Abstract
Background: The objective of this study was to compare the effect of once weekly iron supplementation (IS) versus twice weekly, on hemoglobin (Hb) levels and anemia prevalence.
Methods: In this cluster-randomized clinical trial study, we evaluated infants aged 6–18 months. Length of intervention: 16 weeks. Infants were cluster randomized to either 25 mgelemental iron once weekly (Group-A) or twice weekly (Group-B). Primary outcome variables were change in Hb concentration and anemia prevalence. Two biochemical evaluations were performed to determine Hb concentrations, before and after intervention.
Results: For Group-A, at baseline, mean Hb concentration was 10.8 ± 1.18 g/dl and after intervention 11.2 ± 1.07 g/dl, p = 0.12; anemia prevalence was 52.5% at baseline and 37.5% after intervention, p = 0.18; Group-B, mean baseline Hb was 10.7 ± 1.04 g/dl, and 11.3 ± 0.91 g/dl after intervention, p = 0.002; anemia prevalence reduced from 57.9 to 36.8%.
Conclusions: Both once and twice weekly IS increased mean Hb concentration; however, twice weekly supplementation provided more significant results.
Keywords: anemia, hemoglobins, iron, infants.
INTRODUCTION
Iron deficiency (ID) is the most common and widespread nutritional disorder in the world and a public health problem in developing countries. ID is the result of negative balance of this mineral over time. Iron deficiency anemia (IDA) is the most serious form of ID, occurring after a long period of deficiency, when stores have already been depleted, and after a reduction in biochemical iron [1].
Groups at risk for the development of IDA are children, adolescents, pregnant women and women of reproductive age. This occurs owing to increased nutritional needs during this period in qualitative and/or qualitative manner [2, 3]. Adequate iron intake could be achieved through the consumption of naturally iron-rich foodstuffs [4]. However, this is not always the case, and new approaches to prevent ID in infants are necessary [5].
In a meta-analysis by Ramakrishnan et al. [6], iron supplementation (IS) has proven effective in increasing hemoglobin (Hb) levels and reducing anemia prevalence. The most common form of iron supplements are iron sulfate drops, chewable tablets and sprinkles with or without additional nutrients. Up to date, several studies have investigated different schemes to optimize IS in different populations, some of them with conflicting results [7–12]. As a result, further research is necessary to assess and validate findings.
This study investigated the effects of two different IS regimens for the prevention/treatment of anemia. In this investigation, we compared the effect of ferrous sulfate oral solution given once weekly versus twice weekly, on Hb concentrations, in infants aged 6–18 months.
MATERIALS AND METHODS
This cluster-randomized clinical trial study was conducted in the municipality of Sobral, northeast of Brazil, between September and December 2013. The study population was composed of infants aged 6–18 months, from four randomized public infant education centers; the first two formed Group-A, and the latter Group-B. Group-A was allocated to 25 mg elemental iron once weekly (n = 55), and Group-B to 25 mg elemental iron twice weekly (n = 53).
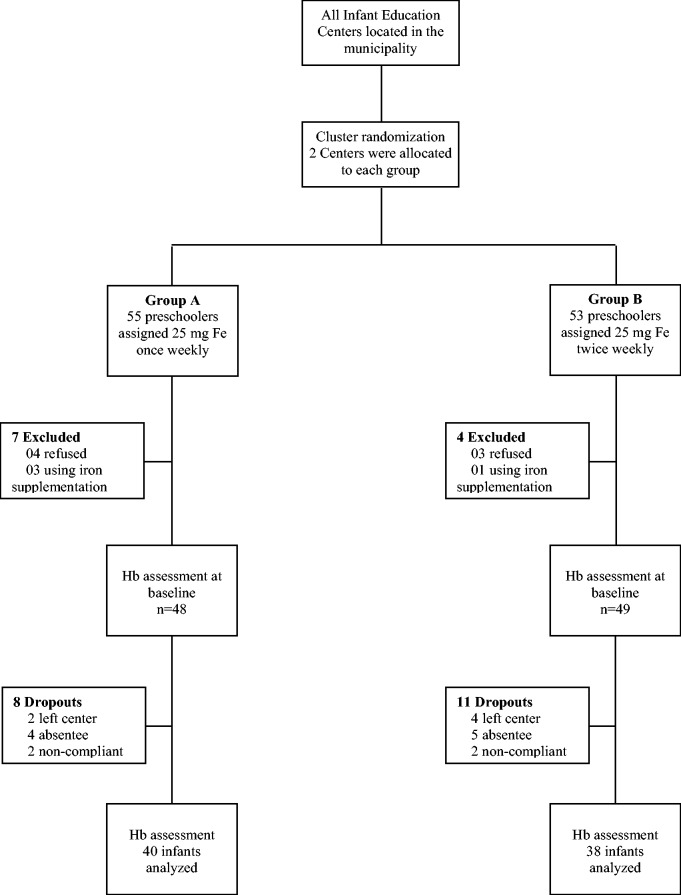
All infants from the centers were invited to participate in our study. Exclusion criteria were parents’ refusal to participate and infants already using IS (Fig. 1).
