A recent study demonstrated widespread substitution of analog for human insulin and rising out-of-pocket costs in privately insured people with type 2 diabetes in the United States.1 Medicaid reimbursements have increased for both human insulin and more costly analog insulins.2 Although studies have described per-person changes in excess medical spending of US adults with diabetes on prescription medications,3 they have not reported trends in expenditures for different classes of antihyperglycemic medications that simultaneously consider changes in use and price.
Methods
We analyzed individual and prescription-level data from the Medical Expenditure Panel Survey (MEPS) to describe and compare trends in expenditure and price of antihyperglycemic medications in the United States from 2002 through 2013. The MEPS involves deidentified, publicly available data of a nationally representative household survey of noninstitutionalized residents.4 The in-person interview response rate ranged from 69.2% to 58.0%. We first described the prevalence of treated patients with diabetes, their characteristics, and use of antihyperglycemic medications. We then estimated inflation-adjusted expenditures per patient for insulin (combining both human and analog) compared with other classes of antihyperglycemic medications. Medications were identified using Multum Lexicon therapeutic class codes. Drug expenditures from all sources (including patient co-payments) and quantity used came from household surveys, with data verified by pharmacies. Relative and absolute mean drug prices were calculated by dividing expenditure per prescription by quantity. All analyses were conducted in Stata (StataCorp), version 13.1, accounting for MEPS sampling weights and the complex survey design. The 95% confidence intervals were calculated and compared to determine statistically significant differences.
Results
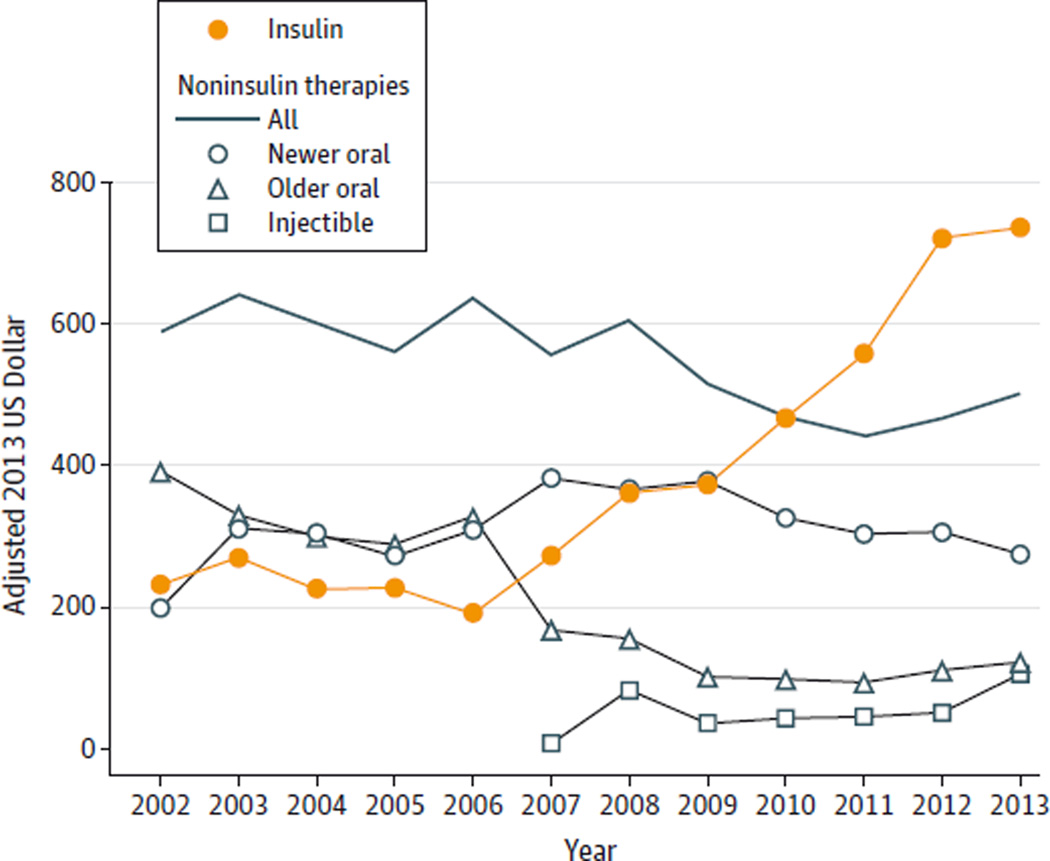
The unweighted analytic sample consisted of 27 878 people treated for diabetes (mean age, 60.4 years [SD, 14.7]; men, 44.4%). During the study period, the prevalence of treated diabetes increased from 5.2% (95% CI, 4.9%–5.4%) in 2002–2004 to 7.7% (95% CI, 7.4%–8.0%) in 2011–2013 (Table). For those with recorded insulin use, the quantity per year increased from 171 mL (95% CI, 160–181) in 2002–2004 to 206 mL (95% CI, 193–220) in 2011–2013; over the same period, estimated spending for insulin per patient increased from $231.48 (95% CI, $190.40–272.55) in 2002 to $736.09 (95% CI, $639.72–$832.47) in 2013 (Figure). In 2013, estimated expenditure per patient amounted to $507.89 (95% CI, $422.34-$593.44) for analog insulin and $228.20 (95% CI, $183.98-$273.42) for human insulin. The total expenditure on insulin in 2013 was significantly greater than the combined expenditure on all other antihyperglycemic medications of $502.57 (95% CI, $430.37–$574.78).
