Abstract
During the clinically silent liver stage of a Plasmodium infection the parasite replicates from a single sporozoite into thousands of merozoites. Infection of humans and rodents with large numbers of sporozoites that arrest their development within the liver can cause sterile protection from subsequent infections. Disruption of genes essential for liver stage development of rodent malaria parasites has yielded a number of attenuated parasite strains. A key question to this end is how increased attenuation relates to vaccine efficacy. Here, we generated rodent malaria parasite lines that arrest during liver stage development and probed the impact of multiple gene deletions on attenuation and protective efficacy. In contrast to P. berghei strain ANKA LISP2(–) or uis3(–) single knockout parasites, which occasionally caused breakthrough infections, the double mutant lacking both genes was completely attenuated even when high numbers of sporozoites were administered. However, different vaccination protocols showed that LISP2(–) parasites protected better than uis3(–) and double mutants. Hence, deletion of several genes can yield increased safety but might come at the cost of protective efficacy.
Malaria is transmitted through the bite of a mosquito, which injects Plasmodium sporozoites into the host. After migration through the skin, sporozoites enter the blood stream and invade hepatocytes to further differentiate into merozoites, which in turn infect red blood cells causing the clinical symptoms of malaria. Successful experimental vaccines that protect from natural infection have first been generated by radiation treatment of Plasmodium sporozoites1. These damaged parasites could still enter hepatocytes but, at the right doses of radiation, did not replicate and failed to develop into merozoites2. When applied in large numbers these defective sporozoites could induce protective immunity from subsequent wild type sporozoite challenge in both rodents and humans1,3,4. Similarly, infection of humans and rodents with wild type sporozoites followed by application of parasite-targeting drugs also generated protective immunity5,6,7,8. Ablation of genes that are essential for liver stage development has expanded the potential for generating experimental whole parasite vaccines, generally called genetically attenuated parasites, GAPs9,10,11,12,13,14. GAPs arresting late during development were found to confer a stronger protective immunity in rodent models potentially due to a broader antigenic repertoire and/or prolonged persistence15. However, independent from the time of arrest GAPs can still cause breakthrough infections such that rodents or humans develop a blood stage infection16,17,18. Thus, in order to produce safer GAPs, multiple genes have been deleted from the respective Plasmodium genomes19,20,21,22. Yet, all of the combined gene deletions lead to an arrest early during liver stage development. While several rodent GAPs have been developed that confer long lasting immunity, this has not yet been completely translated into a genetically attenuated human malaria parasite21. One important difference between the species being that human malaria parasites take over a week to fully develop in hepatocytes, while rodent parasites do so in less than three days23. In a first trial with P. falciparum GAPs, ablation of at least three genes appeared to be necessary to achieve complete arrest21. Yet, how ablation of multiple genes affects vaccine efficacy is not clear. Thus more candidate genes need to be identified and tested in rodent malaria parasites that could then be translated to generate efficient attenuated P. falciparum strains24.
Here we investigated Plasmodium berghei parasites lacking a gene, LISP2 (for liver specific protein 2), which is specifically expressed in liver stages25 and exported from the parasite to the host cell26. The partial disruption of this gene yielded severely impaired, but not completely attenuated liver stages26,27. For this reason the gene would not be listed as a potential GAP candidate. However, we now show that complete disruption of LISP2 caused a growth arrest during liver stage development and yielded a genetically attenuated rodent malaria parasite line. Additional depletion of the uis3 (for up-regulated in infectious sporozoites 3) gene in P. berghei strain ANKA increased the safety of the vaccination strain. However, the level of protection induced by this double knockout strain was lower than that obtained with single LISP2(–) knockout strain. Interestingly, we also observed a marked parasite strain-specific difference upon depletion of the uis3 gene between P. berghei strains NK65 and ANKA. In P. berghei NK65, deletion of uis3 led to early and complete arrest during liver stage development9. Curiously uis3(–) ANKA parasites showed a different phenotype as they were not completely attenuated and arrested later in development than uis3(–) NK65 and P. yoelii parasites, respectively28. This allowed us to test two attenuated parasites and a parasite lacking both genes for attenuation and vaccination efficacy.
