Abstract
Background
Lung cancer has heterogeneous features. It remains unclear whether ALK rearrangement was distributed heterogeneously in tumor from different anatomic sites. To address this issue, we investigate the concordance of ALK rearrangement between primary tumors and paired metastatic lymph nodes in pulmonary adenocarcinoma patients.
Methods
From Sep 2013 to May 2014, resectable lung adenocarcinoma patients with EGFR wildtype and paired metastatic lymph nodes from Tongji University affiliated Shanghai pulmonary hospital were selected into this study. An auto-mated Ventana ALK with clone D5F3 antibody immunohistochemistry (IHC) and reverse transcriptase-polymerase chain reaction (RT-PCR) were used to detected ALK rearrangement. Discordant cases between IHC and RT-PCR were further validated by fluorescence in situ hybridization (FISH).
Results
A total of 101 patients were enrolled into this study with a median age of 60 years old (range, 35–78 years). ALK rearrangement was found in 20 primary lesions, while in 18 paired metastatic lymph nodes. ALK rearrangement was more frequently happened in younger (P<0.001), Nonsmokers (P=0.012), high-stage disease (P=0.021) and predominantly solid growth pattern (P=0.024). The concordance rate between primary tumor and paired metastatic lymph nodes was 98%. Two patients with ALK rearrangement on primary tumor didn’t show ALK gene fusion on paired metastatic lymph nodes. Sixty-eight cases had more than two stations of metastatic lymph nodes. ALK rearrangement in the different station of metastatic lymph nodes of the same patient was consistent.
Conclusions
High concordant rate of ALK rearrangement between primary tumors and paired metastatic lymph nodes were found in this study. The authors concluded that specimens from metastatic lesions and primary tumors are equally suitable for detection ALK rearrangement.
Keywords: ALK rearrangement, heterogeneity, lung adenocarcinoma, immunohistochemistry (IHC), reverse transcriptase-polymerase chain reaction (RT-PCR), fluorescence in situ hybridization (FISH)
Introduction
Lung cancer remains the most prevalent malignant tumor and the leading cause of cancer related death world-widely (1). Non-small cell lung cancer (NSCLC) accounts for 85% of all lung cancer (2). Advances in the understanding of the molecular biology of lung cancer have made individual therapy into clinical practice for advanced NSCLC. As an example, patients with EGFR mutation or ALK rearrangement showed dramatic response to EGFR or ALK tyrosine kinase inhibitor (TKI) and have been recommended to receive EGFR-TKI or Crizotinib as first line setting (3-5). Thus, precise detection of these driver genes mutation will be urgently needed in clinical practice.
ALK rearrangement can be detected by different methods including immunohistochemistry (IHC), reverse transcriptase polymerase chain reaction (RT-PCR), and fluorescence in situ hybridization (FISH). Among them, FISH was approved as a gold standard by the Food and Drug Administration (FDA) with the Vysis ALK Break Apart FISH Probe Kit. ALK rearrangement could also detected by IHC, an auto-mated the Ventana ALK assay kit with D5F3 antibody (Ventana Medical Systems Inc., Tucson, Ariz) coupled with an ultrasensitive amplification and detection system have been verified in ALK protein detection (6,7). Besides that, RT-PCR is a more sensitive and specific method that can allows the detection of even a few molecules of chimeric ALK transcripts. But the accuracy of RT-PCR-based testing ALK rearrangement depends greatly on the RNA quality of specimens. Furthermore, some unknown fusion partners or breakpoint variants may be missed because the primers in the assay are specifically designed to evaluate these known alterations.
As we know, lung cancer is a heterogeneous tumor with diverse molecular and pathological characteristics. For example, several studies have reported the discrepancy of EGFR mutation status between primary tumor and metastatic lesions from the same patient (8,9). However, it remains unclear whether ALK rearrangement between primary and metastatic lesions showed a similar heterogeneous phenomenon as EGFR mutation.
To address this issue, we compare the ALK rearrangement status between primary lesions and paired metastatic lymph nodes in 101 resected patients with adenocarcinoma and EFGR wildtype through the methods of VENTANA IHC and RT-PCR. Discrepancy of ALK rearrangement between IHC and RT-PCR was further confirmed by FISH.
Methods
Clinical samples
The enrolled criteria included primary pulmonary adenocarcinoma patients with metastatic lymph node who received surgical resection in Tongji University affiliated Shanghai pulmonary hospital, the primary and paired metastatic lymphatic section containing more than 100 tumor cells and EGFR wild type. From September 2013 to May 2014, a total of 101 patients were collected. The histological type was confirmed according to the criteria of ERS/ATS/IASLC multidisciplinary classification of lung adenocarcinoma in our routine practice (10). All patients had signed an informed consent for further molecular analysis.
