Abstract
Objective
To identify patients who are at a higher risk of pathologic circumferential resection margin involvement using preoperative magnetic resonance imaging.
Methods
Between October 2008 and November 2012, 165 patients with locally advanced rectal cancer (cT4 or cT3 with <2 mm distance from tumour to mesorectal fascia) who received preoperative chemoradiotherapy were analysed. The morphologic patterns on post-chemoradiotherapy magnetic resonance imaging were categorized into five patterns from Pattern A (most-likely negative pathologic circumferential resection margin) to Pattern E (most-likely positive pathologic circumferential resection margin). In addition, the location of mesorectal fascia involvement was classified as lateral, posterior and anterior. The diagnostic accuracy of the morphologic criteria was calculated using receiver operating characteristic curve analysis.
Results
Pathologic circumferential resection margin involvement was identified in 17 patients (10.3%). The diagnostic accuracy of predicting pathologic circumferential resection margin involvement was 0.73 using the five-scale magnetic resonance imaging pattern. The sensitivity, specificity, positive predictive value and negative predictive value for predicting pathologic circumferential resection margin involvement were 76.5, 65.5, 20.3 and 96.0%, respectively, when cut-off was set between Patterns C and D. On multivariate logistic regression, the magnetic resonance imaging patterns D and E (P= 0.005) and posterior or lateral mesorectal fascia involvement (P= 0.017) were independently associated with increased probability of pathologic circumferential resection margin involvement. The rate of pathologic circumferential resection margin involvement was 30.0% when the patient had Pattern D or E with posterior or lateral mesorectal fascia involvement.
Conclusions
Patients who are at a higher risk of pathologic circumferential resection margin involvement can be identified using preoperative magnetic resonance imaging although the predictability is moderate.
Keywords: rectal cancer, circumferential resection margin, mesorectal fascia, magnetic resonance imaging, chemoradiotherapy
Introduction
In locally advanced rectal cancer, preoperative long-course chemoradiotherapy (CRT) together with total mesorectal excision (TME) is recommended for improved local control (1). Despite the advance in treatment, local recurrence remains a major problem in locally advanced rectal cancer, and the pathologic circumferential resection margin (pCRM) is one of the key factors that determine local recurrence (2).
During long-course CRT, most of the patients receive a standard radiotherapy (RT) dose of 50.4 Gy in 28 fractions, regardless of the extent of mesorectal invasion. Long-term results from prospective trials show a local recurrence rate (LRR) of 25–56% after preoperative long-course CRT in pCRM-positive patients (3,4). Therefore, achieving a negative pCRM is crucial for successful treatment.
Currently, the most accurate imaging modality to clinically evaluate the pCRM status before TME is high-resolution magnetic resonance imaging (MRI). MRI has been shown to accurately predict the pCRM status who undergo primary TME (5). Although MRI is currently the gold standard approach, after long-course CRT, the diagnostic accuracy of MRI in determining pCRM involvement decreases. Previous studies showed moderate accuracy of 64–92% on predicting mesorectal fascia (MRF) invasion and 33–45% on predicting pCRM involvement using post-CRT MRI (5–8).
We hypothesized that RT dose escalation on the site of MRF invasion would confer a lower pCRM-positive rate. As groundwork, the goal of this research was to identify patients who were at a higher risk of pCRM involvement. Herein, we reviewed the patients with threatened or involved MRF at diagnosis who had received preoperative CRT and then correlated the MRI findings with patients’ pCRM status.
Patients and methods
Patients and pretreatment evaluation
Between October 2008 and November 2012, 379 patients received preoperative CRT for histologically confirmed adenocarcinoma of the rectum. Both pre- and post-CRT rectal MRI were available in 327 patients. Patients with tumour invading adjacent pelvic organs or structures (cT4) and mesorectal infiltration of tumour with a distance of <2 mm from tumour to MRF (cT3mrf+) according to the rectal MRI were included. For patients with lower rectal tumours, the distance from the tumour to the levator muscle was required to be <2 mm, and for patients with tumours presenting at or below the level of the puborectalis sling, tumour invasion into the intersphincteric plane or beyond was required to be present. Patients with a distance of >2 mm from the tumour to MRF (n= 145), whose CRM status was not evaluable due to abscess perforation or stent insertion status (n= 3) and who refused surgery (n= 14) were excluded. Finally, 165 patients were retrospectively analysed. The Institutional Review Board approved this retrospective analysis.
