimum temperature and frost period length across habitats. In herbaceous species, there is evidence of enhanced thickness of cold-acclimated leaves (Boese and Huner 1990, Stefanowska et al. 1999). In this issue of Tree Physiology, the study of González-Zurdo et al. (2016) investigated leaf structure, chemistry and photosynthetic traits in five Mediterranean species over a complex environmental gradient characterized by variations in annual average, minimum and maximum temperatures, precipitation and solar radiation. They found that both leaf dry mass per unit area (MA) and leaf thickness were most strongly associated with minimum temperature, with both traits increasing with decreasing minimum winter temperature and with increasing number of frost days in three Mediterranean evergreen species, but not in the deciduous species (Figure 1a; González-Zurdo et al. 2016). Given that soil drought has been traditionally considered as a key limitation in these environments (Zavala et al. 2000, Reichstein et al. 2002, David et al. 2007), this result is surprising and suggests that future research should focus more on the effects of low temperature on foliage differentiation.
Figure 1.
Leaf dry mass per unit area in relation to the number of frost days (a), and correlations of leaf nitrogen (b) and phosphorus (c) content per dry mass with leaf dry mass per unit area in two deciduous (Quercus faginea Lam. and Q. pyrenaica Willd.) and three evergreen (Q. suber L., Q. ilex L. and Pinus pinaster Aiton) Mediterranean species (data from González-Zurdo et al. 2016 in this issue of Tree Physiology). Data were fitted by linear regressions and solid lines correspond to statistically significant and punctuated lines to nonsignificant relationships (see González-Zurdo et al. 2016 for details). In evergreen species Q. ilex and P. pinaster, open symbols correspond to current-year leaves and filled symbols to 1-year-old leaves. Ellipses in (b) emphasize that within the broad nitrogen content vs leaf dry mass per unit area relationship observed across the species, there is a within-species economics spectrum driven by acclimation to cold temperatures (a). In the case of phosphorus content vs leaf dry mass per unit area relationship, significant within-species relationships are observed, while the broad trend collapses (c).
Beating cold by being tough
Apart from changes in leaf thickness, frost-adapted species characteristically also have thicker cell walls, especially thicker epidermal cell walls (Körner et al. 1989). There is evidence that within species, frost acclimation leads to increases in cell wall thickness and cell wall rigidity (Weiser et al. 1990, Rajashekar and Burke 1996, Rajashekar and Lafta 1996, Stefanowska et al. 1999, Solecka et al. 2008, Scholz et al. 2012). Given that freezing and potential desiccation stress lead to excessively low cellular water potentials, increased cell wall rigidity is an important acclimation feature, avoiding collapse of cell walls as a result of associated damage at membrane level (Rajashekar and Burke 1996, Rajashekar and Lafta 1996). High cell wall rigidity also implies that for any given change in cellular water potential, intracellular water content changes less than when the cells had more elastic walls (Rajashekar and Burke 1996, Niinemets 2001). Thus, upon freezing, less water migrates from cells to extracellular space where it freezes, while the water inside the cells remains supercooled at lower temperatures (Rajashekar and Burke 1996, Burr et al. 2001).
How increases in cell wall rigidity are achieved is not fully understood, but studies suggest that this primarily reflects increases in the pectin fraction of cell walls and stronger cross-linking of polymers in the pectin fraction (Solecka et al. 2008, Domon et al. 2013, Baldwin et al. 2014). However, pectins alone do not explain the increase in cell wall mass in cold-acclimated plants (Solecka et al. 2008), and there is also evidence of increases in other cell wall constituents including hemicellulose and cellulose and cell wall proteins such as expansins (Weiser et al. 1990, Le Gall et al. 2015). Although the classical Van Soest method for cell wall fiber measurement used by González-Zurdo et al. (2016) does not measure pectins (Van Soest 1994), important increases in leaf cellulose and hemicellulose contents with decreasing minimum temperature were observed in three evergreen species in their study (González-Zurdo et al. 2016). This evidence indicates that both increases in overall leaf thickness and investment in cell wall material constitute the key structural modifications to cold environments in Mediterranean evergreens.
Is being tough sufficient to survive winter periods?