ALK protein expression
Representative formalin-fixed, paraffin-embedded blocks containing the most tumor cells from primary tumor and paired metastatic lymph node were chosen for this current study by two pathologists. Ten serial tissue sections of each block were prepared to detect ALK rearrangement (two tissue slides for IHC, one for FISH and the rest for RT-PCR). Immunohistochemical staining was performed on a VENTANA BenchMark XT automated slide-processing system at Ventana Medical Systems. Four-µm-thick FFPE tissue sections were deparaffinized with EZ Prep solution and antigen-retrieval with cell conditioning 1 (64 minutes at 100 °C) was performed. Primary antibody (clone D5F3, VMSI) was incubated on tissue sections for 20 minutes. According to the manufacturer’s protocol, OptiView DAB IHC Detection Kit (VMSI) and OptiView Amplification Kit (VMSI) were used. Slides were counterstained with Hematoxylin II and then dehydrated. Tissue slides were protected with coverslips. All immunohistochemical stains were evaluated independently by two pathologists. Specimens were scored positive if strong granular cytoplasmic brown staining in tumor cells (excluding nontumor cells: alveolar macrophages, cells of neural origin, glandular epithelial staining), the absence of strong granular cytoplasmic staining was classified a negative result as described previously (11).
ALK reverse transcriptase-polymerase chain reaction (RT-PCR) detection
The total RNA from tissue sections containing at least 70% tumor cells was extracted and converted to first-strand cDNA according to the manufacturer’s instructions (Amoy Diagnostics, Xiamen, China). This AmoyDx EML4-ALK Fusion Gene Detection kit is designed to qualitatively detect 21 EML4-ALK fusion transcripts in four reaction mixtures (including reference gene beta-actin) with a declared sensitivity of 1% mutant DNA. Real-time PCR was performed on in a 7500 real-time PCR system and analyzed by SDS 2.0 system software (Applied Biosystems, Foster City, CA). As defined by the manufacturer’s instructions, cycle threshold (CT) values ≤30 of the samples detection was interpreted a positive result as described previously (11-13).
ALK fluorescence in situ hybridization (FISH) analysis
When ALK rearrangement was discordant between IHC and RT-PCR, FISH evaluation was further performed according to the guidelines of the LSI ALK Dual Color, Break-apart Rearrangement Probe kit (Abbott Molecular, Abbott Park, Illinois), Briefly, for FISH, tissue sections were baked at 60 °C and deparaffinized, and then pretreated with protease solution at 37 °C for 20 min. A thermoBrite (Abbott Molecular, IL, USA) was used for the denaturation step at 73 °C followed by hybridization at 37 °C overnight for 18 hours. After stringency wash steps, nuclear counterstaining with DAPI was performed. Sections were observed under a ×100 objective with a fluorescence microscope (Leica DM6000, Wetzlar, Germany) equipped with “Applied Imaging 4.0” analysis software (Genetix, England, UK) independently by two experienced pathologists. The positive tumor cells show split signals (at least two signals diameters apart) or a single red signal (deleted green signal) in addition to fused and/or split signals. If the average percentage of positive cells was ≥15% (≥15/100), the sample was considered to be positive.
Statistical analysis
Differences in demographic characteristics including age, gender, smoking history, histological subtype and TNM stage between ALK+ and ALK− patients were analyzed using pearson’ chi-square test or Fisher’s exact test. Two-sided P values <0.05 was considered statistically significant. Statistical calculations were performed using SPSS17.0 software (SPSS Inc., Chicago, IL, USA).
Results
Clinicopathological features of the enrolled patients
One hundred and one patients were enrolled into this study with a median age of 60 years old (range, 35–78 years). Among them, 50 (49.5%) were females, 61 (60.4%) were non-smokers and 20 patients (20/101, 19.8%) were found to have ALK fusion in either of the paired samples. The patients with ALK fusion were more likely in younger (P<0.001), Nonsmokers (P=0.012), high-stage disease (P=0.021) and predominantly solid tumor growth pattern (P=0.024). The clinicopathological characteristics are summarized in Table 1.