MR imaging and protocols
All pre- and post-CRT rectal MRI examinations were acquired on a 3.0-T system (MAGNETOM TrioTim; Siemens, Erlangen, Germany). Patients received an intramuscular injection of 20 mg of scopolamine butylbromide (Buscopan, Boehringer Ingelheim) before the MR imaging in order to reduce the colonic motility. After endorectal administration of ∼80–100 ml of sonography transmission gel using an enema syringe, axial, sagittal and oblique axial and coronal T2-weighted MRI scans were obtained using a respiratory-triggered echo-train spin-echo sequence with the following parameters: echo time (TE)/repetition time (TR) of 98–118/3800–5310 ms, thickness of 3 mm, echo train length of 17–35, matrix of 320 × 320 to 448 × 314 and field of view of 250 × 250 to 199 × 199.
MRI-based tumour assessment
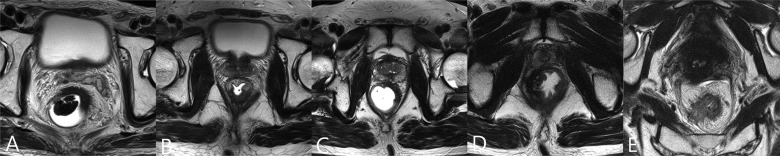
Rectal MRI was performed at a median of 13 days [interquartile range (IQR), 11–17 days] before CRT and a median of 28 days (IQR, 26–29 days) after completing CRT. Two readers with 14 and 7 years of clinical experience as gastrointestinal radiologists retrospectively reviewed the pre- and post-CRT rectal MR images. Each classified their own observations and then they reviewed the images together. Any disagreements were solved by consensus. Although they were aware that the patients had pathologically proven rectal cancer, they were blinded to the pathologic and clinical information. The pre-CRT MR images were referred for the interpretation of post-CRT image findings. The morphologic patterns in post-CRT MRI were divided into five categories by modifying a previously suggested classification (7), as follows (Fig. 1): Pattern A, fat pad larger than 2 mm between the residual tumour mass and the MRF; Pattern B, spiculations extending <2 mm to the MRF; Pattern C, diffuse fibrotic or tumour tissue abutting on the MRF at the initial tumour site without thickening of the MRF itself; Pattern D, diffuse fibrotic or tumour tissue abutting on the MRF with thickening of the MRF itself and Pattern E, diffuse fibrotic or tumour tissue beyond the MRF. The morphologic patterns were evaluated not only by the primary tumour but also by extramural vascular invasion, metastatic mesorectal lymph nodes and tumour deposit.
Figure 1.
The morphologic patterns in transverse T2-weighted magnetic resonance images (MRI) after preoperative chemoradiotherapy. Pattern A is defined as fat pad larger than 2 mm between the residual tumour mass and the mesorectal fascia (MRF). Pattern B is defined as spiculations extending <2 mm to the MRF. Pattern C is defined as diffuse fibrotic or tumour tissue abutting on the MRF at the initial tumour site without thickening of the MRF itself. Pattern D is defined as diffuse fibrotic or tumour tissue abutting on the MRF with thickening of the MRF itself. Pattern E is defined as diffuse fibrotic or tumour tissue beyond the MRF.
The tumour location was defined by a ‘virtual line’ extending from the centre of symphisis pubis to the peritoneal reflection on the sagittal T2-weighted images. Tumours with their lower edges located above the virtual line were defined as upper rectal cancers (9). Another ‘virtual line’ extending from the centre of symphisis pubis to intervertebral junction between the fifth sacral bone and coccyx was drawn to divide the lower and middle rectal cancer, whereas those with their lower edges lying below the line were denoted as lower rectal cancers. In patients with tumours located above the anterior peritoneal reflection, the MRF involvement was evaluated for the non-serosa-covered posterior side.
The involved site of MRF was categorized as the posterior side, defined as the posterior one-fourth of the circumference facing the sacrum; the anterior side, defined as the anterior one-fourth of the circumference or the lateral side, defined as the remaining circumference of the rectum. In cases with more than one site of MRF involvement, they were classified to the more dominantly involved site.