Across Mediterranean Quercus species, greater freezing resistance was observed in species with greater MA and leaf thickness (Cavender-Bares et al. 2005), and González-Zurdo et al. (2016) have further highlighted an important within-species variability in structural traits as driven by site minimum temperature. However, the overall foliage freezing resistance of Mediterranean evergreen Quercus species is relatively moderate. The leaf freezing resistance of Q. ilex subsp. ilex L. growing at the northern limit of Q. ilex distribution is ca −12 °C (Larcher and Mair 1969). In the case of the two evergreen Quercus species studied by González-Zurdo et al. (2016), Q. ilex subsp. ballota (Desf.) Samp., which grows at more continental sites and at the altitudinal limit of Q. ilex, and Q. suber, which has a less continental range, the freezing resistance is ca −17 °C for the former and ca −10 °C for the latter species (González-Zurdo et al. 2016). Yet, several evergreen broad-leaved species can tolerate exceptional freezing temperatures as low as −35 to −40 °C (Nilsen 1991, Rajashekar and Lafta 1996), and the question is how general is the relationship between leaf toughness and freezing resistance. Paradoxically, in temperate Rhododendron species, tougher-leaved species with more rigid cell walls and lower osmotic potentials indeed had lower leaf freezing points and supercooling temperatures, but nevertheless, their leaves had lower frost resistance than in species with less tough leaves (Nilsen 1991), suggesting that extension of frost resistance from ca −10 to −20 °C to lower temperatures relies on traits other than those determining leaf toughness.
In fact, in Rhododendron species, leaf freezing resistance was strongly correlated with the avoidance of winter photoinhibition damage (Bao and Nilsen 1988, Nilsen 1990, Russell et al. 2009). The degree of damage scaled negatively with the capacity for thermonastic leaf moment, including changes in leaf angle and leaf curling (Nilsen 1991, Nilsen and Tolbert 1993). Species with more elastic cell walls and thinner epidermis had greater capacities for changing leaf angle and for leaf curling, and were effectively exposed to lower levels of solar radiation than nonthermonastic species (Nilsen and Tolbert 1993, Wang et al. 2008). Reduction of the exposed leaf area due to curling has also been associated with the decreases in leaf water loss and reduced rate of leaf thawing upon exposure to light (Nilsen 1990). Although the evergreen Rhododendron species have the most iconic changes in leaf shape upon exposure to low temperatures, similar modifications also occur in many other species including Aucuba japonica Thunb., Buxus sempervirens L. and Camellia japonica L., which all have relatively elastic cell walls.
Analogous correlations of chilling and freezing resistance with photoinhibitory damage have also been observed in other species that do not necessarily change leaf shape upon freezing (Boese and Huner 1990, Cavender-Bares et al. 1999, Cavender-Bares 2007, Kurtz et al. 2013), including Mediterranean Quercus species (Cavender-Bares et al. 2005). Obviously, being thicker, especially having a thicker epidermal layer, reduces the average intensity of light reaching the interior of the leaf, thereby reducing the probability for photodamage. Thus, increases in leaf thickness with decreasing site minimum temperature as observed by González-Zurdo et al. (2016) can also underscore the role of thickness in avoidance of photoinhibition. Of course, in addition to thickness per se, leaf surface optical characteristics and accumulation of antioxidants and photoprotective pigments can play a role in photoinhibition resistance (Streb et al. 1998, García-Plazaola and Becerril 2000, Cescatti and Niinemets 2004). So far, there are limited data available for site temperature effects on antioxidants, protective pigments and leaf surface characteristics in Q. ilex (Camarero et al. 2012). In the three sites studied by Camarero et al. (2012), the minimum temperature influence on pigments and leaf optics was not clear-cut, calling for more work on the role of photoinhibition resistance in freezing tolerance in Mediterranean evergreen species over a larger number of sites.
Tolerance of different stresses through leaf life span: frost vs drought
Mediterranean evergreens are exposed to harsh winters and hot and dry summers in their altitudinal and continental limits of dispersal. Traditionally, the sclerophyllous leaf habit of these species has been associated with tolerance of drought periods (Mooney and Dunn 1970, Kummerow 1973, Oertli et al. 1990). In particular, thick rigid cell walls corresponding to high leaf bulk elastic modulus have been associated with a greater capacity to extract water from drying soil at a given change in leaf symplastic water content (Niinemets 2001). Contrary to this expectation, precipitation had a weaker effect on leaf traits than minimum temperature in the evergreens studied by González-Zurdo et al. (2016), suggesting that modifications in leaf robustness primarily reflected adaptation to winter conditions in these species. In a similar manner, species differences in the bulk leaf elastic modulus were primarily associated with freezing tolerance rather than with drought tolerance in Patagonian steppe species (Scholz et al. 2012).
