Abstract
Articular cartilage damage of the knee can cause severe morbidity. Owing to its avascular nature, articular cartilage has limited potential for self-healing and increased propensity to progress to osteoarthritis. Treatment of large, full-thickness cartilage defects is still a challenge for orthopaedic surgeons but has recently achieved high success rates with the use of osteochondral allografts. This article details our technique of osteochondral allograft transplantation for the treatment of articular cartilage defects of the knee.
Treatment of large articular cartilage defects of the knee remains a challenging entity, particularly in young high-demand patients. Damaged articular cartilage has limited potential for self-healing and has an increased propensity to progress to osteoarthritis.1, 2 Among the numerous surgical options for cartilage repair, abrasion arthroplasty, subchondral drilling, and microfracture all focus on the principle of marrow stimulation to promote healing. However, marrow stimulation procedures create a hyaline-like fibrocartilage that is physiologically and mechanically inferior to hyaline cartilage.2, 3 Additionally, they are not recommended for the treatment of large osteoarticular lesions.4 Autologous chondrocyte implantation can be used to treat large lesions but entails a prolonged rehabilitation and a staged procedure.4, 5, 6 Autogenous osteochondral transfer can also be used to repair cartilage defects but is best suited for defects <2.5 cm2 owing to donor site size limitations and morbidity.5 Osteochondral allograft transplantation can be used to repair large, solitary defects through the use of fully mature articular cartilage during a single operation, while avoiding donor site morbidity.6 The purpose of this surgical technique description was to describe the method for osteochondral allograft transplantation for treatment of articular cartilage defects of the knee.
Indications and Contraindications for Surgery
The main indication for allograft resurfacing is the presence of a symptomatic full-thickness articular cartilage defect of >3 cm2. Contraindications to allograft resurfacing include a “kissing” lesion of the corresponding articular cartilage surface, ligamentous instability, malalignment, more than minor peripheral osteophytes, joint-space narrowing, or absence of >50% of the meniscus in the affected compartment. Patients whose weight-bearing axis passes medial or lateral to the tip of the tibial eminences within the affected compartment should undergo a concurrent tibial osteotomy to rectify the mechanical axis. Patients with >50% loss of the meniscus in the ipsilateral compartment should undergo a concurrent meniscal transplantation.6 The main indications and contraindications are summarized in Table 1.
Table 1.
Indications and Contraindications for Osteochondral Autograft Transfer System Procedure
| Indications | Contraindications |
|---|---|
| Symptomatic full-thickness articular cartilage defect of >3 cm2 Localized grade III or IV unipolar lesions of the femoral condyle, trochlea or patella Defects due to trauma, osteochondral dissecans, avascular necrosis, or intra-articular plateau fractures Young, high-demand patients who are not candidates for joint replacement Associated underlying subchondral bone defect |
Absolute
|
Objective Diagnosis
Preoperative radiographs should include standing anteroposterior and lateral films, long-axis weight bearing, 45° posteroanterior flexion weight bearing, and patellofemoral (sunrise) views.3 Sizing markers should be used to allow for appropriate allograft size matching to the recipient sites.6 All compartments should then be evaluated for pathologic changes. Long-axis radiographs are useful for assessing alignment, thereby helping determine the need for concurrent osteotomy. Additionally, preoperative magnetic resonance imaging is helpful to determine meniscal and ligamentous pathology, as well as bone edema and subchondral sclerosis.7 Furthermore, the size of the defect is measured using magnetic resonance imaging. Finally, diagnostic arthroscopy is recommended to further evaluate for the size, location, and severity of the lesion and to verify that the patient is a candidate for a fresh osteoarticular allograft procedure.
Technique
Patient Positioning and Anesthesia
The patient is placed in the supine position on the operating table, and general anesthesia is used for induction. A well-padded high-thigh tourniquet is subsequently placed on the operative leg, and then a bump is placed under the knee so that it rests at approximately 30° of flexion. The contralateral leg is secured to the table in full extension with a pneumatic compression device to help prevent deep vein thrombosis.
