Abstract
Background
The analysis of lung artefacts has gained increasing importance as markers of lung pathology. B-line artefact (BLA), caused by a reverberation phenomenon, is the most important lung artefact. In this review, we discuss the current role of BLA in pneumology and explore open questions of the published consensus.
Methods
We summarized current literature about BLA. Also, we presented observations on healthy subjects and patients with interstitial syndrome (pulmonary fibrosis and edema), to investigate technical factors influencing BLA visualization.
Results
BLA imaging is influenced by more factors than recently assumed. When multiple BLA is visualized in the lung, they represent a sign of increased density due to the loss of aeration in the lung periphery. This condition may indicate different diseases including cardiogenic pulmonary edema, diffuse or focal interstitial lung diseases (ILD), infections and acute respiratory distress syndrome (ARDS). Correct interpretation of BLA in lung ultrasound is strongly influenced by associated sonographic signs and careful integration of all relevant clinical information.
Conclusions
BLA is useful to monitor clinical response, and may become crucial in directing the diagnostic process. Further research is warranted to clarify technical adjustments, different probe and machine factors that influence the visualization of BLA.
Keywords: Guidelines, pleural effusion (PE), consolidations, pneumonia, atelectasis, malignancies, pulmonary thromboembolism, interstitial syndrome
Introduction
Thorax and lung ultrasound has gained importance in daily routine (1-6) which is especially true in the setting of point of care ultrasound (POCUS) (7). To interpret ultrasound findings of the thorax and lung it is crucial to know the current symptoms, clinical condition, medical history, physical examination and imaging findings (8). In addition to conventional lung ultrasound, mainly targeted to the evaluation of real anatomic images of pleural effusion (PE) pleural masses and lung consolidations, the analysis of artefacts has gained growing importance as features of lung disease. Therefore, imaging of the anatomy (direct ultrasound findings) (Figure 1 with pneumonia) and indirect signs (artefacts) (Figure 2) have to be differentiated. The diaphragm can be directly visualized (Figure 3).
Figure 1.

Pneumonia. The value of direct sonographic signs is shown in a young boy with pneumonia. The infiltration resembles the liver (so-called hepatisation, right side of the image). Small abscesses can be identified using contrast enhanced ultrasound (CEUS) (left side, non-enhancing areas).
Figure 2.
Pneumonia. The figure illustrates the need for a sequential approach for obtaining all the imaging information available. The value of indirect sonographic signs is shown in the same young boy with pneumonia as described in Figure 1. The overview with a curved array transducer shows BLA to a depth of 12 cm. A more detailed view with a high frequency transducer shows artifacts similar to BLA with much less depth penetration and additionally ALA. Examination with the same high frequency transducer then focuses on the direct ultrasound findings including pleural effusion, pleural thickening of the parietal and visceral pleura subpleural consolidations and additional the accompanying artefacts. BLA, B-line artifact; ALA, A-line artifact.
Figure 3.
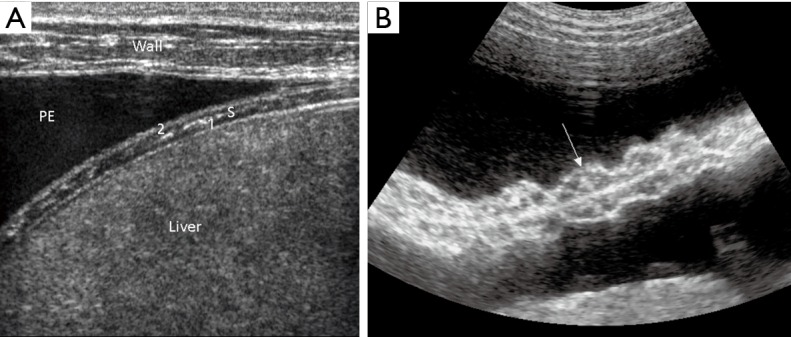
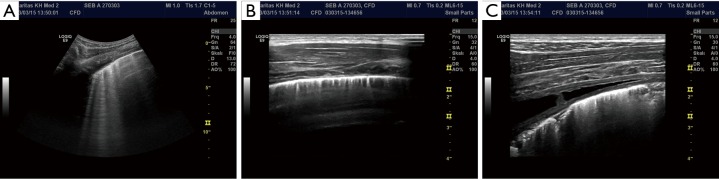
The anatomy of the parietal pleura and diaphragm is shown in a patient with a pleural effusion (PE). Note the thoracic wall (wall), two muscle layers of the diaphragm [1,2] next to the liver, separated by a fibrous septum (S) (A). The parts of the diaphragm are displayed using a curved array transducer (B, arrow).
In the present review, we discuss the current role of B-line artefacts (BLA) in pneumology and explore open questions of the published consensus (1). This review should serve as a discussing paper for future prospective studies.
B-line artefacts (BLA)
Many descriptions of BLA have been published and are contradictory (6,9-11). Lung ultrasound is based on direct visualization of structures such as consolidation, but also on the analysis of artefacts. B-line-like artefacts were first demonstrated by Ziskin and colleagues in 1982 in a patient with an abdominal shotgun wound, who used the term ‘comet tail artefact’ (12,13). The definitions of BLA and nomenclature have changed over time. Comet tails, ultrasound lung comets, and BLA is synonymously used in the literature to describe the same physical artefacts, and not exclusively in connection with lung and pleura (3). To clarify the field, a consensus document suggested that the term BLA should be used when referring to the lung (1).