However, across Rhododendron species, there was a trade-off between drought and frost tolerance; nonthermonastic species with thicker epidermis and less elastic cell walls (e.g., R. ponticum L.) were more drought tolerant and less frost tolerant than thermonastic species with more elastic cell walls (e.g., R. catawbiense Michx.) (Nilsen 1991, Nilsen and Tolbert 1993, Wang et al. 2008). This apparent contradiction among broad-leaved evergreens from mesic habitats vs drought-prone habitats might reflect the overall greater constitutive level of cell wall adaptations to cope with drought stress in species from drought-stressed habitats.
What currently remains unclear is to what extent the strong effect of minimum site temperature on leaf traits in the study of González-Zurdo et al. (2016) is driven by ecotypic (genetic) and plastic components of phenotypic variance. Common garden studies have demonstrated that altitudinal variation in leaf traits is driven by both components of variance, whereas the importance of the ecotypic and plastic sources of variation is different for different traits and can vary among species (Kitayama et al. 1997, Cordell et al. 1998, Bresson et al. 2011, Thomas 2011). The other question is to what extent the capacity for plastic modifications varies for different ecotypes within the species climatic range. There is evidence that it is primarily the plastic response rather than the genotypic differentiation that is responsible for frost and drought tolerance of Q. ilex at its specific growth locations (Gimeno et al. 2009). Nevertheless, across the whole area of dispersal of Q. ilex, leaf traits were driven by precipitation and temperature in a complex manner, with the minimum temperature not always being a significant predictor of leaf structural and/or photosynthetic characteristics (Niinemets 2015).
Does enhanced leaf cost in cold climates limit canopy formation?
Classical studies have suggested that extension of leaf life span allows for amortization of leaf cost over a longer time period and thus, for construction of a more extensive canopy for light interception (Schulze et al. 1977, 1986, Kikuzawa 1995). In a competitive situation, greater leaf area provides an important means for shading out neighbors and capturing more environmental resources (Givnish 1978, Schieving and Poorter 1999, Anten 2002). Although increases in leaf thickness and MA are typically associated with increased leaf longevity, leaf longevity did not change with decreasing minimum temperatures in the study of González-Zurdo et al. (2016). This contrasts the observed and theoretically predicted worldwide trends in MA and leaf longevity relationships that become steeper with decreasing temperature, i.e., greater longevity at given MA in colder environments (Kikuzawa et al. 2013). However, these global predictions do not consider other potentially interacting stresses in tougher environments, e.g., increases in abrasive wind damage at higher elevation sites that could limit the extension of leaf longevity.
Furthermore, there was evidence of reduction of foliage photosynthetic activity in colder sites, reflecting reduced nitrogen investment in the components of photosynthetic machinery (González-Zurdo et al. 2016). In addition to constrained investment of nitrogen in photosynthetic machinery that can result from greater fractions of nitrogen associated with cell walls (Hikosaka and Shigeno 2009), tougher leaves are generally characterized by greater restriction of photosynthesis due to stomatal and mesophyll diffusion constraints than more mesophytic leaves (Hikosaka and Shigeno 2009, Niinemets et al. 2011). This evidence collectively indicates that in the evergreens studied by González-Zurdo et al. (2016), leaves became increasingly costly at colder sites without associated longevity benefits, altogether implying longer leaf payback times that could ultimately lead to reduced carbon availability for foliage construction. In fact, Ogaya and Peñuelas (2007) demonstrated that leaf area index (LAI) of Q. ilex canopies decreased with decreasing minimum temperature, indicating that plants in colder sites indeed support lower leaf areas.
In general, very few species can simultaneously tolerate frost, drought and shade (Laanisto and Niinemets 2015). Becoming tougher and gaining an improved capacity to cope with frost and drought, but losing the capacity to support extensive leaf area indices, might ultimately limit evergreen species dispersal in competitive situations where shading by deciduous neighbors with higher LAI can curb the carbon availability for leaf construction in evergreens.
