Abstract
Background
To investigate whether gross tumor volume (GTV) defined on radiotherapy planning scans a prognostic factor for esophageal squamous cell carcinoma (ESCC) patients treated with definitive radiotherapy.
Methods
From 2008 to 2011, 187 ESCC patients who were treated with definitive radio(chemo)therapy were analyzed retrospectively. Tumor volumes such as GTV, gross tumor volume of primary esophageal cancer (GTV-P), and gross tumor volume of metastases lymph nodes (GTV-N) were computed by Philips Healthcare radiation therapy planning system (Pinnacle 8.0). Kaplan-Meier cumulative probability and Cox proportional hazards regression models were used to assess the effect of the clinical factors along with tumor volume on progression-free survival (PFS) and overall survival (OS).
Results
In the univariate analysis, fraction dose, TNM stage, total radiation dose, GTV, GTV-P, and GTV-N were all significantly associated with both OS and PFS (P<0.05). While in multivariate analysis, GTV and fraction dose were significantly associated with both OS and PFS (adjusted P<0.05) with adjustment for age, sex, smoking status, chemotherapy, fraction dose, GTV, and radiation dose.
Conclusions
GTV may serve as a good prognostic factor for ESCC patients treated with definitive radiotherapy. Larger prospective studies are needed to validate these findings.
Keywords: Radiation therapy, tumor volume, prognostic factor, esophageal cancer
Introduction
Esophageal squamous cell carcinoma (ESCC) is a common thoracic carcinoma with high incidence rate in China. Radio(chemo)therapy as a definitive modality is reserved for esophageal cancer patients who are unresectable and those who cannot tolerate esophagectomy because of comorbidity and old age. Unfortunately, the overall survival (OS) rate of esophageal cancer patients is not optimistic. In 2014 global cancer statistics (1), esophageal cancer was the 7th among the top ten leading cancer types for estimated deaths. TNM stage classification acts as a common prognostic factor for esophageal carcinoma for many years. It contains the information for presence of nodal metastasis and primary tumor invasion. Nevertheless, in recent years, more and more researches consider TNM stage classification not sufficient to present the prognostic information for the reason that it does not take into account the tumor volume that varies in either T or N classification. Indeed, a few studies have suggested that other clinical factor such as sex, age, BMI, platelet count, tumor length, total dose, concurrent chemotherapy, and weight loss, et al. may function as additional prognostic factors (2-6).
Recently, many scientific studies indicated that the clinical parameters such as the anterior-posterior extent of primary tumor, and the volume of the metastasis lymph node which was currently named tumor burden are correlated with the overall final results (5,7,8). Modern deliver of intensity-modified radiotherapy (IMRT) is based on computed tomography (CT) simulation planning system and target contouring system, which makes it possible to get data of volume of the primary tumor and the metastasis lymph nodes. In this study, we investigated whether measure the volume in both the primary esophageal cancer and the metastasis lymph node could provide a good prognostic factor for ESCC.
Methods
This retrospective study included 187 ESCC patients treated at Fudan University Shanghai Cancer Center between 2008 and 2011. The clinical results have been previously published (9). These patient were all newly diagnosed and cytologically or histpathologically confirmed ESCC with pretreatment imaging work up, including esophageal barium radiography, endoscopic and CT. All patients were re-staged with the 6th edition of the American Joint Committee on Cancer’s atlas (10).
Radio(chemo)therapy
All patients were treated with IMRT. Definitive radiotherapy alone or in combination with chemotherapy was intended to be administered (total radiation dose ≥50 Gy). All treatments were planned based on CT simulation planning system with 5 mm thickness scan slice throughout the entire neck and thorax. Criteria for metastasis lymph nodes were as follows: pathologic confirmation or short axis of ≥10 mm in mediastinum and cervix or short axis of ≥5 mm in tracheo-esophageal groove.
