Abstract
Background: Safety of oral sucrose, commonly used procedural analgesic in neonates, is questioned.
Aim: To evaluate the effect of sucrose analgesia, for repeated painful procedures, on short-term neurobehavioral outcome of preterm neonates.
Methods: Stable preterm neonates were randomized to receive either sucrose or distilled water orally, for every potentially painful procedure during the first 7 days after enrollment. Neurodevelopmental status at 40 weeks postconceptional age (PCA) measured using the domains of Neurobehavioral Assessment of Preterm Infants scale.
Results: A total of 93 newborns were analyzed. The baseline characteristics of the groups were comparable. No statistically significant difference was observed in the assessment at 40 weeks PCA, among the groups. Use of sucrose analgesia, for repeated painful procedures on newborns, does not lead to any significant difference in the short-term neurobehavioral outcome.
Keywords: procedural pain, neurobehavior, sucrose analgesia
INTRODUCTION
Clinicians have emerged from a period of relative neglect of neonatal pain and are now recognizing the need to alleviate pain effectively. The procedural pain is likely to be experienced by all at-risk newborns, more commonly by ill infants who need neonatal intensive care [1, 2]. Most of the potentially painful procedures are often carried out without any pain alleviation, and the pain thus caused can lead to decreased oxygenation, hemodynamic instability or increased intracranial pressure [3]. Recent research has shown that even short-term pain can have long-lasting negative effects [4]. Mounting evidence from preliminary studies raises a growing concern over the deleterious effects of repeated procedural pain on the neurodevelopment of neonatal intensive care unit graduates [5, 6].
Sucrose has been used as an analgesic in neonates and has found place in almost all international position papers and recommendations. However, concerns now are being raised over the risks associated with the use of multiple doses of sucrose especially in preterm neonates [7]. The cumulative effect of sucrose on neurobehavior is expected to be a result of interplay between its analgesic property and potential direct adverse effect, if any.
Neurobehavioral Assessment of Preterm Infant (NAPI) measures the developmental maturity of preterm infants. It is a reliable tool to identify lags in development, as early as 32 weeks postconceptional age (PCA), providing an opportunity for early interventions [8–10].
The present study was planned to evaluate the effect of sucrose analgesia, for repeated painful procedures, on short-term neurobehavioral outcome of preterm neonates, assessed at 40 weeks PCA using NAPI.
MATERIALS AND METHODS
Setting and subjects
The study was conducted at the neonatal care units of a tertiary-level teaching hospital in North India between April 2010 and April 2011. All consecutively born clinically stable preterm newborns, between completed 32 weeks and 37 weeks of gestational age, were eligible for the study. Those with risk factors (perinatal sepsis, maternal sedatives or opiates exposure, major congenital anomalies, etc.) or symptoms and signs of any condition (asphyxia, sepsis, intraventricular bleed, respiratory distress, trauma, etc), potentially affecting hemodynamic stability or neurological status, were excluded. The parents of eligible newborns were offered enrollment within first 48 h of birth of the newborn and those who were willing were enrolled after obtaining an informed consent. The relevant antenatal (maternal morbidities, medication exposure, socioeconomic status, etc.), intrapartum (birth weight, Apgar score, resuscitation details, etc.) and neonatal (morbidities, required interventions and outcome) information was recorded in a predesigned pro forma.
The neonates were randomly assigned to either intervention (sucrose) or control (distilled water) group.
Randomization and allocation concealment
Block randomization using computer-generated random sequences was used with a static block size of six each. Allocation sequence was generated and maintained confidentially by the co-investigator from department of Pharmacology. At the time of enrollment, the group allocation was telephonically conveyed to the research candidate, to ensure allocation concealment. Identically looking packets carrying sucrose and the double-distilled water, prepared and serially labeled according to confidential randomization code by pharmacy, were available at neonatal units. The primary care team members were responsible for administrating the intervention/control to the enrolled newborn according to the allocated serially numbered packet, unaware of the randomization code. The participants, the research candidate as well as the primary care team members assessing the painful response were blinded to the group assignment. Randomization codes and allocation sequences were disclosed subsequent to completion of data analysis.