Treatment
Preoperative CRT was delivered for a median of 39 days (IQR, 37–41 days). All patients underwent three-dimensional conformal RT- and CT-based simulation using a custom-made belly board and bladder filling in the prone position (10). The total RT dose was 50.4 Gy in 28 fractions consisting of whole pelvis RT, 45 Gy in 25 fractions followed by boost RT and 5.4 Gy in 3 fractions. The gross tumour and the involved mesorectum were included in the boost volume. The preoperative chemotherapy regimens used were 5-fluorouracil combined with leucovorin (n= 126), irinotecan combined with S-1 (n= 23) (11), and capecitabine (n= 15). Only one patient refused boost RT, and another refused concurrent chemotherapy during RT.
Surgery was performed 6–8 weeks after the completion of CRT. Our surgical techniques have been described previously (12,13). In brief, either lower anterior resection or abdominoperineal resection was performed depending on the tumour location. All patients received complete TME, but in some cases, extended TME was applied for complete removal in patients with tumours penetrating the MRF and/or invading the pelvic organs according to preoperative MRI. The decision to use extended TME was made based on multidisciplinary conferences. In some cases, the adherence was difficult to differentiate from tumour invasion or fibrosis after CRT, and the surgeon had to decide during surgery whether to resect the whole organ or only a part of the affected organ. A positive pCRM was defined as having <1 mm distance between the tumour and the resection margin. Tumour regression was classified according to the Mandard regression grading system (14).
Follow-up evaluation
After surgery, all patients were followed at 3-month intervals for the first 3 years, 6-month intervals for the next 2 years and annually thereafter. Follow-ups included physical examination, endoscopy, chest radiography, serum carcinoembryonic antigen, abdominal pelvic CT and toxicity evaluation. When recurrence was suspected, histological confirmation, MRI or FDG-PET was performed for further assessment. Local recurrence was defined as a recurrent tumour within the pelvis, and all other recurrences were defined as distant metastases.
Statistical analysis
The Pearson's χ2 test or Fisher's exact test, as appropriate, was used to compare characteristics or to evaluate the correlation between the pCRM-positive rate and post-CRT MRI factors. The diagnostic accuracy for determining pCRM involvement using the five patterns was evaluated by a receiver operating characteristic curve (ROC) analysis, and the area under the curve (AUC) was calculated with 95% confidence intervals (CIs). Optimal cut-off values were set where the Youden Index was maximized. The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were also calculated. The cumulative LRR was defined as the time from the start of preoperative CRT to local recurrence. The LRR was calculated using the competing risk analysis considering death as a competing risk and was compared using the Gray's test (15). Univariate and multivariate odds ratios (ORs) associating preoperative clinical variables with pCRM were calculated via logistic regression. Univariate and multivariate Fine and Gray's regression analysis was used to analyse prognostic factors for LRR (16). For the multivariate model, we used stepwise regression with variables entered into the multivariate models at P< 0.15 and removed if P> 0.15. All tests were two sided and considered significant at P< 0.05. Statistical analyses were performed using IBM SPSS version 20.0 (SPSS, Chicago, IL) and the open-source statistical software R (version 2.12.1, R Development Core Team, Vienna, Austria).
Results
Patient characteristics
The patient characteristics are summarized in Table 1. Preoperative CRT was well tolerated. After CRT, 57 patients were down-staged from cT3 to ypT0-2 and 19 patients were down-staged from cT4 to ypT0-3. Adjacent organ or structure invasion was observed radiologically in 24 patients: vagina (n= 4), uterus (n= 4), seminal vesicle (n= 6), prostate (n= 5) and levator ani muscle (n= 5). Among the 19 patients with pelvic organ invasion, 11 patients received resection of the affected organ and eight patients did not due to lack of evidence of pelvic organ involvement during surgery. All five patients with levator muscle invasion received extended TME along the invaded levator muscle.
Table 1.
