Abstract
Purpose
Cardiovascular surgery-associated acute kidney injury (AKI-CS) contributes to mortality and morbidity. However, risk factors accelerating its development are unclear. We identified risk factors for AKI-CS in patients with cardiopulmonary bypass in the hospital surgical intensive care unit to predict and minimize renal complication in future cardiac surgery.
Methods
We analyzed data from 14 case-control studies published prior to June 2014 and indexed in Science Citation Index, PubMed, and other databases to determine the major risk factors for AKI-CS.
Results
Analyzed risk factors were divided into three groups: preoperative, intraoperative and postoperative. Preoperative factors included: age (OR, 4.87; 95% CI, 3.50-6.24), NYHA class III/IV (OR, 2.53; 95% CI, 1.32-4.86), hypertension (OR, 1.68; 95% CI, 1.44-1.97), preoperative creatinine (OR, 0.66; 95% CI, 0.18-1.14), peripheral vascular disease (OR, 1.31 95% CI, 1.09-1.57), respiratory system disease (OR, 1.29; 95% CI, 1.10-1.50), diabetes mellitus (OR, 1.52; 95% CI, 1.07-2.16), and cerebrovascular disease (OR, 2.13; 95% CI, 1.11-4.09). Intraoperative factors were: cardiopulmonary bypass time (OR, 33.78; 95% CI, 23.15-44.41), aortic clamping time (OR, 13.24; 95% CI, 7.78-18.69), use of intra-aortic balloon pump (OR, 4.44; 95% CI, 2.37-8.30), and type of surgery (OR, 1.01; 95% CI, 0.43-2.39). Postoperative factors were: infection (OR, 3.58; 95% CI, 1.43-8.97), redo operation (OR, 2.57; 95% CI, 1.75-3.78), emergency surgery (OR, 4.76; 95% CI, 3.05-7.43), and low cardiac output (OR, 2.30; 95% CI, 1.05-5.04).
Conclusions
Our results support that preoperative, intraoperative, and postoperative factors are associated with AKI-CS. Ejection fraction, BMI, acute myocardial infarction, type of surgery, and congestive heart failure were not absolutely associated with AKI.
Key Words: Risk factors, Acute kidney injury, Cardiovascular surgery, Cardiopulmonary bypass, Meta-analysis
Introduction
Cardiovascular surgery-associated acute kidney injury (AKI-CS) is a complication contributing significantly to mortality, lengthy hospitalization, high costs, and patient and family suffering [1]. The reported prevalence of acute kidney injury (AKI) associated with cardiopulmonary bypass (CPB) varies between 5 and 30% [2,3], with a mortality of 14.5-64% [4,5]. Subsequent dialysis is required in approximately 1-5% of cases [6] and is associated with an increased incidence of infection and length of stay in the intensive care unit [7,8,9]. Moreover, along with further deterioration of the kidney function, multiple organ dysfunction syndrome (MODS) arises [10].
The pathogenesis of AKI-CS includes multiple inflammatory, hemodynamic, and nephrotoxic factors that overlap, leading to kidney injury [11]. Despite continuous advances in surgery, management, monitoring, and hemodialysis technology, postoperative renal injury incidence rates have not significantly decreased [12], and thus, avoidance of AKI-CS is a focus of intense research.
Early studies revealed that renal function deterioration after surgery included preoperative elevated serum creatinine levels, types of cardiac procedures, a history of diabetes mellitus, CPB time, aortic clamping time, postoperative infection, and low cardiac output, among others [13,14,15,16,17,18,19,20,21,22,23,24,25,26]. However, because of differences in study characteristics, research data are prone to error, and accurate information is still lacking. Thus, we performed a meta-analysis to identify risk factors for AKI-CS, which can be used to predict and minimize renal complications following future cardiac surgery.
Methods
Selection Criteria and Search Strategy
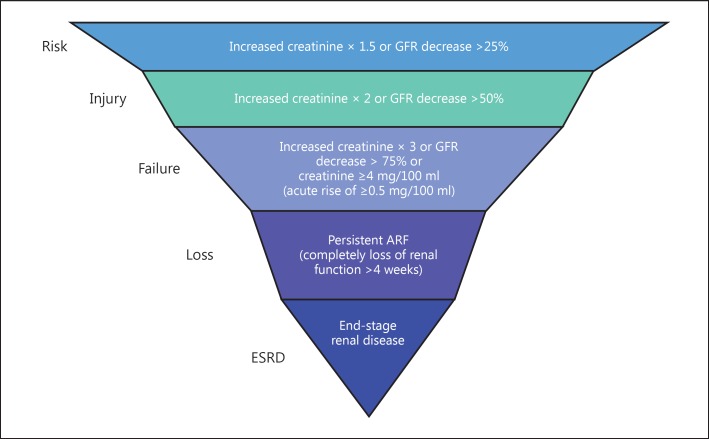
We located studies from June to August 2014 and review articles from the Web of Science databases, PubMed, Google Scholar, Medline, the Cochrane Library, Embase, and Science Citation Index for articles written in either Chinese or English. Two independent reviewers (Q.Y. and K.L.) carried out the literature search. We used the search term ‘acute kidney injury’ or ‘acute renal failure’ or ‘acute renal insufficiency’, ‘cardiovascular surgery’, ‘bypass’, and ‘factor’. Articles were screened according to the RIFLE [27] hierarchical diagnosis standard proposed by the Acute Dialysis Quality Initiative Group of America [28] in 2004 (fig. 1), and the criteria selected in our study were injury, failure, loss and end-stage renal disease. To be eligible for analysis, studies must have had data from individual cases in which risk factors contributed to renal injury and available corresponding author information for acquiring additional data.
Fig. 1.
The RIFLE classification criteria for serum creatinine. RIFLE criteria were used to evaluate the ability of renal function, which was divided into five stages: risk, injury, failure, loss, and end stage. Each phase is defined in the figure. GFR = Glomerular filtration rate; ARF = acute renal failure; ESRD = end-stage renal disease.