Table 1. Clinicopathological features of the enrolled 101 patients.
| Characteristics | ADC cases (n=101) | ALK rearrangement | P |
|---|---|---|---|
| Primary site | 101 | 20 | |
| Paired metastatic lymph nodes | 101 | 18 | |
| Sex | 0.122 | ||
| Women | 50 | 13 | |
| Men | 51 | 7 | |
| Age (y) | <0.001 | ||
| Median | 60 | 52 | |
| Range | 35–78 | 35–69 | |
| Smoking historya | 0.012 | ||
| Smokers | 40 | 3 | |
| Non-smokers | 61 | 17 | |
| Clinical stage | 0.021 | ||
| II | 26 | 1 | |
| III–IV | 75 | 19 | |
| Predominant pattern | 0.024 | ||
| Solid | 22 | 9 | |
| Acinar/papillary | 76 | 11 | |
| Micropapillary | 3 | 2 |
Note: a, definition of smoking history: nonsmokers had smoked less than 100 cigarettes in their lifetime. The rest were categorized as smokers. ADC, Adenocarcinoma.
The concordance rate of ALK rearrangement between primary tumor and paired metastatic lymph nodes by immunohistochemistry (IHC) and reverse transcriptase-polymerase chain reaction (RT-PCR)
A total of 101 paired specimens (primary tumor site and paired metastatic lymph nodes) have to be confirmed as ALK rearranged. Of 101 paired specimens detected by IHC and RT-PCR, 81 paired specimens were both IHC and RT-PCR negative, 18 paired specimens were both IHC and RT-PCR positive (as shown in Table 2). Sixty-eight cases showed more than two stations of metastatic lymph nodes. ALK rearrangement was totally concordant between different stations of metastatic lymph nodes in the same patient (Table S1). The concordance rate of ALK rearrangement between primary tumors and paired metastatic lymph nodes was 98.0%.
Table 2. ALK rearrangement concordance between primary tumor and paired metastatic lymph nodes by IHC and RT-PCR (99 of 101 cases).
| Methods | Primary site (No.) | Paired metastatic lymph nodes (No.) | ALK rearrangement |
|---|---|---|---|
| IHC− | 81 | 81 | Negative |
| IHC+ | 18 | 18 | Positive |
| RT-PCR− | 81 | 81 | Negative |
| RT-PCR+ | 18 | 18 | Positive |
IHC, immunohistochemistry; RT-PCR, reverse transcriptase-polymerase chain reaction.
The discordance of ALK rearrangement between primary tumor and metastatic lymph nodes may derive from the intra-tumoral heterogeneity of primary lesions
Two patients were confirmed with discordance of ALK rearrangement between primary tumors and paired metastatic lymph nodes requiring verification by a third methodology, FISH. One patient (Case #15) was IHC, RT-PCR and FISH positive in primary tumor, triples negative in paired metastatic lymph nodes (Figures 1,2). The other (Case #21) who was IHC and FISH positive in both primary tumor and paired metastatic lymph nodes was RT-PCR positive in primary tumor and negative in paired metastatic lymph nodes (Table S2). By IHC analysis of discordant Cases, We found that most of tumor cells were stained with strong granular cytoplasm in some areas of primary tumor section. In other areas of the same section, some tumor cells were negative in anti-ALK (D5F3) staining. ALK rearrangement showed intra-tumoral heterogeneity in different areas of the same tissue section (Figure 3). For case #21, RT-PCR was enough sensitive to detect the ALK rearrangement in the fewer fraction of tumor cells in metastatic lymph nodes, in which most of the metastatic tumor cells didn’t contain ALK rearrangement by IHC and FISH. Two patients were both female, non-smokers and were diagnosed as adenocarcinoma with predominant solid growth pattern (as shown in Table 3).
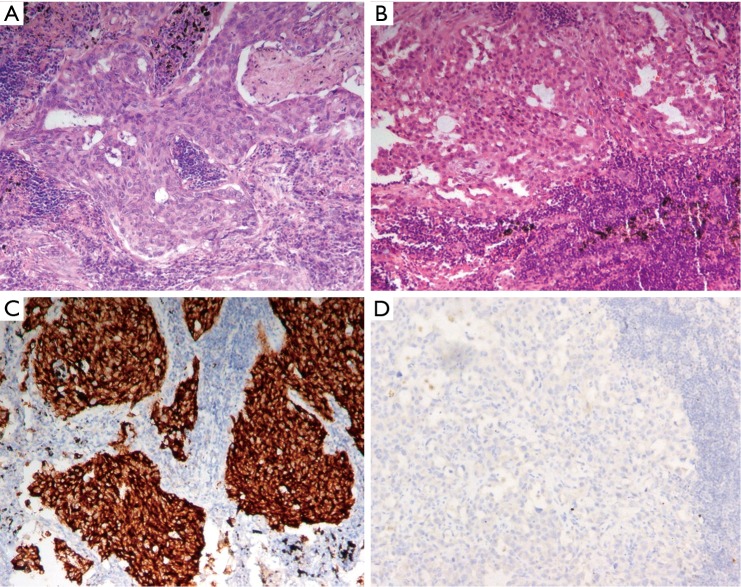
Figure 1.