Patient characteristics
| Characteristic | n | % |
|---|---|---|
| Age, mean (±SD), year | 57.8 (±11.9) | |
| Sex | ||
| Male | 113 | 68.5 |
| Female | 52 | 31.5 |
| ECOG performance status | ||
| 0 | 37 | 22.4 |
| 1 | 128 | 77.6 |
| Location | ||
| Upper | 10 | 6.1 |
| Middle | 85 | 51.5 |
| Lower | 70 | 42.4 |
| Clinical T stage | ||
| cT3mrf+ | 141 | 85.5 |
| cT4 | 24 | 14.5 |
| Clinical N stage | ||
| cN0 | 15 | 9.1 |
| cN+ | 150 | 90.9 |
| Pathological T stage | ||
| ypT0 | 24 | 14.5 |
| ypT1 | 4 | 2.4 |
| ypT2 | 33 | 20.1 |
| ypT3 | 98 | 59.4 |
| ypT4b | 6 | 3.6 |
| Pathological N stage | ||
| ypN0 | 109 | 66.1 |
| ypN1 | 49 | 29.7 |
| ypN2 | 7 | 4.2 |
| Pathologic CRM | ||
| Negative | 148 | 89.7 |
| Positive | 17 | 10.3 |
| Lymphovascular invasion | ||
| No | 153 | 92.7 |
| Yes | 12 | 7.3 |
| Perineural invasion | ||
| No | 159 | 96.4 |
| Yes | 6 | 3.6 |
| Mucinous component | ||
| No | 143 | 86.7 |
| Yes | 22 | 13.3 |
| Histologic grade | ||
| WD | 25 | 15.2 |
| MD | 129 | 78.2 |
| PD | 6 | 3.6 |
| SRC | 3 | 1.8 |
| Unknown | 2 | 1.2 |
| Tumour regression grade | ||
| TRG 1 | 23 | 13.9 |
| TRG 2 | 43 | 26.1 |
| TRG 3 | 67 | 40.6 |
| TRG 4 | 32 | 19.4 |
| Type of resection | ||
| LAR | 135 | 81.8 |
| APR | 30 | 18.2 |
| Adjuvant chemotherapy | ||
| Administered | 148 | 89.7 |
| Not administered | 17 | 10.3 |
ECOG, Eastern Cooperative Oncology Group; cT3mrf+, mesorectal infiltration with a distance of <2 mm from tumour to mesorectal fascia; CRM, circumferential resection margin; WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated; SRC, signet ring cell carcinoma; TRG, tumour regression grade; LAR, low anterior resection; APR, abdominoperineal resection.
Predictors for pCRM involvement following preoperative CRT
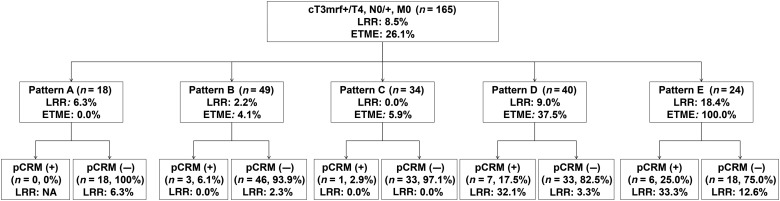
The patients were categorized as Pattern A (n= 18), B (n= 49), C (n= 34), D (n= 40) or E (n= 24). pCRM involvement was identified in 17 patients (10.3%). The rate of pCRM involvement was stratified by MRI patterns and an increasing frequency of pCRM involvement was observed from Pattern A to Pattern E (Fig. 2, P= 0.014). There was a sharp increase in pCRM-positive rate from Pattern C (2.9%) to Pattern D (17.5%). The diagnostic accuracy (AUC) of MRI patterns for predicting a positive pCRM was 0.73 (95% CI, 0.61–0.85). Using ROC analysis, the optimal cut-off was set between Patterns C and D. By using the optimal cut-off, the sensitivity, specificity, PPV and NPV for predicting pCRM involvement were 76.5, 65.5, 20.3 and 96.0%, respectively.
Figure 2.
The pathologic circumferential resection margin (pCRM)-positive rate, 3-year local recurrence rate (LRR) and rate of extended total mesorectal excision (ETME) performed stratified by magnetic resonance imaging patterns.