Results
P. berghei strain ANKA sporozoites lacking uis3 arrest during mid-liver stage development and can cause breakthrough infections
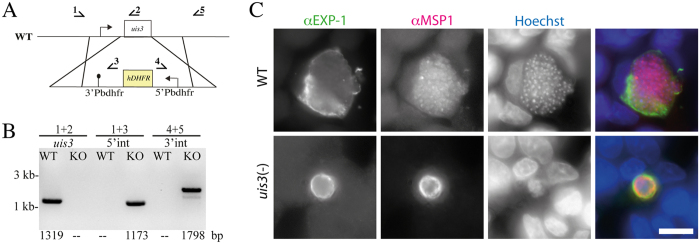
P. berghei parasites of the NK65 strain that lack uis3 arrest early during hepatic development, yet they confer complete protection from subsequent infection in a classical 3-shot vaccination regimen9. The P. berghei ANKA strain undergoes a more rapid development in the liver stage than P. berghei NK6529,30. To investigate potential differences in development and protective capacity of NK65 and ANKA, we generated an ANKA parasite line that lacks uis3 (Fig. 1A,B). Like in the NK65 strain we could not detect any differences in blood stage or early mosquito stage development and sporozoites accumulated in similar numbers in mosquitoes as they did for the wild type (data not shown). Unexpectedly, the ANKA uis3(–) parasites developed into larger hepatic stages than shown for NK65 uis3(–) parasites9 as revealed by infection of HepG2 cells (Fig. 1C). Yet, their development was attenuated compared to wild type controls as the parasites did not form mature merozoites within 65 h as shown by altered merozoite surface protein 1 (MSP1) staining restricted to the surrounding parasite plasma membrane (Fig. 1C). However, ANKA uis3(–) sporozoites caused breakthrough infections in 6 out of 46 mice infected with a total of over 2.4 million sporozoites (Supplementary Table S2). Genotyping of these blood stage parasites confirmed that they were uis3(–) (data not shown). We also immunized mice intravenously with 50.000 ANKA uis3(–) sporozoites followed by two boosts with 20.000 ANKA uis3(–) sporozoites in a 3-dose regimen9. Mice with breakthrough infection were excluded from the experiment. When blood stage parasite free mice were challenged 14 days later with 10.000 wild type sporozoites all mice were protected (Table 1) as was reported for the NK65 uis3(–) parasites9.
Figure 1. Generation and characterization of P. berghei ANKA uis3(–) parasites.
(A) Schematic of the uis3 replacement strategy using the 5′ and 3′UTRs of uis3 to integrate a resistance cassette by double crossover into the P. berghei strain ANKA genome. Locations of primers used for PCR in (B) are indicated. (B) Diagnostic PCR to investigate generation of uis3(–) parasites. WT: wild type, KO: uis3(–) parasite line. Numbers below the gel indicate expected amplicon sizes. (C) Liver stages in HepG2 cells formed by WT and uis3(–) sporozoites 65 hours post infection labeled with antibodies against EXP-1 and MSP1; Hoechst reveals host and parasite DNA. Images on the right show the merged images. Scale bar: 10 μm.
Table 1. Protection of C57BL/6 mice immunized in a 3-shot regimen with the indicated strains against challenge with wildtype P. berghei ANKA sporozoites or blood stages).
| Exp. | Attenuated parasite line (P. berghei ANKA) | 1st shot | 2nd and 3rd shots* (day of shots) | Challenge dose# (day of challenge) |
Protected/challenged animals (pre-patency)+ | |
|---|---|---|---|---|---|---|
| Challenge 1 | Challenge 2 | |||||
| 1 | LISP2(–) | 10.000 | 10.000 (d14, d28) | 1.000 (d42) | 10.000 (d116) | C1: 3/4 (d 6), C2: 1/1 |
| 5.000 bs (d116) | C2: 0/2 (d 4.5) | |||||
| 1.000 | 0/4 (d 3) | |||||
| 2 | LISP2 (–) | 50.000 | 20.000 (d14, d28) | 10.000 (d42) | 10.000 (d102) | C1 : 4/4, C2 : 2/2 |
| 10.000 bs(d102) | C2 : 0/2 | |||||
| uis3(–) | 50.000 | 20.000 (d14, d28) | 10.000 (d42) | 10.000 (d102) | C1: 6/6**, C2: 6/6 | |
| LISP2(–)/uis3(–) | 50.000 | 20.000 (d14, d28) | 10.000 (d42) | 10.000 (d102) | C1: 8/8, C2: 8/8 | |
| 10.000 | 0/8 (d 3.1) | |||||
Groups of C57BL/6 age-matched mice were used for different immunizations. Blood stages were included to probe stage specific protection.
*Data are presented as numbers of sporozoites for first, second and third shot; days of shots are indicated in parentheses.
#mice are challenged with PbANKA wild type sporozoites or blood stages (bs); days of challenge after prime are indicated in parentheses.
+C1: challenge 1, C2: challenge 2; day of blood stage emergence (pre-patency) is indicated in parentheses.