HE staining and ALK VENTANA D5F3 for detecting ALK rearrangement on primary tumor and paired metastatic lymph nodes. (A,B) HE staining on primary tumor and paired metastatic lymph node (100×); (C,D) ALK positive on primary tumor and negative on paired metastatic lymph node by ALK VENTANA D5F3 (100×).
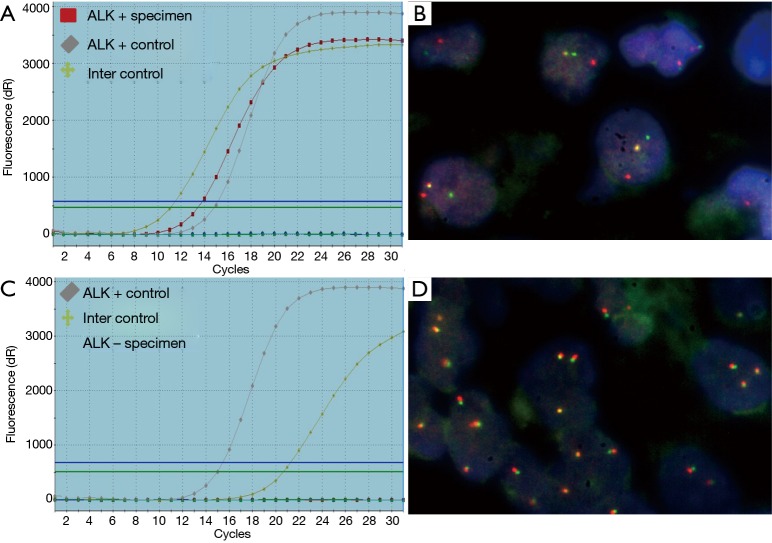
Figure 2.
RT-PCR and FISH for detecting ALK rearrangement on primary tumor and paired metastatic lymph nodes in the same patients. (A,B) ALK positive on primary tumor by RT-PCR and FISH, respectively; (C,D) ALK negative on paired metastatic lymph node by RT-PCR and FISH in the same patient, respectively. RT-PCR, reverse transcriptase-polymerase chain reaction; FISH, fluorescence in situ hybridization.
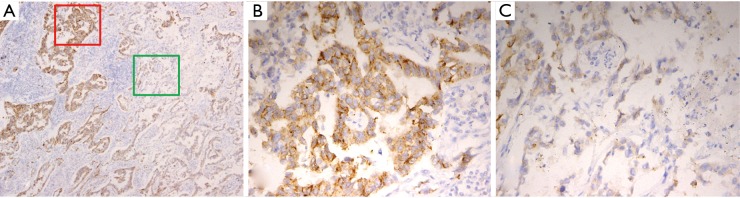
Figure 3.
The heterogeneous strong granular cytoplasmic staining in some tumor cells of different primary tumor areas by IHC. (A) Heterogeneous granular cytoplasmic staining pattern throughout the section (40×); (B) strong granular cytoplasmic staining in most tumor cells (red square area’s magnification in Figure 3A, 200×); (C) most tumor cells without strong granular cytoplasmic staining pattern (green square area’s magnification in Figure 3A, 200×). IHC, immunohistochemistry.
Table 3. Characteristics of patients with discordance between ALK rearrangement results in primary tumor and paired metastatic lymph nodes.
| Patients (No.) | IHC | RT-PCR | FISH | ALK fusion | Age (years) | Gender | Smoking history | Predominant pattern |
|---|---|---|---|---|---|---|---|---|
| No. 15 | 50 | Female | No | Solid | ||||
| PS | + | + | + | Positive | ||||
| PMLN | − | − | − | Negative | ||||
| No. 21 | 48 | Female | No | Solid | ||||
| PS | + | + | + | Positive | ||||
| PMLN | − | − | − | Negative |
PS, primary tumor; PMLN, paired metastatic lymph node. IHC, immunohistochemistry; RT-PCR, reverse transcriptase-polymerase chain reaction; FISH, fluorescence in situ hybridization.