Table.
Weighted Characteristics of Treated Patients With Diabetes in the Medical Expenditure Panel Survey (MEPS), 2002–2013
| Characteristics | MEPS Survey Years | |||
|---|---|---|---|---|
| 2002–2004 (n = 5799)a |
2002–2004 (n = 5799)a |
2008–2010 (n = 7237) |
2011–2013 (n = 8356) |
|
| Treated diabetes, % (95% CI)b |
5.2 (4.9–5.4) | 6.2 (5.9–6.5) | 7.1 (6.8–7.4) | 7.7 (7.4–8.0) |
| Age, mean (SD), y | 60.2 (15.0) | 60.3 (14.6) | 60.3 (14.8) | 60.7 (14.6) |
| Men, No. (%) | 2496 (47.7) | 2850 (48.3) | 3182 (47.9) | 3845 (50.0) |
| Race, No. (%)c | ||||
| White | 2951 (65.3) | 3209 (65.0) | 3089 (64.9) | 3210 (62.0) |
| Black | 1202 (16.2) | 1350 (15.1) | 1805 (15.0) | 2197 (15.5) |
| Hispanic | 1334 (12.5) | 1533 (13.5) | 1699 (12.9) | 2202 (15.1) |
| Others | 312 (6.1) | 394 (6.5) | 644 (7.2) | 747 (7.4) |
| Use of medications, % (95% CI) |
||||
| Insulin | 28.1 (26.2–29.8) | 24.1 (22.4–25.8) | 25.3 (23.7–27.0) | 29.2 (27.6–30.8) |
| Metformin | 36.1 (34.2–38.0) | 43.6 (41.6–45.5) | 47.3 (45.4–49.2) | 51.5 (49.8–53.1) |
| Sulfonylureas | 38.2 (36.2–40.1) | 35.1 (33.2–36.9) | 30.7 (28.9–32.4) | 27.5 (25.8–29.3) |
| Thiazolidinediones | 21.1 (19.5–22.7) | 23.2 (21.5–24.9) | 13.0 (11.6–14.3) | 5.8 (5.0–6.6) |
| α-Glucosidase inhibitors and nonsulfonylurea secretagogues |
2.6 (2.0–3.2) | 2.8 (2.2–3.4) | 1.4 (1.0–1.8) | 0.7(0.5–1.0) |
| DPP-4 inhibitors | 1.2 (0.8–1.5) | 5.6 (4.7–6.5) | 7.7 (6.8–8.7) | |
| Combinations | 6.8 (5.8–7.7) | 8.9 (7.8–9.9) | 8.0 (7.0–9.0) | 6.0 (5.1–6.9) |
| All oralsd | 68.9 (66.9–70.8) | 72.6 (70.9–74.4) | 70.8 (69.2–72.5) | 69.5 (67.9–71.1) |
| Amylin analogs | 0.1 (0–0.1) | 0.2 (0.1–0.4) | 0.1 (0–0.2) | |
| GLP-1 receptor agonists |
2.2 (1.6–2.8) | 2.7 (2.1–3.4) | ||
| All noninsulin injectablese |
2.4 (1.8–3.1) | 2.8 (2.1–3.4) | ||
| Quantity of medications (95% CI)f |
||||
| Insulin, mL | 171 (160–181) | 150 (137–164) | 205 (191–218) | 206 (193–220) |
| All orals, tablets | 611 (580–641) | 632 (607–657) | 775 (746–804) | 800 (772–828) |
| All noninsulin injections, mL |
21 (16–25) | 36 (30–42) | ||
Abbreviations: DPP-4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1.
The reported statistics were based on a pooled sample across 3 waves of MEPS.
Percentage of all survey respondents. People treated for diabetes were identified using 3-digit International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes.
Race was included as part of the descriptive analysis. As defined by MEPS, classification by race and ethnicity was mutually exclusive and based on information reported for each family member. All persons whose main national origin or ancestry was reported as Hispanic, regardless of racial background, were classified as Hispanic.
Included metformin, sulfonylureas, thiazolidinediones, α-glucosidase inhibitors, and nonsulfonylurea secretagogues, combinations, and DPP-4 inhibitors.