**Two mice became positive after priming; thus challenge was only performed with 6 mice.
P. berghei strain ANKA sporozoites lacking LISP2 arrest during liver stage development and cause rare breakthrough infection
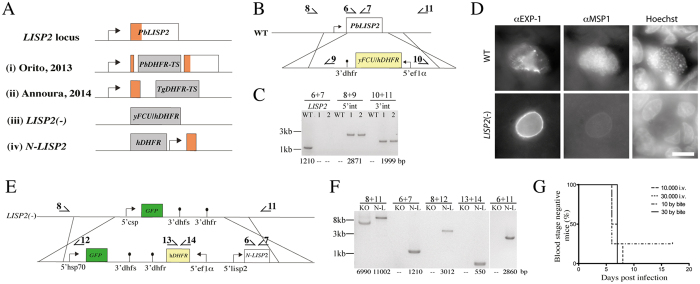
LISP2 is a 6-cys domain-containing protein with conserved gene structure across different Plasmodium species (Supplementary Figs S1 and S2). Previously, two laboratories generated P. berghei ANKA parasites lacking LISP226,27. Curiously, both labs targeted the gene in a way that did not completely disrupt the gene (Fig. 2A). We thus generated a P. berghei ANKA parasite line lacking the entire coding sequence of LISP2 (Fig. 2B,C). Like in the previous reports we found no difference in blood stage development. ANKA LISP2(–) parasites infected mosquitoes at similar rates as wild type parasites and accumulated in the salivary glands at comparable numbers (data not shown). However, in contrast to the previously published results, we found that only one mouse out of 60 inoculated with a total of over 2.2 million ANKA LISP2(–) sporozoites developed a blood stage infection (Supplementary Table S2). Similar to ANKA parasites lacking uis3, we found that ANKA LISP2(–) parasites developed into large intrahepatic stages (Fig. 2D). Yet, immunofluorescence analysis of infected HepG2 cells revealed that there were no merozoites developing in ANKA LISP2(–) intrahepatic stages in vitro, similar to what was observed with ANKA uis3(–) parasites and little MSP1 was detectable in these parasites (Fig. 2D). After immunization of C57BL/6 mice with ANKA LISP2(–) sporozoites following the same 3-dose regimen as for ANKA uis3(–) sporozoites we also observed that all immunized mice were protected from subsequent challenge with wild type parasites (Table 1).
Figure 2. Generation and characterization of ANKA LISP2(–) and N-LISP2 parasites.
(A) LISP2 locus and differently modified gene loci as generated in previous studies (i, ii) and in the current study (iii, iv). The region encoding the N-terminus is colored in orange. Boxes not drawn to scale. (B) Schematic of the LISP2 replacement strategy using the 5′ and 3′UTRs of LISP2 to integrate a resistance cassette by double crossover into the genome of the P. berghei strain ANKA. Note that this resistance cassette (yellow) included a negative selection marker to enable recycling of the cassette. The locations of primers used for PCR in (C) are indicated. (C) Diagnostic PCR to investigate generation of LISP2(–) parasites. Numbers below the gel indicate expected amplicon sizes. (D) Liver stages in HepG2 cells formed by ANKA WT and LISP2(–) sporozoites 65 hours post infection labeled with antibodies against EXP-1 and MSP1; Hoechst reveals host and parasite DNA. Scale bar: 10 μm. (E) Schematic of the strategy used to integrate the N-terminus of LISP2 into the LISP2(–) parasite. The locations of primers used for PCR in (F) are indicated. The N-terminal fragment contained a start and stop codon. (F) Diagnostic PCR to investigate generation of N-LISP2 parasites. Numbers below the gel indicate expected amplicon sizes. KO: LISP2(–) parasites, N-L: N-LISP2 parasites. (G) Meyer-Kaplan plot to indicate the number of blood stage positive mice after injection of sporozoites i.v. and by bite. 4 groups of 4 mice each were infected; of these 15 became blood stage patent.
The N-terminus of LISP2 is sufficient for full liver stage development
We next aimed to address the hypothesis that the N-terminus of LISP2 could account for the differences between our own (Fig. 2) and previous observations26,27. To this end we used negative selection17 to remove the resistance marker from one of the ANKA LISP2(–) parasite lines (Supplementary Fig. S3). Into the resulting parasite line we transfected a construct that led to the integration of the N-terminal 1210 base pairs of LISP2, which could still be expressed in one of the previously published parasite lines (Fig. 2E,F)27. We termed this parasite line ANKA N-LISP2. As expected and already observed with the previous LISP2(–) parasite lines the development of these parasites in the blood and mosquito stages was normal. When mice were infected with 10.000 N-LISP2 sporozoites intravenously or by the bites of 10 mosquitoes we found that 15 out of 16 mice developed a blood stage infection, albeit with a delayed prepatency (Fig. 2G and Supplementary Table S2). These data show that the N-terminus of LISP2 can partially rescue the defect in liver stage development of ANKA LISP2(–) parasites.
Parasites lacking uis3 and LISP2
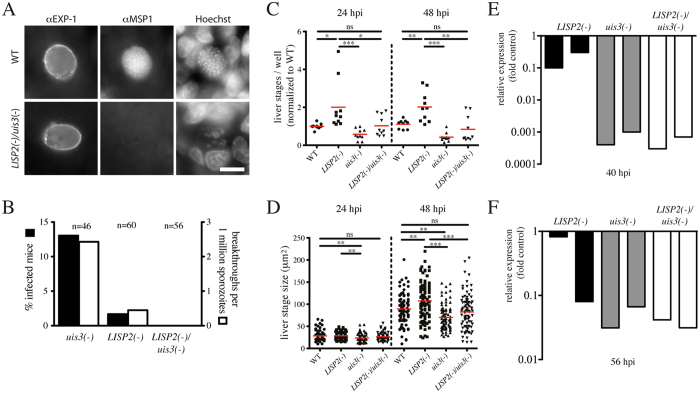
We next generated a parasite line lacking the genes uis3 and LISP2 (Supplementary Fig. S3). These ANKA LISP2(–)/uis3(–) parasites showed no phenotypic or growth differences until the liver stage (data not shown). They readily infected HepG2 hepatocytes in vitro and developed into large liver stages but like the parasites lacking the individual genes never formed recognizable merozoites and showed little MSP1 staining (Fig. 3A). To assess the rate of breakthrough infection we infected 56 mice intravenously with a total of almost 5 million ANKA LISP2(–)/uis3(–) sporozoites. None of these mice developed blood stage parasitemia over the observation time of up to 150 days (Supplementary Table S2, Fig. 3B) suggesting a complete arrest of parasite development in the liver. These parasites also conferred complete protection from wild type challenge in a 3-shot regime (Table 1).
