Abstract
Background
This study was designed to evaluate the effect of chronic bronchitis (CB) symptoms and degree of emphysema in a multicenter Korean cohort.
Methods
From April 2012 to May 2015, patients diagnosed with chronic obstructive lung disease (COPD) who were aged above 40 years at 46 hospitals throughout Korea were enrolled. All of the patients were classified according to CB symptoms and the diffusing capacity of the lung for carbon monoxide (DLCO); demographic data, symptom scores, and the result of lung function tests and exacerbations were then analyzed.
Results
A total of 812 patients were enrolled. Among these patients, 285 (35.1%) had CB symptoms. A total of 51% of patients had high DLCO without CB symptoms [CB (−) high DLCO], 24.9% had CB symptoms only [CB (+) high DLCO], 14.2% had low DLCO only [CB (−) low DLCO], and 10.2% had both low DLCO and CB [CB (+) low DLCO]. Patients with CB (+) low DLCO showed a significantly lower post-bronchodilator (BD) forced expiratory volume for 1 second (FEV1) and more severe dyspnea than patients with CB (−) high DLCO. On multivariate analysis, the risk of acute exacerbation was two times higher [odds ratio (OR) 2.06; 95% confidence interval (CI): 1.18–3.62; P=0.01] in the CB (+) low DLCO group than in the CB (−) high DLCO group.
Conclusions
In this COPD cohort, patients showed distinct clinical characteristics and outcomes according to the presence of CB and degree of DLCO. CB and low DLCO were associated with the risk of acute exacerbation.
Keywords: Chronic bronchitis (CB), diffusing capacity, chronic obstructive lung disease (COPD), acute exacerbation
Introduction
Chronic obstructive lung disease (COPD) is a major disease that leads to impaired quality of life and imposes a significant worldwide socio-economic burden (1-3). The prevalence of COPD is increasing worldwide, and the rate was 9–10% in subjects aged ≥40 years in 2006 (1). Additionally, it has been estimated that COPD will become the third leading cause of death by 2020 (3).
According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2012, the treatment objectives of COPD are the relief of symptoms and reduction in the risk of future adverse health events (4). The current GOLD guidelines have evolved such that treatment of COPD is determined based on a combination of the risk of acute exacerbation, symptoms and forced expiratory volume by 1 second (FEV1). Recently, treatment strategies have been developed ranging from simply assessing disease severity by FEV1 to discussing endotypes and clinical phenotypes due to the complexity and heterogeneity of the disease (5). Specifically, the traditional approach, which divided COPD into chronic bronchitis (CB) or emphysematous types has been challenged by various clinical phenotypes. For example, Turner et al. classified COPD into frequent exacerbation, CB, α1-antitrypsin deficiency, upper zone dominant emphysema or bullous emphysema, eosinophilic COPD and biomass COPD subgroups (6). These groupings not only aid identification of clusters of characteristics related to clinically meaningful outcomes, but also allow for differentiated therapeutic approaches.
There are many studies concerning COPD phenotypes, but no validated consensus has been reached (7). Among the phenotypes, CB is the most widely studied and has been suggested to be associated with increased mortality and frequent exacerbations (8,9). However, there are controversies concerning whether chronic cough and sputum production alone can be a clinical COPD phenotype because conflicting data exist regarding the association between COPD and clinical manifestations and outcomes (10-12). Moreover, the definition of CB remains to be confirmed, such that CB prevalence differs according to the definition used (13).
Recent studies have reported that an emphysematous lung was associated with acute exacerbation of COPD and comorbidities including atherosclerosis and osteoporosis (14-16). However, whether the severity of emphysema, as designated by the emphysema index on computed tomography (CT) scans, is a parameter associated with severe respiratory symptoms and prognosis, or is independent to airflow limitation, remains unclear. Moreover, although high-resolution CT (HRCT) accurately assesses the degree of emphysema, measuring the emphysema score is still not routinely performed in clinical practice. Patients who have airflow obstruction, decreased diffusing capacity of the lung for carbon monoxide (DLCO) suggests emphysema (17) and DLCO correlate well with the degree of emphysema, as assessed by HRCT (18,19). Measuring DLCO is feasible in clinical practice, and there is no radiation exposure (in contrast to HRCT).
