Abstract
Gestational age is a critical factor in the management, decision-making, prognostication and follow-up of newborn infants. It is also essential for research and epidemiology. In the absence of an early assessment of fetal gestation by abdominal ultrasound, many neonatal units in developing countries determine gestational age by neonatal scores and last menstrual period—both of which are highly inaccurate. The aim of this pilot study was to determine whether postnatal foot length measurement could accurately determine gestational age in a specified South African hospitalized neonatal population. Foot length was measured with a plastic Verniere's caliper. Foot length was shown to correlate well with gestational age (r = 0.919, p < 0.001). Intra-observer and inter-observer variability of foot length measurements was low. Foot length can therefore be used with high accuracy to determine the gestational age in a population where there is poor access to or utilization of antenatal sonar.
Keywords: postnatal foot length, gestational age.
INTRODUCTION
Gestational age (GA) is a critical factor in the management, decision-making, prognostication and follow-up of newborn infants, especially preterm infants. An accurate GA is also essential with regard to research and epidemiology [1]. The misclassification of infants as term, preterm and post-term can lead to the inaccurate description of neonatal growth and jeopardize statistical analyses [2], thereby reducing important outcomes to mere observed associations or artifacts [3]. In the absence of an early assessment of fetal gestation by abdominal ultrasound, many neonatal units in developing countries determine GA by the neurological and physical characteristics of the infant. The search for an accurate method of GA estimation remains an important goal.
GA can be estimated from the last menstrual period (LMP), antenatal ultrasound or neonatal estimates. LMP dating is a simple and low-cost method, but assumes the menstrual cycle to be 28 days and does not take into consideration any delay of ovulation and may cause an inaccuracy of 1–4 weeks [4]. Ultrasound-based dating, if performed early in pregnancy (<20–22 weeks GA), is considered the gold standard, with random errors of ±10 days [5]. Values are based on age-specific reference values but may be biased when symmetrically large or small fetuses are evaluated. Neonatal estimates of GA, including the Ballard [19] (12 clinical factors) and Dubowitz (22 clinical factors) scores, are standardized postnatal scoring systems based on a variety of physical and neurological maturity factors. They are time-consuming and have been shown to be highly inaccurate, overestimating GA by 1.3–11.9 weeks [24].
Foot length has been used in antenatal ultrasound examinations as an alternative marker for GA when other markers are unreliable, e.g. in the presence of hydrocephalus, anencephaly and limb dysplasia [7]. Streeter [8] first analyzed foot length as a determinant of GA in 1920, and his data have been revalidated in numerous antenatal [2, 9–11] and postnatal [12–15] studies (Table 1). Streeter's study population comprised postmortem specimens already fixated in formalin, which may have affected foot lengths. Ultrasound-determined foot length remains operator dependant, and only one study determined the correlation between antenatal and postnatal foot length measurements [6].
Table 1.
Summary of neonatal foot length studies and models
| Description | Streeter [8] | Usher [13] | Jordaan [15] | Chatterjee [10] | Mercer [7] | Mhaskar [11] | Merz [12] | Meirowitz [16] |
|---|---|---|---|---|---|---|---|---|
| Year | 1920 | 1969 | 1982 | 1986 | 1987 | 1988 | 2000 | 2000 |
| n | 704 | 300 | 132 live born, 94 abortus | 53 | 223 | 105 | 610 | 5372 |
| Purpose | Determine weight, sitting height, head size and foot length | Determine seven anthropometric measurements in normal newborns | Compare FL and BW with Streeter data; estimate BW from FL | Fetal foot length as determinant of GA | Compare fetal and postnatal foot lengths | Correlate FL with GA | Reference range for fetal foot growth | Evaluate effect of growth disturbances in SGA and LGA fetuses |
| Population | America-Caucasian | Canada, Caucasian | South Africa | Mexico | USA | India | Germany | American 85%, Caucasian 10%, African American 10.9% SGA incidence |
| GA (weeks) | 8.5–40, pathology specimens (formalin) | 25–40 | Live born: 23–44, abortus: 16–20 | 14–40 | 11–43 | 13–42 | 12–42 | 15–37 |
| Method of confirm GA | LMP | LMP | Clinical estimates and antenatal ultrasound | EUS <14 weeks | Secure dates and EUS | Not specified | EUS <12 weeks | Two week concordance between LMP and US |
| Method FL measurement | Sliding compass | Metal millimetre rule | Specially constructed sliding calliper | Ultrasound | Ultrasound | Ultrasound;101 measured postnatally | Ultrasound | Ultrasound |