Inclusion and Exclusion Criteria
Articles meeting the following criteria were included: those which (a) focused on risk factors for AKI after CPB; (b) were case-controlled; (c) provided sufficient information to calculate case numbers and risk estimates with 95% confidence intervals (CIs) and odds ratios (ORs), and (d) where diagnostic criteria were in accordance with the RIFLE classification criteria including injury, failure, loss and end-stage renal disease [29] (fig. 1). Studies were excluded if (1) they provided data but no means of calculating AKI incidence; (2) they were derived from non-peer-reviewed sources; (3) they were animal experiments; (4) the diagnosis was unclear, or (5) assessing specific causes of AKI such as rapidly progressive glomerulonephritis, hemolytic-uremic syndrome, or hepatorenal syndrome. Studies must have excluded end-stage kidney disease patients. We selected the most recent study with the largest number of participants for multiple studies with the overlapping data published.
Data Extraction
The following data were screened from each study: first author's surname, year, country, participant characteristics, source (hospital or community), study design (case-controlled), ORs, 95% CIs, and adjusted covariates, among other information. If data could be acquired from the literature search results, they were extracted into 2 × 2 tables by two independent reviewers (Q.Y. and K.L.), based on the inclusion criteria described above. Continuous data from AKI and control groups are reported as means ± standard deviations, and dichotomous data are reported as number of cases. If information was incomplete or unclear, reviewers would contact the original authors for verification. Disagreements were resolved through discussion between the two reviewers (table 1).
Table 1.
Characteristics of 14 studies analyzing risk factors associated with cardiac surgery with CPB
| First author [Ref.] | Year | Country | Race | Source | Study type | Sample size (case/control) | Risk factor | Quality evaluation |
|---|---|---|---|---|---|---|---|---|
| Suen [13] | 1998 | China Hong Kong | Asia | hospital | case-controlled | 67/380 | 1, 2, 7, 11, 13, 14, 16, 20 | 6 |
| Sirvinskas [14] | 2008 | US | US | hospital | case-controlled | 19/160 | 1, 6, 10, 12 | 6 |
| Haase [15] | 2012 | English | Europe | hospital | case-controlled | 179/741 | 1–11, 15, 16, 20 | 6 |
| Rodrigues [16] | 2009 | Brazil | South America | hospital | case-controlled | 78/691 | 2, 4, 6–10, 13, 14, 16, 17, 19, 21 | 7 |
| Ilknur [17] | 2005 | Turkey | Asia | hospital | case-controlled | 168/14,269 | 2, 3, 6, 10–13, 15, 16, 18, 20 | 7 |
| Bove [18] | 2004 | Italy | Europe | hospital | case-controlled | 171/4,897 | 1, 2, 5, 6, 8, 10, 11, 14–16 | 7 |
| Thakar [19] | 2005 | US | US | hospital | case-controlled | 269/15,569 | 1, 2, 6, 8–11, 14–16, 18, 19 | 6 |
| Loef [20] | 2005 | Holland | Europe | hospital | case-controlled | 145/698 | 1–7, 9–11, 14–20 | 7 |
| Lin [21] | 2009 | China | Asia | hospital | case-controlled | 340/4,760 | 10, 12 | 6 |
| Han [22] | 2006 | China | Asia | hospital | case-controlled | 108/152 | 7, 10, 12, 15 | 6 |
| Yang [23] | 2013 | China | Asia | hospital | case-controlled | 83/129 | 1–4, 6, 7, 11, 17–19, 21 | 6 |
| Englberger [24] | 2013 | Mayo | Europe | hospital | case-controlled | 115/836 | 2–4, 6, 8–10, 12, 13, 17–20, 21 | 7 |
| Karkouti [25] | 2009 | Canada | US | hospital | case-controlled | 347/3,460 | 1–6, 8–11, 13, 15, 16, 19 | 7 |
| Landoni [26] | 2007 | Italy | Europe | hospital | case-controlled | 68/3,035 | 2, 5, 6, 8, 10, 11, 13, 15, 16, 19, 20 | 7 |
Risk factors: 1 = any type of cardiac surgery; 2 = diabetes mellitus; 3 = hypertension; 4 = acute myocardial infarction; 5 = low cardiac output; 6 = age; 7 = NYHA class III/IV; 8 = respiratory system disease; 9 = peripheral vascular disease; 10 = CPB time; 11 = use of IABP; 12 = aortic clamping time; 13 = postoperative infection; 14 = congestive heart failure; 15 = redo operation; 16 = emergency operation; 17 = BMI; 18 = preoperative creatinine; 19 = cerebrovascular disease; 20 = mortality; 21 = EF.
Quality Evaluation
We evaluated the studies to determine their quality based on the Newcastle-Ottawa quality assessment scale [30]. A ‘star system’ was used to judge data quality based on selection, comparability, and exposures and to calculate scores. Scores with 0-9 stars were summed to compare study quality. Two reviewers (Q.Y. and K.L.) independently cross-checked the study quality and assessed study bias. An open discussion was held to confirm scores of studies that caused disagreements between the reviewers.
Statistical Methods
All statistical tests were performed using the Cochrane Collaboration's Revman 5.1. Extensive effort was made to eliminate duplicate data and to include all studies published to date. Measurement data regarding average standard deviations and publication bias in outcomes were estimated and managed using standard methodology. We classified epidemiological studies of the relationships between AKI and risk factors as case-control studies. The association between AKI and risk factors was evaluated by comparing the ORs and the corresponding 95% CIs between exposed and nonexposed groups.
Analysis of Clinical Heterogeneity
Study heterogeneity was measured using the χ2-based Q test and I2 statistics. We used the I2 values to quantify the proportion of observed inconsistency across study results not explained by chance [31]; p < 0.05 and I2 >50% indicated severe heterogeneity. We proposed predefined subgroup analyses (preoperative, intraoperative, and postoperative) based on our a priori hypotheses to explain potential heterogeneity across studies on the strength of association due to different research types considering a significant interaction when p < 0.05. In addition, we used the Newcastle-Ottawa quality assessment scale scores and sensitivity analyses to evaluate the impact of studies with poor methodological quality. Comparisons of risk estimates between subgroups and sensitivity analyses were made with a test of interaction. Publication bias for each of the study groups was assessed with a funnel plot. A two-tailed test was used to assess funnel plot asymmetry (significance was set at the p < 0.05 level) [32].
