Abstract
Objective
The upper gastrointestinal characteristics in Japanese familial adenomatous polyposis patients have not yet been clarified. The aim of the present study was to elucidate these characteristics in Japanese familial adenomatous polyposis patients.
Methods
This study was conducted by the study group for familial adenomatous polyposis in the Japanese Society for Cancer of the Colon and Rectum. Familial adenomatous polyposis patients who underwent surgical resection from 2000 to 2012 were included in the study.
Results
In total, 303 familial adenomatous polyposis patients were enrolled, with 265 cases of classical familial adenomatous polyposis (≥100 adenomas) and 38 cases of attenuated familial adenomatous polyposis (<100 adenomas). Fundic gland polyps were significantly more common in classical familial adenomatous polyposis than in attenuated familial adenomatous polyposis; however, gastric cancer was significantly less common in classical familial adenomatous polyposis than in attenuated familial adenomatous polyposis. Gastric cancer and duodenal adenoma were significantly more common in familial adenomatous polyposis patients with gastric adenoma than in those without gastric adenoma. Duodenal cancer was detected in 7 of 72 familial adenomatous polyposis patients with duodenal adenoma. The median tumour risk in 50-year-old familial adenomatous polyposis patients was 55.3, 21.8, 3.8, 39.2 and 7.7% for fundic gland polyp, gastric adenoma, gastric cancer, duodenal adenoma and duodenal cancer, respectively.
Conclusions
Upper gastrointestinal tumours/polyps were frequently found in familial adenomatous polyposis patients, and their incidences were correlated; however, the frequency of gastric cancer in Japanese familial adenomatous polyposis patients was similar to that in the general population.
Keywords: familial adenomatous polyposis, fundic gland polyp, gastric adenoma, gastric cancer, duodenal adenoma, duodenal cancer
Introduction
Familial adenomatous polyposis (FAP) is an autosomal dominant disease caused by germline mutations in the APC gene (1–4). FAP is characterized by the development of numerous adenomas because of tumour formation caused by inactivation of the APC gene through germline mutations plus somatic mutations of the normal allele or loss of the normal allele. Genotype–phenotype correlations have been reported with respect to the mutation site and the number of colorectal adenomas (5,6). The profuse type of FAP is characterized by the development of numerous adenomas, and these patients have germline APC mutations between codons 1250 and 1464. The sparse type of FAP is characterized by the development of 100–1000 adenomas, and these patients have germline mutations in other regions of the APC gene. Further, the attenuated type of FAP is characterized by the development of few (<100) adenomas, with a later age onset of the symptoms, and these patients have germline mutations located at the 5′ end (7) at exon 9 or at the 3′ distal end (8). FAP patients also develop upper gastrointestinal manifestations, including gastric and duodenal tumours, at high rates, which can cause serious morbidity in patients. Fundic gland polyps (FGPs) are the most common gastric tumours/polyps in FAP patients, and the frequency of FGPs ranges from 20 to 88% in FAP patients (9–11). Gastric adenoma is also common in FAP patients and develops in 9–50% of these patients (12–15). Gastric cancer is more frequently found in Asian FAP patients than in Western FAP patients, which is similar to the corresponding rates in the general population. Particularly, gastric cancer is observed in 2–4% of Asian FAP patients (12,13,16,17), whereas gastric cancer is reportedly rare in Western FAP patients (14,15,18,19). Duodenal adenomatosis is the most common upper gastrointestinal tumour, and adenomatosis-related cancer is the main cause of death in FAP patients. Almost all FAP patients develop duodenal adenomatosis, and the death rate from duodenal cancer has increased from 1.5% before 1990 to 5.6% after 1990 (20).
As mentioned above, the frequencies of upper gastrointestinal tumours in FAP patients change according to the regions and the times. Therefore, to clarify the upper gastrointestinal features in Japanese FAP patients at the current point in time, we analysed the data obtained by the study group for FAP in the Japanese Society for Cancer of the Colon and Rectum (JSCCR).
Patients and methods
The multicentre retrospective cohort study of FAP in Japan was conducted by the study group for FAP in the JSCCR. In this study, we aimed to clarify the following characteristics of Japanese FAP patients:
the prognosis,
the surgical procedure,
the clinical outcome and
the extra-colonic features.