This study was designed to evaluate the clinical, physiological characteristics of COPD patients presenting with CB symptoms and variable degrees of emphysema, represented by DLCO, and to validate the usefulness of this new parameter in a COPD cohort.
Methods
Study design
This study used the KOCOSS (Korean COPD Subgroup Study) database and an observational, multi-center cohort design. Patients diagnosed with COPD at 46 referral hospitals (345–2,806 beds) throughout Korea were recruited (20). Patients diagnosed with COPD at the Department of Pulmonology were enrolled if they were aged above 40 years and the post-bronchodilator (BD) ratio of FEV1 to forced vital capacity was <0.7. This cohort was initiated in April 2012 and is currently ongoing; the data included in the present analysis were obtained in May 2015. At the initial visit, demographic and clinical data, including age, sex, smoking history, the duration of cough or sputum, severity of dyspnea, quality of life, previous exacerbation within one year before enrollment, and the results of pulmonary function and the 6-minute walking test were collected. Dyspnea was assessed using the modified Medical Research Council Dyspnea scale (mMRC) and the COPD assessment test (CAT). St George’s Respiratory Questionnaire (SGRQ)-C was used to assess health-related quality of life. Lung function was measured using spirometry and DLCO. During the lung function test, multiple forced expiratory efforts were performed to meet American Thoracic Society (ATS) acceptability criteria (21) by trained examiners. Additionally, the 6-minute walking test was performed according to the ATS guidelines (22). During the follow-up periods, lung function, scores on the dyspnea scale and quality of life questionnaire, and exacerbation history were measured. The histories of acute exacerbation were checked using a questionnaire that recorded whether patients visited an out-patient clinic or emergency department due to increased sputum, change in sputum characteristics or aggravation of dyspnea for previous 12 months at initial visit. Ethics approval was obtained from the Ethics Committee at each site. All of the subjects provided written informed consent. We obtained approval to use the patients’ records from each institution and patient confidentiality was maintained (Institutional Review Board of Seoul St. Mary’s Hospital, No. KC12OIMI0163).
Classification of patients
All of the patients were classified according to the presence of CB and level of diffusing capacity of the lung. Patients who had phlegm on most days for at least 3 months per year, and a DLCO less than 60% of the predictive value (23) were classified into the CB with Low DLCO group. If patients did not present with phlegm but DLCO was greater than 60% of the predictive value, they were classified into the High DLCO without CB group. Additionally, patients who did not have phlegm but had a DLCO of less than 60% were grouped into the Low DLCO without CB group. If patients had phlegm and a DLCO of more than 60%, they were grouped into the CB and High DLCO group. Acute exacerbation was defined as worsening of respiratory symptoms such as an increased amount of sputum, purulent color change or aggravation of dyspnea requiring a visit to an emergency room or outpatient clinic.
Statistical analysis
Data are provided as means ± SEM for continuous variables, and as proportions for categorical variables. Analyses of the differences between exacerbators and non-exacerbators were performed using Student’s t-test for continuous variables and χ2 test or Fisher’s exact test for categorical variables. Odds ratios (ORs) and their 95% confidence intervals (CIs) were computed. All tests were two-sided, and a P value <0.05 was taken to indicate statistical significance. Simple and multiple logistic regression analyses were performed on significant variables from a previous analysis of the comparison between exacerbators and non-exacerbators. Variables that were associated with a risk of acute exacerbation (P<0.05) from simple logistic regression models were selected for inclusion in multiple backward stepwise logistic regression models. All statistical analyses were performed using the SPSS for Windows software package (ver. 18.0; SPSS, Inc., Chicago, IL, USA).
Results
Baseline characteristics of all patients
During the study period, 1,148 patients were enrolled, of whom 42 were excluded due to missing data on bronchitis symptoms, and 294 patients were excluded due to the absence of diffusing capacity measurements. Table 1 shows the baseline demographic and clinical characteristics of the remaining 812 patients. At the initial visit, 750 (92.7%) were male, and their mean age was 71.6 years. Among these 812 patients, 35.1% (n=285) presented with CB symptoms. The mean mMRC dyspnea scale score was 1.6±0.03, the mean CAT score was 15.2±0.3, and the mean SGRQ-C score was 34.0±0.7. The mean 6-minute walk test result was 371.0±4.3 m. The severity of airflow limitation was classified according to GOLD staging. Most (58.7%) of the patients were GOLD stage 2 (50%≤ FEV1 <80%), 29.3% were stage 3 (30%≤ FEV1 <50%), 5.7% were stage 4 (FEV1 ≤30%), and 6.3% were stage 1 (FEV1 ≥80%). The mean DLCO was measured as 75.3%±0.9%, and 198 (24.4%) patients had low DLCO.