According to the international consensus conference, BLA is defined as discrete laser-like vertical hyperechoic reverberation artefacts that arise from the pleural line (previously described as “comet tails”), which extend to the bottom of the screen without fading, and move synchronously with lung sliding. The artefact consists of a trail of dense echoes that resembles a distally oriented comet-tail (1,14).
The ultrasound examination of BLA has been standardized (1,2,15). Brattain et al. developed algorithms to evaluate the feasibility of diagnostic assistants to reliably quantify BLA in a sample set of clips from one machine (16). The normal lung is characterized by the absence, or presence of very few BLA (less than three per field of view). BLA were found in 37% of elderly subjects, but only 10% of young healthy subjects (17). In a recently performed pilot study (personal data of Christoph F Dietrich et al., not published) we identified distinctive influencing factors on the detection and characterisation of BLA, which is summarized in Table 1. The reduction of impedance between lung parenchyma and soft tissues of the chest wall and the increased thickness of interlobular septa might explain these findings (17). Three or more BLA between two ribs in a single scan indicates a subpleural component of the interstitial syndrome (18).
Table 1. Table of possible influencing factors on BLA visualization. During a pilot study we found the following parameters influencing the detection, number, size and shape of BLA (personal observation of Christoph Dietrich and his group).
| Manufacturer |
| Imaging techniques, e.g., tissue harmonic imaging (THI) |
| Compound techniques |
| Transducer type (e.g., cardiac, convex, linear, microconvex probes) |
| Transducer shape |
| Transducer size (image size, width) |
| Transducer frequency, multi-frequency |
| Depth penetration (transmit zone from the transducer, from the pleura) |
| Focus level setting, single or multiple focuses (kind of focus, e.g., electronic) |
| Gain, depth gain compensation (DGC) |
| Dynamic range |
| Persistence |
| Tissue equalization technology |
| Frame rate |
| Line density |
| Smoothing parameters [depending on the equipment used, e.g., “edge” (sharper or smoother border), “delta” (to control the degree of contrast resolution within an image), space time (temporal resolution, spatial resolution)] |
| Other preprocessing parameters |
| Post processing parameters |
| Patient’s position, depth and circle of respiration |
| Area of examination |
| Focal changes of the pleura with or without pleural effusion |
| Examiner |
| Time of examination |
| Inter- and intra-observer variability |
| Pulmonary and extra-pulmonary disease |
BLA, B-line artifact.
Case series
We present here the personal observations (Christoph F. Dietrich) on healthy subjects and patients with interstitial syndrome (pulmonary fibrosis and edema), to investigate technical factors influencing BLA visualization. Analysis was based on lung ultrasound studies performed on the same subject utilizing different equipment (Siemens Acuson Sequoia, Hitachi Ascendus, Hitachi 8500, GE Logiq E9, Siemens S2000, Siemens Acuson 300, Supersonic Aixplorer, Mindray M9, Mindray Resona, VScan Dual probe, Toshiba Aplio). A variety of possible influencing factors on BLA were investigated (Table 1). Some of the influencing factors summarized in Table 1 are illustrated in the Figures 4 and 5.
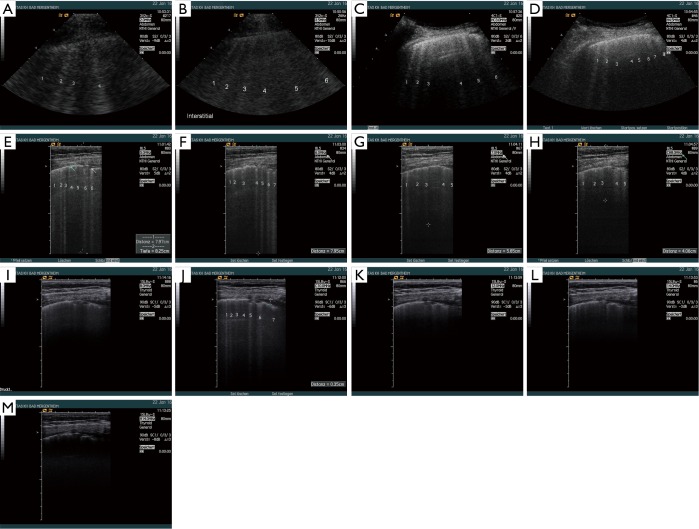
Figure 4.