Intervention
The enrolled neonates were administered either a sterile solution of 24% sucrose or double-distilled water (0.5 ml each in 1 ml syringe) orally depending on the randomization code, for every potentially painful procedure during the first 7 days of study. Strict asepsis and confidentiality was maintained in handling, labeling and transporting these identical-looking packets. Fresh solutions were prepared daily and unused solutions were discarded at the end of the day to be replaced with identically numbered solution from the lab the next time they were required. All study solutions were stored in the refrigerator at 2–8°C until they were used; 2 min before each potentially painful procedure, 0.5 ml of the solution marked with the patient’s serial number was administered orally to the patient by the personnel carrying out the procedure using the prefilled syringes. This intervention was carried out for each potentially painful procedure for the period of 7 days since enrollment, and each procedure and intervention was recorded in a predesigned record form kept at the bedside of the patient.
Outcome measures
Primary outcome was score of motor development and vigor (MDV) and alertness and orientation (AO) domains of Neurobehavioral Assessment of Preterm Infants (NAPI) scale performed at 40 weeks PCA. It was carried out by the research candidate as per the recommended procedure and scoring system provided with the standard NAPI kit. Higher scores are associated with more mature behavior. In addition, the highest heart rate and lowest SpO2 obtained during the procedure were recorded till 30 s after the prick, for newborns in both the groups.
Statistical analysis
All the results were analyzed using the windows SPSS software version 17. For descriptive statistics, mean and standard deviation were calculated. Unpaired and paired student’s t-test was used for comparison of means between different groups and means of two sets of readings within the same group, respectively. For comparisons of proportions, chi-square test was used. A p-value <0.05 was considered statistically significant. Assuming that sucrose analgesia leads to an improvement in the neurobehavioral outcome by 15%, an alpha error of 5% and a power of 90%, 46 subjects were required to be analyzed in each limb. The study protocol was approved by institutional ethics committee.
RESULTS
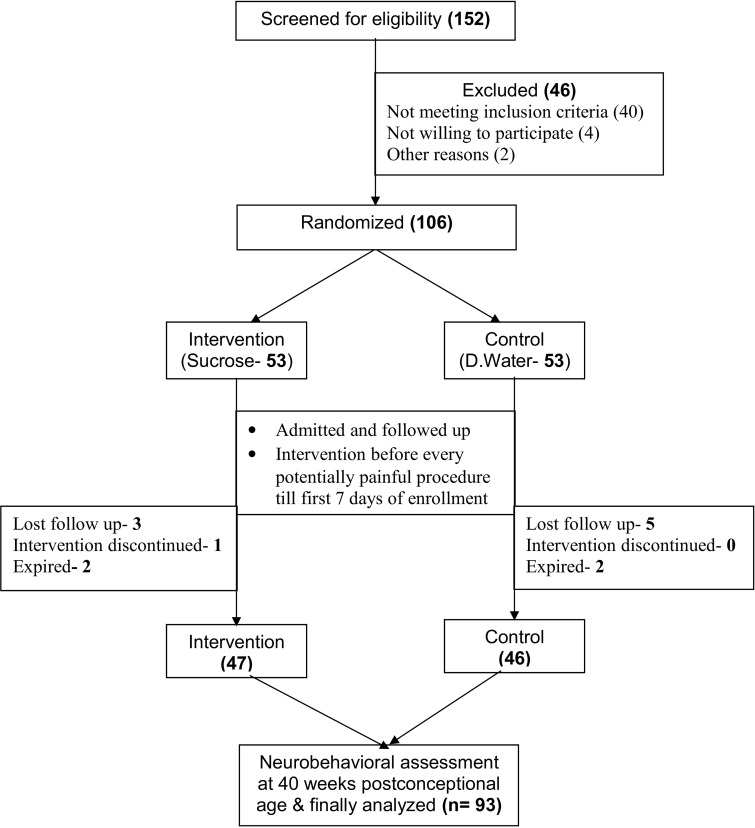
Of the total 152 neonates who were screened for enrollment, 46 were excluded, 106 were randomized, of which, 93 (47 in the sucrose and 46 in the placebo group) were available for final analysis (Fig. 1). Table 1 shows the baseline characteristics of the subjects enrolled in the two study groups. Mean (SD) number of procedures in the intervention group, 6.15 (1.55), was statistically similar to that, 6.15 (1.28), in the placebo group. Similarly, no difference was observed in the cumulative dose of sucrose and placebo across the two study groups. The mean NAPI scores at 40 weeks of corrected gestational age across the two study groups are shown in Table 2. No statistically significant difference was detected in both the domains of MDV and AO between the two study groups. There was no significant difference in the frequency of adverse effects (fall in heart rate or oxygen saturation) across the two groups.