Table 1. Baseline characteristics of total patients (n=812).
| Characteristics | Mean ± SEM or No. (%) |
|---|---|
| Male, n (%) | 750 (92.7) |
| Age (yr) | 71.6±0.3 |
| Chronic bronchitis, n (%) | 285 (35.1) |
| mMRC | 1.6±0.03 |
| CAT | 15.2±0.3 |
| SGRQ-C | 34.0±0.7 |
| Six-minute walk test (m) | 371.0±4.3 |
| Post-BD FVC (%) | 84.2±0.6 |
| Post-BD FEV1 (%) | 57.3±0.6 |
| GOLD 1, n (%) | 51 (6.3) |
| GOLD 2, n (%) | 477 (58.7) |
| GOLD 3, n (%) | 238 (29.3) |
| GOLD 4, n (%) | 46 (5.7) |
| Post-BD FEV1/FVC (%) | 49.3±0.005 |
| DLCO (%) | 75.3±0.9 |
| DLCO <60% | 198 (24.4) |
| Current smoker, n (%) | 226 (27.8) |
mMRC, modified Medical Research Council Dyspnea scale; CAT, COPD assessment test; SGRQ-C, St George’s Respiratory Questionnaire (SGRQ)-C; Post-BD FVC, post bronchodilator forced vital capacity; Post-BD FEV1, post bronchodilator forced expiratory volume for 1 second; GOLD, global initiative for chronic obstructive lung disease; Post-BD FEV1/FVC, post bronchodilator forced expiratory volume for 1 second/forced vital capacity ratio; DLCO, diffusing capacity of the lung for carbon monoxide.
Comparison of clinical characteristics and parameters among the four groups
Figure 1 describes the distribution of the 812 patients. A total of 412 (50.7%) patients were grouped into the high DLCO without CB group [CB (−) high DLCO], 202 (24.9%) were grouped into the high DLCO with CB group [CB (+) high DLCO], 115 (14.2%) were grouped into the low DLCO without CB group [CB (−) low DLCO], and 83 (10.2%) were grouped into the low DLCO with CB group [CB (+) low DLCO].
Figure 1.
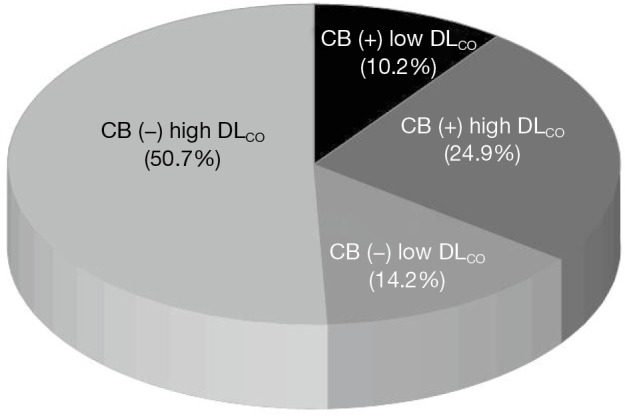
Distribution of patients according to chronic bronchitis symptom and DLCO. DLCO, diffusing capacity of the lung for carbon monoxide.