Figure 3. Characterization of a ANKA LISP2(–)/uis3(–) double knockout parasite line and comparison with single knockout lines.
(A) Liver stages in HepG2 cells formed by WT and LISP2(–)/uis3(–) sporozoites 65 hours post infection as revealed by labelling with antibodies against EXP-1, and MSP1; Hoechst reveals host and parasite DNA. Scale bar: 10 μm. (B) Percentage of breakthrough infections (black) and infected mice per 1 million sporozoites injected (white) of the different parasite lines, see also Appendix Table SII. (C) Number of liver stages 24 and 48 hours post infection (hpi) of HepG2 cells with WT, LISP2(–), uis3(–) and LISP2(–)/uis3(–) sporozoites. All data normalized to the mean of WT duplicates in each individual experiment. Raw data are shown in Supplementary Fig. S4. (D) Sizes of liver stages 24 and 48 hours post infection (hpi) of HepG2 cells with WT, LISP2(–), uis3(–) and LISP2(–)/uis3(–) sporozoites. (E,F) Relative liver load of two mice 40 and 56 hours post infection (hpi) of LISP2(–), uis3(–) and LISP2(–)/uis3(–) sporozoite infected C57BL/6 mice. 18S rRNA abundance was normalized to the average value of 2 PbANKA infected mice for each time point. Note that at 56 hpi some WT parasites have already emerged from the liver, hence the lower relative levels.
To investigate potential subtle differences in the protective capacity of the different strains we additionally tested all parasite lines in a single (one-shot) and a double (2-shot) immunization trial. For the one-shot trial we injected either 20.000 or 30.000 sporozoites of the respective parasite lines intravenously into mice and challenged these animals with 10.000 wild type sporozoites 14 days later. Curiously, this revealed that mice immunized with ANKA LISP2(–) parasites consistently showed a better protection than mice immunized with ANKA uis3(–) or the ANKA LISP2(–)/uis3(–) parasites (Tables 2 and 3). This suggests that ANKA uis3(–) parasites are not as protective as ANKA LISP2(–) parasites and that this uis3(–) phenotype is dominant in the parasites lacking both genes. Intriguingly, we found higher numbers of infected HepG2 cells and larger liver stages for ANKA LISP2(–) compared to WT parasites (Fig. 3C,D). In contrast, we found lower numbers of ANKA uis3(–) infected HepG2 cells at 24 and 48 hours post infection and smaller liver stage parasites. In the ANKA LISP2(–)/uis3(–) parasites both numbers of infected cells and liver stage size were comparable to WT. qRT-PCR analysis of infected livers after injection of 10.000 sporozoites showed a small decrease of 18S rRNA in ANKA LISP2(–) infected mice 40 and 56 hours post infection, while 18S rRNA in ANKA uis3(–) and LISP2(–)/uis3(–) infected livers was barely detectable (Fig. 3E,F). This might explain the decreased efficacy of ANKA uis3(–) and ANKA LISP2(–)/uis3(–) parasites compared to ANKA LISP2(–) parasites.
Table 2. Protection of C57BL/6 mice immunized in a 2-shot regimen with the indicated P. berghei ANKA knockout lines against challenge with wild type P. berghei ANKA sporozoites or blood stages.
| Exp. | Attenuated parasite line (P. berghei ANKA) | 1st shot | 2nd shot* (day14) | Challenge dose# (day of challenge) | Protected/challenged animals (pre-patency) |
|---|---|---|---|---|---|
| 3 | LISP2(–) | 10.000 | 10.000 | 1.000 (d42) | 1/4 (d 5.75) |
| 1.000 (d42) | 0/4 (d 3) | ||||
| 4 | LISP2(–) | 20.000 | 20.000 | 10.000 (d28) | 8/8 |
| uis3 (–) | 20.000 | 20.000 | 10.000 (d28) | 3/7** (8.75 d) | |
| LISP2(–)/uis3(–) | 20.000 | 20.000 | 10.000 (d28) | 3/8 (8.6 d) | |
| 10.000 (d28) | 0/4 (3.75 d) |
*Numbers of sporozoites for first boost at day 14; d0 corresponds to date of first shot.
#Mice were challenged with PbANKA wild type sporozoites; day of challenge after prime in parentheses.