The target volumes were defined as follows: gross tumor volume (GTV), primary esophageal tumor and involved metastasis lymph nodes; clinical target volume (CTV), GTV +3 cm margins in the esophageal long axis superiorly and inferiorly to encompass potential submucosal invasions; planning target volume (PTV) 1, CTV +1 cm margin; PTV 2, GTV +1 cm margin. Images were retrieved from the patients’ database, and primary esophageal tumor and metastasis lymph nodes were recontoured separately as gross tumor volume of primary esophageal cancer (GTV-P) and gross tumor volume of metastases lymph nodes (GTV-N) based on the initial targets contouring for this study on Philips healthcare radiation therapy planning system (Pinnacle 8.0). Two radiation oncologists reviewed these new volumes for accuracy and consistency.
The dosimetric data were calculated from the Pinnacle 8.0 system for each patient, including GTV, GTV-P, GTV-N and the length of gross tumor volume of primary esophageal cancer (LGTV-P).
Toxicity
Acute esophagitis and acute pneumonitis were graded according to the Common Terminology Criteria for Adverse Events v4.0 (11).
Follow-up
Patients were followed up with CT scans, esophagogram, and images from endoscopic evaluations starting at 1 month after the completion of therapy and then every 3 months for the first 2 years of follow-up and every 6 months afterwards until death or loss of follow-up.
Statistics
Volumetric and length parameters were analyzed as both continuous and categorical variables. Receiver operating characteristic (ROC) curve method was used to identify the best cut-off values of these continuous parameters. The Youden index (sensitivity + specificity-1), defined as the value with the highest average of sensitivity and specificity, was used to assess the tumor volume threshold value, and it corresponds to the furthest point on the ROC curve from the identity line. Univariate Cox proportion hazards regression analysis was performed to estimate the hazards ratio (HR) and confidence interval (CI) to evaluate the effect of each clinical or dosimetric parameter on progression-free survival (PFS) and OS. In addition, multivariate Cox hazards regression analysis was performed to adjust for age, sex, smoking status, chemotherapy history, fraction dose, radiation dose, LGTV-P and GTV. Kaplan-Meier curve was used to estimate PFS and OS. The observed difference would be statistically significant if the P value was <0.05. All statistical analyses were performed using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA).
Statement of ethics approval
The data involved in this study was collected retrospectively, which was not required the statement of ethics approval.
Results
Clinical characteristics
A total of 187 ESCC patients between April 2008 and December 2011 were included in this study. A majority of patients were male (73.80%). The median age of all patients was 64 years. Of the patients, two patients (1.07%) had stage I disease, 65 patients (34.76%) had stage II disease, 54 patients (28.88%) had stage III disease, and 66 patients (35.29%) had stage IV disease. The details of patients’ characteristics were provided in Table 1.
Table 1. Characteristics of 187 ESCC patients (staging based on AJCC 6th edition).
| Patient characteristics | No. of patients (%) |
|---|---|
| Sex | |
| Male | 138 (73.80) |
| Female | 49 (26.20) |
| Age, years | |
| Median | 64 |
| Range | 37–88 |
| No. of pack yearsa | |
| None | 91 (48.66) |
| ≤18 | 21 (11.23) |
| >18 | 70 (37.43) |
| Stage | |
| I | 2 (1.07) |
| II | 65 (34.76) |
| III | 54 (28.88) |
| IV | 66 (35.29) |
| T classification | |
| 1 | 5 (2.67) |
| 2 | 72 (38.50) |
| 3 | 56 (29.95) |
| 4 | 54 (28.88) |
| N classification | |
| 0 | 78 (41.71) |
| 1 | 109 (58.29) |
a, smoking status of 5 patients were missing. ESCC, esophageal squamous cell carcinoma.
There were 138 ESCC patients treated with radiation combined with chemotherapy. The chemotherapeutic agents during radiotherapy were cisplatin-based or fluorouracil-based, including: cisplatin and 5-fluorouridine every 4 weeks in 118 patients; paclitaxel and cisplatin every 4 weeks in 18 patients; Tegafur, Gimeracil and Oteracil Potassium Capsulesn (S-1) in one patient; and paclitaxel, cisplatin and 5-fluorouridine every 4 weeks in one patient. The details of chemotherapy were provided in Table 2.