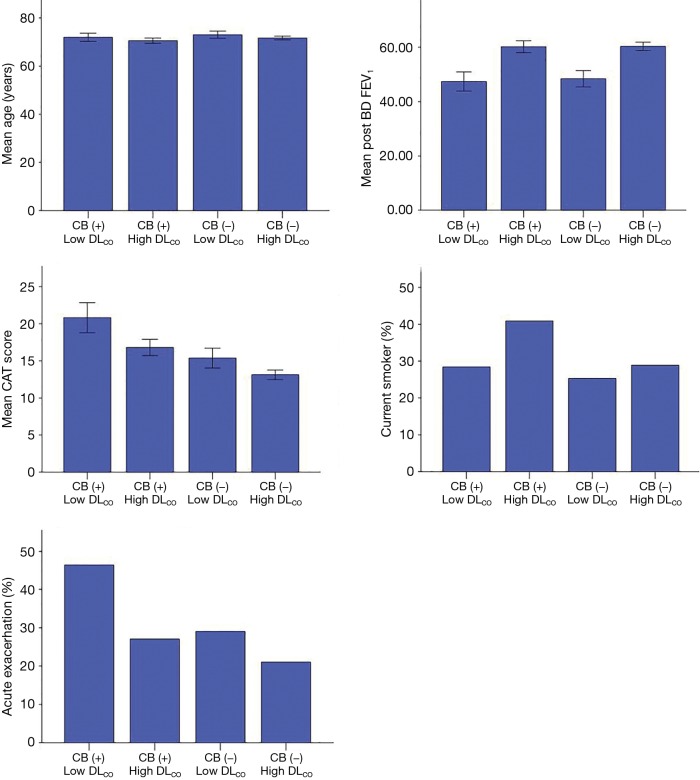
After dividing the patients into the four groups, we compared the clinical parameters among groups (Table 2, Figure 2). The post-BD FEV1, CAT score and history of acute exacerbation for previous 12 months were significantly different among groups (P<0.001). Compared with patients without CB and high DLCO [CB (−) high DLCO], patients who had CB and low DLCO [CB (+) low DLCO] showed a significantly lower post-BD FEV1 and higher dyspnea symptom scores. The percentage of patients who had acute exacerbation was highest in the CB (+) low DLCO group, and was about two times higher than that in the CB (−) high DLCO group. Patients with CB (−) low DLCO showed a lower post-BD FEV1 than patients with CB (+) high DLCO (Table 2). The mean age did not differ significantly among groups.
Table 2. Comparison of clinical parameters between the groups classified according to the bronchitis and DLCO.
| Clinical parameters | Mean ± SEM (min–max) or N (%) | P |
|---|---|---|
| Age | 0.06 | |
| CB (+) low DLCO | 71.9±0.86 [53–88] | |
| CB (+) high DLCO | 70.5±0.54 [52–90] | |
| CB (−) low DLCO | 73.0±0.74 [44–89] | |
| CB (−) high DLCO | 71.6±0.38 [48–93] | |
| Post-BD FEV1 (%) | <0.001 | |
| CB (+) low DLCO | 47.4±1.75 [19–99] | |
| CB (+) high DLCO | 60.2±1.12 [24–109] | |
| CB (−) low DLCO | 48.4±1.51 [23–108] | |
| CB (−) high DLCO | 60.4±0.75 [25–123] | |
| CAT | <0.001 | |
| CB (+) low DLCO | 20.8±1.00 [2–40] | |
| CB (+) high DLCO | 16.8±0.55 [2–36] | |
| CB (−) low DLCO | 15.4±0.67 [0–32] | |
| CB (−) high DLCO | 13.1±0.31 [0–34] | |
| Current smoker, n (%) | 0.01 | |
| CB (+) low DLCO | 23 (28.4) | |
| CB (+) high DLCO | 74 (40.9) | |
| CB (−) low DLCO | 25 (25.3) | |
| CB (−) high DLCO | 104 (28.9) | |
| Patients with exacerbation during 12 months, n (%) | <0.001 | |
| CB (+) low DLCO | 38 (46.3) | |
| CB (+) high DLCO | 54 (27.0) | |
| CB (−) low DLCO | 33 (29.0) | |
| CB (−) high DLCO | 87 (21.1) | |
CB (+) low DLCO, patients with chronic bronchitis and DLCO <60% of predictive value; CB (+) high DLCO, patients with chronic bronchitis and DLCO ≥60% of predictive value; CB (−) low DLCO, patients without chronic bronchitis and DLCO <60% of predictive value; CB (−) high DLCO, patients without chronic bronchitis and DLCO ≥60% of predictive value; Post-BD FEV1, post bronchodilator forced expiratory volume for 1 second; CAT, COPD assessment test.
Figure 2.
Comparison of clinical parameters between the groups. DLCO, diffusing capacity of the lung for carbon monoxide.