| FL measurement technique | Longest toe (first or second) to heel | Longest toe to heel | Heel to outstretched longest toe | Heel to end of big toe | Longest toe to heel | heel to tip of big toe; Postnatal measurements with a ruler | heel to tip of big toe | Not specified |
| Growth curve FL/GA | Curvilinear correlation | Curvilinear correlation | Curvilinear correlation | Linear | Curvilinear | Linear | Curvilinear | Curvilinear |
| Results | Growth curves for various parameters studied, including FL | Mean foot length values documented: ± SD 4.2–8.8% difference AGA vs. SGA; correlation FL and BW r = 0.95 and FL/COH 0.96 | FL and BW r = 0.8152; insignificant difference with Streeter's data | r2 = 0.89, p << 0.0001 between GA and FL, only 5th, 50th and 95th centile | Similar to Streeter's data; FL/GA r2 = 0.98, reference range | GA/FL correlation r2 = 0.85, p < 0.001, BPD and FL, r4 = 0.91, p < 0.001. Antenatal FL correlated well with postnatal FL, measured within 3 days Correlated well with Streeter and Mercer data | FL leveled off slightly at 24/52—continued linear growth, values for 5th, 50th and 95th centile only; results for half week | Smoothed normogram for 5th, 10th, 50th, 90th and 95th centile |
Notes. FL = foot length; BW = birth weight; COH = circumference of head; EUS = early ultrasound; BPD = biparietal diameter; US = ultrasound.
In the World Health Organization's 2012 ‘Born too soon', it was stated that ‘simplified approaches to identify preterm babies such as foot size' were required for the early identification and management of preterm babies. There is, however, no single standardized foot length chart available to determine GA, especially not for a population as diverse as found in South Africa.
AIM
The aim of this pilot study was to determine whether postnatal foot length measurement, compared with an existing model, accurately determined GA in a specified South African hospitalized neonatal population.
METHOD
Infants admitted to Tygerberg Children’s Hospital neonatal units were prospectively enrolled in a larger study group between 2009 and 2010. Entry criteria for the larger study were infants that developed respiratory distress and required a chest X-ray in the first 48 h of life as part of their routine care. Infants were excluded from the study if there were indications of antenatal or postnatal structural chest deformities, limb deformities, assumed or confirmed genetic abnormalities, a neuromuscular condition or a congenital infection.
From the larger study population, a group of infants who had an early antenatal ultrasound, performed before 23 weeks of gestation (Western Cape Province Policy [17]), were enrolled. To ensure a uniform patient group, only appropriate-for-gestational-age (AGA) infants were selected for analysis. The Fenton growth chart [18] for infants was used to determine growth appropriateness. Small-for-gestational-age (SGA) infants (defined as weight <10th percentile for the GA according to the antenatal ultrasound) or large-for-gestational-age (LGA) infants (weight >90th centile for GA as determined by the antenatal ultrasound) were excluded, as were twins and triplets.
Foot lengths were measured using a plastic Verniere’s sliding caliper within the first 24 h of life (Fig. 1). The infant’s foot was measured from the midpoint of the heel to the longest toe, ensuring that no pressure was exerted on the soft tissue. The foot was placed in a lateral position while the ankle was held and a finger placed on the foot dorsum so as not to elicit a grasp reflex, which would shorten the measurement. Measurements were performed by the researchers only (L.V.W., J.S.) to ensure a consistent measurement technique. Both feet were measured. GA was noted from the obstetric admission notes—GA as calculated by the last menstrual age (GALMP) and early ultrasound (GAUS) was noted. A Ballard score [18] (GAB) was also performed within the first 24 h of life.
Fig. 1.
Verniere's caliper to measure neonatal foot length.
Ethical approval for the larger study was obtained from the University of Stellenbosch, Human Research committee (IRB 0005239, N10/07/219).
Statistical analysis was performed using NTSS. Numerical data were presented as the mean, standard deviation, percentages and 95% confidence intervals (95% CIs). Student's independent t-test, the paired t-test, Pearson’s correlation coefficient and least square regression were used to compare measurements.
RESULTS
From the larger study group, 200 infants (31.1%) had early antenatal ultrasounds available for analysis. The following infants were excluded:
15 (7.5%) on clinical grounds—myelomeningocele, short humerus and femurs noted on antenatal ultrasound, congenital cardiac anomaly, nonspecific congenital abnormalities;
34 (17%) were classified as SGA;
2 (1%) were classified as LGA;
64 (32%) were multiple pregnancy infants of which 27 (42.1%) were SGA and 3 (4.6%) were LGA.