Table 2. Treatment characteristics of 187 ESCC patients.
| Treatment characteristics | No. of patients (%) |
|---|---|
| Radiation dose, Gy | |
| <60 | 9 (4.81) |
| 60 | 12 (6.42) |
| 61.2 | 66 (35.29) |
| 62–64.8 | 79 (42.25) |
| >65 | 21 (11.23) |
| Fraction dose, Gy | |
| ≤2 | 141 (75.40) |
| >2 | 46 (24.60) |
| Chemotherapy | |
| Induction + concurrent | 13 (6.95) |
| Concurrent | 35 (18.72) |
| Concurrent + consolidation | 90 (48.13) |
| None | 49 (26.20) |
The median follow-up time for surviving patients was 59.30 months ranged from 45.30 to 77.50 months. The median PFS was 14.36 months and the 1-, 3-, and 5-year PFS rate was 54.80%, 27.12%, and 21.34% respectively. The median OS was 21.33 months and the 1-, 3-, and 5-year OS rate was 69.83%, 35.20%, and 25.70% respectively (Figure 1).
Figure 1.
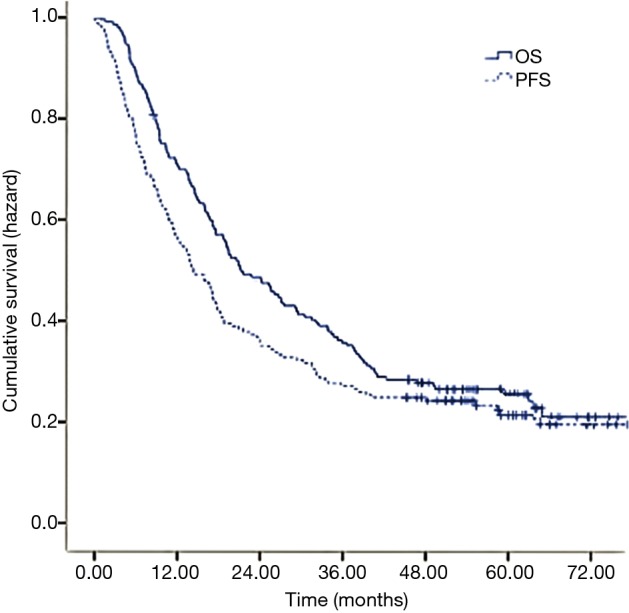
Kaplan-Meier survival curves for study patients. Progression-free survival (PFS) curve and overall survival (OS) curve for ESCC patients treated with radio(chemo)therapy.
In this study, 57 (30.5%) patients exhibited no acute esophagitis, while grades 1, 2, 3, and 4 acute esophagitis were observed in 88 (47.1%), 33 (17.6%), 6 (3.2%) and 3 (1.6%) patients, respectively. In addition, 111 patients (59.4%) exhibited no acute pneumonitis, while grades 1, 2, 3, and 4 acute pneumonitis were observed in 48 (25.7%), 23 (12.3%), 5 (2.7%) and 0 (0%) patients, respectively. None of the patients suffered death caused by acute radiation-induced toxicity.
Dosimetric and volumetric characteristics
The median total dose prescribed to PTV1 was 62.00 Gy (range, 50.00–68.00 Gy) based on physician preference. Radiotherapy was delivered using conventional fractions of 1.80–2.25 Gy given 5 days per week over 4 to 7 weeks. A total of 57 patients were treated with the prescription of 50.40 Gy to PTV1 and 63.00 Gy to PTV2, while the others were treated without PTV2.
The median values of GTV, GTV-P, and GTV-N were 39.16 cm3 (range, 4.46–287.20 cm3), 28.30 cm3 (range, 4.24–287.20 cm3), and 3.88 cm3 (range, 0.00–128.88 cm3), respectively. The best cut-off value of GTV, GTV-P, and GTV-N was 39.41 cm3, 32.17 cm3, and 4.47 cm3 respectively.