Comparison of exacerbators and non-exacerbators for previous 12 months at initial visit
At the initial visit, 212 (26.1%) patients had acute exacerbation during the past 12 months. Simple logistic regression analysis showed that acute exacerbation was associated with the presence of CB symptoms, low post-BD FEV1 (%), low DLCO (%) and high CAT scores and reversely associated with current smoking (Table 3). Moreover, the parameter generated by the combination of CB symptoms and level of DLCO was statistically associated with acute exacerbation (P<0.001). In the CB (+) low DLCO group, the percentage of exacerbators was higher than that of non-exacerbators. By contrast, in the CB (−) high DLCO group, the percentage of non-exacerbators was higher than that of exacerbators. Age, the proportion of males did not differ significantly between the exacerbators and non-exacerbators.
Table 3. Comparison between exacerbator and non-exacerbator for previous 12 months at initial visit.
| Parameters | Exacerbator (n=212) | Non-exacerbator (n=596) | Odds ratio | 95% CI | P |
|---|---|---|---|---|---|
| Age (y) | 72.0±7.4 | 71.4±7.9 | 0.37 | ||
| Male, n (%) | 197 (92.9) | 549 (92.6) | 1.05 | 0.57–1.93 | 0.87 |
| Chronic bronchitis, n (%) | 92 (43.4) | 190 (31.9) | 1.63 | 1.19–2.26 | <0.01 |
| Post-BD FEV1 (%) | 52.1±17.7 | 59.2±15.6 | <0.001 | ||
| DLCO (%) | 70.1±25.0 | 77.1±23.5 | <0.001 | ||
| CB_DLCO, n (%) | <0.001 | ||||
| CB (+) low DLCO | 38 (17.9) | 44 (7.4) | |||
| CB (+) high DLCO | 54 (25.5) | 146 (24.5) | |||
| CB (−) low DLCO | 33 (15.6) | 81 (13.6) | |||
| CB (−) high DLCO | 87 (41.0) | 325 (54.5) | |||
| CAT | 16.9±8.3 | 14.5±7.2 | <0.001 | ||
| Current smoker, n (%) | 44 (23.9) | 181 (34.0) | 0.61 | 0.41–0.90 | 0.01 |
Post-BD FEV1, post bronchodilator forced expiratory volume for 1 second; DLCO, diffusing capacity of the lung for carbon monoxide; CB_DLCO, the parameter which was generated by combination of chronic bronchitis symptom and level of DLCO; CB (+) low DLCO, patients with chronic bronchitis and DLCO <60% of predictive value; CB (+) high DLCO, patients with chronic bronchitis and DLCO ≥60% of predictive value; CB (−) low DLCO, patients without chronic bronchitis and DLCO <60% of predictive value; CB (−) high DLCO, patients without chronic bronchitis and DLCO ≥60% of predictive value; mMRC, modified Medical Research Council Dyspnea scale; CAT, COPD assessment test.
On multiple logistic regression analysis, post-BD FEV1 (%) (OR: 0.98; 95% CI: 0.96–0.99; P<0.001), and the CB and DLCO parameters, were associated with acute exacerbation. Interestingly, the CB (+) low DLCO group showed a 2.06-fold higher risk of acute exacerbation than the CB (−) high DLCO group (95% CI: 1.18–3.62; P=0.01). The CAT score and current smoking were not significantly associated with acute exacerbation in multivariate analysis (Table 4).
Table 4. Multivariate analysis between exacerbator and non-exacerbator.
| Parameters | Odds ratio | 95% CI | P |
|---|---|---|---|
| Post-BD FEV1 (%) | 0.98 | 0.96–0.99 | <0.001 |
| CB_DLCO | |||
| CB (−) high DLCO [1] | |||
| CB (+) low DLCO vs. [1] | 2.06 | 1.18–3.62 | 0.01 |
| CB (+) high DLCO vs. [1] | 1.35 | 0.86–2.12 | 0.19 |
| CB (−) low DLCO vs. [1] | 1.02 | 0.59–1.76 | 0.96 |
| CAT | 1.02 | 1.00–1.05 | 0.11 |
| Current smoker | 1.46 | 0.98–2.17 | 0.06 |
Goodness of fit (Hosmer-Lemeshow) χ2 P=0.095. Post-BD FEV1, post bronchodilator forced expiratory volume for 1 second; CB_DLCO, the parameter which was generated by combination of chronic bronchitis symptom and level of DLCO; CB (−) high DLCO, patients without chronic bronchitis and DLCO ≥60% of predictive value; CB (+) low DLCO, patients with chronic bronchitis and DLCO <60% of predictive value; CAT, COPD assessment test.