The influence of ultrasound transducers and transducer frequency on BLA was examined in a patient with rheumatoid arthritis and lung fibrosis being treated with methotrexate. The examinations were performed using Acuson Sequoia with different multifrequency transducers, 3V2c-S (cardiac), 4C1-S (curved array abdominal), 8L5 (linear), 15L8w-S (linear), using tissue equalisation modus (TEQ) for all sequences. The sequences allow comparison of findings using these different techniques. The fibrotic changes of the pleura could be delineated only using high frequency transducers. Influencing factors examined were the transducer itself, the frequency used, harmonic imaging (HI), depth penetration, focus zone, location, and others (not shown). Both, 3V2c-S [2 MHz (A) and 3.5 MHz (B)] and 4C1-S [3 MHz (C) and 4.5 MHz (D)] showed multiple BLA indicating typical signs of lung fibrosis. The size, shape, depth penetration and other features were somewhat comparable similar to the findings with the 8L5 transducer examined with 5 (E), 6 (F), 7 (G), and 8 MHz (H). The higher the frequency, the lower the penetration. The 15L8w-S transducer with 8, 10, 12 and 14.0 MHz without and with HI revealed significant lower depth penetration and different amounts of BLA (I-M). Less BLA was observed in the higher frequencies. The figures illustrate that high frequency transducer information on direct pleura findings is important for correct interpretation since pleural irregularities, subpleural consolidation and the very small amount of pleural effusion could only be seen using high frequency transducers. BLA, B-line artifact.
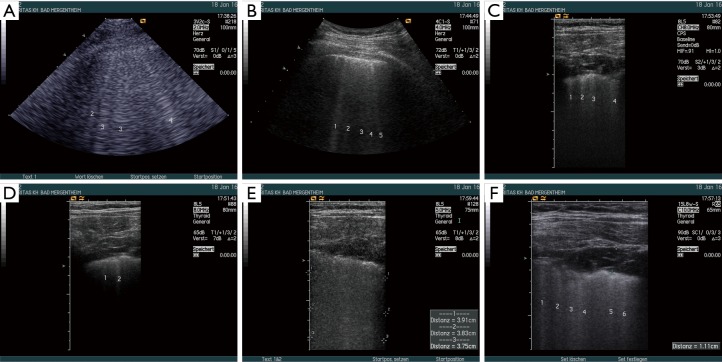
Figure 5.
The influence of ultrasound transducers and transducer frequency on BLA visualization was examined in a patient with mixed lung fibrosis and edema and focal pleural irregularities. The examinations were performed using Acuson Sequoia with different multifrequency transducers, 3V2c-S (cardiac), 4C1-S (curved array abdominal), 8L5 (linear), 15L8w-S (linear). The images demonstrate that the focal pleural irregularity was only detected using the high frequency probes, which might influence the imaging characteristics and accompanying signs in BLA patterns. The sequence demonstrates that not all transducers show the same findings. Influencing factors were the transducer itself, frequency used, harmonic imaging (HI), depth penetration, focus zone. The 3V2c-S (2 MHz) (A) and 4C1-S (4 MHz) (B) transducers showed multiple BLA indicating both fibrosis and edema. The amount of BLA and the surrounding artefacts were different. Multiple BLA were detected using 8.0 MHz with harmonic imaging (C) and less without harmonic (D). The “sound of lung water” (pulmonary edema) is best shown by 8L5 using 5.0 MHz (E). The 15L8w-S transducer revealed multiple BLA with 10.0 MHz (F). BLA, B-line artifact.
Review of the literature
Since BLA are artefacts caused by physiologic changes in the lung parenchyma, they are potentially influenced by machine settings and signal processing. Sophisticated pre- and post-processing should be turned off if possible or limited to a minimum to allow comparability. Sperandeo et al. compared the BLA examinations done with a low-medium frequency (3.5–5.0 MHz) convex probe and a high-frequency (8–12.5 MHz) linear probe. Counts of BLA were higher when convex probes were used (14). However, other more accepted studies performed with convex probe (15), linear probe (19), cardiac probe (20) and microconvex probe (21) showed similar findings on the visualization of BLA in a variety of settings and patients and by using different machines. Microconvex 2–5 MHz transducer was recommended by Lichtenstein et al. because this transducer provides an extended view of the pleural surface and penetrated deeply enough to verify the characteristic of vertical artefacts. The abdominal probe at 3–5 MHz has the advantage of coupling a wider field of view of the pleura and detection of deep structures (15). The additional use of high frequency transducers to identify pleural and subpleural changes was generally neglected. The higher the frequency the lower the penetration but other factors also influence the depth of penetration, e.g., harmonic imaging.
We conclude that BLA looks different at different levels, depending on the frequency and transducer shape used. The depth of penetration should be standardised to 4–8 cm starting from the pleural line (depending on the frequency used). The focus of the image should be set at the level of the pleural line, focusing the most energy for reflection and reverberating. Tissue harmonic imaging, compound imaging, different pre- and post-processing techniques, filters and interpolation algorithms can alter the appearance of BLA.
Comments
Indeed, there is the possibility that visualization of BLA may vary by changing technical adjustments, machines and probes. However, there are two points to consider regarding this hypothesis. The first is that the normal adjustment of the lung image should be optimized simply by regulating the gain and all the other basic settings. In the aerated lung, the pleural line should be well visualized. The focus should be set at the level of the pleural line and the subpleural zone adjusted for optimal imaging showing the typical granular echoic aspect, regardless of the probe and frequency used. The second consideration is that a slight variability in the number of visible BLA may have no influence on the final diagnostic adjudication (6). However, new studies are needed to verify the exact influence of diverse adjustments, machines, probes and frequencies on the image generation of BLA, focalized on whether these changes may effectively bias the diagnostic criteria in lung ultrasound. In the specific pneumology setting, such as the high resolution imaging study of pulmonary fibrosis, these influencing factors may be of more importance than in the emergency setting.