Eighty-five infants were AGA and were available for analysis (Table 2).
Table 2.
Study population characteristics
| GA | n | Foot length | Birth weight | Head circumference | Length |
|---|---|---|---|---|---|
| mm | g | cm | cm | ||
| Weeks | 85 | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD |
| 24 | 1 | 50 | 840 | 24 | 34 |
| 25 | 0 | ||||
| 26 | 3 | 48.3 ± 3.05 | 856.6 ± 136.5 | 24.6 ± 0.57 | 33.3 ± 1.52 |
| 27 | 2 | 45.5 ± 0.70 | 739.5 ± 84.14 | 24.5 ± 0.70 | 32.5 ± 2.12 |
| 28 | 14 | 53.2 ± 2.75 | 1029.57 ± 189.09 | 26.07 ± 1.29 | 36 ± 2.39 |
| 29 | 7 | 56.8 ± 4.78 | 1192.8 ± 244.11 | 27 ± 1.38 | 37.5 ± 1.94 |
| 30 | 6 | 59 ± 6.78 | 1323.3 ± 347.31 | 27.5 ± 2.1 | 38 ± 3.6 |
| 31 | 7 | 61.4 ± 1.99 | 1491.4 ± 149.6 | 28.3 ± 1.15 | 39.6 ± 2.93 |
| 32 | 9 | 62.2 ± 6.32 | 1668.9 ± 254.43 | 29.5 ± 1.1 | 41.7 ± 5.08 |
| 33 | 5 | 68.0 ± 4.24 | 2032.0 ± 227.09 | 30.6 ± 2.04 | 44.8 ± 1.64 |
| 34 | 6 | 64.8 ± 2.23 | 1845.0 ± 139.53 | 31.2 ± 0.99 | 42.1 ± 3.13 |
| 35 | 4 | 66.0 ± 7.53 | 2181.0 ± 632.92 | 31.5 ± 2.07 | 45.1 ± 2.66 |
| 36 | 2 | 71 ± 7.07 | 2290 ± 296.98 | 32.5 ± 0.70 | 42.5 ± 0.70 |
| 37 | 4 | 72.5 ± 2.36 | 3266.7 ± 269.13 | 34.2 ± 1.26 | 48.7 ± 4.03 |
| 38 | 5 | 75.0 ± 4.79 | 3468.4 ± 714.72 | 33.9 ± 0.89 | 49.7 ± 5.11 |
| 39 | 4 | 75.2 ± 2.21 | 3007.7 ± 386.58 | 34 ± 1.41 | 49 ± 2.16 |
| 40 | 3 | 80.0 ± 4.0 | 3293.3 ± 340.78 | 34.7 ± 1.53 | 50.8 ± 3.25 |
| 41 | 2 | 76 ± 0 | 3610 ± 183.85 | 34.7 ± 1.06 | 53.5 ± 2.12 |
| 42 | 1 | 75 | 3480 | 35 | 56 |
Inter-observer (L.V.W. and J.S.) correlation of foot length measurement was high (r = 0.984, r2 = 0.969). Intra-observer (L.V.W.) correlation of foot length measurement was high (r = 0.993, r2 = 0.987). Right and left foot length correlation coefficient was high (r = 0.968).
The male : female ratio of the study population was 47 : 38. The majority of the infants were of mixed ethnic origin (65.9%) and African origin (33.9%). There were no Caucasian or Asian infants in the study group. Ethnicity had no statistically significant effect on foot length (p = 0.961).
There was significant correlation between foot length and GA (r = 0.887), birth weight (r = 0.920), length (r = 0.906) and head circumference (r = 0.903).