Fig. 1.
Study profile.
The study included two primary outcome variables: change in Hb concentration; and anemia prevalence before and after intervention. Hb concentration <11.0 g/dl was used as cutoff point to define anemia [1]. According to information provided by parents, a standardized data sheet was filled in containing information on (other study variables) age, gender, exclusive breastfeeding (EBF) <6 months of age, mother’s schooling and family income.
Two biochemical evaluations were performed, to determine Hb concentrations, before and after intervention, with portable HemoCue B-hemoglobin photometer (Hb301-HemoCue AB, Ängelholm, Sweden). Finger prick capillary blood was collected under aseptic conditions. Data collection team was blinded to different interventions.
In this study, we used ferrous sulfate oral solution (orange flavor) (Far-Manguinhos/Fiocruz). Intervention was administered using a plastic medical syringe to gently squirt solution into the side of the child's mouth. Length of intervention: 16 weeks, beginning/ending on the same date for both groups.
Anemia prevalence in study population was estimated at 40%. To achieve a reduction in global anemia prevalence from 50 to 25%, with 80% power, two-sided, type I error of 5%, accounting for 10% losses to follow-up, each group required a minimum of 43 participants [13].
At baseline, to identify statistical significance between groups, we used unpaired t-test for age and mean Hb concentration, and Fisher’s exact test for gender, EBF, mother’s schooling and family income.
To compare means, we used paired student’s t-test to assess difference in Hb concentration within the groups, and Fisher’s exact test to assess the difference between good and bad outcomes (absence or presence of anemia). Data had normal distribution. The statistical software package SPSS for Windows, version 17.0, was used for all analyses (SPSS Inc., Chicago, IL). Limit for statistical significance was set at p < 0.05. Analyses were by intention to treat.
This study was approved by the ethics committee for research at the State University Vale do Acaraú following the ethical principles established by the National Health Council Resolution #466/2012, with necessary prior written consent from school directors and parents/guardians. Medical support was available on request. After intervention, anemic children were referred for treatment.
RESULTS
At baseline, the other study variables were analyzed. However, there were no statistically significant differences between the groups (Table 1). Before second biochemical evaluation, there were 19 dropouts (Fig. 1).
Table 1.
Baseline characteristics of study participants, by intervention group
| Variables | Group A (n = 48) | Group B (n = 49) | p |
|---|---|---|---|
| Age (months) Mean ± SD | 11.3 ± 2.47 | 12.1 ± 2.74 | 0.16a |
| Gender M:F | 21:27 | 25:24 | 0.54b |
| EBF | 20 | 18 | 0.68b |
| Mother with ≤ 9 year schooling | 28 | 33 | 0.40b |
| Family income ≤ 300 USD | 38 | 35 | 0.48b |
| Hb (g/dl) | 10.8 ± 1.25 | 10.6 ± 0.99 | 0.60a |
Note. All numbers are absolute.
SD = standard deviation; M:F = male:female; EBF = exclusively breastfed up to 6 months of age.
aBased on unpaired student t-test.
bBased on Fisher’s exact test (two-tailed).
In Group-A, mean Hb concentration before intervention was 10.8 ± 1.18 g/dl and 11.2 ± 1.07 g/dl after intervention, p = 0.12; anemia prevalence was 52.5% (21 of 40) at baseline and 37.5% (15 of 40) after intervention, without statistical significance, p = 0.18. In the twice weekly group (Group-B), mean baseline Hb concentration was 10.7 ± 1.04 g/dl, and after intervention mean Hb concentration improved significantly to 11.3 ± 0.91 g/dl, p = 0.002; anemia prevalence reduced from 57.9 to 36.8%, without statistical difference (Table 2).
Table 2.