Discussion
This study demonstrates a high concordance rate (98%) in the ALK rearrangement between primary tumors and paired metastatic lymph nodes. Two patients with ALK rearrangement on primary tumor didn’t show ALK gene fusion on paired metastatic lymph nodes. In addition, ALK rearrangement was totally 100% concordant between different stations of metastatic lymph nodes in the same patient by IHC, RT-PCR or FISH.
EML4-ALK rearrangement was reported as a relatively low frequent rearrangement ranging from 1.5% to 6.7% among unselected Caucasian NSCLC patients (14-17). It seems to have higher frequency in Asian population, and was reported as high as 5.1–10% (18-21). Part of the explanation was that in those studies, patients were selected from EGFR wild population. Our previous study has shown ALK rearrangement could be as high as 32.3% among patients who have never smoked, and had adenocarcinoma harboring wild-type EGFR (13). In line with these results, we found the incidence of ALK rearrangement was 19.8% in patients with wild-type EGFR. The subgroup analysis showed that ALK rearrangement was more likely to occur in patients who were younger, never smokers and with adenocarcinoma histology, all of which were consistent with previous studies (22-24).
Intratumor heterogeneity has been described recently and poses a significant challenge to personalized cancer medicine. It was reported that activating EGFR mutations were found to display intratumor heterogeneity and spatial discordance in 6.3–30% of cases in lung cancer (25,26). However, intratumor heterogeneity of ALK rearrangement was rarely evaluated. Rossi et al. firstly reported that the discrepancy of ALK rearrangement in a heavy smoking 54-year-old man with pulmonary adenocarcinoma. This patient showed ALK rearrangement negative on primary lesion while harboring ALK fusion in metastatic peritoneal biopsy specimen 8 month later (27). In the present study, the primary and paired samples were collected simultaneously after surgical resection. Unlike EGFR mutation, our result showed a high concordance between primary tumors and paired metastatic lymph nodes. What’s more, Two patients with discordant ALK rearrangement showed an ALK fusion positive in primary tumor while not in paired metastatic lymph nodes. Recent study reported that ALK rearrangement was discordant among spatially separated tumor areas in the same primary tumor of ALK positive patient by RT-PCR. It therefore seems reasonable to infer that metastatic tumor cells may come from the ALK negative tumor cells in primary lesion because of the genetic intratumoral heterogeneity (28). Different clones and innate tumor heterogeneity of samples may attribute to the discrepancy (29-31).
Previous studies reported that IHC assay was a valuable tool for the screening of patients with ALK rearrangement in clinical practice (7,32,33), IHC with new antibody and modified protocols will extend its serviceable range for detecting ALK rearrangement (34). Although FISH was approved by Food and Drug Administration as a companion diagnostics, FISH required specialized equipment and highly trained and skilled readers for interpretation because of variability between inter-observers. However, surgical samples were only available in minority patients with NSCLC. Several studies investigated the feasibility of cytological sample obtaining from minimally invasive procedures like EBUS-TBNA or CT-guided fine needle biopsy for molecular testing (35-40). A substantial part of patients had insufficient tumor cell number for FISH testing after successful differential diagnosis for NSCLC subtype by IHC marker. RT-PCR is a sensitive method for detecting ALK rearrangement and had an advantage to utility of cytological samples. Thus, RT-PCR is also regarded as an acceptable method to detect ALK rearrangement by Chinese FDA (41,42). Our study also found that FISH, Ventana IHC and RT-PCR have good concordance though one case showed different results. The efficacy of ALK inhibitors detected by RT-PCR need be further evaluated in clinical trials or clinical practice (43).
Several limitations have to be mentioned in this study. Firstly, all the enrolled patients were resected, thus the finding from this study may not represent the whole population. Secondly, most of the patients are still not received the treatment of ALK inhibitor, thus we could not observe the response to ALK inhibitor from the patients who showed a discordant result on primary and metastatic lymph nodes. Thirdly, the samples of this study are still small though enrolling more than 100 patients. Similar results were observed in previous study which showed ALK arrangement was heterogeneous in 67 paired NSCLC specimens between primary tumors and their corresponding metastatic lesions by FISH (44), Thus, large scale study with the data of efficacy to ALK inhibitor is warranted to further support the findings in this study.
In conclusion, we have demonstrated that high concordant rate of ALK rearrangement between primary tumors and paired metastatic lymph nodes through three different detection methods. Though ALK rearrangement was found positive in the primary tumor while not in metastatic lymph nodes in the two discordant patients, primary tumor and metastatic lesions were equally suitable to perform ALK rearrangement testing for therapeutic strategies in patients with advanced NSCLC.