Surgical Technique
A small medial or lateral parapatellar arthrotomy is performed (Video 1), depending on the location of the defect. The defect should be identified (Fig 1) and templated. Next a guide pin is placed in the center of the defect and the edges of the defect are scored (Fig 2). The defect is then reamed until bleeding, healthy bone is encountered (Fig 3), with care not to exceed a maximum of 7 to 8 mm of overall bone depth. This can be achieved by frequently checking the calibrated coring reamer (Arthrex, Naples, FL, U.S.A.), along with a final measurement (Fig 4). While reaming, liberal amounts of irrigation fluid at room temperature are used to avoid heat necrosis of the surrounding articular cartilage and subchondral bone. The recipient site is then dilated with a smooth cylinder (Arthrex) several times to ensure the donor plug can be inserted without the need to apply too much pressure. In order to accomplish a perfect fit between the donor graft and the host socket, a compass reference is created on the prepared defect and measures are taken from each main coordinate (north/south/east/west). These measurements will be used later at the time of graft trimming. The fresh (15 to 28 days) allograft (JRF Ortho, Centennial, CO, U.S.A.) is then warmed by soaking it in room temperature saline. Next, the corresponding area on the allograft is outlined with methylene blue to match the dimensions of the patient's knee defect. The whole donor specimen is then secured within an allograft workstation (Arthrex) to ensure precision during harvest (Fig 5). The osteochondral donor plug is then harvested from the allograft with the use of a coring reamer while using copious amounts of irrigation to prevent heat necrosis. The subchondral bone of the donor plug is then trimmed according to previous measurements to match the corresponding depths of the host location, and the surfaces are smoothed with a rasp. The depth of the recipient site and donor plug is measured several times to make sure there are no areas that will be too proud. Prior to implantation of the donor bone plug, the subchondral bone is subjected to pulse lavage (Arthrex) to eradicate any remaining bone marrow elements to minimize the chance of immune reaction. The bone plug is then gently press-fit into the socket to match the exact height of the surrounding articular cartilage (Fig 6). Maximum bone depth should be 8 to 10 mm. The ideal depth of the bone plug is 7 to 9 mm. If >0.5 mm proud, consider removal of the plug with a small blunt elevator and smoothing off the small elevated area with a rasp. If the recipient site is too deep (>1 mm), then remove the allograft, insert a small amount of bone graft into the areas that are too deep, and then slowly reinsert the osteochondral allograft so that it fits anatomically. If a subchondral cyst is encountered at the recipient site, bone autograft is obtained with a curette from the proximal tibia and used to bone graft the cyst rather than ream the tunnel deeper to get rid of the cyst.
Fig 1.
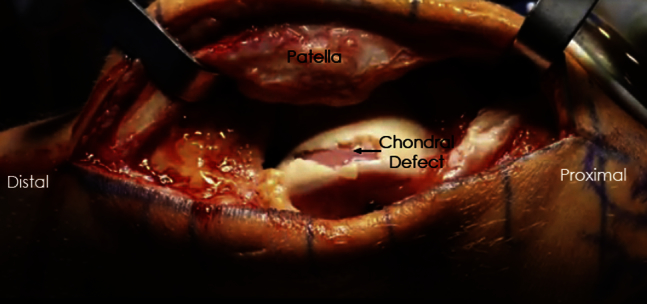
Intraoperative photograph of a left knee demonstrating a large full-thickness chondral defect on the lateral trochlea.
Fig 2.
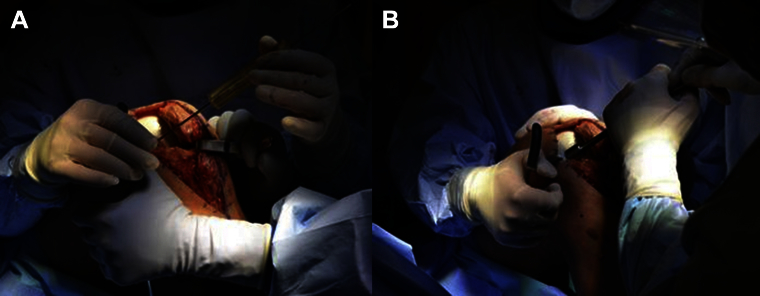
Photographs displaying (A) guide pin placement in the center of an osteochondral defect on the medial femoral condyle and (B) scoring of the edges of the defect (left knee).