Included amylin analogs and GLP-1 receptor agonists from 2008.
Quantities of medication used were means per patient per year, conditional on some recorded use of the drug over the given period.
Figure. Mean Expenditure per Patient for Antihyperglycemic Medications, 2002–2013.
Medications were classified as follows: insulin (human and analog); newer oral therapies (thiazolidinediones, dipeptidyl peptidase-4 inhibitors, and combinations); older oral therapies (metformin, sulfonylureas, α-galactosidase inhibitors, and nonsulfonylurea secretagogues); noninsulin-based injectable therapies (glucagon-like peptide-1 receptor agonists and amylin analogs).
The mean price per milliliter of insulin increased by 197% from $4.34 per milliliter (95% CI, $4.19-$4.51) in 2002 to $12.92 per milliliter (95% CI, $12.34-$13.50) in 2013, whereas the mean price of dipeptidyl peptidase-4 (DPP-4) inhibitors increased by 34% from $6.67 (95% CI, $6.26–$7.09) per tablet in 2006 to $8.92 (95% CI, $8.43-$9.41) in 2013. The mean price of metformin decreased by 93% from $1.24 per tablet (95% CI, $1.19–$1.29) in 2002 to $0.31 per tablet (95% CI, $0.25–$0.36) in 2013.
Discussion
Based on a nationally representative survey, the mean price of insulin increased from $4.34 per milliliter in 2002 to $12.92 in 2013. The estimated expenditure per patient for insulin in the United States in 2013 was greater than all other antihyperglycemic medications combined. Another factor contributing to the rise in expenditures on insulin was increased treatment intensity.
The mean price of insulin increased at a much faster rate than oral medications including DPP-4 inhibitors. We were unable to separate out generics from branded medications; however, unlike oral therapies, the mean price of insulin is unlikely to decline as a result of generic competition5 because of the stringent regulations and substantial costs of bringing biosimilar insulins to market.
Limitations of our study included changes in editing rules for improved price benchmarking of the MEPS prescribed medicines data from 2007.6 This may have artificially increased the reported drug expenditures by an estimated 10%.6 Our reported estimates of expenditure and price did not include the cost of the various insulin delivery devices except prefilled pens.
Significant changes in mean price of insulin, relative to comparator therapies, suggest a need to reassess the effectiveness and cost-effectiveness of alternative antihyperglycemic therapies.
Acknowledgments
Dr Herman reports receiving personal fees from Merck Sharp & Dohme, Lexicon Pharmaceuticals, and Profil Institute for Clinical Research.
Funding/ Support: This research was partly supported by grants 5R01 DK090435-02, P30 DK092949, and K24 DK105340 from the National Institutes of Health and grants 1028335 and 1079621 from the National Health and Medical Research Council.
Role of the Funder/Sponsor: The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Hua and Clarke had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Tew, Herman, Clarke.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Hua, Carvalho, Herman, Clarke.
Critical revision of the manuscript for important intellectual content: Tew, Huang, Herman, Clarke.
Statistical analysis: Hua, Carvalho, Tew.
Administrative, technical, or material support: Tew, Huang.
Study supervision: Herman, Clarke.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. No other disclosures were reported.
References
- 1.Lipska KJ, Ross JS, Van Houten HK, Beran D, Yudkin JS, Shah ND. Use and out-of-pocket costs of insulin for type 2 diabetes mellitus from 2000 through 2010. JAMA. 2014;311(22):2331–2333. doi: 10.1001/jama.2014.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luo J, Avorn J, Kesselheim AS. Trends in Medicaid reimbursements for insulin from 1991 through 2014. JAMA Intern Med. 2015;175(10):1681–1686. doi: 10.1001/jamainternmed.2015.4338. [DOI] [PubMed] [Google Scholar]
- 3.Zhuo X, Zhang P, Kahn HS, Bardenheier BH, Li R, Gregg EW. Change in medical spending attributable to diabetes: national data from 1987 to 2011. Diabetes Care. 2015;38(4):581–587. doi: 10.2337/dc14-1687. [DOI] [PubMed] [Google Scholar]
- 4.Agency for Health Care Research and Quality. MEPS HC-155: 2012 full year consolidated data file. [Accessed September 23, 2015]; http://meps.ahrq.gov/mepsweb/data_stats/download_data/pufs/h155/h155doc.pdf.
- 5.Greene JA, Riggs KR. Why is there no generic insulin? historical origins of a modern problem. N Engl J Med. 2015;372(12):1171–1175. doi: 10.1056/NEJMms1411398. [DOI] [PubMed] [Google Scholar]
- 6.Agency for Health Care Research and Quality. Comparison of retail drug prices in the MEPS and MarketScan: implications for MEPS editing rules. [Accessed July 29, 2015]; http://meps.ahrq.gov/mepsweb/data_files/publications/workingpapers/wp_10001.pdf.