GA model construction
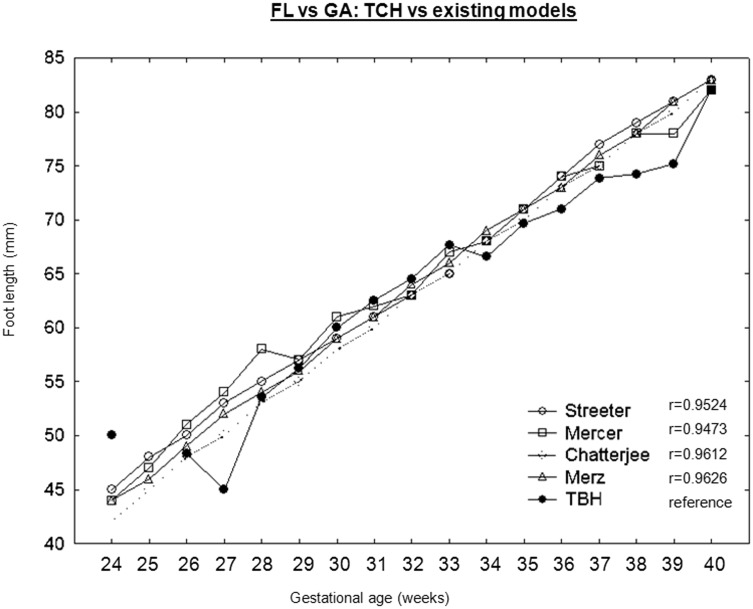
Existing foot length-derived GA models were identified from the literature [6–11, 20]. Studies were excluded because of clear ethnic differences [9] from the Tygerberg population, no available data for >37 weeks of GA [20] and incomplete data for <28 weeks of GA [11]. The median foot lengths were available in all models and were used for comparison with the Tygerberg study group. Least square regression showed that the Merz model [10] most closely resembled the Tygerberg data (r = 0.9611) (Fig. 2).
Fig. 2.
Comparison of Tygerberg's foot length data with existing foot length models.
A modified Merz model (Table 3) was then used to determine the gestational age (GAFL) of the study infants and compare with the gestational age as determined by Ballard (GAB), LMP (GALMP) and antenatal ultrasound (GAUS). Gestational age, as determined by early ultrasound (GAUS), was taken as the gold standard.
Table 3.
Model used to determine GA
| GA | Foot length (mm) |
|---|---|
| <24 | <44 |
| 24 | 44.1–45.9 |
| 25 | 46–48.9 |
| 26 | 49–51.9 |
| 27 | 52–53.9 |
| 28 | 54–55.9 |
| 29 | 56–58.9 |
| 30 | 59–60.9 |
| 31 | 61–63.9 |
| 32 | 64–65.9 |
| 33 | 66–68.9 |
| 34 | 69–70.9 |
| 35 | 71–72.9 |
| 36 | 73–75.9 |
| 37 | 76–77.9 |
| 38 | 78–80.9 |
| 39 | 81–82.9 |
| 40 | 83–4.9 |
| 40+ | >85 |
Adapted from Merz data [10].
t-tests showed that GALMP was the least accurate when compared with GAUS (p = 0.0004, 95% CI 0.233; 0.743). GAB caused a severe underestimation of GA (p = 0.005, 95% CI −0.854; −0.157) when compared with GAUS. GAFL was more accurate than the GAB or GALMP (p = 0.05, 95% CI −0.0005; 0.871).
DISCUSSION
In this study, foot length-derived GA was found to be more accurate than LMP or Ballard-determined GA. In a comparison of GA determination methods in a low-resource setting [21], it was found that the Ballard score underestimated the GA, while the Dubowitz score overestimated the GA in a preterm infant population in Bangladesh. The clinical use of these neonatal scores is compromised by the need for sufficient training and clinical skills needed by the health care workers to accurately apply these scores. Foot length measurement with the Verniere's caliper requires minimal training, is faster and can therefore be used by all levels of medical personnel. In contrast to the neonatal scores, foot length measurements also cause minimal disturbance to the infant.
In this study, only one-third of mothers had accurate pregnancy dating with antenatal ultrasound before 23 of weeks gestation. In an Indian study [20], it was found that antenatal ultrasound was available for 10–70% of women. In a South African study [22], it was shown that ultrasound rates before 18 weeks were low (37.3%) and only slightly better when performed at 24 weeks of gestation (65.1%).
Foot length was shown to correlate well with GA, birth weight, length and head circumference in the present study. It was shown not to be influenced by sex or race.
A commercially available plastic sliding Verniere’s caliper was used in this study. Different measurement techniques have been used to measure the fetal/infant foot (sonar [2, 4–6], calipers [3, 8], footprints [10] or specifically designed instruments [9]), thereby not allowing a single method of measurement to stand out as the ideal. This study showed a high degree of inter- and intra-observer agreement, making this method of measurement easy to apply and rapid to perform.