Fig 3.
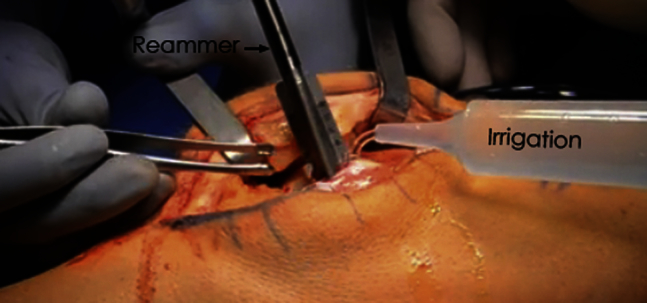
Photograph displaying reaming of a lateral trochlear lesion of a left knee. Copious amounts of irrigation are used to avoid heat necrosis of the surrounding articular cartilage and subchondral bone.
Fig 4.
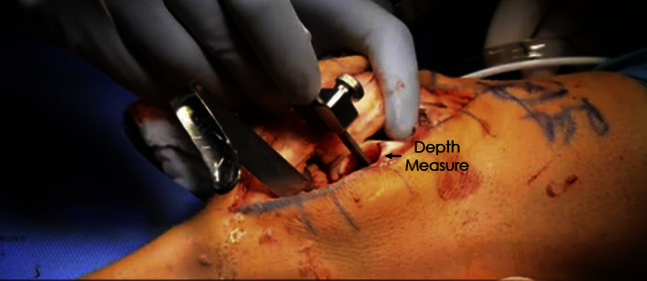
Photograph showing the depth measurement of the freshly reamed defect of a trochlear lesion of a left knee. Maximum bone depth should be 8 to 10 mm.
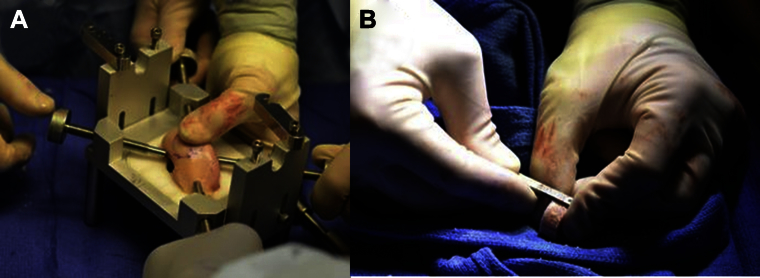
Fig 5.
Photographs of (A) osteochondral allograft with outlined template being prepared for reaming within allograft workstation and (B) measuring allograft depth after reaming. The ideal depth of the bone plug is 7 to 9 mm.
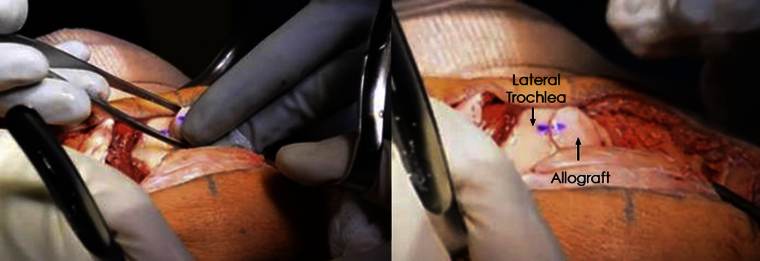
Fig 6.
Photographs of a left knee demonstrating gentle press-fit allograft insertion. To ensure proper orientation of the allograft with the recipient, the dye marking on the recipient site and donor graft should align.
Postoperative Rehabilitation
The patient should remain non–weight bearing for the first 8 weeks. A supervised rehabilitation program should start immediately postoperatively. Quadriceps exercises and straight-leg raises with the patient wearing a knee immobilizer should be performed 4 times daily. For the first 8 weeks, patients should use a continuous passive-motion machine at a minimum time interval of 2 hours, for a minimum of 10 hours per day. Low-impact activities are recommended for the first 12 months to allow complete healing and incorporation of the graft. All patients are advised to perform low-impact activities and avoid high-impact activities as much as possible after this time period. Pearls and pitfalls are found in Table 2.