Fig. 1.
Flow diagram depicting flow of the study from enrollment to analysis.
Table 1.
Baseline characteristics of participants in both the study groups
| Baseline characteristic | Intervention (n = 53) | Control (n = 53) |
|---|---|---|
| Age at enrollment (hours) (Mean, SD, 95% CI) | 3.78, 2.92, 2.98–4.59 | 3.16, 2.18, 2.56–3.76 |
| Birth weight (grams) (Mean, SD, 95% CI) | 1555.58, 242.79, 1488.66–1622.51 | 1575.24, 241.89, 1508.57–1641.92 |
| Apgar score at 5 min (Mean, SD, 95% CI) | 8.51, 0.70, 8.32–8.70 | 8.55, 0.50, 8.41–8.69 |
| Number of males (%) | 26 (49) | 31 (58) |
| Gestational age distribution (n) | ||
| 32–34 weeks | 42 | 38 |
| >34 weeks | 11 | 15 |
| Vertex presentation (n) | 49 | 44 |
Table 2.
Scores [Mean, SD (95% CI)] of motor development and vigor (MDV) and alertness and orientation (AO) domains of neurobehavioral assessment of preterm infant tool, performed at baseline and at 40 weeks postconceptional age among two study groups
| Intervention group (47) | Control group (46) | ‘p’ value | ||
|---|---|---|---|---|
| Baseline | MDV score | 58.49, 15.51 (54.23–62.77) | 57.46, 16.53 (52.90–62.02) | 0.740 |
| AO score | 63.79, 22.52 (57.59–70.00) | 55.19, 21.90 (49.15–61.23) | 0.049 | |
| 40 weeks postconceptional age | MDV score | 74.65, 17.13 (69.93–79.38) | 76.48, 16.13 (72.03–80.92) | 0.573 |
| AO score | 70.86, 20.86 (65.11–76.61) | 67.77, 25.93 (60.63–74.92) | 0.501 |
DISCUSSION
This study on the role of sucrose analgesia for repeated procedural pain on short-term neurobehavioral outcome of preterm neonates revealed that there was no difference in the neurobehavioral scores between the two study arms with respect to the mean score of MDV and AO at follow up (40 weeks). This implies that repeated painful procedures conducted without sucrose analgesia do not lead to a detectable difference in the neurobehavioral status at 40 weeks of corrected gestational age when compared with procedures conducted with sucrose analgesia.
We planned to carry out an early neurobehavioral assessment at 40 weeks in our study using the NAPI score to pick up subtle deviations in neurodevelopment early. The therapeutic implications of this early identification of deviant neurodevelopment would be immense in terms of instituting early intervention and rehabilitation for these affected neonates. NAPI has been shown to correlate well both with Bayley Scale of Infant Development and Bayley Infant Neurodevelopmental Screener in predicting neurodevelopmental deficits early [8–11]. The absence of a measurable difference in NAPI scores in our study subjects across the two study arms is difficult to explain. Our study subjects were relatively more mature (33–34 weeks) babies who were clinically stable, and an adverse neurobehavioral outcome per se, is relatively infrequent in this subclass of neonates. In other studies, the number of procedures varied from 24 to 125. As compared with other studies, the numbers of procedures carried out were fewer in our study. Also, a single dose of sucrose was used before each procedure. These factors may have had an important forbearing on the neurobehavioral outcome.