Effect of iron supplementation, 25 mg elemental iron once weekly and twice weekly, and anemia prevalence before and after intervention
| Variables | Group-A—Once Weekly (n = 40) |
Group-B—Twice Weekly (n = 38) |
||||
|---|---|---|---|---|---|---|
| Before | After | p | Before | After | p | |
| Hb (g/dl) Mean ± SD | 10.8 ± 1.18 | 11.2 ± 1.07 | 0.12a | 10.7 ± 1.04 | 11.3 ± 0.91 | 0.002a |
| CI | 10.46, 11.21 | 10.84, 11.52 | 10.32, 11.01 | 10.98, 11.58 | ||
| Anemiab | 21 (52.5) | 15 (37.5) | 0.26c | 22 (57.9) | 14 (36.8) | 0.11c |
Note. All numbers are absolute except numbers in brackets, which represent percentages.
SD = standard deviation.
aBased on paired Student’s t-tests.
bAnemia defined as Hb concentration < 11.0 g/dl.
cBased on Fisher’s exact test (two-tailed).
Considering only anemic participants, both interventions significantly increased mean Hb levels. In Group-A, mean Hb concentration was 9.93 ± 0.79 g/dl at baseline and 10.92 ± 0.84 after intervention, p = 0.001; from the 21 participants who were anemic at baseline, 12 were no longer anemic after intervention, p < 0.0001. In Group-B, mean Hb concentration was 9.93 ± 0.67 at baseline and 11.06 ± 0.92 after intervention, p < 0.0001; at baseline, 22 participants were anemic, and after intervention this number reduced to 11, p = 0.0002 (Table 3).
Table 3.
Effect of iron supplementation in anemic participants, 25 mg elemental iron once weekly and twice weekly on hemoglobin levels, and anemia prevalence before and after intervention
| Variables | Group-A—once weekly (n = 21) |
Group-B—twice weekly (n = 22) |
||||
|---|---|---|---|---|---|---|
| Before | After | p | Before | After | p | |
| Hb (g/dl) Mean ± SD | 9.93 ± 0.79 | 10.92 ± 0.84 | 0.001a | 9.93 ± 0.67 | 11.06 ± 0.92 | <0.0001a |
| CI | 9.47, 10.29 | 10.54, 11.31 | 9.63, 10.22 | 10.65, 11.47 | ||
| Anemiab | 21 | 9 | <0.0001c | 22 | 11 | 0.0002c |
Note. All numbers are absolute.
Hb = hemoglobin; SD = standard deviation; CI 95% = confidence interval.
Comparison between the groups for anemia based on Fisher’s exact test (two-tailed) p = 0.76.
aBased on paired Student’s t-tests.
bAnemia defined as Hb concentration < 11.0 g/dl.
cBased on Fisher’s exact test (two-tailed).
In this study, we compared once weekly with twice weekly IS, for a favorable (absence of anemia) or adverse (anemic) outcome. After intervention, adverse outcome was present in 37.5% of participants in Group-A and 36.8% of participants in Group-B. The difference, the reduction of absolute risk, was 0.66%. The 95% confidence interval for this difference ranges from −20.80 to 22.11%. The number needed to treat was 153.
DISCUSSION
The objective of this study was to compare two different IS regimens, once weekly and twice weekly, in infants aged 6–18 months. In both groups, participants presented an increase in mean Hb concentration and a reduction in anemia rates; however, the increase in Hb levels was only statistically significant in the twice weekly supplementation group. Furthermore, there was a greater reduction in anemia prevalence in the twice weekly group; however, this reduction was not significant.
If we consider only anemic participants, both interventions significantly increased mean Hb concentrations. Additionally, the number of anemic individuals also reduced, from 21 to 9 (Group-A), and 22 to 11 (Group-B). These results suggest that the two interventions were efficacious in the treatment of anemia.
Several studies have compared the effectiveness of IS in infants. Three clinical trials in preschoolers have demonstrated that supervised weekly IS had the same effectiveness as daily supplementation [14–16].
Meta-analyses by Beaton and McCabe [17] and De-Regil et al. [18] compared the effectiveness of daily and weekly IS regimens; it was observed that both supplementation regimens were effective in favorable conditions, with daily supplementation providing better results. Greater effectiveness from daily supplementation was witnessed from higher Hb and serum ferritin values, and a reduction in relative risk for anemia. In these studies, weekly supplementation was effective when compared with placebo; nevertheless, effectiveness was inferior to that of daily supplementation. De-Regil et al. [18] concluded that weekly supplementation should only be considered for preschoolers and schoolchildren when there is a strong guarantee of supervision and high adherence, or when daily supplementation cannot be implemented.