Species-specific leaf economics spectra within the universal worldwide economics spectrum
The worldwide leaf economics spectrum characterizes coordinated changes in suites of leaf traits through resource-limited to resource-rich environments (Wright et al. 2004). Species growing in resource-limited environments typically have high leaf longevity, low photosynthetic capacity and nitrogen content per dry mass (Nm), and high MA, while species in resource-rich habitats have the opposite combination of these traits (Wright et al. 2004). However, the universal leaf trait spectrum consists of within-species trait spectra that are shaped by plastic and ecotypic sources of trait variation (Niinemets 2015). As the study of González-Zurdo et al. (2016) shows, localized adjustment to given environmental pressures can produce within-species trait combinations that apparently contradict the trends within the universal trait spectrum, including low and high MA vs invariant Nm and leaf life span, high MA and high phosphorus content per dry mass (Pm; Figure 1).
Previously, it has been demonstrated that shade is a low resource environment that can result in trait variations that are opposite to those predicted by the worldwide economics spectrum (Lusk et al. 2008, Hallik et al. 2009). The results of González-Zurdo et al. (2016) and the evidence outlined above indicate that adjustment to frost can differently shape various functional traits with potential implications for broad trait scaling. While the invariance of Nm across habitats with varying temperature had a minor effect on the broad scaling of Nm with MA (Figure 1b), the strong positive scaling of both MA and Pm with minimum temperature resulted in significant intraspecific correlations, but the general trend across species collapsed (Figure 1c). Whether the scaling of Pm with the freezing period length reflects nutritional changes across the sites (e.g., Maire et al. 2015) or physiological acclimation responses such as accumulation of phospholipids to enhance cryotolerance of membranes (Siminovitch et al. 1975, Willemot 1975) is not clear. Nevertheless, this discrepancy among within-species vs across-species leaf trait relationships provides important evidence of how environmental factors can reshape the trait correlation networks. Given the contrasting strategies of structural adjustment to frost in evergreen species with elastic (e.g., rhododendrons and allies) and nonelastic cell walls (e.g., Mediterranean evergreens), further studies are called for to gain insight into the effects of such divergent responses on leaf trait relationships in evergreens.
References
- Allen DJ, Ort DR (2001) Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci 6:36–42. doi:10.1016/S1360-1385(00)01808-2 [DOI] [PubMed] [Google Scholar]
- Anten NPR. (2002) Evolutionarily stable leaf area production in plant populations. J Theor Biol 217:15–32. doi:10.1006/jtbi.2002.3022 [DOI] [PubMed] [Google Scholar]
- Atkin OK, Botman B, Lambers H (1996) The causes of inherently slow growth in alpine plants: an analysis based on the underlying carbon economies of alpine and lowland Poa species. Funct Ecol 10:698–707. doi:10.2307/2390504 [Google Scholar]
- Baldwin L, Domon J-M, Klimek JF et al. (2014) Structural alteration of cell wall pectins accompanies pea development in response to cold. Phytochemistry 104:37–47. doi:10.1016/j.phytochem.2014.04.011 [DOI] [PubMed] [Google Scholar]
- Ball MC, Wolfe J, Canny M, Hofmann M, Nicotra AB, Hughes D (2002) Space and time dependence of temperature and freezing in evergreen leaves. Funct Plant Biol 29:1259–1272. doi:10.1071/FP02037 [DOI] [PubMed] [Google Scholar]
- Bao Y, Nilsen ET (1988) The ecophysiological significance of leaf movements in Rhododendron maximum. Ecology 69:1578–1587. doi:10.2307/1941655 [Google Scholar]