The use of sucrose for single painful event has been reported to be safe. However, there have been concerns regarding the safety profile of repeated administration of sucrose for frequently performed painful procedures. Wallace et al. reported that small volume of 20% sucrose and calcium lactate given via nasogastric tube was associated with increased incidence of necrotizing enterocolitis (NEC) in very low birth weight (VLBW) infants [12]. Johnston et al. in their study have evaluated the repeated use of sucrose over 7 days [7]. Here, the total number of study doses given per infant during the week ranged from 24 to 125 with a mean of 63 in the sucrose group. No significant adverse effects either immediate or late related to the administration were reported. Similar findings were reported by Stevens et al. who while using neurobiological risk score in neonates did not find any difference in the scores across the study limbs, namely, water and pacifier versus sucrose, pacifier and standard care when evaluating preterms less than 36 weeks of age [8]. In our study, there was no significant difference in the rate of adverse effects either immediate or long term across the two study arms. We can conclude from our observations that use of repeated doses of sucrose for procedural pain relief in preterm neonates is devoid of any significant immediate or long-term adverse effects.
Our study had few limitations; with the study population being more mature, the likelihood of adverse neurodevelopmental outcome was theoretically low. Also, lesser number of procedures were carried out and a single dose of sucrose was used before each procedure. These factors per se may have had an important forbearing on the neurobehavioral outcome of the study population. In view of the above, we recommend further studies using the same study design and a longer follow up period of at least 12–18 months for detecting neurodevelopmental delays. These studies should enroll extremely low birth weight and preterm neonates <32 weeks, as this subgroup is most susceptible to the effects of procedural pain [7].
We conclude that use of oral sucrose analgesia, in comparison with distilled water as placebo, does not lead to a poor neurobehavioral outcome in preterm neonates when assessed by the NAPI at 40 weeks PCA. The use of sucrose for procedural pain over 7 days appears free of immediate and long-term adverse effects.
REFERENCES
- 1.Anand KJS, Carr DB. The neuroanatomy, neurophysiology and neurochemistry of pain, stress, and analgesia in newborns and children. Pediatrics Clin North Am 1989;36:795–822. [DOI] [PubMed] [Google Scholar]
- 2.Anand KJ, Hickey PR. Pain and its effects in the human neonate and fetus. N Engl J Med 1987;317:1321–9. [DOI] [PubMed] [Google Scholar]
- 3.Anand KJ. Clinical importance of pain and stress in preterm neonates. Biol Neonate 1998;73:1–9. [DOI] [PubMed] [Google Scholar]
- 4.Grunau RE, Holsti L, Peters JW. Long-term consequences of pain in human neonates. Semin Fetal Neonatal Med 2006;11:268–75. [DOI] [PubMed] [Google Scholar]
- 5.Anand KJS, Scalzo FM. Can adverse neonatal experiences alter brain development and subsequent behaviour? Biol Neonate 2000;77:69–82. [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald M, Millard C, McIntosh N. Cutaneous hypersensitivity following peripheral tissue damage in newborn infants and its reversal with topical anaesthesia. Pain 1989;39:31–6. [DOI] [PubMed] [Google Scholar]
- 7.Johnston CC, Filion F, Snider L, et al. Routine sucrose analgesia during the first week of life in neonates younger than 31 weeks' postconceptional age. Pediatrics 2002;110:523–8. [DOI] [PubMed] [Google Scholar]
- 8.Stephens BE, Liu J, Lester B, et al. Neurobehavioral assessment predicts motor outcome in preterm infants. J Pediatr 2010;156:366–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majnemer A, Snider L. A comparison of developmental assessments of the newborn and young infant. Ment Retard Dev Disabil Res Rev 2005;11:68–73. [DOI] [PubMed] [Google Scholar]
- 10.Korner AF, Constantinou J, Dimiceli S, et al. Establishing the reliability and developmental validity of a neurobehavioral assessment for preterm infants: a methodological process. Child Dev 1991;62:1200–8. [PubMed] [Google Scholar]
- 11.Constantinou JC, Adamson-Macedo EN, Mirmiran M, et al. Neurobehavioral assessment predicts differential outcome between VLBW and ELBW preterm infants. J Perinatol 2005;25:788–93. [DOI] [PubMed] [Google Scholar]
- 12.Schultz M, Loughran-Fowlds A, Spence K. Neonatal pain: a comparison of the beliefs and practices of junior doctors and current best evidence. J Paediatr Child Health 2010;46:23–8. [DOI] [PubMed] [Google Scholar]