**1 breakthrough infection after priming with 20.000 PbANKA uis3(–) sporozoites.
Table 3. Protection of C57BL/6 mice immunized in a 1-shot regime with the indicated P. berghei ANKA knockout lines against challenge with wild-type P. berghei ANKA sporozoites.
| Exp. | Attenuated parasite line (P. berghei ANKA) | 1st shot | Challenge dose (day 14) | Protected/challenged animals (pre-patency) |
|---|---|---|---|---|
| 5 | LISP2(–) | 30.000 | 10.000 | 7/15* (d6.25) |
| uis3 (–) | 30.000 | 10.000 | 4/16 (d5) | |
| LISP2(–)/uis3(–) | 30.000 | 10.000 | 0/16 (d5.1) | |
| 10.000 | 0/16 (d3.2) | |||
| 6 | LISP2(–) | 20.000 | 10.000 | 4/6 (d7) |
| uis3 (–) | 20.000 | 10.000 | 0/4* (d6) | |
| LISP2(–)/uis3(–) | 20.000 | 10.000 | 1/6 (d5.4) | |
| 10.000 | 0/4 (d4.25) |
*1 breakthrough infection after injection of 30.000 LISP2(–) sporozoites and 2 breakthrough infections after injection of 20.000 uis3(–) sporozoites.
Discussion
Our work revealed several interesting and relevant findings about ANKA LISP2(–) and uis3(–) parasites. Two previous papers have addressed the function of LISP2 by only partially disrupting the LISP2 gene (Fig. 2A) resulting in a severe reduction of liver stage growth but not in a complete liver stage arrest26,27. Our work revealed an almost complete liver stage developmental arrest of the ANKA LISP2(–) parasites and showed that the N-terminus plays an important role in liver stage biology. The 403 amino acid long N-terminal region is conserved (>60% identity) within rodent malaria species (P. berghei, P. yoelii and P. chabaudi) and within the human parasite species P. vivax and P. knowlesii (Supplementary Fig. S1). However, it is rather diverse (<20% identity) between rodents and human species and absent in P. falciparum. This N-terminal region contains a PEXEL motif (aa 373–377 in PbLISP2), which might play a role in secretion of the protein. Curiously our ANKA LISP2(–) parasites grew to larger size than the wild type in vitro, while they did not express large amounts of MSP1 (Figs 2D and 3D). This suggests that the size of the parasite and the developmental stage are not necessarily linked. The larger size might be due to the lack of merozoite formation and possibly an excess of membrane being deposited in the plasma membrane thus leading to a larger size, although this is currently just speculation.
Deletion of uis3 in P. berghei strain NK65 led to an early arrest during liver stage development and attenuated parasites9. In contrast, the P. berghei strain ANKA uis3(–) parasite arrests later in what might be called a mid-stage (Fig. 3D) and causes breakthrough infections (Fig. 3B, Supplementary Table 2). Part of this difference might be due to a faster liver stage development of P. berghei ANKA parasites29,30. A clearer definition of the timing of arrest, also for the other parasite lines, should take into account the parasite size, the developmental stage of arrest as can be determined by electron microscopy and a gene expression profile.
Although vaccination with radiation-attenuated sporozoites (RAS) is still considered the gold standard for experimental malaria immunization (Seder et al.3), a first clinical trial demonstrated partial safety of PfGAP-vaccination in humans31. Compared to RAS, GAPs arrest at more uniform time points during intrahepatic development2,32 either early9,33,34 or late13,35. Protective immunity appears to rely on CD8+ T cells and IFN-γ as key mediators of protection in all the different attenuation models28,36. However a GAP leading to an arrest in late liver stage development appears to elicit a larger number of CD8+ memory T cells with a broader antigenic repertoire as compared to early arresting GAP and/or RAS15. Whether this can be generalized to all GAPs arresting at the late liver stage however, remains to be determined. It is also not clear how the broader antigen repertoire and the increased size of the late GAP individually contribute to increased protection. The enhanced immune responses induced by these late arresting parasites correlate with enhanced protection suggesting that growth arrest during late liver stage development may confer superior protection than earlier arresting attenuated parasites15. As some single and double gene deletions can cause breakthrough infections16,27,37, it is important to find novel candidate genes that can be included in an attenuated parasite line lacking several genes as shown by various groups21,22,24,36. For example, early arresting NK65 uis3(–) parasites cause no breakthrough, while NK65 uis4(–) parasites show frequent breakthroughs. The mutant lacking both genes causes no breakthroughs and results in complete protection after immunizations, as do both individual uis3(–) and uis4(–) in a 3-shot regimen. However it is not clear if these multi-gene deletions affect protective efficacy.