Results
Study Descriptions
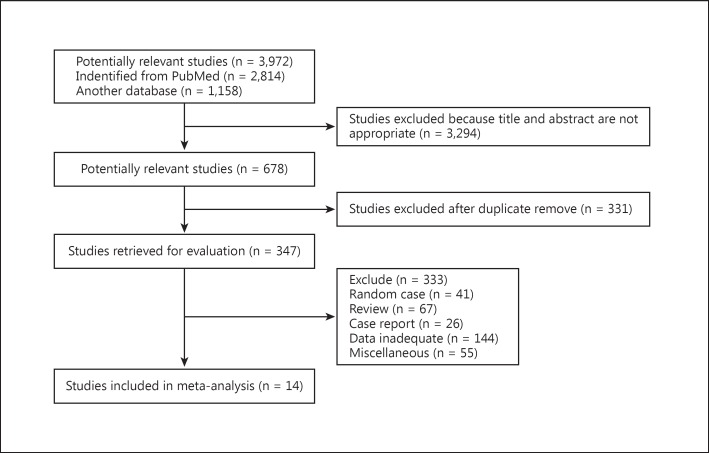
The search process is shown in figure 2. First, 3,972 potentially relevant papers published up to June 2014 were systematically ascertained through the previously mentioned databases. Next, 3,294 studies were excluded because they either failed to meet the inclusion criteria or did not provide adequate information for our study, as evidenced either by title or abstract. Among the remaining 678 articles, 331 articles were excluded because they were duplicate reports. We received full-text reviews of the remaining 347 articles; 333 articles were excluded because they were not complete full texts or they were reviews, case reports, miscellaneous reports, or the data were inadequate. Finally, 14 case-controlled studies [13,14,15,16,17,18,19,20,21,22,23,24,25,26]were used for our meta-analysis. Detailed characteristics of the 14 studies are listed in table 1. A total of 2,157 cases and 49,777 controls were included. Results ranged from a star rating of 6-9 (mean = 7), with a higher value indicating better methodology (table 2).
Fig. 2.
Flowchart of article screening and selection process.
Table 2.
Assessment of study quality
| First author [Ref.] | Selection |
Comparability |
Exposure |
Score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5a | 5b | 6 | 7 | 8 | ||
| Charuhas [19] | yes | yes | no | no | yes | yes | yes | yes | no | 6 |
| Ilknur [17] | yes | yes | no | yes | yes | yes | yes | yes | no | 7 |
| Sirvinskas [14] | yes | yes | no | yes | yes | yes | yes | no | no | 6 |
| Haase [15] | yes | yes | no | no | yes | yes | yes | yes | no | 6 |
| Loef [20] | yes | yes | no | yes | yes | yes | yes | yes | no | 7 |
| Rodrigues [16] | yes | yes | no | yes | yes | yes | yes | yes | no | 7 |
| Suen [13] | yes | yes | no | no | yes | yes | yes | yes | no | 6 |
| Bove [18] | yes | yes | no | yes | yes | yes | yes | yes | no | 7 |
| Lin [21] | yes | yes | no | no | yes | yes | yes | yes | no | 6 |
| Han [22] | yes | yes | no | yes | no | yes | no | yes | no | 5 |
| Yang [23] | yes | yes | no | yes | no | yes | yes | yes | no | 6 |
| Englberger [24] | yes | yes | no | yes | yes | yes | yes | yes | no | 7 |
| Karkouti [25] | yes | yes | no | yes | yes | yes | yes | yes | no | 7 |
| Landoni [26] | yes | yes | no | yes | yes | yes | yes | yes | no | 7 |
For case-control studies: 1 = cases were independently validated; 2 = cases are consecutive or representative of a population; 3 = community controls; 4 = controls had no history of renal insufficiency; 5a = study controlled for age and CPB time; 5b = study controlled for any additional factor(s); 6 = ascertainment of exposure by secure record or structured interview where the interviewer was blinded to case/control status; 7 = same method of ascertainment for cases and controls; 8 = same nonresponse rate for cases and controls.