The inclusion criteria of this cohort study comprised FAP patients who underwent surgical resection from January 2000 to December 2012 and who had at least one of the following: (i) >100 adenomas in the colon and rectum, (ii) a family history of FAP if there were <100 polyps in the colon and rectum and (iii) a germline mutation of the APC gene. The cohort study protocol was approved by the JSCCR Ethics Committee and the Institutional Review Board of each centre.
Clinical information, such as personal and family cancer history, was collected either from medical records or directly from the patients. We defined patients with ≥100 adenomas in the colon and rectum as having classical FAP and those with <100 adenomas as having attenuated FAP (AFAP). Mutation data were not collected because it was not possible because of the retrospective nature of this cohort study.
The aim of this upper gastrointestinal cohort study was to clarify the following upper gastrointestinal features in Japanese FAP patient at the current point in time:
the incidences of upper gastrointestinal polyps/tumours,
the correlations of the incidences among the different types of upper gastrointestinal polyps/tumours,
the correlations between specific colonic phenotypes and the incidences of different types of upper gastrointestinal polyps/tumours and
the cumulative incidence of upper gastrointestinal polyps/tumours.
The data are presented as totals, medians (range or standard deviation) or percentages (95% confidence intervals). Statistical analysis was performed using Fisher's exact test and the Mann–Whitney U test. The cumulative cancer risks were calculated using the Kaplan–Meier method, and the log-rank test was used to compare the risk of each type of cancer between classical FAP and AFAP. Statistical significance was defined at the level of P < 0.05. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan; http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmedEN.html; Kanda, 2014), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria; version 3.1.0). This interface is a modified version of R Commander (version 2.1-3) that was designed to add statistical functions frequently used in biostatistics.
Results
In total, 303 FAP patients from 23 institutions were enrolled in the multicentre retrospective cohort study of FAP in Japan, with 265 cases of classical FAP (≥100 adenoma) and 38 cases of AFAP (<100 adenoma). All of the patients were eligible for inclusion in this upper gastrointestinal analysis study. The baseline characteristics of the patients are summarized in Table 1, and the frequencies of the different types of upper gastrointestinal polyps/tumours were 50.2, 14.7, 4.2, 31.7 and 2.8% for FGP, gastric adenoma, gastric cancer, duodenal adenoma and duodenal cancer, respectively.
Table 1.
Baseline characteristics of the patients
| Clinical features | Number of patients |
|---|---|
| Gender (male : female) | 156 : 147 |
| Age at diagnosis (range) | 32.4 (10–75) |
| Colonic phenotype (classical FAP : AFAP) | 265 : 38 |
| Fundic gland polyp (present : absent) | 114 : 113 (50.2%) |
| Gastric adenoma (present : absent) | 33 : 192 (14.7%) |
| Gastric cancer (present : absent) | 11 : 250 (4.2%) |
| Duodenal adenoma (present : absent) | 72 : 155 (31.7%) |
| Duodenal cancer (present : absent) | 7 : 247 (2.8%) |
FAP, familial adenomatous polyposis; AFAP, attenuated familial adenomatous polyposis.
The frequencies of some upper gastrointestinal polyps/tumours were correlated with each other in these Japanese FAP patients (Table 2); particularly, the frequency of FGP was significantly higher in FAP patients with gastric adenoma (P = 0.0003) and duodenal adenoma (P < 0.0001), and the frequency of gastric adenoma was significantly higher in FAP patients with duodenal adenoma (P = 0.0091). As expected, gastric adenoma was correlated with gastric cancer, and similarly, duodenal adenoma was correlated with duodenal cancer. Gastric cancer was significantly frequent in FAP patients with gastric adenoma (P = 0.046), and duodenal cancer was significantly frequent in FAP patients with duodenal adenoma (P = 0.0042).
Table 2.