Univariate and multivariate analysis of clinical and volumetric characteristics related to PFS and OS
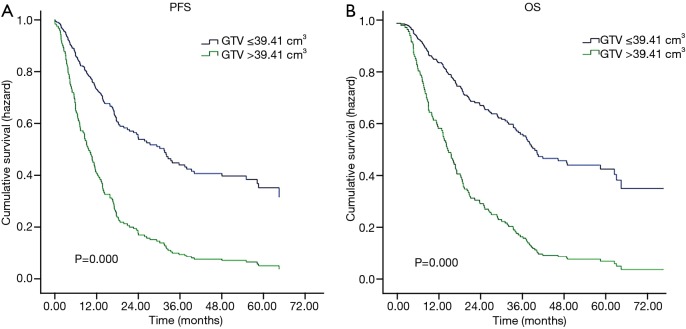
Table 3 shows univariate and multivariate analyses of clinical, volumetric characteristics and PFS and OS. In the univariate analysis, radiation dose showed significantly associated with PFS (P=0.034), while sex, fraction dose, TNM stage, LGTV-P, GTV, GTV-P, and GTV-N were all significantly associated with both PFS and OS (P<0.050). We use GTV substitute TNM stage in the multivariate analysis. The results showed that patients treated with fraction dose >2 Gy had significantly better PFS and OS than those treated with fraction dose ≤2 Gy (adjusted P=0.006 and P=0.012 respectively). Patients whose GTV >39.41 cm3 had significantly worse PFS and OS than those GTV ≤39.41 cm3 (adjusted P=0.000 and P=0.000 respectively; Figure 2) with adjustment for age, sex, smoking status, chemotherapy, fraction dose, GTV, and radiation dose.
Table 3. Associations between patient-, tumor-, and therapy-related characteristics and OS.
| Parameter | No. | PFS | OS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
| HR (95% CI) | P | HR (95% CI) | Pa | HR (95% CI) | P | HR (95% CI) | Pa | |||||
| Sex | ||||||||||||
| Male | 138 | 1.000 | – | 1.000 | – | 1.000 | – | 1.000 | – | |||
| Female | 49 | 0.651 (0.434–0.977) | 0.038 | 0.751 (0.482–1.169) | 0.205 | 0.646 (0.428–0.975) | 0.037 | 0.817 (0.522–1.279) | 0.376 | |||
| Age, years | ||||||||||||
| ≤65 | 112 | 1.000 | – | 1.000 | – | 1.000 | – | 1.000 | – | |||
| >65 | 75 | 1.153 (0.822–1.617) | 0.409 | 1.240 (0.862–1.783) | 0.246 | 1.192 (0.846–1.678) | 0.315 | 1.319 (0.913–1.904) | 0.140 | |||
| No. of pack yearsb | ||||||||||||
| ≤18 | 112 | 1.000 | – | 1.000 | – | 1.000 | – | 1.000 | – | |||
| >18 | 70 | 1.228 (0.869–1.734) | 0.244 | 0.936 (0.644–1.360) | 0.729 | 1.291 (0.911–1.832) | 0.152 | 0.982 (0.675–1.429) | 0.924 | |||
| Chemotherapy | ||||||||||||
| No | 49 | 1.000 | – | 1.000 | – | 1.000 | – | 1.000 | – | |||
| Yes | 138 | 0.903 (0.621-1.311) | 0.591 | 0.729 (0.468–1.135) | 0.162 | 0.861 (0.594–1.276) | 0.479 | 0.684 (0.436–1.073) | 0.098 | |||
| Fraction dose, Gy | ||||||||||||
| ≤2 | 141 | 1.000 | – | 1.000 | – | 1.000 | – | 1.000 | – | |||
| >2 | 46 | 0.547(0.361-0.831) | 0.005 | 0.532 (0.325-0.870) | 0.012 | 0.522 (0.337-0.810) | 0.004 | 0.485 (0.291-0.809) | 0.006 | |||
| Stagec | ||||||||||||
| I–II | 67 | 1.000 | – | – | – | 1.000 | – | – | – | |||
| III–IV | 120 | 2.076 (1.432–3.008) | 0.000 | – | – | 2.094 (1.434-3.059) | 0.000 | – | – | |||
| Radiation dose, Gy | ||||||||||||