Our data on 3 different parasite lines, which arrest during liver stage development showed a similar reduction in breakthrough infections but revealed interesting differences in their capacity to induce protective immune responses. Both ANKA uis3(–) and LISP2(–) parasites caused breakthrough infections during the immunization experiments. To quantify the rate of breakthrough we injected in total over 9 million sporozoites into over 160 mice (Fig. 3B, Supplementary Table S2). While our breakthrough rates are not problematic for experimental approaches similar rates in P. falciparum would clearly prevent use in human trials, let alone wider applications. To estimate if there is an additive safety effect of deleting both genes, we injected over 4.8 million of the LISP2(–)/uis3(–) sporozoites in 56 mice (Fig. 3B, Supplementary Table S2). In these mice we observed no breakthrough infections. This suggests that deletion of two genes leading to mid-stage arrest and possibly be involved in different biological functions can indeed add an additional level of safety as has been suggested previously for genes causing early arrest31. We would expect that in the best case this scaling would be the product of the infection rates of the individual KOs. Hypothetically this would result in an expected breakthrough rate of one per trillion sporozoites, which is far beyond the testable range. Ironically, this implies that the relationship of additive protection is most easily assessed with parasites showing very high breakthrough rates.
To test efficacy we turned to different vaccination schemes that generally lower protection from challenge. In a simple ‘one-shot’ immunization scheme, where mice were immunized just once and then challenged 14 days later with 10.000 wild type sporozoites, mice immunized with LISP2(–) parasites were better protected against wild type challenge than mice immunized with either uis3(–) or the LISP2(–)/uis3(–) sporozoites (Table 3). Similar data were obtained in ‘2-shot’ vaccination schemes (Table 2). These differences suggest a possible trade-off between achieving a fully save genetically attenuated parasite and a maximally protective one as the less protective gene deletion appears to dominate the efficacy of protection. This suggests, that the strains, while equally effective at establishing a hepatocyte infection, differ in their capacity to elicit protection during vaccination. A similar finding with just one gene deleted was shown for early arresting uis3(–) and uis4(–) P. yoelii parasites infecting BALB/c mice28. While uis3(–) but not uis4(–) P. berghei NK65 showed complete block in liver stage development9,16, deletion of either gene in P. yoelii led to a complete arrest28. Yet, immunization with uis4(–) P. yoelii parasites was more protective than with uis3(–) parasites28. However, there is no observable correlation in these experiments between time/safety of arrest, and efficacy of protection.
We suggest that many more genes that, upon deletion, arrest parasite development at defined points during infection should be identified. Their combination in multiple knockouts should then be tested to see which achieve totally abrogation of breakthrough events while keeping maximum protective efficacy. These should be tested in similar assays as shown here and also in assays that correlate the overall number of sporozoites used per immunization with protective capacity. Once more strains are available a careful dissection of the exact time of arrest in developmental terms as well as an immunological dissection of the different protective efficacies should be determined. Furthermore, alternative approaches to generate parasites that are attenuated at the liver stage such as the expression of foreign toxins38 or the induction of DNA double strand breaks39 should also be explored and tested towards their protective capacity prior to translation to P. falciparum.
Materials and Methods
Animals and parasites
C57BL/6 and NMRI mice were purchased from Charles River Laboratories. For all experiments six to eight weeks old female mice were used unless stated otherwise. P. berghei ANKA parasites were maintained by passaging via intra-peritoneal injections in NMRI mice and Anopheles stephensi mosquitoes. All animal experiments were performed according to FELASA standard guidelines and were approved by German authorities (Regierungspräsidium Karlsruhe).
Generation of LISP2(–), uis3(–) and LISP2(–)/uis3(–) P. berghei ANKA mutants
To delete the genes encoding LISP2 (Gene ID: PBANKA_100300) and uis3 (Gene ID: PBANKA_140080) in P. berghei ANKA, the corresponding 5′UTR and 3′UTR regions were amplified from genomic DNA of mixed blood stages by polymerase chain reaction (PCR) using Phusion® High-Fidelity DNA Polymerase (NEB, USA). For primers see Supplementary Table S1. The amplified fragments of LISP2 were ligated into Pb262 vector that contains the human dihydrofolate reductase and the yeast bifunctional enzyme cytosine deaminase as well as uracil phosphoribosyl transferase (hdhfr-yFCU), as a positive-negative selection cassette17. The 5′UTR and 3′UTR fragments of uis3 were ligated into the b3D-vector containing the hDHFR resistance cassette. The linearized vectors were transfected into P. berghei ANKA merozoites using the Nucleofector™ kit in the Amaxa (Lonza) electroporator as previously described40 followed by intravenous injection into NMRI mice. Mutant parasites were selected by adding pyrimethamine (0.07 mg/ml, Sigma-Aldrich) to the drinking water (PbANKA LISP2(–)) or by subcutaneous injection of WR99210 (16 mg/ kg body weight, Sigma-Aldrich) (PbANKA uis3(–)) and clones were derived by limiting dilution. The integration of the vector into the genome was confirmed by PCR using test primer pairs as shown in the figures and Supplementary Table S1. Two different transfections were performed to obtain independent clones for LISP2(–) and uis3(–) ANKA parasites. Negative selection of Pb ANKA LISP2(–) parasites was performed by adding 5-Fluorocytosine (1 mg/ml, Sigma-Aldrich) in the drinking water. The LISP2(–)/uis3(–) ANKA parasite line was generated by transfecting the linearized uis3(–) knock-out vector (b3D containing TgDHFR resistance cassette) into a negatively selected LISP2(–) parasite line. Similarly the N-terminus of LISP2 was integrated into a negatively selected LISP2(–) parasite line using Pb238 vector containing the corresponding 5′UTR and 3′UTR regions flanking the hDHFR resistance cassette.