Subgroup Analyses
Analysis of Preoperative Indices
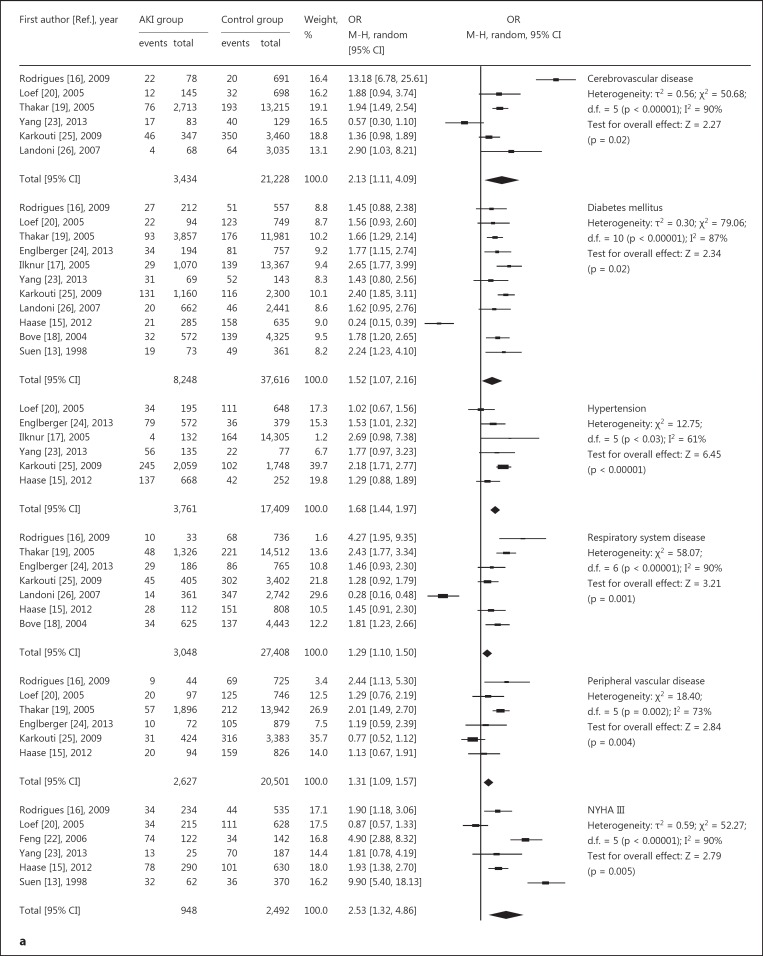
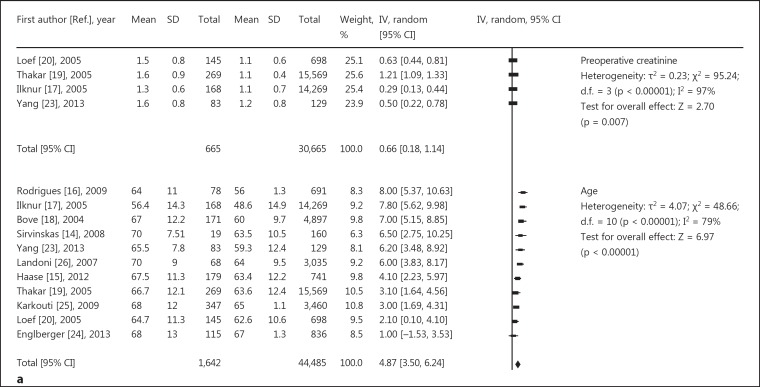
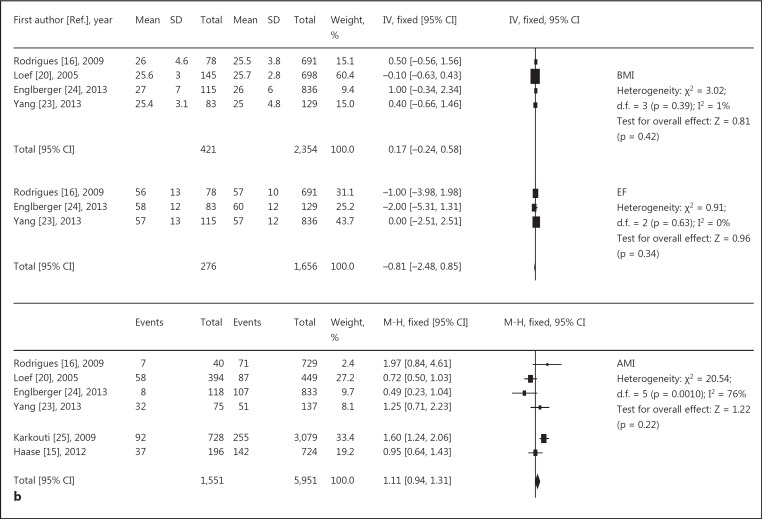
The following data are represented in figure 3a, b: four studies [16,20,23,24] suggested no association between BMI and AKI, and three studies [16,23,24] revealed no association between ejection fraction (EF) and AKI. Six studies confirmed no association between acute myocardial infarction and AKI, and eleven studies [14,15,16,17,18,19,20,23,24,25,26] suggested that age was a significant risk factor. In general, the older the patient, the greater the incidence of AKI. The results for preoperative heart function [13,15,16,20,22,23] and serum creatinine level [17,19,20,23] was also a significant risk factor for AKI. Overall, besides the reason of surgery, basic diseases and complications such as hypertension [15,17,20,23,24,25], peripheral vascular disease [15,16,19,20,24,25], respiratory system disease [15,16,18,19,24,25,26], and cerebrovascular disease [16,19,20,23,25,26] were also related to AKI. More severe lesions were associated with a greater incidence of AKI.
Fig. 3.
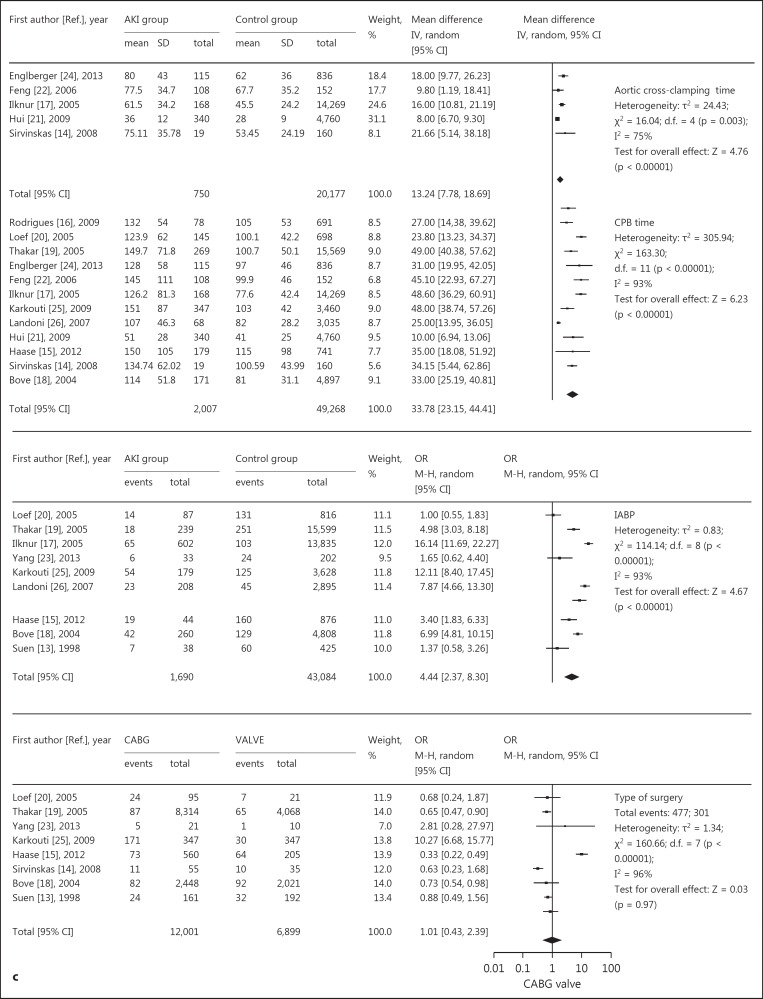
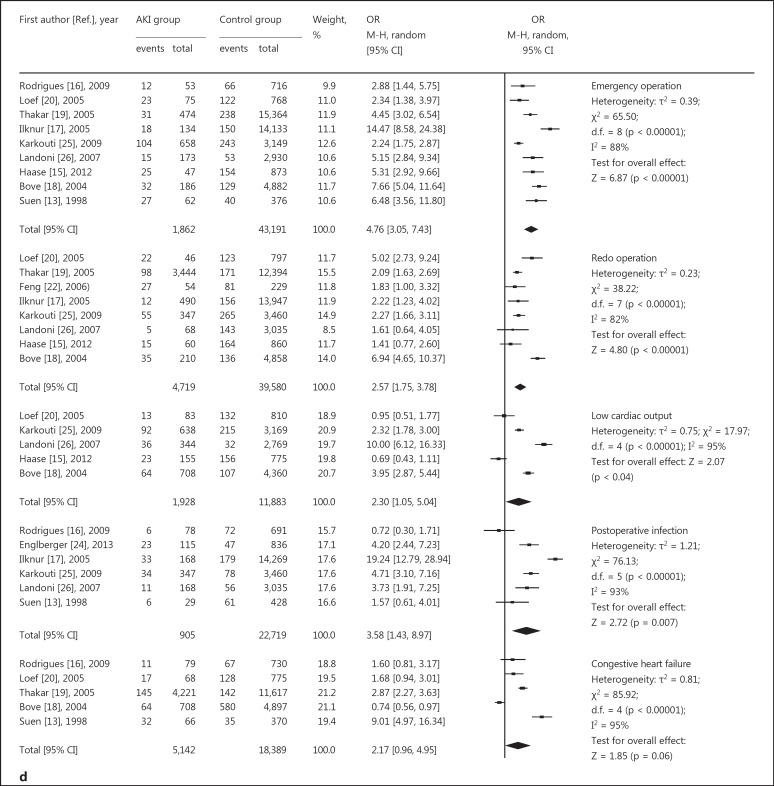
Relative risk of AKI and cardiac surgery during CPB. a, b Risk factors for preoperative basic diseases, showing positive (a) and negative results (b). c Risk factors for intraoperative issues. d Risk factors for postoperative complications. CABG = Coronary artery bypass grafting.
Analysis of Intraoperative Indices