| ≤61.2 | 87 | 1.000 | – | 1.000 | – | 1.000 | – | 1.000 | – | |||
| >61.2 | 100 | 0.695 (0.497–0.973) | 0.034 | 0.777 (0.497–1.216) | 0.270 | 0.713 (0.506–1.005) | 0.053 | 0.830 (0.531–1.298) | 0.414 | |||
| LGTV-P, cm | ||||||||||||
| ≤7 | 133 | 1.000 | – | 1.000 | – | 1.000 | – | 1.000 | – | |||
| >7 | 52 | 1.491 (1.036–2.147) | 0.032 | 0.968 (0.635–1.477) | 0.881 | 1.669 (1.159–2.404) | 0.006 | 1.080 (0.706–1.652) | 0.723 | |||
| GTV, cm3 | ||||||||||||
| ≤39.41 | 93 | 1.000 | – | 1.000 | – | 1.000 | – | 1.000 | – | |||
| >39.41 | 92 | 2.508 (1.768–3.557) | 0.000 | 2.865 (1.897–4.326) | 0.000 | 2.796 (1.961–3.988) | 0.000 | 3.152 (2.066–4.807) | 0.000 | |||
| GTV-P, cm3 | ||||||||||||
| ≤32.17 | 102 | 1.000 | – | – | – | 1.000 | – | – | – | |||
| >32.17 | 83 | 2.332 (1.655–3.287) | 0.000 | – | – | 2.839 (2.003–4.023) | 0.000 | – | – | |||
| GTV-N, cm3 | ||||||||||||
| ≤4.47 | 95 | 1.000 | – | – | – | 1.000 | – | – | – | |||
| >4.47 | 90 | 1.852 (1.316–2.606) | 0.000 | – | – | 1.851 (1.307–2.621) | 0.001 | – | – | |||
a, P values were calculated with adjustment for age, sex, smoking status, chemotherapy history, fraction dose, radiation dose, LGTV-P and GTV; b, smoking status of 5 patients were missing; c, UICC 6th edition. OS, overall survival; PFS, progression-free survival; HR, hazards ratio; CI, confidence interval; LGTV-P, length of gross tumor volume of primary esophageal cancer; GTV, gross tumor volume; GTV-P, gross tumor volume of primary esophageal cancer; GTV-N, gross tumor volume of metastases lymph nodes.
Figure 2.
Multivariate Cox survival curves for study patients. (A) Progression-free survival (PFS) curve for ESCC patients with stratified by gross tumor volume; (B) overall survival (OS) curve for ESCC patients with stratified by gross tumor volume.
Discussion
Radio(chemo)therapy is the standard treatment for ESCC patients who were inoperable or locally advanced (12). The current TNM staging system in place for esophageal carcinoma is based on 4,627 surgical data in 13 institutions from five countries and three continents (13). Nevertheless, for those non-surgical cases, it’s hard to identify the exact number of metastasis lymph nodes with the current UICC 7th TNM staging system which is surgical pathology based (14). Therefore, radiation oncologists prefer to use the UICC 6th TNM staging system to classify patients’ outcomes. However, in clinical practice, we found that tumor volume may affect prognostic outcomes since it varies even in the same TNM stage. Our study demonstrated that larger GTV did predict a poorer prognosis in ESCC patients treated with definitely radio(chemo)therapy.