Upper gastrointestinal polyps and tumours in FAP patients
| FGPs+ | FGPs− | P value | GA+ | GA− | P value | GCa+ | GCa− | P value | DA+ | DA− | P value | DCa+ | DCa− | P value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | 63 | 59 | 0.69 | 17 | 104 | 0.85 | 7 | 127 | 0.54 | 41 | 82 | 0.67 | 5 | 128 | 0.45 |
| Female | 51 | 54 | 16 | 88 | 4 | 123 | 31 | 73 | 2 | 119 | |||||
| Median age at diagnosisa | 33.6 (±10.0) | 38.5 (±14.5) | 0.030 | 34.9 (±9.5) | 36.3 (±13.0) | 0.93 | 50.3 (±15.0) | 35.5 (±12.0) | 0.0022 | 35.6 (±10.5) | 36.7 (±13.5) | 0.99 | 37.9 (±7.0) | 36.2 (±12.7) | 0.36 |
| Classical | 104 | 89 | 0.0094 | 27 | 164 | 0.61 | 7 | 219 | 0.045 | 66 | 127 | 0.071 | 6 | 213 | 0.99 |
| Attenuated | 10 | 24 | 6 | 28 | 4 | 31 | 6 | 28 | 1 | 34 | |||||
| FGPs+ | 24 | 84 | 0.0003 | 5 | 103 | 0.99 | 51 | 56 | <0.0001 | 5 | 97 | 0.27 | |||
| FGPs− | 6 | 107 | 6 | 106 | 16 | 97 | 2 | 107 | |||||||
| GA+ | 24 | 6 | 0.0003 | 4 | 26 | 0.046 | 15 | 13 | 0.0091 | 1 | 29 | 0.99 | |||
| GA− | 84 | 107 | 7 | 184 | 54 | 138 | 6 | 175 | |||||||
| GCa+ | 5 | 6 | 0.99 | 4 | 7 | 0.046 | 4 | 7 | 0.74 | 0 | 11 | 0.99 | |||
| GCa− | 103 | 106 | 26 | 184 | 65 | 144 | 7 | 231 | |||||||
| DA+ | 51 | 16 | <0.0001 | 15 | 54 | 0.0091 | 4 | 65 | 0.74 | 6 | 61 | 0.0042 | |||
| DA− | 56 | 97 | 13 | 138 | 7 | 144 | 1 | 146 | |||||||
| DCa+ | 5 | 2 | 0.27 | 1 | 6 | 0.99 | 0 | 7 | 0.99 | 6 | 1 | 0.0042 | |||
| DCa− | 97 | 107 | 29 | 175 | 11 | 231 | 61 | 146 |
FAP, familial adenomatous polyposis; FGP, fundic gland polyp; GA, gastric adenoma; GCa, gastric cancer; DA, duodenal adenoma; DCa, duodenal cancer; the numbers in parentheses represent the standard deviation.
aIf the patients did not have a diagnosis of upper gastrointestinal polyps/tumours, the median age at the final gastroduodenoscopy is listed.
When comparing the colonic phenotype with the different types of upper gastrointestinal polyps/tumours, FGP and gastric cancer were significantly frequent in the classical FAP patients (P = 0.00094), and duodenal cancer tended to be frequent in the classical FAP patients (P = 0.071). In contrast, gastric cancer was significantly less frequent in the classical FAP patients (P = 0.045).