Mosquito stage development and sporozoite infectivity
Sporozoite numbers were determined in the midguts and salivary glands by mosquito dissection 12 and 17/18 days post feeding, respectively. Gliding motility of sporozoites was analyzed in glass bottom 96 well plates (Greiner) using freshly dissected salivary gland sporozoites as described before41.
Confluent HepG2 cells were infected with freshly isolated salivary gland sporozoites, cultivated in DMEM supplemented with 10% fetal calf serum, 1 mM glutamine and 1% Anti-anti (Gibco) at 37 °C and 5% CO2 and fixed with 4% paraformaldehyde followed by ice cold methanol. Primary antibodies used for staining: monoclonal mouse anti-PbHSP7042, rat anti-PbMSP143, mouse anti-PbEXP1; secondary antibodies: Alexa-Fluor® 488 goat anti-mouse IgG antibody, Alexa-Fluor® 546 goat anti-rat IgG antibody; DNA was revealed by Hoechst 33342 (all Life Technologies). Size measurements were carried out by acquisition of images with a Zeiss Axiovert 200 M microscope followed by analysis using Volocity (PerkinElmer).
In vivo infectivity of mutant sporozoites and immunizations
10 infected mosquitoes (on day 17 post infection) were allowed to bite anaesthetized mice or isolated salivary gland sporozoites in 100 μL PBS were injected intravenously per mouse. Parasitemia was monitored by daily Giemsa stained blood smears starting at day 3 after infection.
For immunization C57BL/6 mice were intravenously injected with sporozoites according to the different immunization protocols described in Tables 1–3. Immunized mice were monitored for absence of blood stage parasites by daily Giemsa stained blood smears. Challenge of all immunized and naïve control mice with wild type Pb ANKA sporozoites was performed as depicted in Tables 1–3. Blood stage parasitemia was monitored from day 3 onward up to 21 days after the challenge.
Real-time PCR analysis
C57BL/6 mice were infected by intravenous injection of 20.000 sporozoites of the respective parasite strain. 40 h or 56 h post-infection mice were sacrificed, livers were homogenized in 4 ml QIAzol (QIAGEN) and 0.5 ml aliquots were frozen at –80 °C immediately. RNA extraction followed by DNA digest using the TURBO DNA-free Kit (Ambion) was performed according to the manufacturer’s instructions. cDNA was synthesized with the First Strand cDNA Synthesis Kit (ThermoFisher) and checked afterwards for gDNA contaminations via RT-PCR. Real-time PCR was performed in real time using triplicates on a CFX96 Touch Real-Time PCR Detection System (BIO-RAD) using Power SYBR Green PCR Master Mix (Applied Biosystems) and gene-specific primers (P18-21, see Supplementary Table S1). Relative copy numbers of the different parasite lines were calculated by applying the ∆∆Ct methodology.
Statistical analysis
1-way ANOVA with Bonferroni post test was performed with Prism software (version 5.0b, GraphPad). A p-value of <0.05 was considered significant.
Additional Information
How to cite this article: Kumar, H. et al. Protective efficacy and safety of liver stage attenuated malaria parasites. Sci. Rep. 6, 26824; doi: 10.1038/srep26824 (2016).
Supplementary Material
Acknowledgments
We thank Ulrike Böhme for help with gene annotation, Catherine Moreau, Dennis Klug, Gunnar Mair, Kartik Bane and Ross Douglas for discussions and comments on the manuscript. The work was further supported by grants from the Chica and Heinz Schaller Foundation, the European Research Council (StG 281719), the Deutsche Forschungsgemeinschaft (SFB 1129) and the Heidelberg University cluster of excellence CellNetworks. HK was the recipient of a predoctoral fellowship from the German Academic Exchange Service (DAAD). VH and FF were members of the EU FP7 network of excellence EVIMalaR. AKM is a member of the German center for infection research DZIF.
Footnotes
Author Contributions H.K., J.M.S., M.S. and F.F. planned the experiments, H.K., J.M.S., M.S., K.H., M.R. and C.H.-K. performed the experimental work, H.K., J.M.S., M.S. and F.F. analyzed results, H.K., J.M.S., M.S., V.H., A.-K.M. and FF discussed and wrote the paper.