- Boese SR, Huner NPA (1990) Effect of growth temperature and temperature shifts on spinach leaf morphology and photosynthesis. Plant Physiol 94:1830–1836. doi:10.1104/pp.94.4.1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresson CC, Vitasse Y, Kremer A, Delzon S (2011) To what extent is altitudinal variation of functional traits driven by genetic adaptation in European oak and beech? Tree Physiol 31:1164–1174. doi:10.1093/treephys/tpr084 [DOI] [PubMed] [Google Scholar]
- Burr KE, Hawkins CDB, L’Hirondelle SJ, Binder WD, George MF, Repo T (2001) Methods for measuring cold hardiness of conifers. In: Bigras FJ, Colombo SJ (eds) Conifer cold hardiness. Kluwer Academic Publishers, Dordrecht, pp 369–401. [Google Scholar]
- Camarero JJ, Olano JM, Arroyo Alfaro SJ, Fernández-Marín B, Becerril JM, García-Plazaola JI (2012) Photoprotection mechanisms in Quercus ilex under contrasting climatic conditions. Flora 207:557–564. doi:10.1016/j.flora.2012.06.003 [Google Scholar]
- Cavender-Bares J. (2007) Chilling and freezing stress in live oaks (Quercus section Virentes): intra- and inter-specific variation in PS II sensitivity corresponds to latitude of origin. Photosynth Res 94:437–453. doi:10.1007/s11120-007-9215-8 [DOI] [PubMed] [Google Scholar]
- Cavender-Bares J, Apostol S, Moya I, Briantais JM, Bazzaz FA (1999) Chilling-induced photoinhibition in two oak species: are evergreen leaves inherently better protected than deciduous leaves? Photosynthetica 36:587–596. doi:10.1023/A:1007000406399 [Google Scholar]
- Cavender-Bares J, Cortes P, Rambal S, Joffre R, Miles B, Rocheteau A (2005) Summer and winter sensitivity of leaves and xylem to minimum freezing temperatures: a comparison of cooccurring Mediterranean oaks that differ in leaf lifespan. New Phytol 168:597–612. doi:10.1111/j.1469-8137.2005.01555.x [DOI] [PubMed] [Google Scholar]
- Cescatti A, Niinemets Ü (2004) Sunlight capture. Leaf to landscape. In: Smith WK, Vogelmann TC, Chritchley C (eds) Photosynthetic adaptation. Chloroplast to landscape. Springer, Berlin, pp 42–85. [Google Scholar]
- Cordell S, Goldstein G, Mueller-Dombois D, Webb D, Vitousek PM (1998) Physiological and morphological variation in Metrosideros polymorpha, a dominant Hawaiian tree species, along an altitudinal gradient: the role of phenotypic plasticity. Oecologia 113:188–196. doi:10.1007/s004420050367 [DOI] [PubMed] [Google Scholar]
- David TS, Henriques MO, Kurz-Besson C et al. (2007) Water-use strategies in two co-occurring Mediterranean evergreen oaks: surviving the summer drought. Tree Physiol 27:793–803. doi:10.1093/treephys/27.6.793 [DOI] [PubMed] [Google Scholar]
- Domon J-M, Baldwin L, Acket S et al. (2013) Cell wall compositional modifications of Miscanthus ecotypes in response to cold acclimation. Phytochemistry 85:51–61. doi:10.1016/j.phytochem.2012.09.001 [DOI] [PubMed] [Google Scholar]
- Fall R, Karl T, Jordan A, Lindinger W (2001) Biogenic C5 VOCs: release from leaves after freeze-thaw wounding and occurrence in air at a high mountain observatory. Atmos Environ 35:3905–3916. doi:10.1016/S1352-2310(01)00141-8 [Google Scholar]
- García-Plazaola JI, Becerril JM (2000) Photoprotection mechanisms in European beech (Fagus sylvatica L.) seedlings from diverse climatic origins. Trees 14:339–343. [Google Scholar]
- Gimeno TE, Pias B, Lemos-Filho JP, Valladares F (2009) Plasticity and stress tolerance override local adaptation in the responses of Mediterranean holm oak seedlings to drought and cold. Tree Physiol 29:87–98. doi:10.1093/treephys/tpn007 [DOI] [PubMed] [Google Scholar]
- Givnish TJ. (1978) Ecological aspects of plant morphology: leaf form in relation to environment. Acta Biotheor 27:83–142. [Google Scholar]
- González-Zurdo P, Escudero A, Babiano J, García-Ciudad A, Mediavilla S (2016) Costs of leaf reinforcement in response to winter cold in evergreen species. Tree Physiol 36:273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray GR, Chauvin L-P, Sarhan F, Huner NPA (1997) Cold acclimation and freezing tolerance. A complex interaction of light and temperature. Plant Physiol 114:467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallik L, Niinemets Ü, Wright IJ (2009) Are species shade and drought tolerance reflected in leaf-level structural and functional differentiation in Northern Hemisphere temperate woody flora? New Phytol 184:257–274. doi:10.1111/j.1469-8137.2009.02918.x [DOI] [PubMed] [Google Scholar]