Discussion
The present study demonstrated a CB prevalence of 35% in a COPD cohort. Patients with CB and low DLCO showed a significantly lower post-BD FEV1, more severe dyspnea and a higher risk of acute exacerbations than those without CB and low DLCO. We found that approximately 51% of the patients had neither CB nor a low diffusing capacity, and 10% of patients had both CB and low diffusing capacity. This categorization system produced group differences in symptom scores, post-BD FEV1 and acute exacerbation events, indicating that patients with COPD cannot be simply divided into two categories (i.e., CB type or emphysematous type).
The prevalence of CB in COPD varies among several studies in accordance with differences in the definition and study populations used. The reported prevalence of CB in COPD was 14–30% in previous population-based studies when CB was defined as having a chronic cough and/or sputum production at least 3 months per year in two consecutive years (24-26). In the Proyecto Latinoamericano de Investigación en Obstructión Pulmonar (PLATINO) study, CB was 14.4% among COPD patients when the definition of CB was “presence of phlegm” for at least 3 months per year for ≥2 years, but 7.4% when using “presence of cough and phlegm” as a different definition of CB (24). In our study, we investigated CB symptoms using the definition “presence of phlegm for at least 3 months per year”; the prevalence was 35%, which is similar to that of the Genetic Epidemiology of COPD (COPDGene) study (26), and to Lu’s study conducted in China (25), although they used the classic definition of CB. The mean age of our cohort was 71.6 years, which is older compared to previous cohorts. For our patients, the question pertaining to the classical definition of CB appeared to be difficult to understand. Moreover, the term “2 consecutive years” is not intuitive for Koreans, because there is no word in Korean that equates precisely to “consecutive”. Thus, in the present study, we defined patients as having CB if they responded to a simpler and easier question (presence of phlegm for at least 3 months per year).
There could be some ambiguity concerning the relationship between CB symptoms and smoking history: CB could occur in smokers with normal lung function. There were 27.8% of current smokers in our study, and the percentage of current smokers was higher in patients with CB compared to those without CB (37.0% vs. 28.1%; P=0.02; data not shown). This result is compatible with that of a previous cohort study (9,13,24,26).
In addition to the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) cohort that demonstrated COPD heterogeneity independent of FEV1 (27), investigators make robust efforts to identify clinical, genetic and biochemical markers that correlate with patients’ symptoms and predict the risks of frequent exacerbations. CB has become a very important issue. In one recent study, Meek et al. (28) analyzed the Lovelace Smokers’ Cohort and COPDGene cohort and reported that subjects with COPD and CB only had worse quality of life, symptoms and mental well-being than those with chronic airway obstruction (CAO). This study was the first to examine the difference between patients with CB only and CAO only. Additionally, it is well-known that COPD patients with CB have a worse lung function and quality of life, more respiratory symptoms and frequent acute exacerbations (29). Our result accords well with those of previous studies.
Using another approach, the severity of emphysema has been shown to correlate positively with lung function decline and frequency of acute exacerbation (14,16,30). In those studies, emphysema was measured primarily on CT. However, at present emphysema score on CT is not routinely obtained in clinical practice. Moreover, there are risks of radiation exposure associated with undergoing CT regularly. By contrast, quantifying the severity of emphysema by DLCO is a more feasible method that can be performed simply at out-patient clinics with no harm to patients. As expected, previous data have shown that DLCO corresponded well with the CT emphysema score and predicted the risk of acute exacerbation, as well as the patients’ exercise capacity (16,31). Our results also demonstrated that lower DLCO value was associated with acute exacerbation in univariate analysis (Table 3).
To the best of our knowledge, the combination of the two parameters CB and DLCO has never been reported on previously. Because CB and emphysema are already known to be indicators of poor prognosis, the idea that their combination could be associated with patients’ symptoms or acute exacerbation appeared reasonable. Our study revealed that patients with CB and low DLCO had severe respiratory symptoms, low FEV1 and a history of acute exacerbations.