Another recent meta-analysis analyzed the effectiveness of daily and weekly regimens of ferrous sulfate supplementation for the prophylaxis of IDA in children <5 years. This study also showed that the daily dose of ferrous sulfate was more efficient in increasing Hb levels than the weekly administration of this supplement; however, there was no difference between the reductions in the prevalence of anemia [19].
Yet, despite these meta-analyses, other studies in infants aged 6–18 months, using a similar dose to our 25 mg of elemental iron weekly, during a 4 month period, did not obtain significant increases in mean Hb concentrations [20–22].
Nevertheless, Coutinho et al. [23] and Nogueira Arcanjo et al. [24] in randomized controlled trials were able to significantly increase Hb concentration; the first study used 25 mg of elemental iron once weekly, during 12 weeks, and the second used either 25 mg of elemental iron once weekly or 12.5 mg daily, both groups increased Hb levels when compared with control; it is important to acknowledge that in this study mean baseline Hb concentrations were low.
In contrast, studies conducted by Ferreira et al. [25] and Monteiro et al. [26] obtained favorable results with the use of 50 mg weekly supplementation regimens in infants. The first before-and-after study supplemented 293 children aged 24 months during a 24 week period, and the intervention was able to increase mean Hb concentration from 10.1 to 11.1 g/dl. However, in the study by Monteiro et al. [26] with 1158 children using a 5 mg/kg/week dose of iron (approximately 50 mg/week), mean Hb levels increased significantly (>1 g/dl) from 11.1 to 12.1 g/dl.
Differing from the previous studies, there are researchers such as Zlotkin et al. [27] in a clinical trial with 40 mg of iron during 24 weeks, and Brunken et al. [28] in a before-and-after study with 6 mg/kg/week of iron during 16 weeks did not witness statistical differences after interventions; this in part may be explained by the high Hb values at the beginning of the studies, 11.0 and 11.2 g/dl, respectively. In an Iranian study by Khandemloo et al. [29], a dose of approximately 3–4 mg/kg/week was able to significantly increase Hb levels after 12 weeks (p = 0.005, mean Hb > 0.4 g/dl).
In Brazil, there is already a National Iron Supplementation Program, which distributes free iron supplements at health units within the Unified Health System to infants aged 6–18 months, who meet criteria for inclusion in the program. The program is based on a 25 mg dose of elemental iron weekly for infants <18 months [30]. However, the program has had problems with coverage: 1 year after implementation in 2006, the program covered only 19.4% of national territory, rising to 27.2% in 2010 [31]. In 2013, the program was reformulated with the decentralization of acquisition of supplements to municipal, district and state spheres [32]; another problem in the program concerns low adhesion—many mothers do not know of the program or the benefits that IS provides [33].
Some limitations need to be acknowledged and addressed regarding the present study. Many confounding factors affect the outcome of Hb concentrations and anemia prevalence, such as illness, inconsistent eating habits, periods of rapid growth. Furthermore, the number of children in each group was small; a larger number of participants in each group could have led to more expressive results, especially in the once weekly group. Another important fact is that the period of intervention was short (limited by the school semester); a longer period may have presented more expressive results in both groups. Another possible limitation is that this study depended exclusively on Hb concentrations to measure outcomes, without serum ferritin levels, which measure iron stores in the organism. However, the inclusion of these measurements would have implied operational difficulties and possibly a lower number of participants in the study. However, despite these limitations, our study was able to identify significant results.
Although mean Hb concentration increased significantly in the twice weekly group, the decrease in anemia prevalence was not statistically significant. This may have been caused by the fact that several children in the study were on the borderline between anemia and non-anemia (10.8/10.9 g/dl) after intervention; in other words, with a minimal increase in Hb concentration, more children would no longer have been classified as anemic; this may have been achieved if the intervention had lasted a little longer. However, both interventions were efficacious in increasing mean Hb concentrations in anemic infants.
IDA is a public health problem, which if left untreated can cause irreversible sequels especially in this age range. Therefore, there is an important and urgent need to find the best regimen to treat and/or prevent anemia. In our study, we witnessed that both once and twice weekly, IS with 25 mg of elemental iron increased mean Hb concentration; however, twice weekly supplementation provided more significant results. Furthermore, both interventions were similar in the treatment of anemic individuals. Simple and inexpensive IS programs constitute a useful strategy to treat highly anemic populations in public schools.