Are BLA reproducible?
BLA is reproducible and identifying them is easy to learn by operators with different skill and expertise (15,20,22-28). In a recently published large multicenter study, Pivetta et al. obtained a Kappa statistic for agreement of 0.94 on 1,200 scans performed by several operators, reviewed by an expert and two residents with limited training. In the same study, intraobserver agreement was 0.97 for the expert operator and 0.92 for the physicians with limited training (29).
The use of BLA in clinical practice
It is crucial to incorporate the current complaints, clinical condition, medical history, physical examination and imaging results to interpret ultrasound findings of the thorax and lung. One should always ask first, does the severity of the patient’s clinical condition correlate with the extension and diffuse pattern of BLA? Only then should bedside decisions be made on lung ultrasound BLA findings (30) as the meaning of BLA, by definition a low specificity sign, can dramatically change.
Which diseases can be differentiated using BLA?
BLA diagnoses a loss of peripheral lung aeration due to interstitial disease involvement, but considering only BLA it is not possible to differentiate the cause. BLA appears with pulmonary edema (diffusely and homogeneously distributed) and congestive heart failure (31-36) lung contusions, pneumonia and acute respiratory distress syndrome (ARDS) most commonly (typically focally or inhomogeneously distributed). If ARDS is diffuse, it can appear like pulmonary edema and potentially bilateral lung contusions. While they have been reported in pulmonary edema, pulmonary fibrosis, pneumonia, or ARDS, single focal BLA can also be observed in healthy individuals (31). However when integrated with clinical decision making, one can differentiate pulmonary edema or ARDS from interstitial pneumonia, pulmonary fibrosis, lung contusions and other conditions.
The presence of multiple diffuse bilateral BLA indicates the interstitial syndrome and the number of lines increases with decreasing air content and increasing lung density. Causes of the interstitial syndrome include the following conditions (1):
Pulmonary edema of various causes (including cardiogenic pulmonary edema and ARDS);
Interstitial pneumonia or pneumonitis;
Diffuse parenchymal lung disease (pulmonary fibrosis).
Some studies indicate good correlation between the number of BLA and the grade of pulmonary edema (15,37,38). Increased extravascular lung water (EVLW) is the main determinant of multiple and diffuse BLA on chest ultrasonography (39,40). BLA resolution appears to occur in real time as fluid is removed from the body. Together these data support thoracic ultrasound as a useful and potentially superior method for evaluating physiologic response to the removal of fluid (20,23,41).
Interstitial lung diseases (ILD) (diffuse, focal)
The ultrasound assessment of ILD is determined by the presence and semi-quantification of BLA (Figure 6). The distance between each of the two adjacent BLA correlates with the severity of disease (42,43). A BLA cut-off value of BLA ≥4 was suggested as the criteria for pulmonary fibrosis in transthoracic lung ultrasound (44). A focal sonographic pattern of the interstitial syndrome may be seen in pleurisy (45) and at the margin of pneumonia, contusion, in pulmonary infarction, atelectasis and neoplasia (46-48). Therefore, it is necessary to consider the focal interstitial syndrome within the context of the history and clinical findings. Lung ultrasound might prove a suitable method for screening patients with systemic sclerosis for incipient pulmonary structural changes (49,50).
Figure 6.
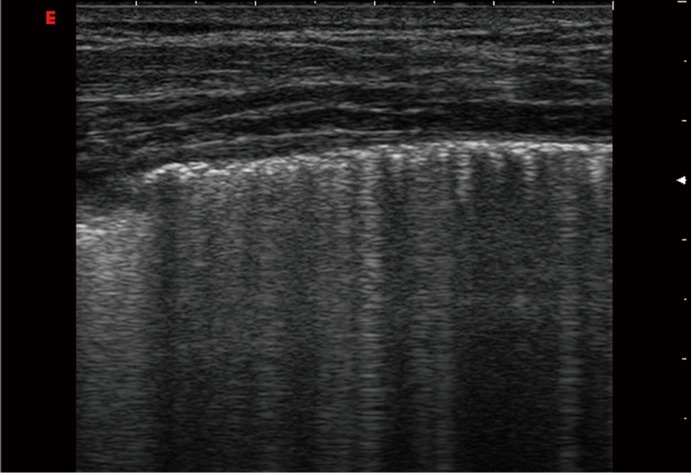
Sarcoidosis. A 26-year-old man got a cold by biking. Dry cough, left sided pleuritic pain and dry rales on auscultation was observed. Ultrasound shows an interrupted visceral pleura reflex with small subpleural consolidations and multiple irregular BLA. Extrapulmonary manifestations of sarcoidosis are often observed. BLA, B-line artifact.
Pneumonia
Meta-analyses confirm that pneumonia can be diagnosed using lung ultrasound (2,11). BLA is often seen in the areas adjacent to the consolidation, likely as an expression of inflammatory perilesional edema. Pleural line abnormalities and PEs were consistently associated with areas of confluent BLA and/or lung consolidation (51).