- Herrick GT, Friedland AJ (1991) Winter desiccation and injury of subalpine red spruce. Tree Physiol 8:23–36. doi:10.1093/treephys/8.1.23 [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Shigeno A (2009) The role of Rubisco and cell walls in the interspecific variation in photosynthetic capacity. Oecologia 160:443–451. doi:10.1007/s00442-009-1315-z [DOI] [PubMed] [Google Scholar]
- Huner NPA, Öquist G, Sarhan F (1998) Energy balance and acclimation to light and cold. Trends Plant Sci 3:224–230. doi:10.1016/S1360-1385(98)01248-5 [Google Scholar]
- Ivanov AG, Sane PV, Zeinalov Y, Malmberg G, Gardeström P, Huner NPA, Öquist G (2001) Photosynthetic electron transport adjustments in overwintering Scots pine (Pinus sylvestris L.). Planta 213:575–585. doi:10.1007/s004250100522 [DOI] [PubMed] [Google Scholar]
- Kikuzawa K. (1995) The basis for variation in leaf longevity of plants. Vegetatio 121:89–100. doi:10.1007/BF00044675 [Google Scholar]
- Kikuzawa K, Onoda Y, Wright IJ, Reich PB (2013) Mechanisms underlying global temperature-related patterns in leaf longevity. Glob Ecol Biogeogr 22:982–993. doi:10.1111/geb.12042 [Google Scholar]
- Kitayama K, Pattison R, Cordell S, Webb D, Mueller-Dombois D (1997) Ecological and genetic implications of foliar polymorphism in Metrosideros polymorpha Gaud. (Myrtaceae) in a habitat matrix on Mauna Loa, Hawaii. Ann Bot 80:491–497. doi:10.1006/anbo.1996.0473 [Google Scholar]
- Koppel A, Heinsoo K (1994) Variability in cuticular resistance of Picea abies(L.) Karst. and its significance in winter desiccation. Proc Estonian Acad Sci Ecol 4:56–63. [Google Scholar]
- Körner C, Bannister P, Mark AF (1986) Altitudinal variation in stomatal conductance, nitrogen content and leaf anatomy in different plant life forms in New Zealand. Oecologia 69:577–588. doi:10.1007/BF00410366 [DOI] [PubMed] [Google Scholar]
- Körner C, Neumayer M, Pelaez Menendez-Riedl S, Smeets-Scheel A (1989) Functional morphology of mountain plants. Flora 182:353–383. [Google Scholar]
- Kummerow J. (1973) Comparative anatomy of sclerophylls of Mediterranean climatic areas: origin and structure. In: di Castri F, Mooney HA (eds) Mediterranean-type ecosystems. Springer, Berlin, Heidelberg, New York, pp 157–167. [Google Scholar]
- Kurtz CM, Savage JA, Huang I-Y, Cavender-Bares J (2013) Consequences of salinity and freezing stress for two populations of Quercus virginiana Mill. (Fagaceae) grown in a common garden. J Torrey Bot Soc 140:145–156. doi:10.3159/TORREY-D-12-00060.1 [Google Scholar]
- Laanisto L, Niinemets Ü (2015) Polytolerance to abiotic stresses: how universal is the shade-drought tolerance trade-off in woody species? Glob Ecol Biogeogr 24:571–580. doi:10.1111/geb.12288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamontagne M, Margolis H, Bigras F (1998) Photosynthesis of black spruce, jack pine, and trembling aspen after artificially induced frost during the growing season. Can J For Res 28:1–12. doi:10.1139/x97-184 [Google Scholar]
- Larcher W. (1985) Winter stress in high mountains. In: Turner H, Tranquillini W (eds) Establishment and tending of subalpine forest: research and management. Eidgenössische Anstalt für forstliches Versuchswesen, Birmensdorf, pp 11–19. [Google Scholar]
- Larcher W, Mair B (1969) Die Temperaturresistenz als ökophysiologisches Konstitutionsmerkmal. 1. Quercus ilex und andere Eichenarten des Mittelmeergebietes. Oecol Plant 4:347–375. [Google Scholar]