ACKNOWLEDGEMENTS
The author would like to thank the infants and teachers at the infant education centers for their participation and cooperation during this study, and the Secretariat of Education and Secretariat of Health at the Municipal City Hall—Sobral-Ceará for their support during the project.
FUNDING
This project was funded by The Federal University of Ceará, Sobral Unit—Research Initiative Grant.
REFERENCES
- 1.WHO. Iron deficiency anaemia: assessment, prevention, and control: a guide for programme managers. Geneva: WHO, 2001. [Google Scholar]
- 2.Viteri FE. A new concept in the control of iron deficiency: community-based preventive supplementation of at-risk groups by the weekly intake of iron supplements. Biomed Environ Sci 1998;11:46–60. [PubMed] [Google Scholar]
- 3.International Nutrition Foundation and Micronutrient Initiative UNICEF/UNU/WHO/MI Technical Workshop. Preventing Iron Deficiency in Women and Children: Background and Consensus on Key Technical Issues and Resources for Advocacy, Planning and Implementing National Programmes. New York: INF, 1998. [Google Scholar]
- 4.Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium and Zinc: A Report of the Panel on Micronutrients Subcommittees on Upper Reference Levels of Nutrients and of Interpretation and Uses of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Washington, DC: National Academic Press, 2001. [Google Scholar]
- 5.Lutter CK. Iron deficiency in young children in low-income countries and new approaches for its prevention. J Nutr 2008;138:2523–8. [DOI] [PubMed] [Google Scholar]
- 6.Ramakrishnan U, Nguyen P, Martorell R. Effects of micronutrients on growth of children under 5 y of age: meta-analyses of single and multiple nutrient interventions. Am J Clin Nutr 2009;89:191–203. [DOI] [PubMed] [Google Scholar]
- 7.Arcanjo FP, Arcanjo CC, Amancio OM, et al. Weekly iron supplementation for the prevention of anemia in pre-school children: a randomized, double-blind, placebo-controlled trial. J Trop Pediatr 2011;57:433–8. [DOI] [PubMed] [Google Scholar]
- 8.Coutinho GG, Cury PM, Cordeiro JA. Cyclical iron supplementation to reduce anemia among Brazilian preschoolers: a randomized controlled trial. BMC Public Health 2013;13:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawamdeh HM, Rawashdeh M, Aughsteen AA. Comparison between once weekly, twice weekly, and daily oral iron therapy in Jordanian children suffering from iron deficiency anemia. Matern Child Health J 2013;17:368–73. [DOI] [PubMed] [Google Scholar]
- 10.Schümann K, Longfils P, Monchy D, et al. Efficacy and safety of twice-weekly administration of three RDAs of iron and folic acid with and without complement of 14 essential micronutrients at one or two RDAs: a placebo-controlled intervention trial in anemic Cambodian infants 6 to 24 months of age. Eur J Clin Nutr 2009;63:355–68. [DOI] [PubMed] [Google Scholar]
- 11.Tavil B, Sipahi T, Gökçe H, et al. Effect of twice weekly versus daily iron treatment in Turkish children with iron deficiency anemia. Pediatr Hematol Oncol 2003;20:319–26. [PubMed] [Google Scholar]
- 12.Thu BD, Schultink W, Dillon D, et al. Effect of daily and weekly micronutrient supplementation on micronutrient deficiencies and growth in young Vietnamese children. Am J Clin Nutr 1999;69:80–6. [DOI] [PubMed] [Google Scholar]
- 13.Lwanga SK, Lemesshow S. Sample Size Determination in Health Studies: A Practical Manual. Geneva: World Health Organization, 1991. [Google Scholar]
- 14.Liu XN, Kang J, Zhao L, et al. Intermittent iron supplementation in Chinese pre-school children is efficient and safe. Food Nutr Bull 1995;16:139–46. [Google Scholar]
- 15.Schultink W, Gross R, Gliwitzki M, et al. Effect of daily vs twice weekly iron supplementation in Indonesian preschool children with low iron status. Am J Clin Nutr 1995;61:11–15. [DOI] [PubMed] [Google Scholar]
- 16.Thu B, Schultink W, Dillon D, et al. Effect of daily and weekly nutrient supplementation on micronutrient deficiencies and growth in young Vietnamese children. Am J Clin Nutr 1999;69:80–6. [DOI] [PubMed] [Google Scholar]