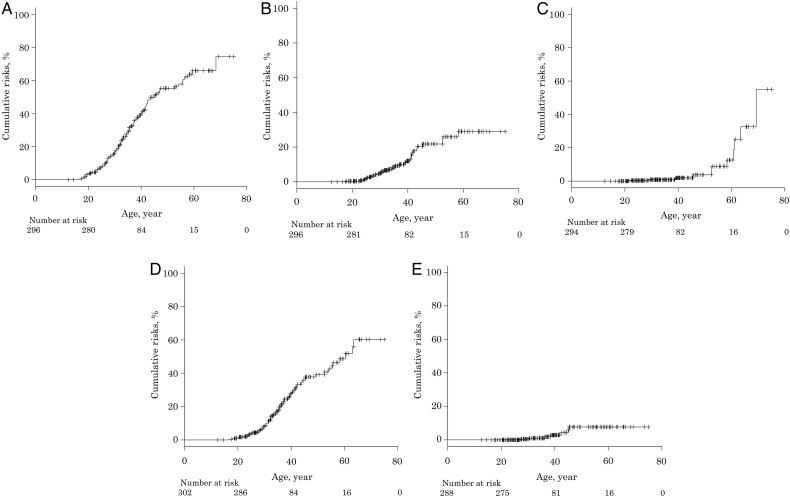
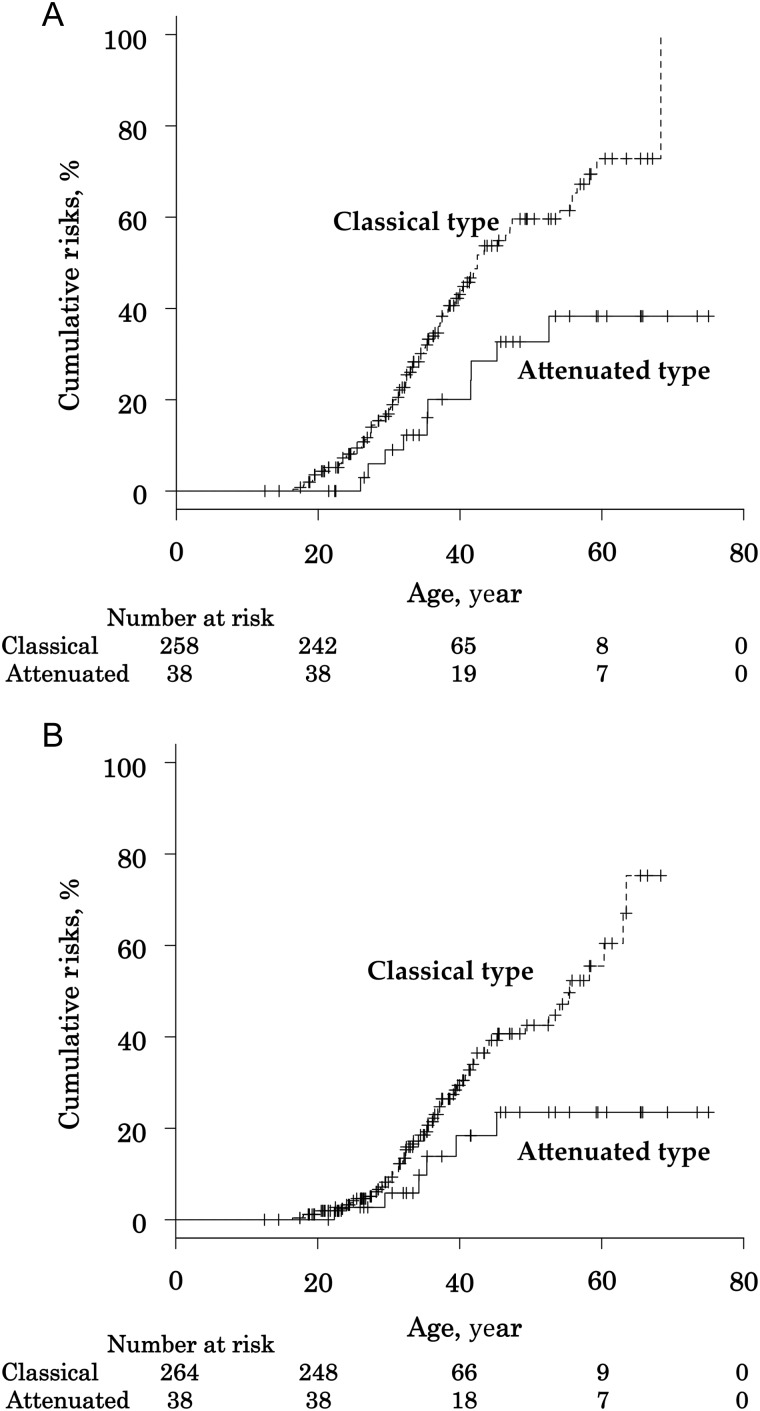
We estimated that the median tumour risk in 50-year-old FAP patients was 55.3% (47.6–63.3%) for FGP, 21.8% (15.1–30.8%) for gastric adenoma, 3.8% (1.2–11.5%) for gastric cancer, 39.2% (31.4–48.2%) for duodenal adenoma and 7.7% (3.5–16.5%) for duodenal cancer (Fig. 1). When comparing the colonic phenotypes, the median tumour risk of FGP [59.5% (51.0–68.4%) for classical FAP; 32.7% (19.3–54.0%) for AFAP; P = 0.0045] and duodenal adenoma [42.5% (33.6–52.7%) for classical FAP; 23.5% (11.0–45.9%) for AFAP; P = 0.013] were significantly higher for classical FAP than for AFAP (Fig. 2); conversely, there were no significant differences of the cumulative incidences of gastric adenoma, gastric cancer and duodenal cancer between classical FAP and AFAP.