Chronic obstructive pulmonary disease (COPD)
Lung ultrasound appears to be particularly useful in differentiating exacerbations of COPD, a condition that does not show BLA, from decompensated heart failure (52).
Acute respiratory failure
The primary diagnosis of pulmonary interstitial fluid in the emergency setting is crucial for the differentiating between cardiogenic and non-cardiogenic factors determining acute respiratory failure (53). BLA has been demonstrated as a useful primary diagnostic test in this context. In patients with acute lung injury (ALI)/ARDS, a given degree of lung aeration (referring to well defined CT scan entities) corresponds to a specific ultrasound pattern (7,21,54,55). Lung ultrasound is also a useful, non-invasive tool in predicting hydration status in mechanically ventilated patients (56).
Monitoring fluid overload
Monitoring of different states, e.g., fluid overload in hemodialysis, semi-quantification of EVLW and pulmonary aeration has been studied as well. The change in BLA is rapid and BLA responds quickly to changes in lung water content. Thus, as a fluid overloaded patient is dialyzed, it is possible to track BLA resolution in real time as fluid, and therefore EVLW, is removed from the body (25,41,57-59). In patients with cardiogenic pulmonary edema, evaluation of BLA and a change (decrease) in their number enables noninvasive monitoring of response to therapy (15).
Pulmonary embolism
Severe pulmonary embolism with acute respiratory failure shows the A profile (regular sliding with absence of BLA) with a 95% sensitivity (60). The best diagnostic strategy to confirm or exclude suspected pulmonary embolism is the combination of clinical assessment, plasma D-dimer measurement and computed tomographic pulmonary angiography (CTPA). Lung US looking for lung consolidation cannot be considered as the first imaging test but a possible alternative to CTPA when the latter is contraindicated or not available (61). In the emergency department and intensive care setting, a combined strategy based on a multiorgan ultrasound (veins, heart, lung) was shown to improve diagnostic accuracy compared to lung ultrasound alone (60,62).
Open questions
Future research protocols on lung artefacts should focus on:
The role of different transducers in the evaluation of BLA;
The possible influencing factors (Table 1) in the visualization of BLA and whether these may be of any practical importance in changing the diagnostic criteria for the first diagnosis of interstitial syndromes and in monitoring techniques of pulmonary congestion and aeration;
The clinical significance of subtle pleural fluid, pleural irregularities, pleural nodules and small subpleural consolidations, studied by higher resolution ultrasound imaging;
The role of endoscopic ultrasound in evaluating BLA has not been examined so far (63-66).
Conclusions
BLA has been incorporated into the diagnosis of lung diseases and gained importance. BLA are reverberation artefacts, displayed on the screen as vertical echogenic lines, which are easily identified. BLA are signs of diffuse or focal interstitial lung involvement and in general is considered as a sign of partial loss of aeration and increased density of peripheral lung parenchyma. In conjunction with comprehensive review of other clinical information including the clinical setting and patient’s condition, BLA assessment may become crucial in directing the diagnostic process. BLA is useful to monitor clinical response, in critically ill ICU patients and outpatients in rheumatology, pulmonology, cardiology and nephrology settings. Further research is warranted to clarify technical adjustments, different probe and machine factors that influence the visualization of these artefacts in the normal lung and in diseases, and in definitions to increase the specificity of BLA in the myriad of different settings it can be applied to.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 2012;38:577-91. 10.1007/s00134-012-2513-4 [DOI] [PubMed] [Google Scholar]
- 2.Volpicelli G. Lung sonography. J Ultrasound Med 2013;32:165-71. [DOI] [PubMed] [Google Scholar]
- 3.Dietrich CF, Gebhard Mathis G, Cui XW, et al. Ultrasound of the pleurae and lungs. Ultrasound Med Biol 2015;41:351-65. 10.1016/j.ultrasmedbio.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 4.Mathis G. Why look for artifacts alone when the original is visible? Chest 2010;137:233; author reply 233-4. 10.1378/chest.08-2601 [DOI] [PubMed] [Google Scholar]