- 17.Beaton GH, McCabe GP. Efficacy of intermittent iron supplementation in the control of iron deficiency anaemia in developing countries: an analysis of experience. Final report to the micronutrient initiative. Toronto, Canada: GHB Consulting, 1999. [Google Scholar]
- 18.De-Regil LM, Suchdev PS, Vist GE, et al. Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age. Cochrane Database Syst Rev 2011;9:CD008959. [DOI] [PubMed] [Google Scholar]
- 19.Cembranel F, Corso AC, González-Chica DA. Coverage and adequacy of ferrous sulfate supplementation in the prevention of anemia among children treated at health centers of Florianopolis, Santa Catarina. Rev Paul Pediatr 2013;31:315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engstrom EM, Castro IRR, Portela M, et al. Efetividade da suplementação diária ou semanal com ferro na prevenção da anemia em lactentes. Rev Saúde Pública 2008;42:786–95. [DOI] [PubMed] [Google Scholar]
- 21.Azeredo CM, Cotta RMM, Sant’Ana LFR, et al. Efetividade superior do esquema diário de suplementação de ferro em lactentes. Rev Saúde Pública 2010;44:230–9. [DOI] [PubMed] [Google Scholar]
- 22.Silva DG. Prevenção da anemia e da deficiência de ferro no segundo semestre de vida com diferentes suplementações de ferro. Doctorate thesis. Universidade Federal de São Paulo, São Paulo, 2007. [Google Scholar]
- 23.Coutinho GG, Goloni-Bertollo EM, Pavarino-Bertelli EC. Effectiveness of two programs of intermittent ferrous supplementation for treating iron-deficiency anemia in infants: randomized clinical trial. Sao Paulo Med J 2008;126:314–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nogueira Arcanjo FP, Santos PR, Costa Arcanjo CP, et al. Daily and weekly iron supplementations are effective in increasing hemoglobin and reducing anemia in infants. J Trop Pediatr 2013;59:175–9. [DOI] [PubMed] [Google Scholar]
- 25.Ferreira MLM, Ferreira LOC, Silva AA, et al. Efetividade da aplicação do sulfato ferroso em doses semanais no Programa Saúde da Família em Caruaru, Pernambuco, Brasil. Cad Saúde Pública 2003;19:375–81. [DOI] [PubMed] [Google Scholar]
- 26.Monteiro CA, Szarfarc SC, Brunken GS, et al. A prescrição semanal de sulfato ferroso pode ser altamente efetiva para reduzir níveis endêmicos de anemia na infância. Rev Bras Epidemiol 2002;5:71–83. [Google Scholar]
- 27.Zlotkin S, Antwi KY, Schauer C, et al. Use of microencapsulated iron (II) fumarate sprinkles to prevent recurrence of anaemia in infants and young children at high risk. Bull World Health Organ 2003;81:108–15. [PMC free article] [PubMed] [Google Scholar]
- 28.Brunken GS, Muniz PT, Silva SM. Weekly iron supplementation reduces anemia prevalence by 1/3 in preschool children. Rev Bras Epidemiol 2004;7:210–19. [Google Scholar]
- 29.Khademloo M, Karami H, Ajami A, et al. Comparison of the effectiveness of weekly and daily iron supplementation in 6 to 24 months old babies in urban health centers of Sari, Iran. Pak J Biol Sci 2009;12:195–7. [DOI] [PubMed] [Google Scholar]
- 30.Brasil. Ministério da Saúde. Secretaria de Atenção à Saúde. Departamento de Atenção Básica. Manual operacional do Programa Nacional de Suplementação de Ferro / Ministério da Saúde, Secretaria de Atenção à Saúde, Departamento de Atenção Básica. Brasília: Ministério da Saúde, 2005. [Google Scholar]
- 31.Brasil - Ministério da Saúde - PNAN. Programa Nacional de Suplementação de Ferro: quantitativo da população assistida. http://nutricao.saude.gov.br/ferro_relatorio.php?ferro_tipo_relatorio=3 (31 October 2014, date last accessed).
- 32.Brasil. Ministério da Saúde. Secretaria de Atenção à Saúde. Programa Nacional de Suplementação de Ferro: manual de condutas gerais. Brasília: Ministério da Saúde, 2013. [Google Scholar]
- 33.Bortolini GA, Vitolo MR. Baixa adesão à suplementação de ferro entre lactentes usuários de serviço público de saúde. Pediatria (São Paulo) 2007;29:176–82. [Google Scholar]