Figure 1.
The cumulative incidences of upper gastrointestinal polyps and tumours in familial adenomatous polyposis (FAP) patients. (A) fundic gland polyps, (B) gastric adenoma, (C) gastric cancer, (D) duodenal adenoma and (E) duodenal cancer.
Figure 2.
The cumulative incidences of fundic gland polyps and duodenal adenoma in FAP patients by colonic phenotype. (A) Fundic gland polyps and (B) duodenal adenoma.
Discussion
The present study demonstrated the following: (i) upper gastrointestinal polyps/tumours are common in FAP patients, (ii) the incidences of some of the types of upper gastrointestinal polyps/tumours were correlated with each other as well as with the colonic phenotype of the patients and (iii) the cumulative incidence of upper gastrointestinal polyps/tumours in FAP patients.
Further, the present study revealed FGPs in half of the FAP patients, and the occurrence of FGPs was more frequent in the classical FAP patients than in the AFAP patients. FGPs are considered to be non-neoplastic tumours and are common in FAP patients, with a frequency of 20–88% (9–11).
Some reports have been published concerning the correlation between the upper gastrointestinal phenotype and the APC genotype. Almost all FGPs correspond to somatic mutations of p.T1556Nfs*3 or p.R1450X of the APC gene, whereas FGPs with a germline mutation of p.N1546Lfs*11 of the APC gene demonstrate 5qLOH as second hit in FAP patients (21). Thus, the development of FGPs is related to mutations around codons 1450–1560 in the APC gene. Miyaki et al. (22) analysed gastric adenoma and duodenal adenoma in FAP patients and demonstrated that the truncated APC proteins of these tumours retained two or three 20-amino-acid repeats, in which the APC gene is mutated in codons from 1450 to 1564. Thus, the development of gastric adenoma and duodenal adenoma is related to mutations around codons from 1450 to 1564 in the APC gene. APC germline mutations in this region are associated with the classical colonic phenotype (5,22,23). The above evidence shows that some upper gastrointestinal polyps/tumours, including FGP, gastric adenoma and duodenal adenoma, are more frequently found in classical FAP patients than in AFAP patients; further, the incidences of some upper gastrointestinal polyps/tumours were correlated with each other.
In this study, gastric cancer developed in 4% of Japanese FAP patients. Although Western researchers reported that the risk of gastric cancer is similar between FAP patients and non-FAP patients (19), gastric cancer occurred frequently in Asian FAP patients (16,17). The development of gastric cancer is closely associated with Helicobacter pylori infection (24), which is common in Asia (25). Similar to sporadic cases, H. pylori infection increases the risk of gastric adenoma, the precursor lesion of gastric cancer, in FAP patients (26). These findings suggest that gastric cancer is frequent in Japanese FAP patients.
Duodenal tumour is the most common upper gastrointestinal tumour and occurs in almost all FAP patients. The cumulative incidence of duodenal adenoma at 70 years of age is reportedly 90% in FAP patients (27). In the present study, the cumulative incidence of duodenal adenoma at 50 years of age was 39% in FAP patients. Unlike gastric tumours, the incidence of duodenal adenoma in Asian FAP patients was similar to that in Western FAP patients. With the exception of colorectal cancer, duodenal cancer is one of the most frequent causes of death in FAP patients (20,28). Duodenal adenoma is the precursor lesion of duodenal cancer, and the Spigelman classification has been proposed for assessment of the risk of duodenal cancer in duodenal adenoma patients (29). Age has been reported as being the most important risk factor for duodenal cancer (30). The standardized incidence ratio of duodenal cancer was reported to be 250–330 in FAP patients (16,19), and the cumulative incidence rate of duodenal cancer is reportedly 4.5% at 57 years of age in FAP patients (27). In the present study, we estimated that the cumulative incidence of duodenal cancer was 7.7% at 50 years of age in FAP patients, which was found to increase with age.