- Le Gall H, Philippe F, Domon J-M, Gillet F, Pelloux J, Rayon C (2015) Cell wall metabolism in response to abiotic stress. Plants 4:112–166. doi:10.3390/plants4010112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusk CH, Reich PB, Montgomery RA, Ackerly DD, Cavender-Bares J (2008) Why are evergreen leaves so contrary about shade? Trends Ecol Evol 23:299–303. doi:10.1016/j.tree.2008.02.006 [DOI] [PubMed] [Google Scholar]
- Maire V, Wright IJ, Prentice IC et al. (2015) Global effects of soil and climate on leaf photosynthetic traits and rates. Glob Ecol Biogeogr 24:706–717. doi:10.1111/geb.12296 [Google Scholar]
- Mooney HA, Dunn EL (1970) Convergent evolution of Mediterranean-climate evergreen sclerophyll shrubs. Evolution 24:292–303. doi:10.2307/2406805 [DOI] [PubMed] [Google Scholar]
- Niinemets Ü. (2001) Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology 82:453–469. doi:10.1890/0012-9658(2001)082[0453:GSCCOL]2.0.CO;2 [Google Scholar]
- Niinemets Ü. (2015) Is there a species spectrum within the world-wide leaf economics spectrum? Major variations in leaf functional traits in the Mediterranean sclerophyll Quercus ilex. New Phytol 205:79–96. doi:10.1111/nph.13001 [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Flexas J, Peñuelas J (2011) Evergreens favored by higher responsiveness to increased CO2. Trends Ecol Evol 26:136–142. doi:10.1016/j.tree.2010.12.012 [DOI] [PubMed] [Google Scholar]
- Nilsen ET. (1990) Why do Rhododendron leaves curl? Arnoidia 50:30–35. [Google Scholar]
- Nilsen ET. (1991) The relationship between freezing tolerance and thermotropic leaf movement in five Rhododendron species. Oecologia 87:63–71. doi:10.1007/BF00323781 [DOI] [PubMed] [Google Scholar]
- Nilsen ET, Tolbert A (1993) Does winter leaf curling confer cold stress tolerance in Rhododendron? J Am Rhododendron Soc 47:98–104. [Google Scholar]
- Oertli JJ, Lips SH, Agami M (1990) The strength of sclerophyllous cells to resist collapse due to negative turgor pressure. Acta Oecol 11:281–289. [Google Scholar]
- Ogaya R, Peñuelas J (2007) Leaf mass per area ratio in Quercus ilex leaves under a wide range of climatic conditions. The importance of low temperatures. Acta Oecol 31:168–173. doi:10.1016/j.actao.2006.07.004 [Google Scholar]
- Öquist G, Huner NPA (1993) Cold-hardening-induced resistance to photoinhibition of photosynthesis in winter rye is dependent upon an increased capacity for photosynthesis. Planta 189:150–156. doi:10.1007/BF00201355 [Google Scholar]
- Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R (2009) Tansley review. Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol 182:565–588. doi:10.1111/j.1469-8137.2009.02830.x [DOI] [PubMed] [Google Scholar]
- Rajashekar CB, Burke MJ (1996) Freezing characteristics of rigid plant tissues. Development of cell tension during extracellular freezing. Plant Physiol 111:597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajashekar CB, Lafta A (1996) Cell-wall changes and cell tension in response to cold acclimation and exogenous abscisic acid in leaves and cell cultures. Plant Physiol 111:605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichstein M, Tenhunen JD, Roupsard O, Ourcival JM, Rambal S, Dore S, Valentini R (2002) Ecosystem respiration in two Mediterranean evergreen holm oak forests: drought effects and decomposition dynamics. Funct Ecol 16:27–39. doi:10.1046/j.0269-8463.2001.00597.x [Google Scholar]
- Russell RB, Lei TT, Nilsen ET (2009) Freezing induced leaf movements and their potential implications to early spring carbon gain: Rhododendron maximum as exemplar. Funct Ecol 23:463–471. doi:10.1111/j.1365-2435.2008.01534.x [Google Scholar]
- Schieving F, Poorter H (1999) Carbon gain in a multispecies canopy: the role of specific leaf area and photosynthetic nitrogen-use efficiency in the tragedy of the commons. New Phytol 143:201–211. doi:10.1046/j.1469-8137.1999.00431.x [Google Scholar]
- Scholz FG, Bucci SJ, Arias N, Meinzer FC, Goldstein G (2012) Osmotic and elastic adjustments in cold desert shrubs differing in rooting depth: coping with drought and subzero temperatures. Oecologia 170:885–897. doi:10.1007/s00442-012-2368-y [DOI] [PubMed] [Google Scholar]