The following data are depicted in figure 3c: eight studies [13,14,15,18,19,20,23,25] compared coronary artery bypass grafting and valve operations, and suggested no association between cardiac surgery types and AKI. The CPB times [14,15,16,17,18,19,20,21,22,24,25,26]for subjects with AKI and controls in all studies were reported in minutes. Nine studies [13,15,17,18,19,20,23,25,26] confirmed that the use of an intra-aortic balloon pump (IABP) was a risk factor, and a greater duration of IABP use correlated with a higher rate of AKI-CS. Five studies [14,17,21,22,24] revealed that aortic clamping time was a significant risk factor for AKI, with longer aortic clamping times associated with a higher incidence of AKI-CS.
Analysis of Postoperative Indices
These data are given in figure 3d. Five studies [13,16,18,19,20]suggested no association between congestive heart failure and AKI. Six studies [13,16,17,24,25,26]confirmed that severe postoperative infection correlated with a greater likelihood of AKI. To summarize the reported data, emergency operations [13,15,16,17,18,19,20]and redo operations [15,17,18,19,20,22,25,26] were also independent risk factors for AKI-CS. Meanwhile, the risk of mortality [13,15,17,20,24,26] was statistically higher in the AKI-CS groups.
Publication Bias and Sensitivity Analysis of the Studies
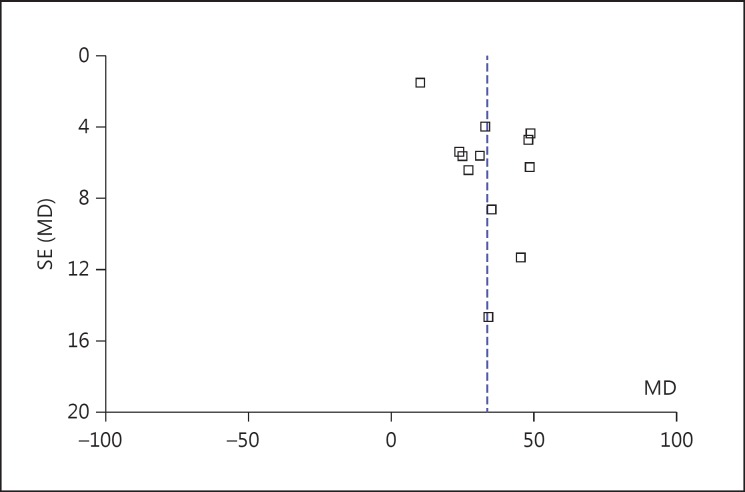
We depict the data related to publication bias and sensitivity (table 3) using reported CPB time funnel plots (fig. 4). The shape of the funnel plots was nearly symmetrical on both sides of the perpendicular line (real value), indicating no obvious publication bias for these studies. To investigate data reliability, fixed-effects and random-effects models were applied to measure sensitivity. The differences in the standardized means and the 95% CIs between the two methods were small. Therefore, both sensitivity testing and the publication bias analysis indicated that the results of our meta-analysis were reliable.
Table 3.