There are several clinical data supporting that tumor volume significantly influences radiation therapy outcome in carcinomas such as Hodgkin’s lymphoma, lung cancer and nasopharyngeal carcinoma (7,8,15). However, there is little evidence for the influence of tumor volume on PFS and OS in esophageal carcinoma. In 2006, Créhange et al. first reported that tumor volume affects outcomes of esophageal cancer (16). They retrospectively analyzed 148 esophageal cancer patients treated with radiotherapy and indicated that patients with tumor volume ≥100 cm3 had significantly worse OS than those with tumor volume <100 cm3 (adjusted P=0.041). However, without the help of treatment planning system, this study calculated the tumor volume in the way that assimilated and represented the esophageal tumor as two opposing truncated cones. So, it had the shortcoming of potentially inaccurate calculation. Since the development of the radiotherapy technique, Chen et al. evaluated 153 ESCC patients treated with three-dimensional conformal radiotherapy and suggested an optimum cut-off point for GTV in ESCC 20 cm3 for survival prediction (5). A recent study investigated 67 locally advanced esophageal cancer patients treated with chemoradiotherapy followed by esophagectomy, and it also highlighted the prognostic importance of GTV which showed more powerful prediction for patient outcomes than traditional TNM staging (17). Our data from 187 ESCC patients further supports this conclusion. In the present study, we determined the prognostic value of tumor volume in ESCC patients treated with definitive radio(chemo)therapy. The results showed that GTV is a good predictive factor for ESCC patients. Patients who suffered the large tumor burden (GTV >39.41 cm3) had significantly worse OS than those who suffered small tumor burden (GTV ≤39.41 cm3). Although GTV-N reaches statistically significance in univariate Cox proportion hazards regression analysis, we did not use GTV-N as a factor for adjustment for multiple Cox proportion hazards regression analysis for the consideration that there was a proportion of patients with difficulty in segregated nodal volume delineation due to lymph nodes conglomerated with primary tumor.
RTOG 9405 reported the high dose of 64.8 Gy did not show better prognosis than standard dose of 50.4 Gy in esophageal carcinoma (18), however, there were several clinical studies showed that higher radiation doses may be associated with increased OS and decreased local failure (19,20). Furthermore, a recent radiobiological study confirmed this hypothesis, and dose escalation of the esophageal GTV showed potential of increasing tumor control with acceptable lung or heart toxicity (21). Since we found GTV may serve as a good prognostic factor for ESCC patients treated with definitive radiotherapy, we could prescribe more radiation doses to those patients who have larger tumor bulk with endurable normal tissue toxicities.
Conclusions
Our study showed that tumor volume is a prognostic factor for ESCC patients treated with definitive radiotherapy. The optimum cut-off point for tumor volume is 39.41 cm3 in predicting survival prognosis in patients with ESCC. Larger prospective studies are needed to confirm these preliminary results and determine the optimum cut-off point.
Acknowledgements
Funding: This study was financially supported by the National Natural Science Foundation of China Research, China (grant number 21172043).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. 10.3322/caac.21208 [DOI] [PubMed] [Google Scholar]
- 2.Feng JF, Huang Y, Lu WS, et al. Preoperative platelet count in esophageal squamous cell carcinoma: is it a prognostic factor? Langenbecks Arch Surg 2013;398:1115-122. 10.1007/s00423-013-1111-4 [DOI] [PubMed] [Google Scholar]
- 3.Semrau R, Herzog SL, Vallböhmer D, et al. Prognostic factors in definitive radiochemotherapy of advanced inoperable esophageal cancer. Dis Esophagus 2012;25:545-54. 10.1111/j.1442-2050.2011.01286.x [DOI] [PubMed] [Google Scholar]