Foot length has also been used to identify very low birth weight (VLBW) babies and was able to decrease the mortality rate in Tanzania [23] by enabling the identification of at-risk infants. The study used foot lengths of <7 cm and <8 cm to identify VLBW and low birth weight (LBW)/preterm infants, respectively. The sensitivity of foot length was 75%, 87% and 93% for VLBW, LBW and prematurity, respectively. Specificity was 99%, 60% and 58%, respectively. Positive predictive values were low (43%), but negative predictive values were high (96% for LBW and 99% for VLBW). Foot length measurement therefore is a simple, acceptable and inexpensive screening tool to improve neonatal care.
LMP and ultrasound comparisons [21] of GA determination have shown ethnical discrepancies, with more non-Caucasians being falsely identified as preterm. The effect of ethnicity on foot length also varies in published literature. Muskhar et al. [5] suggested that there was a relationship between race and foot length. Their study population's (mostly Indian) foot lengths were consistently 1 cm less than the data previously published by Streeter. The authors determined that this was because of the lower birth weight of their population. Munsnick et al. [25], however, found no ethnical differences.
Previous studies have shown a 4–8% difference in foot lengths in SGA infants [7]. In this present study, one-third of the infants were SGA, with the known range for the study population varying from 12.1% [20] to 42% [25]. The effects of fetal growth can be expected to affect foot length measurements, as foot measurements incorporate bone and soft tissue. Soft tissue stores of subcutaneous fat are decreased in SGA infants and may be increased in LGA infants [26]. This may affect the accuracy of foot length measurements in these populations.
Genetic and anatomical abnormal infants were excluded from this study. Sherwood et al. [27] showed that foot length estimations caused a bias of 2–3 weeks in GA in various genetic and chromosomal abnormalities (trisomy 18 and 21, Turner syndrome, anencephaly, spina bifida and renal agenesis)
We acknowledge various weaknesses of this study. There is a lack of ethnical representivity, and a larger study is required to determine if the Merz model is applicable to areas where more Indian and Caucasian infants are prominent. This study’s sample size precluded further analyses to define the effect of antenatal steroids, multiple pregnancies and SGA/LGA on foot length. Training for foot length measurements was not undertaken, and further research is required to determine the applicability of this measurement in areas with less-skilled medical staff.
Despite the weaknesses of the study, there are obvious advantages. The method of measurement is easy to teach and uses a cheap and easily acquired piece of equipment, making it applicable for a low-resource setting. It can be easily inserted through incubator port holes, thereby requiring less manipulation and causing less distress to premature or ill infants. The method is easy to perform with a low inter-observer variability.
CONCLUSION
Foot length can be used with high accuracy to determine the GA in a population where there is poor access to or utilization of antenatal sonar. A larger cross-sectional study is required to facilitate the building of a South African foot length model and to confirm this pilot study.
ACKNOWLEDGEMENTS
The authors thank Prof L Geerts (Department of Obstetrics and Gynecology, University of Stellenbosch and Tygerberg Hospital), Dr C.J.M. Muller (Department of Statistics, University of Stellenbosch) and Prof Maritz (statistician, retired).
REFERENCES
- 1.Ananth CV. Menstrual versus clinical estimate of gestational age dating in the United States: temporal trends and variability in indices of perinatal outcome. Paediatr Perinat Epidemiol 2007;21(Suppl. 2):22–30. [DOI] [PubMed] [Google Scholar]
- 2.Tentoni S, Astolfi P, De Pasquale A, et al. Birthweight by gestational age in preterm babies according to a Gaussian mixture model. BJOG 2004;111:31–7. [DOI] [PubMed] [Google Scholar]
- 3.Lynch CD, Zhang J. The research implications of the selection of a gestational age method. Pediatr Perinat Epidemiol 2007;21:86–96. [DOI] [PubMed] [Google Scholar]