Sensitivity analysis
| Risk factor | Number of studies included | OR or mean difference [95% CI] | p value for interaction | Inconsistency I2, % |
|---|---|---|---|---|
| Preoperative | ||||
| Age | 11 | 4.87 [3.50, 6.24] | <0.00001 | 79 |
| Diabetes | 11 | 1.52 [1.07, 2.16] | 0.02 | 87 |
| Hypertension | 6 | 1.68 [1.44, 1.97] | <0.00001 | 61 |
| Cerebrovascular disease | 6 | 2.13 [1.11, 4.09] | 0.02 | 90 |
| Respiratory system disease | 7 | 1.29 [1.10, 1.50] | 0.001 | 90 |
| Peripheral vascular disease | 6 | 1.31 [1.09, 1.57] | 0.004 | 73 |
| NYHA III/IV | 6 | 2.53 [1.32, 4.86] | 0.005 | 90 |
| Serum creatinine | 4 | 0.66 [0.18, 1.14] | 0.007 | 97 |
| BMI | 4 | 0.17 [–0.24, 0.58] | 0.42 | 1 |
| EF | 3 | −0.81 [–2.48, 0.85] | 0.34 | 0 |
| Acute myocardial infarction | 6 | 1.11 [0.94, 1.31] | 0.22 | 76 |
| Operative | ||||
| Aortic cross-clamping time | 5 | 13.24 [7.78, 18.69] | <0.00001 | 75 |
| CPB time | 12 | 33.78 [23.15, 44.41] | <0.00001 | 93 |
| Use of IABP | 9 | 4.44 [2.37, 8.30] | <0.00001 | 93 |
| Type of surgery | 8 | 1.01 [0.43, 2.39] | 0.97 | 96 |
| Postoperative | ||||
| Infection | 6 | 3.58 [1.43, 8.97] | 0.007 | 93 |
| Emergency operation | 9 | 4.76 [3.05, 7.43] | <0.00001 | 88 |
| Redo operation | 8 | 2.57 [1.75, 3.78] | <0.00001 | 82 |
| Low cardiac output | 5 | 2.30 [1.05, 5.004] | 0.04 | 95 |
| Congestive heart failure | 5 | 2.17 [0.96, 4.96] | 0.06 | 95 |
| Mortality | 6 | 0.19 [0.08, 0.30] | 0.0004 | 93 |
Fig. 4.
Funnel plot for CPB time. SE = Standard error; MD = mean difference.
Discussion
AKI-CS is a serious complication of CPB-induced pathophysiologic changes, and is significantly associated with higher mortality, length of stay in the intensive care unit, and health care costs [4,33,34]. According to a recent report, nearly half of the estimated 7,406 patients who died after cardiac surgery had a diagnosis of AKI [35]. Many factors contribute to AKI after cardiac surgery, but the pathological process is complex and may include (a) renal hypoperfusion ischemic injury due to CPB duration and hemodynamic changes; (b) inflammation caused by the CPB pump, as well as the formation of free radicals, complement activation, and cytotoxicity aggravate renal injury; (c) thrombosis, platelet aggregation, and dislodged aortic athermanous plaques, leading to renal ischemia, and (d) the use of nephrotoxic drugs during surgery. Ischemia-reperfusion injury may be associated with pathologic features of acute tubular necrosis. Thus, even if the postoperative serum creatinine level was only elevated by 20%, the patient's renal recovery may be compromised, perhaps leading to the need for renal dialysis [35].
Preoperative Disease Enhanced AKI-CS Susceptibility
Most patients undergoing CPB have risk factors that predispose to renal failure [36], such as advanced age, respiratory system disease, serum creatinine levels, and NYHA class III/IV disease. Our meta-analysis indicated that BMI, EF, and acute myocardial infarction are not immediately related to AKI development. However, respiratory system disease, such as chronic obstructive pulmonary disease (COPD), was confirmed to be an independent predictor of AKI-CS [11,12]. Small airway obstruction is the basic link in the pathogenesis of COPD, while patients with COPD are more prone to episodes of severe hypoxemia-hypercapnia during the perioperative period, which may worsen respiratory dysfunction and lead to pulmonary infection, and decreased cardiac and renal function [37]. AKI requiring dialysis was associated with conditions that cause occult renal ischemia, such as reduced EF, peripheral vascular disease, and other conditions [5]. Stallwood et al. [38] reported that peripheral vascular disease is a risk factor for AKI. Advanced age correlates with a reduction in the glomerular filtration rate, which often begins after the fourth decade of life [17]. Elderly patients with physiological renal dysfunction and decreased renal reserve and compensatory ability associated with various chronic diseases may have less tolerance to surgery and CPB operations and may be more prone to MODS.
CPB and Aortic Clamping Time Is Critical for AKI-CS
AKI-CS is associated with a significant increase in postoperative mortality, especially if patients require renal replacement therapy [39]. Additionally, CPB obstructs oxygen transport, and within minutes inflammatory mediators are released [5] via endothelial cell and leukocyte activation, which can contribute to MODS (primarily cardiac or renal insufficiency) due to postoperative AKI. Meanwhile, mean arterial pressure often remains at a lower autoregulatory limit or descends below it, especially during periods of hemodynamic instability [40]. In such patients with preexisting kidney function impairment, even a mean arterial pressure within the normal range could be implicated in AKI-CS [41]. Prolonged aortic clamping time increases the risk of low cardiac output, and the exact ‘safe’ period of time for clamping remains undefined. Reports suggest that risk increases 10- to 15-fold after 60 min of CPB [42,43]. Our results support this finding (fig. 3c). Thus, it appears that shorter CPB and aortic cross-clamping times and lower circulation perfusion pressure could reduce perioperative inflammatory response measures. Long-term use of IABP causes thrombus formation, which can precipitate renal ischemia, even though it is considered to be a device that augments perfusion pressure and thus considerably improves perfusion.
Effective Postoperative Management Can Prevent AKI-CS
Postoperative factors associated with kidney damage after cardiac surgery include infection, redo operations, emergency surgery, congestive heart failure, and low cardiac output. Explanatory mechanisms may include exacerbation of hemodynamic instability and surgical trauma [3]. Surgical reexploration was also associated with red blood cell transfusion and anemia. Congestive heart failure is also generally considered a risk factor, but there was insufficient evidence from our meta-analysis to confirm this. Identifying patients at high risk is a logical first step in preventing it. Subsequently, targeting adjusted risk factors should be a therapeutic aim for minimizing the incidence of AKI-CS.