- 4.Cincibuch J, Neoral C, Aujeský R, et al. Prognostic factors in patients with esophageal carcinoma treated with chemoradiation: single center experience. Hepatogastroenterology 2010;57:1145-9. [PubMed] [Google Scholar]
- 5.Chen CZ, Chen JZ, Li DR, et al. Long-term outcomes and prognostic factors for patients with esophageal cancer following radiotherapy. World J Gastroenterol 2013;19:1639-44. 10.3748/wjg.v19.i10.1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohanes P, Yang D, Chhibar RS, et al. Influence of sex on the survival of patients with esophageal cancer. J Clin Oncol 2012;30:2265-72. 10.1200/JCO.2011.38.8751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander BM, Othus M, Caglar HB, et al. Tumor volume is a prognostic factor in non-small-cell lung cancer treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys 2011;79:1381-7. 10.1016/j.ijrobp.2009.12.060 [DOI] [PubMed] [Google Scholar]
- 8.Gobbi PG. Tumor burden in Hodgkin's lymphoma: much more than the best prognostic factor. Crit Rev Oncol Hematol 2014;90:17-23. 10.1016/j.critrevonc.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Zhu M, Zhang Z, et al. A NEIL1 single nucleotide polymorphism (rs4462560) predicts the risk of radiation-induced toxicities in esophageal cancer patients treated with definitive radiotherapy. Cancer 2013;119:4205-11. 10.1002/cncr.28338 [DOI] [PubMed] [Google Scholar]
- 10.Greene FL. American Joint Committee on Cancer. AJCC cancer staging atlas. New York: Springer, 2006. [Google Scholar]
- 11.Van Meter EM, Garrett-Mayer E, Bandyopadhyay D. Dose-finding clinical trial design for ordinal toxicity grades using the continuation ratio model: an extension of the continual reassessment method. Clin Trials 2012;9:303-13. 10.1177/1740774512443593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999;281:1623-7. 10.1001/jama.281.17.1623 [DOI] [PubMed] [Google Scholar]
- 13.Rice TW. Esophageal Cancer Staging. Korean J Thorac Cardiovasc Surg 2015;48:157-63. 10.5090/kjtcs.2015.48.3.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rice TW, Rusch VW, Ishwaran H, et al. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer 2010;116:3763-73. [DOI] [PubMed] [Google Scholar]
- 15.Guo R, Sun Y, Yu XL, et al. Is primary tumor volume still a prognostic factor in intensity modulated radiation therapy for nasopharyngeal carcinoma? Radiother Oncol 2012;104:294-9. 10.1016/j.radonc.2012.09.001 [DOI] [PubMed] [Google Scholar]
- 16.Créhange G, Bosset M, Lorchel F, et al. Tumor volume as outcome determinant in patients treated with chemoradiation for locally advanced esophageal cancer. Am J Clin Oncol 2006;29:583-7. 10.1097/01.coc.0000242346.25229.48 [DOI] [PubMed] [Google Scholar]
- 17.Boggs DH, Hanna A, Burrows W, et al. Primary Gross Tumor Volume is an Important Prognostic Factor in Locally Advanced Esophageal Cancer Patients Treated with Trimodality Therapy. J Gastrointest Cancer 2015;46:131-7. 10.1007/s12029-015-9699-y [DOI] [PubMed] [Google Scholar]
- 18.Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol 2002;20:1167-74. 10.1200/JCO.20.5.1167 [DOI] [PubMed] [Google Scholar]
- 19.Hurmuzlu M, Monge OR, Smaaland R, et al. High-dose definitive concomitant chemoradiotherapy in non-metastatic locally advanced esophageal cancer: toxicity and outcome. Dis Esophagus 2010;23:244-52. 10.1111/j.1442-2050.2009.00999.x [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z, Liao Z, Jin J, et al. Dose-response relationship in locoregional control for patients with stage II-III esophageal cancer treated with concurrent chemotherapy and radiotherapy. Int J Radiat Oncol Biol Phys 2005;61:656-64. 10.1016/j.ijrobp.2004.06.022 [DOI] [PubMed] [Google Scholar]
- 21.Warren S, Partridge M, Carrington R, et al. Radiobiological determination of dose escalation and normal tissue toxicity in definitive chemoradiation therapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2014;90:423-9. 10.1016/j.ijrobp.2014.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]