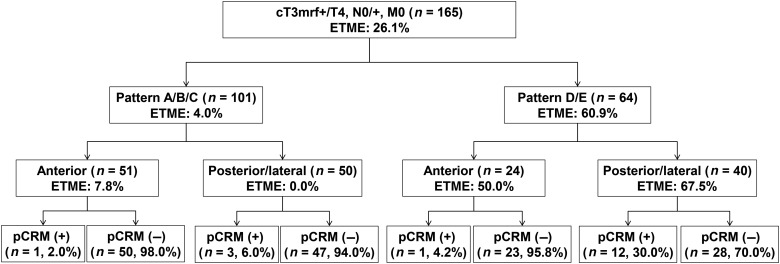
The circumferential location of the site of MRF involvement on MRI was on the anterior side in 75 patients (45.4%) and posterior/lateral side in 90 patients (54.6%). Six patients who showed both lateral and anterior side involvement were classified as having lateral side involvement. The pCRM-positive rates were 2.7 and 16.7% for anterior and posterior/lateral side involved tumours, respectively (P= 0.003). Using a multivariable model, Pattern D or E (OR, 5.52; 95% CI, 1.67–18.21) and posterior/lateral MRF involvement on MRI (OR, 6.41; 95% CI, 1.38–29.70) were independently associated with a higher risk of pCRM involvement (Table 2). By stratifying patients according to both MRI pattern and site of MRF invasion, the pCRM-positive rate was 30% in patients with both Pattern D or E and posterior/lateral MRF involvement (Fig. 3).
Table 2.
Univariate and multivariate logistic regression analyses for pathologic circumferential resection margin involvement
| Variable | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age (>60 vs. ≤60) | 0.87 | 0.31–2.48 | 0.796 | |||
| Sex (female) | 1.60 | 0.57–4.48 | 0.369 | |||
| Clinical T stage (T4 vs. T3mrf+) | 1.97 | 0.58–6.64 | 0.275 | |||
| Clinical N stage (N1 vs. N0) | 1.67 | 0.21–13.57 | 0.631 | |||
| Mucinous component (yes vs. no) | 0.85 | 0.18–4.02 | 0.841 | |||
| Tumour location (upper vs. mid/lower) | 0.43 | 0.08–2.21 | 0.311 | |||
| Type of resection (APR vs. LAR) | 2.82 | 0.95–8.35 | 0.062 | |||
| Chemotherapy regimen (TI vs. FL/capecitabine) | 1.25 | 0.27–5.87 | 0.777 | |||
| MRI pattern (D/E vs. A/B/C) | 6.18 | 1.92–19.93 | 0.002 | 5.52 | 1.67–18.21 | 0.005 |
| Site of MRF involvement (posterior/lateral vs. anterior)a | 7.30 | 1.61–33.05 | 0.010 | 6.41 | 1.38–29.70 | 0.017 |
OR, odds ratio; CI, confidence interval; T3mrf+, mesorectal infiltration with a distance of <2 mm from tumour to mesorectal fascia; APR, abdominoperineal resection; LAR, low anterior resection; TI, irinotecan and S-1; FL, 5-fluorouracil and leucovorin; MRI, magnetic resonance imaging; MRF, mesorectal fascia.
aEvaluated according to the preoperative magnetic resonance imaging.
Figure 3.
The pCRM-positive rate and rate of ETME performed stratified by magnetic resonance imaging based patterns and site of mesorectal fascia involvement.
Local recurrence
At a median follow-up time of 36.8 months (IQR, 28.5–48.6 months), the 3-year LRR was 8.5%. Eight patients had recurrence at the local site only and two patients had synchronous local and distant recurrence. Local recurrences were confirmed pathologically by surgery or biopsy in five patients and clinically by sequential radiologic follow-up in five patients. Among the 10 patients with local recurrences, 2 were not related to the CRM. The two non-CRM-related local recurrences were pelvic lymph node recurrence and mucosal recurrence at the anastomosis site. The two local recurrences in patients with Patterns A and B were non-CRM-related local recurrences, and CRM-related local recurrences were only evident in patients with Pattern D or E. The 3-year LRR was significantly higher in patients with Patterns D and E (Fig. 2, Table 3). However, among the patients with a negative pCRM, the 3-year LRR of Patterns A (6.3%), B (2.3%), C (0.0%), D (3.3%) and E (12.6%) were not statistically significant (Gray's P= 0.290). The circumferential location of the site of MRF involvement and pCRM status was also a significant predictor of local recurrence along with the MRI patterns, but only pCRM status remained significant after multivariate analysis (Table 3).