Our study had several strengths compared with previous cohort studies. First, the total sample was large, and included patients from hospitals of various sizes throughout Korea. Additionally, all of the enrolled patients were confirmed to have COPD using spirometry data. Second, most of the patients were in GOLD group B or D, with moderate to severe airflow obstruction (58.7% were GOLD 2, 35% were GOLD 3 or 4). Using these proportions of patients should aid the development of treatment strategies for at-risk patients with severe respiratory symptoms and low FEV1 before future exacerbations. Third, the combination of CB and DLCO is a novel parameter. CB and DLCO successfully classified the patients such that differences in dyspnea symptoms and risk of acute exacerbation could be assessed in multivariate analysis.
This study also had several limitations. First, the definition of CB that we used was not the classical definition (i.e., cough and phlegm for 3 months, for at least two consecutive years); our definition of CB (i.e., phlegm for more than 3 months per year) could have resulted in overestimation of the population. Second, because this cohort is relatively new, risk of acute exacerbation could not be evaluated prospectively. Further study to validate the parameters of CB and DLCO, to predict acute exacerbation using a prospective observation period, is planned.
Conclusions
COPD patients were classified into four subgroups depending on CB symptoms and DLCO. Patients showed distinct clinical characteristics and outcomes according to the presence of CB and degree of DLCO. Combined CB and low DLCO in patients were independent risk factors of acute exacerbation.
Acknowledgements
None.
Footnotes
Conflicts of Interest: CK Rhee has received honoraria for lectures and/or consulting from MSD Korea, AstraZeneca Korea, Novartis Korea, Takeda Korea, GlaxoSmithKline Korea, Mundipharma Korea, Sandoz Korea, and Boehringer-Ingelheim Korea. The other authors have no conflicts of interest to declare.
References
- 1.Halbert RJ, Natoli JL, Gano A, et al. Global burden of COPD: systematic review and meta-analysis. Eur Respir J 2006;28:523-32. 10.1183/09031936.06.00124605 [DOI] [PubMed] [Google Scholar]
- 2.Ferrer M, Alonso J, Morera J, et al. Chronic obstructive pulmonary disease stage and health-related quality of life. The Quality of Life of Chronic Obstructive Pulmonary Disease Study Group. Ann Intern Med 1997;127:1072-9. 10.7326/0003-4819-127-12-199712150-00003 [DOI] [PubMed] [Google Scholar]
- 3.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006;3:e442. 10.1371/journal.pmed.0030442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global initiative for Chronic Obstructive Lung disease. Global strategy for the diagnosis, management and Prevent of chronic obstructive pulmonary disease, updated 2014. Available online: http://www.goldcopd.org/
- 5.Woodruff PG, Agusti A, Roche N, et al. Current concepts in targeting chronic obstructive pulmonary disease pharmacotherapy: making progress towards personalised management. Lancet 2015;385:1789-98. 10.1016/S0140-6736(15)60693-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner AM, Tamasi L, Schleich F, et al. Clinically relevant subgroups in COPD and asthma. Eur Respir Rev 2015;24:283-98. 10.1183/16000617.00009014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinto LM, Alghamdi M, Benedetti A, et al. Derivation and validation of clinical phenotypes for COPD: a systematic review. Respir Res 2015;16:50. 10.1186/s12931-015-0208-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekberg-Aronsson M, Pehrsson K, Nilsson JA, et al. Mortality in GOLD stages of COPD and its dependence on symptoms of chronic bronchitis. Respir Res 2005;6:98. 10.1186/1465-9921-6-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corhay JL, Vincken W, Schlesser M, et al. Chronic bronchitis in COPD patients is associated with increased risk of exacerbations: a cross-sectional multicentre study. Int J Clin Pract 2013;67:1294-301. 10.1111/ijcp.12248 [DOI] [PubMed] [Google Scholar]
- 10.Burgel PR. Chronic cough and sputum production: a clinical COPD phenotype? Eur Respir J 2012;40:4-6. 10.1183/09031936.00022412 [DOI] [PubMed] [Google Scholar]