Table 2.
Osteochondral Autograft Transfer System Procedure: Pearls and Pitfalls
| Pearls | Pitfalls |
|---|---|
| Ideally, bleeding healthy bone should be encountered while reaming the defect. However, it should not exceed 7-8 mm of depth. Frequently check the calibrated coring reamer to avoid over-reaming. | Not addressing concomitant malalignment, ligament instabilities or meniscal issues prior to the transplant can lead to poor result. |
| Using a smooth dilator after reaming the defect facilitates further insertion of the donor plug. | Heat necrosis can occur from high-speed reamers in the edges of the receptor's bed as well as in the donor plug. Copiously irrigate cutting surfaces with room-temperature saline while using reamers and saws to minimize this problem. |
| Reduce the size of the original allograft in order to facilitate its manipulation during osteochondral plug preparation. | When the measured size of the donor plug is already close to the desired size, using a saw to trim it can lead to inadvertent excessive bone loss and destruction of the graft. In this situation, it is preferable to use a manual rasp and check the size frequently. |
| Use a compass reference (north/south/east/west) for measuring the depth of the receptor's bed and have an assistant outside the surgical field take notes to precisely prepare the donor plug. | Avoid strong hits to the osteochondral plug while press-fitting it into the receptor's bed (use a sponge to cushion the chondral surface). Chondrocytes are sensitive to trauma. This could reduce the population of live cells and consequently the quality of the graft. |
| Submitting the subchondral bone to pulse lavage reduces the bone marrow elements and ultimately the chance of immune reaction | Noncompliance with the postoperative rehab can lead to poor results. Clearly disclose to your patient all the necessary cares (specially restrictions for weight bearing and the use of a continuous passive-motion device) before indicating the procedure. |
Discussion
To date, outcome studies show a high rate of success when treating full-thickness articular cartilage defects of the knee with fresh osteochondral allografts.6, 8, 9, 10 A retrospective study of 46 patients by Gracitelli et al.8 reported an 87.4% 10-year graft survivorship. A prospective study of 25 patients by McCulloch et al.9 reported graft incorporation into host bone in 22 of 25 (88%) patients. They also showed significant improvements in all subjective outcome scores. LaPrade et al.6 followed 23 patients over 3 years and showed improvement in Cincinnati scores from 49.2 to 69.0 (P < .02), International Knee Documentation Committee score improvement from 52.0 to 68.3 (P < .03), and evidence of graft healing in 22 of 23 patients (96%). Recently, Levy et al.10 reported a retrospective case series of 122 patients (129 knees) between 1983 and 2011, with a survivorship of 82% at 10 years, 74% at 15 years, and 66% at 20 years. However, the applicability of this study is limited as a result of their allografts being transplanted within 7 days postmortem. Due to increasing safety concerns over potential infection, allografts are now hypothermically stored for a minimum of 14 days, allowing for extensive microbiologic and serologic testing. The viability of chondrocytes has been shown to decrease after 28 days postmortem. Therefore, osteochondral allografts should be used between 15 to 28 days postmortem.6 However, to maximally preserve chondrocyte viability, transplant should be performed as soon as the 14-day testing period is complete, at day 15 to 16.
Treatment of osteoarticular cartilage defects of the knee with osteochondral allografts offers numerous advantages over the other surgical techniques previously described. Owing to the avascular and aneural nature of osteochondral allografts, they are immunoprivileged and ideally suited for allogenic transplantation.11 Moreover, allografts are able to treat large, full-thickness defects using mature hyaline cartilage, while avoiding donor site morbidity and treating any underlying osseous defects.6 Advantages and limitation are summarized in Table 3.
Table 3.