- 5.Gargani L, Pang PS, Frassi F, et al. Persistent pulmonary congestion before discharge predicts rehospitalization in heart failure: a lung ultrasound study. Cardiovasc Ultrasound 2015;13:40. 10.1186/s12947-015-0033-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gargani L. Lung ultrasound: a new tool for the cardiologist. Cardiovasc Ultrasound 2011;9:6. 10.1186/1476-7120-9-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouhemad B, Liu ZH, Arbelot C, et al. Ultrasound assessment of antibiotic-induced pulmonary reaeration in ventilator-associated pneumonia. Crit Care Med 2010;38:84-92. 10.1097/CCM.0b013e3181b08cdb [DOI] [PubMed] [Google Scholar]
- 8.Volpicelli G. Point-of-care lung ultrasound. Praxis (Bern 1994) 2014;103:711-6. [DOI] [PubMed]
- 9.Lichtenstein DA. BLUE-protocol and FALLS-protocol: two applications of lung ultrasound in the critically ill. Chest 2015;147:1659-70. 10.1378/chest.14-1313 [DOI] [PubMed] [Google Scholar]
- 10.Soldati G, Copetti R, Sher S. Sonographic Interstitial Syndrome. J Ultrasound Med 2009;28:163-74. [DOI] [PubMed] [Google Scholar]
- 11.Blaivas M. Lung ultrasound in evaluation of pneumonia. J Ultrasound Med 2012;31:823-6. [DOI] [PubMed] [Google Scholar]
- 12.Thickman DI, Ziskin MC, Goldenberg NJ, et al. Clinical manifestations of the comet tail artifact. J Ultrasound Med 1983;2:225-30. [DOI] [PubMed] [Google Scholar]
- 13.Ziskin MC, Thickman DI, Goldenberg NJ, et al. The comet tail artifact. J Ultrasound Med 1982;1:1-7. [DOI] [PubMed] [Google Scholar]
- 14.Sperandeo M, Varriale A, Sperandeo G, et al. Assessment of ultrasound acoustic artifacts in patients with acute dyspnea: a multicenter study. Acta Radiologica 2012;53:885-92. 10.1258/ar.2012.120340 [DOI] [PubMed] [Google Scholar]
- 15.Volpicelli G, Mussa A, Garofalo G, et al. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am J Emerg Med 2006;24:689-96. 10.1016/j.ajem.2006.02.013 [DOI] [PubMed] [Google Scholar]
- 16.Brattain LJ, Telfer BA, Liteplo AS, et al. Automated B-Line Scoring on Thoracic Sonography. J Ultrasound Med 2013;32:2185-90. 10.7863/ultra.32.12.2185 [DOI] [PubMed] [Google Scholar]
- 17.Chiesa AM, Ciccarese F, Gardelli G, et al. Sonography of the Normal Lung: Comparison Between Young and Elderly Subjects. J Clin Ultrasound 2014;43:230-4. 10.1002/jcu.22225 [DOI] [PubMed] [Google Scholar]
- 18.Bataille B, Rao G, Cocquet P, et al. Accuracy of ultrasound B-lines score and E/Ea ratio to estimate extravascular lung water and its variations in patients with acute respiratory distress syndrome. J Clin Monit Comput 2015;29:169-76. 10.1007/s10877-014-9582-6 [DOI] [PubMed] [Google Scholar]
- 19.Reissig A, Kroegel C. Transthoracic sonography of diffuse parenchymal lung disease: the role of comet tail artifacts. J Ultrasound Med 2003;22:173-80. [DOI] [PubMed] [Google Scholar]
- 20.Agricola E, Bove T, Oppizzi M, et al. "Ultrasound comet-tail images": a marker of pulmonary edema: a comparative study with wedge pressure and extravascular lung water. Chest 2005;127:1690-5. 10.1378/chest.127.5.1690 [DOI] [PubMed] [Google Scholar]
- 21.Lichtenstein D, Meziere G, Biderman P, et al. The comet-tail artifact: an ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med 1997;156:1640-6. 10.1164/ajrccm.156.5.96-07096 [DOI] [PubMed] [Google Scholar]
- 22.Boussuges A, Coulange M, Bessereau J, et al. Ultrasound lung comets induced by repeated breath-hold diving, a study in underwater fishermen. Scand J Med Sci Sports 2011;21:e384-92. 10.1111/j.1600-0838.2011.01319.x [DOI] [PubMed] [Google Scholar]
- 23.Jambrik Z, Monti S, Coppola V, et al. Usefulness of ultrasound lung comets as a nonradiologic sign of extravascular lung water. Am J Cardiol 2004;93:1265-70. 10.1016/j.amjcard.2004.02.012 [DOI] [PubMed] [Google Scholar]
- 24.Liteplo AS, Marill KA, Villen T, et al. Emergency thoracic ultrasound in the differentiation of the etiology of shortness of breath (ETUDES): sonographic B-lines and N-terminal pro-brain-type natriuretic peptide in diagnosing congestive heart failure. Acad Emerg Med 2009;16:201-10. 10.1111/j.1553-2712.2008.00347.x [DOI] [PubMed] [Google Scholar]
- 25.Mallamaci F, Benedetto FA, Tripepi R, et al. Detection of pulmonary congestion by chest ultrasound in dialysis patients. JACC Cardiovasc Imaging 2010;3:586-94. 10.1016/j.jcmg.2010.02.005 [DOI] [PubMed] [Google Scholar]
- 26.Anderson KL, Fields JM, Panebianco NL, et al. Inter-rater reliability of quantifying pleural b-lines using multiple counting methods. J Ultrasound Med 2013;32:115-20. [DOI] [PubMed] [Google Scholar]
- 27.Sperandeo M, Carnevale V, Muscarella S, et al. Clinical application of transthoracic ultrasonography in inpatients with pneumonia. Eur J Clin Invest 2011;41:1-7. 10.1111/j.1365-2362.2010.02367.x [DOI] [PubMed] [Google Scholar]