We also estimated that the median tumour/polyp risk in 50-year-old FAP patients was 55.3, 21.8, 3.8, 39.2 and 7.7% for FGP, gastric adenoma, gastric cancer, duodenal adenoma and duodenal cancer, respectively. These data are similar to the data from Western countries, except for that of gastric cancer (9–20,27). A recent Japanese report of cancer statistics estimated the lifetime risk of gastric cancer to be 11.2% for males and 5.5% for females (31). As gastric cancer is common in the Japanese general population as well as in Japanese FAP patients, it is difficult to conclude whether the frequency of gastric cancer differs in Japanese FAP patients and the general population.
The present study had some limitations. First, although 303 FAP patients are a relatively large number in this field, our sample size was too small for any definitive conclusions to be made; thus, our findings need to be confirmed by a larger study.
Second, the target study population was FAP patients who had received first colectomy for 12 years of the study period in order to avoid overlap of the patients. However, there may be a decrease in the quality of the data on the development of extra-colonic tumours, such as duodenal cancer; a long-term surveillance is required. Therefore, a long-term study is warranted.
Third, we did not perform genetic analysis of the APC and MUTYH genes, because we could not obtain the patients' informed consent. Thus, the definition of AFAP was important in this research. We defined patients with <100 polyps in the colon and rectum as having AFAP; however, we reduced the risk of false-positive diagnosis of patients with other diseases by enrolling the patients with a family history of FAP.
Fourth, it is important to clarify the nature of ampullary tumours in FAP patients because ampullary lesions can cause obstructive jaundice, acute pancreatitis and cancer. However, we did not distinguish ampulla from non-ampulla in duodenal tumours, because there were no sufficient data to differentiate between the two.
Finally, this study did not include data on H. pylori infection rates. Recent reports have demonstrated that H. pylori infection is associated with FGP and gastric adenoma/cancer risk (24–26,32,33). Therefore, further studies are needed, as H. pylori infection is common in Asia, including Japan.
Nonetheless, considering that we revealed upper gastrointestinal features in FAP patients, we believe that our findings will help researchers and physicians to clarify the upper gastrointestinal features in FAP patients.
In conclusion, FGP was significantly more common in classical FAP patients than in AFAP patients; however, gastric cancer was significantly less common in classical FAP patients than in AFAP patients. Compared with other upper gastrointestinal features, the frequencies of gastric cancer and duodenal adenoma were significantly higher in FAP patients with gastric adenoma than those without gastric adenoma. It is hard to conclude whether the frequency of gastric cancer in Japanese FAP patients is similar to that of the general population; however, similar to Western FAP patients, with the exception of gastric cancer, Japanese FAP patients in this study exhibited high rates of upper gastrointestinal tumour/polyps. Thus, a close surveillance of the upper gastrointestinal tract in FAP patients becomes important.
Authors' contributions
The Japanese Society for Cancer of the Colon and Rectum contributed collectively to this study. All authors contributed to this work—conception and design of this study: Hideyuki Ishida, Toshiaki Watanabe and Kenichi Sugihara; collection and assembly of data, Tatsuro Yamaguchi, Hideki Ueno, Hirotoshi Kobayashi, Takao Hinoi, Yasuhiro Inoue, Fumio Ishida, Yukihide Kanemitsu, Tsuyoshi Konishi, Naohiro Tomita and Nagahide Matsubara; statistical analysis: Tatsuro Yamaguchi; drafting of the article: Tatsuro Yamaguchi, Hideyuki Ishida and Hideki Ueno.
Funding
The present study was supported in part by a grant-in-aid for Cancer Research from the Ministry of Health, Labor and Welfare, and by the Japanese Society for Cancer of the Colon and Rectum.
Conflict of interest statement
None declared.