Table 3.
Univariate and multivariate Fine and Gray's regression analyses for cumulative incidence of local recurrence
| Variable | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age (>60 vs. ≤60) | 1.16 | 0.33–4.07 | 0.822 | |||
| Sex (female vs. male) | 0.95 | 0.24–3.800 | 0.947 | |||
| ECOG (1 vs. 0) | 0.81 | 0.22–2.92 | 0.745 | |||
| MRI pattern (D/E vs. A/B/C) | 6.69 | 1.43–31.20 | 0.016 | 3.85 | 0.83–17.90 | 0.086 |
| Site of MRF involvement (posterior/lateral vs. anterior)a | 8.00 | 1.11–57.50 | 0.038 | 4.71 | 0.63–35.30 | 0.131 |
| Pathologic CRM (positive vs. negative) | 9.60 | 2.81–32.8 | <0.001 | 4.32 | 1.07–17.50 | 0.041 |
| Pathologic T stage (pT3/4 vs. pT0-2) | 5.48 | 0.70–42.70 | 0.104 | |||
| Pathologic N stage (pN1/2 vs. pN0) | 1.99 | 0.58–6.79 | 0.272 | |||
| Lymphovascular invasion (yes vs. no) | 1.45 | 0.20–10.70 | 0.716 | |||
| Mucinous component (yes vs. no) | 0.78 | 0.10–6.29 | 0.813 | |||
| Histologic grade (PD vs. WD-MD) | 1.68 | 0.42–6.67 | 0.463 | |||
| Tumour regression grade (TRG 3/4 vs. TRG 1/2) | 1.16 | 0.34–3.95 | 0.811 | |||
| Type of resection (APR vs. LAR) | 1.04 | 0.24–4.58 | 0.961 | |||
HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; MRI, magnetic resonance imaging; MRF, mesorectal fascia; CRM, circumferential resection margin; PD, poorly differentiated; WD, well differentiated; MD, moderately differentiated; TRG, tumour regression grade; APR, abdominoperineal resection; LAR, low anterior resection.
aEvaluated according to the preoperative magnetic resonance imaging.
Details of extended TME
Forty-three patients underwent extended TME, and the post-CRT MRI patterns of these patients were Patterns B (n= 2), C (n= 1), D (n= 16) and E (n= 24). The rate of receiving extended TME was significantly different among the different patterns (Fig. 2; P< 0.001) but did not differ between patients who had anterior MRF invasion (21.3%) and posterior/lateral MRF invasion (30.0%) (Fig. 3; P= 0.207). The three patients with Patterns B and C were suspected of pelvic organ invasion during surgery; however, on histopathology, these patients were found to have fibrosis instead. Among the 43 patients, 14 patients received en bloc tumour resection by resecting the affected pelvic organ; these cases involved hysterectomy (n= 5), seminal vesicle resection (n= 5), vaginectomy (n= 3) and partial resection of the prostate (n= 1). The final histopathological T stages after extended TME were ypT4b (n= 6), ypT3 (n= 32), ypT2 (n= 1) and ypT0 (n= 4). There were no patients with bone invasion, and no patients received combined resection of the sacrum or coccyx. Only 1 of the 14 patients who received resection of the affected pelvic organ had a positive pCRM on the resected pelvic organ side, while 9 of the other 29 patients had a positive pCRM.
Discussion
Our study assessed the performance of post-CRT MRI for the prediction of pCRM involvement in patients who presented with locally advanced rectal cancer. pCRM is a well-established prognostic factor in local recurrence (2) and it was also observed in our study. Although patients with more locally advanced disease on preoperative evaluation (Patterns D and E) showed higher LRR, the LRR of these patients became similar to that of the patients with less locally advanced disease (Patterns A, B and C) as long as a negative pCRM was achieved. Thus, achieving a clear resection margin should be one of the main goals in treating patients with rectal cancer. By using the post-CRT MRI, we were able to identify the patient subgroup that was at a higher risk of pCRM-positive resection.