- Schulze ED, Fuchs M, Fuchs MI (1977) Spacial distribution of photosynthetic capacity and performance in a mountain spruce forest of northern Germany. III. The significance of the evergreen habit. Oecologia 30:239–248. doi:10.1007/BF01833630 [DOI] [PubMed] [Google Scholar]
- Schulze ED, Küppers M, Matyssek R (1986) The roles of carbon balance and branching pattern in the growth of woody species. In: Givnish TJ. (ed.) On the economy of plant form and function. Proceedings of the Sixth Maria Moors Cabot Symposium, “Evolutionary constraints on primary productivity: adaptive patterns of energy capture in plants”, Harvard Forest, August 1983. Cambridge University Press, Cambridge, London, New York, New Rochelle, Melbourne, Sydney, pp 585–602. [Google Scholar]
- Siminovitch D, Singh J, de la Roche IA (1975) Studies on membranes in plant cells resistant to extreme freezing. I. Augmentation of phospholipids and membrane substance without changes in unsaturation of fatty acids during hardening of black locust bark. Cryobiology 12:144–153. doi:10.1016/S0011-2240(75)80006-X [DOI] [PubMed] [Google Scholar]
- Solecka D, Żebrowski J, Kacperska A (2008) Are pectins involved in cold acclimation and de-acclimation of winter oil-seed rape plants? Ann Bot 101:521–530. doi:10.1093/aob/mcm329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanowska M, Kuraś M, Kubacka-Zebalska M, Kacperska A (1999) Low temperature affects pattern of leaf growth and structure of cell walls in winter oilseed rape (Brassica napus L., var. oleifera L.). Ann Bot 84:313–319. doi:10.1006/anbo.1999.0924 [Google Scholar]
- Steffen KL, Arora R, Palta JP (1989) Relative sensitivity of photosynthesis and respiration to freeze-thaw stress in herbaceous species: importance of realistic freeze-thaw protocols. Plant Physiol 89:1372–1379. doi:10.1104/pp.89.4.1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb P, Shang W, Feierabend J, Bligny R (1998) Divergent strategies of photoprotection in high-mountain plants. Planta 207:313–324. doi:10.1007/s004250050488 [Google Scholar]
- Thomas SC. (2011) Genetic vs. phenotypic responses of trees to altitude. Tree Physiol 31:1161–1163. doi:10.1093/treephys/tpr105 [DOI] [PubMed] [Google Scholar]
- Van Soest PJ. (1994) Nutritional ecology of the ruminant. 2nd ed. Cornell University Press, Ithaca, NY, 479 p. [Google Scholar]
- Venema JH, Villerius L, van Hasselt PR (2000) Effect of acclimation to suboptimal temperature on chilling-induced photodamage: comparison between a domestic and a high-altitude wild Lycopersicon species. Plant Sci 152:153–163. doi:10.1016/S0168-9452(99)00228-9 [Google Scholar]
- Wang X, Arora R, Horner HT, Krebs SL (2008) Structural adaptations in overwintering leaves of thermonastic and nonthermonastic Rhododendron species. J Am Soc Hortic Sci 133:768–776. [Google Scholar]
- Weiser RL, Wallner SJ, Waddell JW (1990) Cell wall and extensin mRNA changes during cold acclimation of pea seedlings. Plant Physiol 93:1021–1026. doi:10.1104/pp.93.3.1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemot C. (1975) Stimulation of phospholipid biosynthesis during frost hardening of winter wheat. Plant Physiol 55:356–359. doi:10.1104/pp.55.2.356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward FI. (1979) The differential temperature responses of the growth of certain plant species from different altitudes. II. Analyses of the control and morphology of leaf extension and specific leaf area of Phleum bertolonii D.C. and P. alpinum L. New Phytol 82:397–405. doi:10.1111/j.1469-8137.1979.tb02666.x [Google Scholar]
- Wright IJ, Reich PB, Westoby M et al. (2004) The world-wide leaf economics spectrum. Nature 428:821–827. doi:10.1038/nature02403 [DOI] [PubMed] [Google Scholar]
- Zavala MA, Espelta JM, Retana J (2000) Constraints and trade-offs in Mediterranean plant communities: the case of holm oak-Aleppo pine forests. Bot Rev 66:119–149. doi:10.1007/BF02857785 [Google Scholar]