Study Limitations
This study still has some limitations. First, meta-analyses are inherently weaker when heterogeneous data sets are combined, especially with respect to individual factors; therefore, the possibility exists that the strength of association may be weaker with a multifactorial regression analysis [44]. Another limitation lies in the fact that some potential factors reported in studies could not be evaluated because of a lack of sufficient clinical data, such as atrial fibrillation, preoperative hemoglobin, and low systolic pressure, and this may account for a source of heterogeneity.
In conclusion, this meta-analysis strongly supports that AKI-CS is associated with advanced age, hypertension, preoperative creatinine level, peripheral vascular disease, respiratory system disease, diabetes mellitus, cerebrovascular disease, low cardiac output, NYHA class III/IV, CPB time, aortic clamping time, use of IABP, infection, emergency surgery, and reoperation. Observations from several studies indicate that there are still many unresolved issues concerning AKI-CS. Future research is warranted to standardize AKI risk factors and improve outcome prediction for this important clinical complication after major cardiac surgery.
Disclosure Statement
We declare that all authors have no conflicts of interest.
References
- 1.Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant. 2008;23:1970–1974. doi: 10.1093/ndt/gfm908. [DOI] [PubMed] [Google Scholar]
- 2.Parolari A, Pesce LL, Pacini D, et al. Risk factors for perioperative acute kidney injury after adult cardiac surgery: role of perioperative management. Ann Thorac Surg. 2012;93:584–591. doi: 10.1016/j.athoracsur.2011.09.073. [DOI] [PubMed] [Google Scholar]
- 3.D'Onofrio A, Cruz D, Bolgan I, Auriemma S, Cresce GD, Fabbri A, Ronco C. RIFLE criteria for cardiac surgery-associated acute kidney injury: risk factors and outcomes. Congest Heart Fail. 2010;16(suppl 1):S32–S36. doi: 10.1111/j.1751-7133.2010.00170.x. [DOI] [PubMed] [Google Scholar]
- 4.Bagshaw SM, George C, Bellomo R. Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care. 2008;12:R47. doi: 10.1186/cc6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chertow GM, Lazarus JM, Christiansen CL, Cook EF, Hammermeister KE, Grover F, et al. Preoperative renal risk stratification. Circulation. 1997;95:878–884. doi: 10.1161/01.cir.95.4.878. [DOI] [PubMed] [Google Scholar]
- 6.Billings FT, 4th, Pretorius M, Siew ED, Yu C, Brown NJ. Early postoperative statin therapy is associated with a lower incidence of acute kidney injury after cardiac surgery. J Cardiothorac Vasc Anesth. 2010;24:913–920. doi: 10.1053/j.jvca.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thakar CV, Yared JP, Worley S, Cotman K, Paganini EP. Renal dysfunction and serious infections after open-heart surgery. Kidney Int. 2003;64:239–246. doi: 10.1046/j.1523-1755.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- 8.Arora P, Kolli H, Nainani N, Nader N, Lohr J. Preventable risk factors for acute kidney injury in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2012;26:687–697. doi: 10.1053/j.jvca.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 10.Chukwuemeka A, Weisel A, Maganti M, Nette AF, Wijeysundera DN, Beattie WS, et al. Renal dysfunction in high-risk patients after on-pump and off-pump coronary artery bypass surgery: a propensity score analysis. Ann Thorac Surg. 2005;80:2148–2153. doi: 10.1016/j.athoracsur.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 11.Kochi AC, Martins AS, Balbi AL, Moraes E, Silva MA, Lima MC, Martins LC, et al. Preoperative risk factors for the development of acute renal failure in cardiac surgery. Rev Bras Cir Cardiovasc. 2007;22:33–40. doi: 10.1590/s0102-76382007000100009. [DOI] [PubMed] [Google Scholar]
- 12.Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1:19–32. doi: 10.2215/CJN.00240605. [DOI] [PubMed] [Google Scholar]
- 13.Suen WS, Mok CK, Chiu SW, Cheung KL, Lee WT, Cheung D, et al. Risk factors for development of acute renal failure (ARF) requiring dialysis in patients undergoing cardiac surgery. Angiology. 1998;49:789–800. doi: 10.1177/000331979804900902. [DOI] [PubMed] [Google Scholar]
- 14.Sirvinskas E, Andrejaitiene J, Raliene L, Nasvytis L, Karbonskiene A, Pilvinis V, et al. Cardiopulmonary bypass management and acute renal failure: risk factors and prognosis. Perfusion. 2008;23:323–327. doi: 10.1177/0267659109105251. [DOI] [PubMed] [Google Scholar]
- 15.Haase M, Bellomo R, Story D, Letis A, Klemz K, Matalanis G, et al. Effect of mean arterial pressure, haemoglobin and blood transfusion during cardiopulmonary bypass on post-operative acute kidney injury. Nephrol Dial Transplant. 2012;27:153–160. doi: 10.1093/ndt/gfr275. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues AJ, Evora PR, Bassetto S, Alves Júnior L, Scorzoni Filho A, Araújo WF, Vicente WV. Risk factors for acute kidney injury after cardiac surgery. Rev Bras Cir Cardiovasc. 2009;24:441–446. doi: 10.1590/s0102-76382009000500003. [DOI] [PubMed] [Google Scholar]
- 17.Ilknur B, Ahmet A, Mehmet AO, et al. Acute renal failure following open heart surgery: risk factors and prognosis. Perfusion. 2005;20:317–322. doi: 10.1191/0267659105pf829oa. [DOI] [PubMed] [Google Scholar]