- 11.Burgel PR. Cough and sputum production in COPD patients: clinical phenotype or markers of disease activity? Int J Clin Pract 2013;67:1218-9. 10.1111/ijcp.12296 [DOI] [PubMed] [Google Scholar]
- 12.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010;363:1128-38. 10.1056/NEJMoa0909883 [DOI] [PubMed] [Google Scholar]
- 13.Kim V, Criner GJ. The chronic bronchitis phenotype in chronic obstructive pulmonary disease: features and implications. Curr Opin Pulm Med 2015;21:133-41. 10.1097/MCP.0000000000000145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han MK, Kazerooni EA, Lynch DA, et al. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology 2011;261:274-82. 10.1148/radiol.11110173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bon J, Fuhrman CR, Weissfeld JL, et al. Radiographic emphysema predicts low bone mineral density in a tobacco-exposed cohort. Am J Respir Crit Care Med 2011;183:885-90. 10.1164/rccm.201004-0666OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh YM, Sheen SS, Park JH, et al. Emphysematous phenotype is an independent predictor for frequent exacerbation of COPD. Int J Tuberc Lung Dis 2014;18:1407-14. 10.5588/ijtld.14.0205 [DOI] [PubMed] [Google Scholar]
- 17.McLean A, Warren PM, Gillooly M, et al. Microscopic and macroscopic measurements of emphysema: relation to carbon monoxide gas transfer. Thorax 1992;47:144-9. 10.1136/thx.47.3.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cotton DJ, Soparkar GR, Grahan BL. Diffusing capacity in the clinical assessment of chronic airflow limitation. Med Clin North Am 1996;80:549-64. 10.1016/S0025-7125(05)70453-3 [DOI] [PubMed] [Google Scholar]
- 19.Gould GA, Redpath AT, Ryan M, et al. Parenchymal emphysema measured by CT lung density correlates with lung function in patients with bullous disease. Eur Respir J 1993;6:698-704. [DOI] [PubMed] [Google Scholar]
- 20.Hwang YI, Park YB, Oh YM, et al. Comparison of Korean COPD guideline and GOLD initiative report in term of acute exacerbation: a validation study for Korean COPD guideline. J Korean Med Sci 2014;29:1108-12. 10.3346/jkms.2014.29.8.1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Standardization of Spirometry , 1994 Update. American Thoracic Society. Am J Respir Crit Care Med 1995;152:1107-36. 10.1164/ajrccm.152.3.7663792 [DOI] [PubMed] [Google Scholar]
- 22.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111-7. 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 23.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948-68. 10.1183/09031936.05.00035205 [DOI] [PubMed] [Google Scholar]
- 24.de Oca MM, Halbert RJ, Lopez MV, et al. The chronic bronchitis phenotype in subjects with and without COPD: the PLATINO study. Eur Respir J 2012;40:28-36. 10.1183/09031936.00141611 [DOI] [PubMed] [Google Scholar]
- 25.Lu M, Yao W, Zhong N, et al. Chronic obstructive pulmonary disease in the absence of chronic bronchitis in China. Respirology 2010;15:1072-8. 10.1111/j.1440-1843.2010.01817.x [DOI] [PubMed] [Google Scholar]
- 26.Kim V, Davey A, Comellas AP, et al. Clinical and computed tomographic predictors of chronic bronchitis in COPD: a cross sectional analysis of the COPDGene study. Respir Res 2014;15:52. 10.1186/1465-9921-15-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res 2010;11:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meek PM, Petersen H, Washko GR, et al. Chronic Bronchitis Is Associated With Worse Symptoms and Quality of Life Than Chronic Airflow Obstruction. Chest 2015;148:408-16. 10.1378/chest.14-2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim V, Han MK, Vance GB, et al. The chronic bronchitic phenotype of COPD: an analysis of the COPDGene Study. Chest 2011;140:626-33. 10.1378/chest.10-2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vestbo J, Edwards LD, Scanlon PD, et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med 2011;365:1184-92. 10.1056/NEJMoa1105482 [DOI] [PubMed] [Google Scholar]
- 31.Díaz AA, Pinto-Plata V, Hernández C, et al. Emphysema and DLCO predict a clinically important difference for 6MWD decline in COPD. Respir Med 2015;109:882-9. 10.1016/j.rmed.2015.04.009 [DOI] [PubMed] [Google Scholar]