- 28.Gullett J, Donnelly JP, Sinert R, et al. Interobserver agreement in the evaluation of B lines using bedside ultrasound. J Crit Care 2015;30:1395-9. 10.1016/j.jcrc.2015.08.021 [DOI] [PubMed] [Google Scholar]
- 29.Pivetta E, Goffi A, Lupia E, et al. Lung ultrasound-implemented diagnosis of acute decompensated heart failure in the Emergency Department - A SIMEU multicenter study. Chest 2015;148:202-10. 10.1378/chest.14-2608 [DOI] [PubMed] [Google Scholar]
- 30.Gargani L, Volpicelli G. How I do it: Lung ultrasound. Cardiovasc Ultrasound 2014;12:25. 10.1186/1476-7120-12-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Facchini C, Malfatto G, Giglio A, et al. Lung ultrasound and transthoracic impedance for noninvasive evaluation of pulmonary congestion in heart failure. J Cardiovasc Med (Hagerstown) 2015. [Epub ahead of print]. 10.2459/JCM.0000000000000226 [DOI] [PubMed] [Google Scholar]
- 32.Miglioranza MH, Gargani L, Sant'Anna RT, et al. Lung ultrasound for the evaluation of pulmonary congestion in outpatients: a comparison with clinical as-sessment, natriuretic peptides, and echocardiography. JACC Cardiovasc Imaging 2013;6:1141-51. 10.1016/j.jcmg.2013.08.004 [DOI] [PubMed] [Google Scholar]
- 33.Weber CK, Miglioranza MH, Moraes MA, et al. The five-point Likert scale for dyspnea can properly assess the degree of pulmonary congestion and predict adverse events in heart failure outpatients. Clinics (Sao Paulo) 2014;69:341-6. 10.6061/clinics/2014(05)08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frassi F, Pingitore A, Cialoni D, et al. Chest sonography detects lung water accumulation in healthy elite apnea divers. J Am Soc Echocardiogr 2008;21:1150-5. 10.1016/j.echo.2008.08.001 [DOI] [PubMed] [Google Scholar]
- 35.Edsell ME, Wimalasena YH, Malein WL, et al. High-intensity intermittent exercise increases pulmonary interstitial edema at altitude but not at simulated altitude. Wilderness Environ Med 2014;25:409-15. 10.1016/j.wem.2014.06.016 [DOI] [PubMed] [Google Scholar]
- 36.Volpicelli G, Skurzak S, Boero E, et al. Lung ultrasound predicts well extravascular lung water but is of limited usefulness in the prediction of wedge pressure. Anesthesiology 2014;121:320-7. 10.1097/ALN.0000000000000300 [DOI] [PubMed] [Google Scholar]
- 37.Fagenholz PJ, Gutman JA, Murray AF, et al. Chest ultrasonography for the diagnosis and monitoring of high-altitude pulmonary edema. Chest 2007;131:1013-8. 10.1378/chest.06-1864 [DOI] [PubMed] [Google Scholar]
- 38.Gargani L, Frassi F, Soldati G, et al. Ultrasound lung comets for the differential diagnosis of acute cardiogenic dyspnoea: A comparison with natriuretic peptides. Eur J Heart Fail 2008;10:70-7. 10.1016/j.ejheart.2007.10.009 [DOI] [PubMed] [Google Scholar]
- 39.Frassi F, Gargani L, Gligorova S, et al. Clinical and echocardiographic determinants of ultrasound lung comets. Eur J Echocardiogr 2007;8:474-9. 10.1016/j.euje.2006.09.004 [DOI] [PubMed] [Google Scholar]
- 40.Frassi F, Gargani L, Tesorio P, et al. Diagnostic Value of Extravascular Lung Water Assessed With Ultrasound Lung Comets by Chest Sonography in Patients With Dyspnea and/or Chest Pain. J Card Fail 2007;13:830-5. 10.1016/j.cardfail.2007.07.003 [DOI] [PubMed] [Google Scholar]
- 41.Noble VE, Murray AF, Capp R, et al. Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis. Time course for resolution. Chest 2009;135:1433-9. 10.1378/chest.08-1811 [DOI] [PubMed] [Google Scholar]
- 42.Hasan AA, Makhlouf HA. B-lines: Transthoracic chest ultrasound signs useful in assessment of interstitial lung diseases. Ann Thorac Med 2014;9:99-103. 10.4103/1817-1737.128856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cogliati C, Antivalle M, Torzillo D, et al. Standard and pocket-size lung ultrasound devices can detect interstitial lung disease in rheumatoid arthritis patients. Rheumatology (Oxford) 2014;53:1497-503. 10.1093/rheumatology/keu033 [DOI] [PubMed] [Google Scholar]
- 44.Buda N, Piskunowicz M, Porzezińska M, et al. Lung Ultrasonography in the Evaluation of Interstitial Lung Disease in Systemic Connective Tissue Diseases: Criteria and Severity of Pulmonary Fibrosis - Analysis of 52 Patients. Ultraschall Med 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 45.Gehmacher O, Kopf A, Scheier M, et al. Can pleuricy be detected with ultrasound? Ultraschall Med 1997;18:214-9. 10.1055/s-2007-1000428 [DOI] [PubMed] [Google Scholar]
- 46.Volpicelli G, Caramello V, Cardinale L, et al. Diagnosis of radio-occult pulmonary conditions by realtime chest ultrasonography in patients with pleuritic pain. Ultrasound Med Biol 2008;34:1717-23. 10.1016/j.ultrasmedbio.2008.04.006 [DOI] [PubMed] [Google Scholar]
- 47.Soldati G, Testa A, Silva FR, et al. Chest ultrasonography in lung contusion. Chest 2006;130:533-8. 10.1378/chest.130.2.533 [DOI] [PubMed] [Google Scholar]