- 18.Bove T, Calabro MG, Landoni G, et al. The incidence and risk of acute renal failure after cardiac surgery. J Cardiothorac Vasc Anesth. 2004;18:442–445. doi: 10.1053/j.jvca.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005;16:162–168. doi: 10.1681/ASN.2004040331. [DOI] [PubMed] [Google Scholar]
- 20.Loef BG, Epema AH, Smilde TD, et al. Immediate postoperative renal function deterioration in cardiac surgical patients predicts in-hospital mortality and long-term survival. J Am Soc Nephrol. 2005;16:195–200. doi: 10.1681/ASN.2003100875. [DOI] [PubMed] [Google Scholar]
- 21.Hui LG, Zeng N, et al. Analysis of factors of acute kidney injury after heart operation with cardiopulmonary bypass in 5,100 cases during operation risk. Cent South Univ. 2009;34:861–866. [Google Scholar]
- 22.Feng HG, Zhang JY, et al. Risk factors of acute renal failure after cardiac surgery. Chin J Emerg Med. 2006;15:625–628. [Google Scholar]
- 23.Yang J, Lu C, Yan L, Tang X, Li W, Yang Y, et al. The association between atherosclerotic renal artery stenosis and acute kidney injury in patients undergoing cardiac surgery. PLoS One. 2013;8:e64104. doi: 10.1371/journal.pone.0064104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Englberger L, Suri RM, Connolly HM, Li Z, Abel MD, Greason KL, et al. Increased risk of acute kidney injury in patients undergoing tricuspid valve surgery. Eur J Cardiothorac Surg. 2013;43:993–999. doi: 10.1093/ejcts/ezs515. [DOI] [PubMed] [Google Scholar]
- 25.Karkouti K, Wijeysundera DN, Yau TM, Callum JL, Cheng DC, Crowther M, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. 2009;119:495–502. doi: 10.1161/CIRCULATIONAHA.108.786913. [DOI] [PubMed] [Google Scholar]
- 26.Landoni G, Bove T, Crivellari M, Poli D, Fochi O, Marchetti C, et al. Acute renal failure after isolated CABG surgery: six years of experience. Minerva Anestesiol. 2007;73:559–565. [PubMed] [Google Scholar]
- 27.Englberger L, Suri RM, Li Z, Casey ET, Daly RC, Dearani JA, et al. Clinical accuracy of RIFLE and Acute Kidney Injury Network (AKIN) criteria for acute kidney injury in patients undergoing cardiac surgery. Crit Care. 2011;15:R16. doi: 10.1186/cc9960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int. 2008;73:538–546. doi: 10.1038/sj.ki.5002743. [DOI] [PubMed] [Google Scholar]
- 30.Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aune D, Vieira AR, Chan DS, Navarro Rosenblatt DA, Vieira R, Greenwood DC, et al. Height and pancreatic cancer risk: a systematic review and meta-analysis of cohort studies. Cancer Causes Control. 2012;23:1213–1222. doi: 10.1007/s10552-012-9983-0. [DOI] [PubMed] [Google Scholar]
- 33.Thakar CV, Liangos O, Yared JP, Nelson D, Piedmonte MR, Hariachar S, et al. ARF after open-heart surgery: influence of gender and race. Am J Kidney Dis. 2003;41:742–751. doi: 10.1016/s0272-6386(03)00021-0. [DOI] [PubMed] [Google Scholar]
- 34.Mangano CM, Diamondstone LS, Ramsay JG, Aggarwal A, Herskowitz A, Mangano DT. Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann Intern Med. 1998;128:194–203. doi: 10.7326/0003-4819-128-3-199802010-00005. [DOI] [PubMed] [Google Scholar]
- 35.Lenihan CR, Montez-Rath ME, Mora Mangano CT, Chertow GM, Winkelmayer WC. Trends in acute kidney injury, associated use of dialysis, and mortality after cardiac surgery, 1999 to 2008. Ann Thorac Surg. 2013;95:20–28. doi: 10.1016/j.athoracsur.2012.05.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parida S, Badhe AS. Cardiac surgery-associated acute kidney injury. J Anesth. 2013;27:433–446. doi: 10.1007/s00540-012-1523-2. [DOI] [PubMed] [Google Scholar]
- 37.Wouters EF. Management of severe COPD. Lancet. 2004;364:883–895. doi: 10.1016/S0140-6736(04)16984-5. [DOI] [PubMed] [Google Scholar]
- 38.Stallwood MI, Grayson AD, Mills K, Scawn ND. Acute renal failure in coronary artery bypass surgery: independent effect of cardiopulmonary bypass. Ann Thorac Surg. 2004;77:968–972. doi: 10.1016/j.athoracsur.2003.09.063. [DOI] [PubMed] [Google Scholar]
- 39.Kumar AB, Suneja M, Bayman EO, Weide GD, Tarasi M. Association between postoperative acute kidney injury and duration of cardiopulmonary bypass: a meta-analysis. J Cardiothorac Vasc Anesth. 2012;26:64–69. doi: 10.1053/j.jvca.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 40.Stafford-Smith M, Phillips-Bute B, Reddan DN, Black J, Newman MF. The association of epsilon-aminocaproic acid with postoperative decrease in creatinine clearance in 1,502 coronary bypass patients. Anesth Analg. 2000;91:1085–1090. doi: 10.1097/00000539-200011000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Abuelo JG. Normotensive ischemic acute renal failure. N Engl J Med. 2007;357:797–805. doi: 10.1056/NEJMra064398. [DOI] [PubMed] [Google Scholar]
- 42.Dehne MG, Sablotzki A, Mühling J, Dehne KL, Röhrig R, Hempelmann G. Renal effects of cardiopulmonary bypass in the elderly. Perfusion. 2002;17:205–209. doi: 10.1191/0267659102pf571oa. [DOI] [PubMed] [Google Scholar]
- 43.Bent P, Tan HK, Bellomo R, Buckmaster J, Doolan L, Hart G, et al. Early and intensive continuous hemofiltration for severe renal failure after cardiac surgery. Ann Thorac Surg. 2001;71:832–837. doi: 10.1016/s0003-4975(00)02177-9. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, Liu Y, Su L, Jiang SJ. Recipient-related clinical risk factors for primary graft dysfunction after lung transplantation: a systematic review and meta-analysis. PLoS One. 2014;9:3 e92773. doi: 10.1371/journal.pone.0092773. [DOI] [PMC free article] [PubMed] [Google Scholar]