Previously, Vliegen et al. (7) investigated the accuracy of MRI in detecting MRF invasion by classifying MRF invasion into four categories. In addition to the distance of tumour tissue from MRF, they attempted tissue characterization through the analysis of signal intensity changes after preoperative CRT. Although a diffuse hypointense signal in T2-weighted images represents fibrotic change after CRT, limitations in detecting small tumour foci within the fibrotic tissue are a major concern. Due to the possibility of excluding residual viable tumour foci, we did not incorporate signal intensity changes in the classification. Including the report from the MERCURY Study Group, CRM involvement on MRI was normally defined as a distance from tumour to MRF of <1 mm (5). However, in our study, patients with tumour abutment on the MRF were further divided into either Pattern C or D according to the thickening of the MRF itself. There was a steep increase in the pCRM-positive rate between Patterns C and D, indicating that the thickening of the MRF itself was a strong predictor of pCRM involvement.
In our study, the diagnostic accuracy for predicting pCRM involvement was 0.73 when using the morphologic patterns in post-CRT MRI divided into five categories. The AUC was smaller compared with previous studies, which showed an AUC of 0.80 or as high as 0.89 (7,8). A recent meta-analysis showed a sensitivity and specificity of 76.3 and 85.9%, respectively, for CRM prediction using MRI after preoperative CRT (6). These figures were higher than the data from our study. However, the predictive values may differ according to the extent of surgery and whether the diagnostic end point is to predict the pCRM involvement or MRF invasion. If a patient who showed Pattern E received extended TME and showed MRF invasion with a negative pCRM on pathology, the diagnostic accuracy would increase if the end point is MRF invasion, but it would decrease if the end point is pCRM involvement. The previous study that showed a higher diagnostic accuracy than that of our study focussed on MRF invasion rather than the surgical margin (7). In our study, a majority of patients with suspected MRF invasion on MRI underwent extended TME, and some achieved a negative margin through this extensive surgery even though they had MRF invasion. This resulted in more false-positive patients and eventually weakened the diagnostic accuracy in our study. A recent report from the MERCURY group prospectively validated MRI as assessing the surgical plain of low rectal cancer (17). Only 20.4% of patients who had a suspicious involvement of the surgical resection plane on MRI after preoperative CRT resulted in pCRM involvement. Most of the patients received abdominoperineal resection in this study, which implies that extensive surgery was performed for these patients. As a result, the predictability of pCRM involvement was similar to our study.
The pCRM rate was also influenced by the location of the MRF invasion on MRI. The prognostic importance of the circumferential position of the tumour has been reported by different authors, yet with conflicting results (18,19). Chan et al. (19) reported that an anterior position is an independent negative prognostic factor for both local recurrence and survival, while Garcia-Granero et al. (18) reported that an anterior position did not have prognostic significance. This discrepancy may be due to the definition of tumour location, the inclusion of circumferential tumours and the surgical technique. In our study, the pCRM-positive rate was significantly lower in patients with anterior MRF invasion than in patients with posterior or lateral MRF invasion even though a similar portion of patients received extended TME in both groups. A possible explanation for the lower rate of pCRM involvement in patients with anterior MRF invasion may be the higher possibility of en bloc tumour removal by resection of the affected pelvic organ in patients with anterior MRF invasion. In contrast, a sufficient margin was difficult to be attained in patients with lateral or posterior MRF involvement, even after extended TME, due to the proximity of large vessels and bony structures. In an attempt to reduce the pCRM involvement rate in patients with posterior or lateral MRF invasion, further intensification of preoperative treatment may be necessary due to the limitation of complete surgical removal.
This study has several limitations stemming from its retrospective nature. The classification suggested in this study also needs further external validation. The interobserver agreement was unknown, as the two radiologists performed a consensus review. In addition, we did not review lesion-by-lesion; thus, the site of MRF invasion may have differed from the site of pCRM involvement. However, most of the patients during the study period underwent pre- and post-CRT MRI routinely and a large number of patients were analysed compared with previous reports (4,7,8). Additionally, all images were reviewed by two experienced radiologists specializing in rectal cancer.