Acknowledgements
The authors would like to acknowledge all the patients and their families. In addition to the investigators in the author list, we acknowledge the following investigators who also participated in this study: Koji Komori, Department of Gastroenterological Surgery Aichi Cancer Center Hospital, Aichi; Kenjiro Kotake, Department of Surgery, Tochigi Cancer Center, Tochigi; Takeshi Nagasaka, Department of Gastroenterological Surgery, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama; Hirotoshi Hasegawa, Department of Surgery, Keio University School of Medicine, Tokyo; Motoi Koyama, Department of Gastroenterological Surgery, Hirosaki University Graduate School of Medicine, Aomori; Yoshito Akagi, Department of Surgery, Kurume University School of Medicine, Kurume, Fukuoka; Toshimasa Yatsuoka, Department of Gastroenterological Surgery, Saitama Cancer Center, Saitama; Masataka Ikeda, Department of Surgery, National Hospital Organization, Osaka National Hospital, Osaka; Kensuke Kumamoto, Department of Organ Regulatory Surgery, Fukushima Medical University School of Medicine, Fukushima; Kiyotaka Kurachi, Department of Surgery 2, Hamamatsu University School of Medicine, Shizuoka; Kohji Tanakaya, Department of Surgery, Iwakuni Clinical Center, Yamaguchi; Kazuhiko Yoshimatsu, Department of Surgery, Tokyo Women's Medical University Medical Center East, Tokyo.
References
- 1.Groden J, Thliveris A, Samowitz W et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell 1991;66:589–600. [DOI] [PubMed] [Google Scholar]
- 2.Joslyn G, Carlson M, Thliveris A et al. Identification of deletion mutations and three new genes at the familial polyposis locus. Cell 1991;66:601–13. [DOI] [PubMed] [Google Scholar]
- 3.Kinzler KW, Nilbert MC, Su L-K et al. Identification of FAP locus genes from chromosome 5q21. Science 1991;253:661–5. [DOI] [PubMed] [Google Scholar]
- 4.Nishisho I, Nakamura Y, Miyoshi Y et al. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science 1991;253:665–9. [DOI] [PubMed] [Google Scholar]
- 5.Nagase H, Miyoshi Y, Horii A et al. Correlation between the location of germ-line mutations in the APC gene and the number of colorectal polyps in familial adenomatous polyposis patients. Cancer Res 1992;52:4055–7. [PubMed] [Google Scholar]
- 6.Nieuwenhuis MH, Vasen HF. Correlations between mutation site in APC and phenotype of familial adenomatous polyposis (FAP): a review of the literature. Crit Rev Oncol Hematol 2007;61:153–61. [DOI] [PubMed] [Google Scholar]
- 7.Spirio L, Olschwang S, Groden J et al. Alleles of APC gene: an attenuated form of familial polyposis. Cell 1993;75:951–7. [DOI] [PubMed] [Google Scholar]
- 8.Knudsen AL, Bisgaard ML, Bulow S. Attenuated familial adenomatous polyposis (AFAP). A review of the literature. Fam Cancer 2003;2:43–55. [DOI] [PubMed] [Google Scholar]
- 9.Church JM, McGannon E, Hull-Boiner S et al. Gastroduodenal polyps in patients with familial adenomatous polyposis. Dis Colon Rectum 1992;35:1170–3. [DOI] [PubMed] [Google Scholar]
- 10.Sarre RG, Frost AG, Jagelman DG et al. Gastric and duodenal polyps in familial adenomatous polyposis: a prospective study of the nature and prevalence of upper gastrointestinal polyps. Gut 1987;28:306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe N, Seno H, Nakajima T et al. Regression of fundic gland polyps following acquisition of Helicobacter pylori. Gut 2002;51:742–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iida M, Yao T, Itoh H et al. Natural history of gastric adenomas in patients with familial adenomatosis coli/Gardner's syndrome. Cancer 1988;61:605–11. [DOI] [PubMed] [Google Scholar]