- 48.Wüstner A, Gehmacher O, Hämmerle S, et al. Ultrasound diagnosis in blunt thoracic trauma. Ultraschall Med 2005;26:285-90. [DOI] [PubMed] [Google Scholar]
- 49.Moazedi-Fuerst FC, Kielhauser S, Brickmann K, et al. Sonographic assessment of interstitial lung disease in patients with rheumatoid arthritis, systemic sclerosis and systemic lupus erythematosus. Clin Exp Rheumatol 2015;33:S87-91. [PubMed] [Google Scholar]
- 50.Gigante A, Rossi Fanelli F, Lucci S, et al. Lung ultrasound in systemic sclerosis: correlation with high-resolution computed tomography, pulmonary function tests and clinical variables of disease. Intern Emerg Med 2016;11:213-7. 10.1007/s11739-015-1329-y [DOI] [PubMed] [Google Scholar]
- 51.Caiulo VA, Gargani L, Caiulo S, et al. Lung ultrasound characteristics of community-acquired pneumonia in hospitalized children. Pediatr Pulmonol 2013;48:280-7. 10.1002/ppul.22585 [DOI] [PubMed] [Google Scholar]
- 52.Volpicelli G, Cardinale L, Garofalo G, et al. Usefulness of lung ultrasound in the bedside distinction between pulmonary edema and exacerbation of COPD. Emerg Radiol 2008;15:145-51. 10.1007/s10140-008-0701-x [DOI] [PubMed] [Google Scholar]
- 53.Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound 2008;6:16. 10.1186/1476-7120-6-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shyamsundar M, Attwood B, Keating L, et al. Clinical review: the role of ultrasound in estimating extra-vascular lung water. Crit Care 2013;17:237. 10.1186/cc12710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jambrik Z, Gargani L, Adamicza A, et al. B-lines quantify the lung water content: a lung ultrasound versus lung gravimetry study in acute lung injury. Ultrasound Med Biol 2010;36:2004-10. 10.1016/j.ultrasmedbio.2010.09.003 [DOI] [PubMed] [Google Scholar]
- 56.Enghard P, Rademacher S, Nee J, et al. Simplified lung ultrasound protocol shows excellent prediction of extravascular lung water in ventilated in-tensive care patients. Critical Care 2015;19:36. 10.1186/s13054-015-0756-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Donadio C, Bozzoli L, Colombini E, et al. Effective and timely evaluation of pulmonary congestion: qualitative comparison between lung ultrasound and thoracic bioelectrical impedance in maintenance hemodialysis patients. Medicine (Baltimore) 2015;94:e473. 10.1097/MD.0000000000000473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strnad M, Prosen G, Borovnik Lesjak V. Bedside lung ultrasound for monitoring the effectiveness of prehospital treatment with continuous positive airway pressure in acute decompensated heart failure. Eur J Emerg Med 2016;23:50-5. 10.1097/MEJ.0000000000000205 [DOI] [PubMed] [Google Scholar]
- 59.Bouhemad B, Brisson H, Le-Guen M, et al. Bedside ultrasound assessment of positive end-expiratory pressure-induced lung recruitment. Am J Respir Crit Care Med 2011;183:341-7. 10.1164/rccm.201003-0369OC [DOI] [PubMed] [Google Scholar]
- 60.Lichtenstein DA, Meziere GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest 2008;134:117-25. 10.1378/chest.07-2800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mathis G, Blank W, Reissig A, et al. Thoracic ultrasound for diagnosing pulmonary embolism: a prospective multicenter study of 352 patients. Chest 2005;128:1531-8. 10.1378/chest.128.3.1531 [DOI] [PubMed] [Google Scholar]
- 62.Nazerian P, Vanni S, Volpicelli G, et al. Accuracy of point-of-care multiorgan ultrasonography for the diagnosis of pulmonary embolism. Chest 2014;145:950-7. 10.1378/chest.13-1087 [DOI] [PubMed] [Google Scholar]
- 63.Konge L, Colella S, Vilmann P, et al. How to learn and to perform endoscopic ultrasound and endobronchial ultrasound for lung cancer staging: A structured guide and review. Endosc Ultrasound 2015;4:4-9. 10.4103/2303-9027.151297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harris K, Modi K, Kumar A, et al. Endobronchial ultrasound-guided transbronchial needle aspiration of pulmonary artery tumors: A systematic review (with video). Endosc Ultrasound 2015;4:191-7. 10.4103/2303-9027.162996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harris K, Gomez J, Dhillon SS, et al. Convex probe endobronchial ultrasound placement of fiducial markers for central lung nodule (with video). Endosc Ultrasound 2015;4:156-7. 10.4103/2303-9027.156757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dincer HE, Gliksberg EP, Andrade RS. Endoscopic ultrasound and/or endobronchial ultrasound-guided needle biopsy of central intraparenchymal lung lesions not adjacent to airways or esophagus. Endosc Ultrasound 2015;4:40-3. 10.4103/2303-9027.151332 [DOI] [PMC free article] [PubMed] [Google Scholar]