Advantages and Limitations of Osteochondral Autograft Transfer System Procedure
| Advantages | Limitations |
|---|---|
| No donor site morbidity Live chondroctyes Uses mature hyaline cartilageCan treat large defectsCan treat underlying osseous defectsSingle stage procedureHarvested from matching donor site to create natural smooth contour of recipient site |
Must use fresh allograft (15-28 days) for maximum chondrocyte viabilityOnly for full thickness lesionsCostAvailabilityNot ideal for small lesions |
Further follow-up studies of osteochondral allografts are needed, particularly long-term outcomes in accordance with the recent allograft transplantation guidelines. We recommend our method of osteochondral allograft repair of articular cartilage defects of the knee and encourage additional studies by other groups to assess our surgical technique.
Supplementary Data
Demonstration of fresh osteochondral allograft use for treatment of a lateral trochlear articular cartilage defect of a left knee. The patient is placed supine on the operating table, and a bump is placed under the operative knee so that it rests at approximately 30° of flexion. A lateral parapatellar arthrotomy is performed; the lesion is then identified, reamed, and measured. The allograft is then harvested and inserted into the recipient site.
References
- 1.Felson D.T., Zhang Y., Hannan M.T. The incidence and natural history of knee osteoarthritis in the elderly: The Framingham Osteoarthritis Study. Arthritis Rheum. 1995;38:1500–1505. doi: 10.1002/art.1780381017. [DOI] [PubMed] [Google Scholar]
- 2.Mankin H.J. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64:460–465. [PubMed] [Google Scholar]
- 3.Alford J.W., Cole B.J. Cartilage restoration. Part 1. Basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33:295–306. doi: 10.1177/0363546504273510. [DOI] [PubMed] [Google Scholar]
- 4.Alford J.W., Cole B.J. Cartilage restoration, part 2: techniques, outcomes, and future directions. Am J Sports Med. 2005;33:443–460. doi: 10.1177/0363546505274578. [DOI] [PubMed] [Google Scholar]
- 5.Chow J.C., Hantes M.E., Houle J.B., Zalavras C.G. Arthroscopic autogenous osteochondral transplantation for treating knee cartilage defects: a 2- to 5-year follow-up study. Arthroscopy. 2004;20:681–690. doi: 10.1016/j.arthro.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 6.LaPrade R.F., Botker J., Herzog M., Agel J. Refrigerated osteoarticular allografts to treat articular cartilage defects of the femoral condyles. A prospective outcomes study. J Bone Joint Surg Am. 2009;91:805–811. doi: 10.2106/JBJS.H.00703. [DOI] [PubMed] [Google Scholar]
- 7.DeFranco M.J., McNickle A.G., Cole B.J. Allograft osteoarticular resurfacing. In: Bonnin M., Amendola N.A., Bellemans J., MacDonald S.J., Ménétrey J., editors. The Knee Joint: Surgical Techniques and Strategies. Springer Science & Business Media; 2013. pp. 497–503. [Google Scholar]
- 8.Gracitelli G.C., Meric G., Briggs D.T. Fresh osteochondral allografts in the knee: comparison of primary transplantation versus transplantation after failure of previous subchondral marrow stimulation. Am J Sports Med. 2015;43:885–891. doi: 10.1177/0363546514565770. [DOI] [PubMed] [Google Scholar]
- 9.McCulloch P.C., Kang R.W., Sobhy M.H., Hayden J.K., Cole B.J. Prospective evaluation of prolonged fresh osteochondral allograft transplantation of the femoral condyle: minimum 2-year follow-up. Am J Sports Med. 2007;35:411–420. doi: 10.1177/0363546506295178. [DOI] [PubMed] [Google Scholar]
- 10.Levy Y.D., Görtz S., Pulido P.A., McCauley J.C., Bugbee W.D. Do fresh osteochondral allografts successfully treat femoral condyle lesions? Clin Orthop Relat Res. 2013;471:231–237. doi: 10.1007/s11999-012-2556-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnoczky S.P. The biology of allograft incorporation. J Knee Surg. 2006;19:207–214. doi: 10.1055/s-0030-1248109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Demonstration of fresh osteochondral allograft use for treatment of a lateral trochlear articular cartilage defect of a left knee. The patient is placed supine on the operating table, and a bump is placed under the operative knee so that it rests at approximately 30° of flexion. A lateral parapatellar arthrotomy is performed; the lesion is then identified, reamed, and measured. The allograft is then harvested and inserted into the recipient site.