Acknowledgements
Funding: This work was supported in part by grants from the National Science Foundation of China (No. 81201707), projects of the Science and Technology Commission of Shanghai Municipality (No. 12ZR1426000), Outstanding Young Doctor Program of Shanghai Municipal Commission of Health and Family Planning (No. XYQ2013097) and Shanghai Municipal Commission of Health and Family Planning (No. 201440398) and the Shanghai Science and Technique Committee (No. 134119a3200).
Table S1. ALK rearrangement concordance between different stations of metastatic lymph nodes by IHC and RT-PCR (68 of 101 cases).
| Methods | Cases with N1 (No.) | Cases with N2 (No.) | ALK rearrangement |
|---|---|---|---|
| IHC− | 66* | 58 | Negative |
| IHC+ | 2 | 2 | Positive |
| RT-PCR− | 66* | 58 | Negative |
| RT-PCR+ | 2 | 2 | Positive |
Note: *, 66 cases were diagnosed with metastatic lymph nodes, including 58 cases with N1 and N2 metastatic lymph nodes, 8 cases with 2 stations of metastatic lymph nodes in N1. IHC, immunohistochemistry; RT-PCR, reverse transcriptase-polymerase chain reaction.
Table S2. Average CT value of ALK positive paired tumors of 20 cases using RT-PCR method.
| Patient number | Average CT value (FAM) | |
|---|---|---|
| Primary tumor site | Metastatic lymph nodes | |
| Case #01 | 18.3 | 19.5 |
| Case #9 | 12.8 | 16.6 |
| Case #11 | 23.3 | 24.1 |
| Case #17 | 13.1 | 13.7 |
| Case #20 | 21.6 | 22.3 |
| Case #29 | 17.8 | 18.2 |
| Case #31 | 23.5 | 23.9 |
| Case #35 | 17.3 | 18.6 |
| Case #41 | 18.8 | 20.4 |
| Case #45 | 21.2 | 22.6 |
| Case #47 | 16.4 | 18.8 |
| Case #48 | 23.6 | 24.6 |
| Case #53 | 20.5 | 20.6 |
| Case #61 | 15.1 | 16.8 |
| Case #77 | 23.6 | 24.1 |
| Case #89 | 18.8 | 20.6 |
| Case #90 | 23.2 | 24.3 |
| Case #93 | 21.4 | 22.7 |
| Case #15 | 20.1 | 0 |
| Case #21 | 19.5 | 27.8 |
RT-PCR, reverse transcriptase-polymerase chain reaction; FAM, Reporter Dye.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Shibuya K, Mathers CD, Boschi-Pinto C, et al. Global and regional estimates of cancer mortality and incidence by site: II. Results for the global burden of disease 2000. BMC Cancer 2002;2:37. 10.1186/1471-2407-2-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. 10.1093/jnci/92.3.205 [DOI] [PubMed] [Google Scholar]
- 3.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. 10.1056/NEJMoa1006448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol 2012;13:1011-9. 10.1016/S1470-2045(12)70344-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. 10.1056/NEJMoa1408440 [DOI] [PubMed] [Google Scholar]
- 6.Ying J, Guo L, Qiu T, et al. Diagnostic value of a novel fully automated immunochemistry assay for detection of ALK rearrangement in primary lung adenocarcinoma. Ann Oncol 2013;24:2589-93. 10.1093/annonc/mdt295 [DOI] [PubMed] [Google Scholar]
- 7.Wynes MW, Sholl LM, Dietel M, et al. An international interpretation study using the ALK IHC antibody D5F3 and a sensitive detection kit demonstrates high concordance between ALK IHC and ALK FISH and between evaluators. J Thorac Oncol 2014;9:631-8. 10.1097/JTO.0000000000000115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gow CH, Chang YL, Hsu YC, et al. Comparison of epidermal growth factor receptor mutations between primary and corresponding metastatic tumors in tyrosine kinase inhibitor-naive non-small-cell lung cancer. Ann Oncol 2009;20:696-702. 10.1093/annonc/mdn679 [DOI] [PubMed] [Google Scholar]
- 9.Kalikaki A, Koutsopoulos A, Trypaki M, et al. Comparison of EGFR and K-RAS gene status between primary tumours and corresponding metastases in NSCLC. Br J Cancer 2008;99:923-9. 10.1038/sj.bjc.6604629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. 10.1097/JTO.0b013e318206a221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren S, Hirsch FR, Varella-Garcia M, et al. Atypical negative ALK break-apart FISH harboring a crizotinib-responsive ALK rearrangement in non-small-cell lung cancer. J Thorac Oncol 2014;9:e21-3. 10.1097/JTO.0000000000000013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu C, Zhao C, Yang Y, et al. High Discrepancy of Driver Mutations in Patients with NSCLC and Synchronous Multiple Lung Ground-Glass Nodules. J Thorac Oncol 2015;10:778-83. 10.1097/JTO.0000000000000487 [DOI] [PubMed] [Google Scholar]