- 4.Hunter LA. Issues in pregnancy dating: revisiting the evidence. J Midifery Womens Health 2009;54:184–90. [DOI] [PubMed] [Google Scholar]
- 5.Chervenak FA, Skupski DW, Romero R, et al. How accurate is fetal biometry in the assessment of fetal age? Am J Obstet Gynecol 1998;178:678–87. [DOI] [PubMed] [Google Scholar]
- 6.Donovan EF, Tyson JE, Ehrenkranz RA, et al. Inaccuracy of Ballard scores before 28 weeks’ gestation. J Pediatrics 1999;135:147–52. [DOI] [PubMed] [Google Scholar]
- 7.Mercer BM, Sklar S, Shariatmadar A, et al. Fetal foot length as a predictor of gestational age. Am J Obstet Gynecol 1987;156:350–5. [DOI] [PubMed] [Google Scholar]
- 8.Streeter GL. Weight, sitting height, head size, foot length and menstrual age of the human embryo. Contrib Embryol Carnegie Inst 1920;11:143. [Google Scholar]
- 9.Chatterjee MS, Izquierdo LA, Nevils B, et al. Fetal foot: evaluation of gestational age. In: Proceeding of the WFUMB, Sydney, Australia, July 14-19, 1986, p. 206. [Google Scholar]
- 10.Mhaskar R, Agarwal N, Takkar D, et al. Fetal foot length - a new parameter of gestational age. Int J Gynecol Obstet 1988;29:35–8. [DOI] [PubMed] [Google Scholar]
- 11.Merz E, Oberstein A, Wellek S. Age-related reference ranges for fetal foot length. Ultraschall Med 2000;21:79–85 [DOI] [PubMed] [Google Scholar]
- 12.Usher R, McLean F. Intrauterine growth of live-born Caucasian infants at sea level: standards obtained from measurements in 7 dimensions of infants born between 25 and 44 weeks gestation. J Pediatr 1969;74:901–10. [DOI] [PubMed] [Google Scholar]
- 13.James DK, Dryburgh EH, Chiswick ML. Foot length – a new and potentially useful measurement in the n eonate. Arch Dis Child 1979;54:226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jordaan HV. Fetal foot length. SAMJ 1982;62:473–5. [PubMed] [Google Scholar]
- 15.Meirowitz NB, Ananth CV, Smulian JC, et al. Foot length in fetuses with abnormal growth. J Ultrasound Med 2000;19:201–5. [DOI] [PubMed] [Google Scholar]
- 16.Ho T, Ou S, Huang S, et al. Assessmnet of growth from foot length in Taiwanese neonates. Pediatr Neonatol 2009;50:287–9. [DOI] [PubMed] [Google Scholar]
- 17.Geerts L, Theron AM, Grove D, et al. A community-based obstetric ultrasound service. Int J Obstet Gynecol 2004;84:23–31. [DOI] [PubMed] [Google Scholar]
- 18.Fenton TR. A new growth chart for preterm infants: Babson and Benda’s chart updated with recent data and a new format. BMC Pediatr 2003;3:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasidharan K, Dutta S, Naranq A. Validity of the new Ballard score until 7th day of postnatal life in moderately preterm neonates. Arch Dis Child Fetal Neonatal Ed 2009;94:F39–44. [DOI] [PubMed] [Google Scholar]
- 20.Akbulut-Yuksel M, Rosenblum D. The Indian Ultrasound Paradox. IZA DP No 6273. http://ftp.iza.org/dp6273.pdf (March 2015, date last accessed). [Google Scholar]
- 21.Rosenberg RE, Ahmed NU, Ahmed S, et al. Determining gestational age in a low resource setting: validity of last menstrual period. J HealthPopul Nutr 2009;27:332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geerts L, Poggenpoel E, Theron G. A comparison of pregnancy dating methods commonly used in South Africa: aprospective sudy. SAMJ 2013;103:552–6. [DOI] [PubMed] [Google Scholar]
- 23.Marchant T, Jaribu J, Penfold S, et al. Measuring newborn foot length to identify small babies in need of extra care: a cross sectional hospitl based study with community follow-up in Tanzania. BMC Public Health 2010;106:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sunjoh F, Njamnshi AK, Titche F, et al. Assessment of gestational age in the Cameroonian newborn infant: a comparison of four scoring methods. J Trop Paeds 2004:50:285–91. [DOI] [PubMed] [Google Scholar]
- 25.Munsick RA. Human fetal extremitty lengths in the interval from 9 to 21 menstrual weeks of pregnanacy. Am J Obstet Gynecol 1984;149:883–7. [DOI] [PubMed] [Google Scholar]
- 26.Kirsten GF, Van Zyl JI, Van Zijl F, et al. Infants of women with severe early pre-eclampsia: the effect of absent end-diastolic umbilical artery Doppler flow velocities on neurodevelopmental outcome. Acta Paediatr 2000;89:556–70. [DOI] [PubMed] [Google Scholar]
- 27.Sherwood RJ, Meindl RS, Robinson HB, et al. Feta age: methods of estimation and effects of pathology. Am J Phys Anthropol 2000;113:305–15. [DOI] [PubMed] [Google Scholar]