In summary, patients at a higher risk of pCRM involvement were able to be identified using the pre- and post-CRT MRI findings according to morphologic characteristics and the involved MRF sites. These results can guide decisions regarding which patients may need more intensive preoperative treatment to secure a negative pCRM. For these high-risk patients, particularly for those who are unable to achieve a clear margin even after extended TME, additional boost RT delivered to the site of MRF invasion might induce further tumour regression and reduce the pCRM-positive rate. A prospective trial evaluating the effect of additional boost RT will be started in the near future.
Funding
This work was supported by faculty research grants from Yonsei University College of Medicine (6-2012-0093 and 6-2011-0139).
Conflict of interest statement
None declared.
References
- 1.Sauer R, Becker H, Hohenberger W et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731–40. [DOI] [PubMed] [Google Scholar]
- 2.Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol 2008;26:303–12. [DOI] [PubMed] [Google Scholar]
- 3.Sauer R, Liersch T, Merkel S et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926–33. [DOI] [PubMed] [Google Scholar]
- 4.Patel UB, Taylor F, Blomqvist L et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol 2011;29:3753–60. [DOI] [PubMed] [Google Scholar]
- 5.MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ 2006;333:779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Paardt MP, Zagers MB, Beets-Tan RG, Stoker J, Bipat S. Patients who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic MR imaging: a systematic review and meta-analysis. Radiology 2013;269:101–12. [DOI] [PubMed] [Google Scholar]
- 7.Vliegen RF, Beets GL, Lammering G et al. Mesorectal fascia invasion after neoadjuvant chemotherapy and radiation therapy for locally advanced rectal cancer: accuracy of MR imaging for prediction. Radiology 2008;246:454–62. [DOI] [PubMed] [Google Scholar]
- 8.Kim SH, Lee JM, Park HS, Eun HW, Han JK, Choi BI. Accuracy of MRI for predicting the circumferential resection margin, mesorectal fascia invasion, and tumor response to neoadjuvant chemoradiotherapy for locally advanced rectal cancer. J Magn Reson Imaging 2009;29:1093–101. [DOI] [PubMed] [Google Scholar]
- 9.Chang JS, Lee Y, Lim JS et al. The magnetic resonance imaging-based approach for identification of high-risk patients with upper rectal cancer. Ann Surg 2014;260:293–8. [DOI] [PubMed] [Google Scholar]
- 10.Chang JS, Yoon HI, Cha HJ et al. Bladder filling variations during concurrent chemotherapy and pelvic radiotherapy in rectal cancer patients: early experience of bladder volume assessment using ultrasound scanner. Radiat Oncol J 2013;31:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin SJ, Kim NK, Keum KC et al. Phase II study of preoperative chemoradiotherapy (CRT) with irinotecan plus S-1 in locally advanced rectal cancer. Radiother Oncol 2010;95:303–7. [DOI] [PubMed] [Google Scholar]
- 12.Kim JS, Cho SY, Min BS, Kim NK. Risk factors for anastomotic leakage after laparoscopic intracorporeal colorectal anastomosis with a double stapling technique. J Am Coll Surg 2009;209:694–701. [DOI] [PubMed] [Google Scholar]
- 13.Kim JS, Hur H, Kim NK et al. Oncologic outcomes after radical surgery following preoperative chemoradiotherapy for locally advanced lower rectal cancer: abdominoperineal resection versus sphincter-preserving procedure. Ann Surg Oncol 2009;16:1266–73. [DOI] [PubMed] [Google Scholar]
- 14.Mandard AM, Dalibard F, Mandard JC et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994;73:2680–6. [DOI] [PubMed] [Google Scholar]
- 15.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988;16:1141–54. [Google Scholar]
- 16.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 17.Battersby NJ, How P, Moran B et al. Prospective validation of a low rectal cancer magnetic resonance imaging staging system and development of a local recurrence risk stratification model: the MERCURY II Study. Ann Surg 2015; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Granero E, Faiz O, Flor-Lorente B, Garcia-Botello S, Esclapez P, Cervantes A. Prognostic implications of circumferential location of distal rectal cancer. Colorectal Dis 2011;13:650–7. [DOI] [PubMed] [Google Scholar]
- 19.Chan CL, Bokey EL, Chapuis PH, Renwick AA, Dent OF. Local recurrence after curative resection for rectal cancer is associated with anterior position of the tumour. Br J Surg 2006;93:105–12. [DOI] [PubMed] [Google Scholar]