- 13.Ren S, Chen X, Kuang P, et al. Association of EGFR mutation or ALK rearrangement with expression of DNA repair and synthesis genes in never-smoker women with pulmonary adenocarcinoma. Cancer 2012;118:5588-94. 10.1002/cncr.27603 [DOI] [PubMed] [Google Scholar]
- 14.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. 10.1038/nature05945 [DOI] [PubMed] [Google Scholar]
- 15.Perner S, Wagner PL, Demichelis F, et al. EML4-ALK fusion lung cancer: a rare acquired event. Neoplasia 2008;10:298-302. 10.1593/neo.07878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeuchi K, Choi YL, Soda M, et al. Multiplex reverse transcription-PCR screening for EML4-ALK fusion transcripts. Clin Cancer Res 2008;14:6618-24. 10.1158/1078-0432.CCR-08-1018 [DOI] [PubMed] [Google Scholar]
- 17.Filipits M. Registry study in NSCLC patients with EGFR, ALK, or ROS1 mutations. Transl Lung Cancer Res 2014;3:AB003. [Google Scholar]
- 18.Sun Y, Ren Y, Fang Z, et al. Lung adenocarcinoma from East Asian never-smokers is a disease largely defined by targetable oncogenic mutant kinases. J Clin Oncol 2010;28:4616-20. 10.1200/JCO.2010.29.6038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.An SJ, Chen ZH, Su J, et al. Identification of enriched driver gene alterations in subgroups of non-small cell lung cancer patients based on histology and smoking status. PLoS One 2012;7:e40109. 10.1371/journal.pone.0040109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia N, An J, Jiang QQ, et al. Analysis of EGFR, EML4-ALK, KRAS, and c-MET mutations in Chinese lung adenocarcinoma patients. Exp Lung Res 2013;39:328-35. 10.3109/01902148.2013.819535 [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Dong Y, Cai Y, et al. Clinicopathologic characteristics of ALK rearrangements in primary lung adenocarcinoma with identified EGFR and KRAS status. J Cancer Res Clin Oncol 2014;140:453-60. 10.1007/s00432-014-1584-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong DW, Leung EL, So KK, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer 2009;115:1723-33. 10.1002/cncr.24181 [DOI] [PubMed] [Google Scholar]
- 23.Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247-53. 10.1200/JCO.2009.22.6993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang WY, Liang DN, Yao WQ, et al. Immunohistochemical screening and fluorescence in situ hybridization confirmation of ALK translocation in lung adenocarcinoma and its clinicopathological significance: a single-center large-scale investigation of Chinese patients. Hum Pathol 2014;45:1414-22. 10.1016/j.humpath.2014.02.015 [DOI] [PubMed] [Google Scholar]
- 25.Chen ZY, Zhong WZ, Zhang XC, et al. EGFR mutation heterogeneity and the mixed response to EGFR tyrosine kinase inhibitors of lung adenocarcinomas. Oncologist 2012;17:978-85. 10.1634/theoncologist.2011-0385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bai H, Wang Z, Wang Y, et al. Detection and clinical significance of intratumoral EGFR mutational heterogeneity in Chinese patients with advanced non-small cell lung cancer. PLoS One 2013;8:e54170. 10.1371/journal.pone.0054170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossi A, Galetta D, Bottiglieri L, et al. Evaluation of ALK gene status in primary lung adenocarcinoma and matched metastases. J Thorac Oncol 2011;6:1146. 10.1097/JTO.0b013e31821528fc [DOI] [PubMed] [Google Scholar]
- 28.Cai W, Lin D, Wu C, et al. Intratumoral Heterogeneity of ALK-Rearranged and ALK/EGFR Coaltered Lung Adenocarcinoma. J Clin Oncol 2015;33:3701-9. 10.1200/JCO.2014.58.8293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakurada A, Lara-Guerra H, Liu N, et al. Tissue heterogeneity of EGFR mutation in lung adenocarcinoma. J Thorac Oncol 2008;3:527-9. 10.1097/JTO.0b013e318168be93 [DOI] [PubMed] [Google Scholar]