- 13.Park SY, Ryu JK, Park JH et al. Prevalence of gastric and duodenal polyps and risk factors for duodenal neoplasm in Korean patients with familial adenomatous polyposis. Gut Liver 2011;5:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wood LD, Salaria SN, Cruise MW et al. Upper GI tract lesions in familial adenomatous polyposis (FAP): enrichment of pyloric gland adenomas and other gastric and duodenal neoplasms. Am J Surg Pathol 2014;38:389–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ngamruengphong S, Boardman LA, Heigh RI et al. Gastric adenomas in familial adenomatous polyposis are common, but subtle, and have a benign course. Hered Cancer Clin Pract 2014;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park JG, Park KJ, Ahn YO et al. Risk of gastric cancer among Korean familial adenomatous polyposis patients. Report of three cases. Dis Colon Rectum 1992;35:996–8. [DOI] [PubMed] [Google Scholar]
- 17.Iwama T, Mishima Y, Utsunomiya J. The impact of familial adenomatous polyposis on the tumorigenesis and mortality at the several organs. Its rational treatment. Ann Surg 1993;217:101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coffey RJ Jr, Knight CD Jr, van Heerden JA et al. Gastric adenocarcinoma complicating Gardner's syndrome in a North American woman. Gastroenterology 1985;88:1263–6. [DOI] [PubMed] [Google Scholar]
- 19.Offerhaus GJ, Giardiello FM, Krush AJ et al. The risk of upper gastrointestinal cancer in familial adenomatous polyposis. Gastroenterology 1992;102:1980–2. [DOI] [PubMed] [Google Scholar]
- 20.Iwama T, Tamura K, Morita T et al. A clinical overview of familial adenomatous polyposis derived from the database of the Polyposis Registry of Japan. Int J Clin Oncol 2004;9:308–16. [DOI] [PubMed] [Google Scholar]
- 21.Abraham SC, Nobukawa B, Giardiello FM et al. Fundic gland polyps in familial adenomatous polyposis: neoplasms with frequent somatic adenomatous polyposis coli gene alterations. Am J Pathol 2000;157:747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyaki M, Yamaguchi T, Iijima T et al. Difference in characteristics of APC mutations between colonic and extracolonic tumors of FAP patients: variations with phenotype. Int J Cancer 2008;122:2491–7. [DOI] [PubMed] [Google Scholar]
- 23.Albuquerque C, Breukel C, van der Luijt R et al. The ‘just-right’ signaling model: APC somatic mutations are selected based on a specific level of activation of the beta-catenin signaling cascade. Hum Mol Genet 2002;11:1549–60. [DOI] [PubMed] [Google Scholar]
- 24.Huang JQ, Sridhar S, Chen Y et al. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology 1998;114:1169–79. [DOI] [PubMed] [Google Scholar]
- 25.Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol 2010;7:629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura S, Matsumoto T, Kobori Y, Iida M. Impact of Helicobacter pylori infection and mucosal atrophy on gastric lesions in patients with familial adenomatous polyposis. Gut 2002;51:485–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bülow S, Björk J, Christensen IJ et al. Duodenal adenomatosis in familial adenomatous polyposis. Gut 2004;53:381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galle TS, Juel K, Bülow S. Causes of death in familial adenomatous polyposis. Scand J Gastroenterol 1999;34:808–12. [DOI] [PubMed] [Google Scholar]
- 29.Spigelman AD, Williams CB, Talbot IC et al. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet 1989;334:783–5. [DOI] [PubMed] [Google Scholar]
- 30.Vasen HF, Möslein G, Alonso A et al. Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut 2008;57:704–13. [DOI] [PubMed] [Google Scholar]
- 31.The Editorial Board of the Cancer Statistics in Japan. Cancer statistics in Japan 2014. http://ganjoho.jp/en/professional/statistics/brochure/2014_en.html, March 2015 (15 September 2015, date last accessed). [Google Scholar]
- 32.Dickey W, Kenny BD, McConnell JB. Prevalence of fundic gland polyps in a western European population. J Clin Gastroenterol 1996;23:73–5. [DOI] [PubMed] [Google Scholar]
- 33.Sakai N, Tatsuta M, Hirasawa R et al. Low prevalence of Helicobacter pylori infection in patients with hamartomatous fundic polyps. Dig Dis Sci 1998;43:766–72. [DOI] [PubMed] [Google Scholar]