- 30.Khode R, Larsen DA, Culbreath BC, et al. Comparative study of epidermal growth factor receptor mutation analysis on cytology smears and surgical pathology specimens from primary and metastatic lung carcinomas. Cancer Cytopathol 2013;121:361-9. 10.1002/cncy.21273 [DOI] [PubMed] [Google Scholar]
- 31.Sun PL, Jin Y, Kim H, et al. High concordance of EGFR mutation status between histologic and corresponding cytologic specimens of lung adenocarcinomas. Cancer Cytopathol 2013;121:311-9. 10.1002/cncy.21260 [DOI] [PubMed] [Google Scholar]
- 32.von Laffert M, Warth A, Penzel R, et al. Multicenter immunohistochemical ALK-testing of non-small-cell lung cancer shows high concordance after harmonization of techniques and interpretation criteria. J Thorac Oncol 2014;9:1685-92. 10.1097/JTO.0000000000000332 [DOI] [PubMed] [Google Scholar]
- 33.Wang W, Tang Y, Li J, et al. Detection of ALK rearrangements in malignant pleural effusion cell blocks from patients with advanced non-small cell lung cancer: a comparison of Ventana immunohistochemistry and fluorescence in situ hybridization. Cancer Cytopathol 2015;123:117-22. 10.1002/cncy.21510 [DOI] [PubMed] [Google Scholar]
- 34.Gruber K, Kohlhäufl M, Friedel G, et al. A novel, highly sensitive ALK antibody 1A4 facilitates effective screening for ALK rearrangements in lung adenocarcinomas by standard immunohistochemistry. J Thorac Oncol 2015;10:713-6. 10.1097/JTO.0000000000000427 [DOI] [PubMed] [Google Scholar]
- 35.Savic S, Bode B, Diebold J, et al. Detection of ALK-positive non-small-cell lung cancers on cytological specimens: high accuracy of immunocytochemistry with the 5A4 clone. J Thorac Oncol 2013;8:1004-11. 10.1097/JTO.0b013e3182936ca9 [DOI] [PubMed] [Google Scholar]
- 36.Tanaka H, Tone K, Hayashi A, et al. Clinical application of immunocytochemical detection of ALK rearrangement on cytology slides for detection or screening of lung adenocarcinoma. Lung Cancer 2013;80:289-92. 10.1016/j.lungcan.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 37.Neat MJ, Foot NJ, Hicks A, et al. ALK rearrangements in EBUS-derived transbronchial needle aspiration cytology in lung cancer. Cytopathology 2013;24:356-64. 10.1111/cyt.12060 [DOI] [PubMed] [Google Scholar]
- 38.Jurado J, Saqi A, Maxfield R, et al. The efficacy of EBUS-guided transbronchial needle aspiration for molecular testing in lung adenocarcinoma. Ann Thorac Surg 2013;96:1196-202. 10.1016/j.athoracsur.2013.05.066 [DOI] [PubMed] [Google Scholar]
- 39.Yarmus L, Akulian J, Gilbert C, et al. Optimizing endobronchial ultrasound for molecular analysis. How many passes are needed? Ann Am Thorac Soc 2013;10:636-43. 10.1513/AnnalsATS.201305-130OC [DOI] [PubMed] [Google Scholar]
- 40.Betz BL, Dixon CA, Weigelin HC, et al. The use of stained cytologic direct smears for ALK gene rearrangement analysis of lung adenocarcinoma. Cancer Cytopathol 2013;121:489-99. 10.1002/cncy.21286 [DOI] [PubMed] [Google Scholar]
- 41.Han XH, Zhang NN, Ma L, et al. Immunohistochemistry reliably detects ALK rearrangements in patients with advanced non-small-cell lung cancer. Virchows Arch 2013;463:583-91. 10.1007/s00428-013-1472-7 [DOI] [PubMed] [Google Scholar]
- 42.Zhang YG, Jin ML, Li L, et al. Evaluation of ALK rearrangement in Chinese non-small cell lung cancer using FISH, immunohistochemistry, and real-time quantitative RT- PCR on paraffin-embedded tissues. PLoS One 2013;8:e64821. 10.1371/journal.pone.0064821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu YC, Chang IC, Wang CL, et al. Comparison of IHC, FISH and RT-PCR methods for detection of ALK rearrangements in 312 non-small cell lung cancer patients in Taiwan. PLoS One 2013;8:e70839. 10.1371/journal.pone.0070839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim H, Xu X, Yoo SB, et al. Discordance between anaplastic lymphoma kinase status in primary non-small-cell lung cancers and their corresponding metastases. Histopathology 2013;62:305-14. 10.1111/j.1365-2559.2012.04356.x [DOI] [PubMed] [Google Scholar]