References
- Nussenzweig R. S., Vanderberg J., Most H. & Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. Nature 216, 160–162 (1967). [DOI] [PubMed] [Google Scholar]
- Silvie O. et al. Effects of irradiation on Plasmodium falciparum sporozoite hepatic development: implications for the design of pre-erythrocytic malaria vaccines. Parasite Immunol. 24, 221–223 (2002). [DOI] [PubMed] [Google Scholar]
- Seder R. A. et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 341, 1359–1365 (2013). [DOI] [PubMed] [Google Scholar]
- Hoffman S. L. et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J. Infect. Dis. 185, 1155–1164 (2002). [DOI] [PubMed] [Google Scholar]
- Beaudouin R. L., Strome C. P. A., Mitchell F. & Tubergen T. A. Plasmodium berghei: Immunization of mice against the ANKA strain using the unaltered sporozoite as an antigen. Exp. Parasitol. 42, 1–5 (1977). [DOI] [PubMed] [Google Scholar]
- Belnoue E. et al. Protective T cell immunity against malaria liver stage after vaccination with live sporozoites under chloroquine treatment. J. Immunol. 172, 2487–2495 (2004). [DOI] [PubMed] [Google Scholar]
- Roestenberg M. et al. Protection against a malaria challenge by sporozoite inoculation. N. Engl. J. Med. 361, 468–477 (2009). [DOI] [PubMed] [Google Scholar]
- Friesen J. et al. Natural immunization against malaria: causal prophylaxis with antibiotics. Sci. Transl. Med. 2, 40ra49 (2010). [DOI] [PubMed] [Google Scholar]
- Mueller A. K., Labaied M., Kappe S. H. & Matuschewski K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature 433, 164–167 (2005). [DOI] [PubMed] [Google Scholar]
- Hafalla J. C., Silvie O. & Matuschewski K. Cell biology and immunology of malaria. Immunol. Rev. 240, 297–316 (2011). [DOI] [PubMed] [Google Scholar]
- Borrmann S. & Matuschewski K. Protective immunity against malaria by ‘natural immunization’: a question of dose, parasite diversity, or both? Curr. Opin. Immunol. 23, 500–508 (2011). [DOI] [PubMed] [Google Scholar]
- Khan S. M., Janse C. J., Kappe S. H. & Mikolajczak S. A. Genetic engineering of attenuated malaria parasites for vaccination. Curr. Opin. Biotechnol. 23, 908–916 (2012). [DOI] [PubMed] [Google Scholar]
- Vaughan A. M. et al. Type II fatty acid synthesis is essential only for malaria parasite late liver stage development. Cell. Microbiol. 11, 506–520 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijpma S. R. et al. Multidrug ABC transporters are essential for hepatic development of Plasmodium sporozoites. Cell. Microbiol., 18, 369–383 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler N. S. et al. Superior antimalarial immunity after vaccination with late liver stage-arresting genetically attenuated parasites. Cell Host Microbe 9, 451–462 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller A. K. et al. Plasmodium liver stage developmental arrest by depletion of a protein at the parasite–host interface. Proc. Natl. Acad. Sci. USA 102, 3022–3027 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr R. Y., Philip N. & Waters A. P. Improved negative selection protocol for Plasmodium berghei in the rodent malarial model. Malar. J. 11, 103 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishino T. et al. LISP1 is important for the egress of Plasmodium berghei parasites from liver cells. Cell. Microbiol. 11, 1329–1339 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labaied M. et al. Plasmodium yoelii sporozoites with simultaneous deletion of P52 and P36 are completely attenuated and confer sterile immunity against infection. Infect. Immun. 75, 3758–3768 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanBuskirk K. M. et al. Preerythrocytic, live-attenuated Plasmodium falciparum vaccine candidates by design. Proc. Natl. Acad. Sci. USA 106, 13004–13009 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolajczak S. A. et al. A next-generation genetically attenuated Plasmodium falciparum parasite created by triple gene deletion. Mol. Ther. 22, 1707–1715 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schaijk B. C. et al. A genetically attenuated malaria vaccine candidate based on P. falciparum b9/slarp gene-deficient sporozoites. Elife 3, e03582 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R. & Diggs C. L. In Parasitic Protozoa Vol. 3 (ed Kreier J. P.) 359–465 (Academic Press, 1977). [Google Scholar]
- Annoura T. et al. Assessing the adequacy of attenuation of genetically modified malaria parasite vaccine candidates. Vaccine 30, 2662–2670 (2012). [DOI] [PubMed] [Google Scholar]
- Helm S. et al. Identification and characterization of a liver stage-specific promoter region of the malaria parasite Plasmodium. PLoS ONE 5, e13653 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orito Y. et al. Liver-specific protein 2: a Plasmodium protein exported to the hepatocyte cytoplasm and required for merozoite formation. Mol. Microbiol. 87, 66–79 (2013). [DOI] [PubMed] [Google Scholar]
- Annoura T. et al. Two Plasmodium 6-Cys family-related proteins have distinct and critical roles in liver-stage development. FASEB J. 28, 2158–2170 (2014). [DOI] [PubMed] [Google Scholar]
- Tarun A. S. et al. Protracted sterile protection with Plasmodium yoelii pre-erythrocytic genetically attenuated parasite malaria vaccines is independent of significant liver-stage persistence and is mediated by CD8 + T cells. J. Infect. Dis. 196, 608–616 (2007). [DOI] [PubMed] [Google Scholar]
- Bafort J., Timperman G. & Delbar T. Observations on tissue schizogony and sporogony of rodent malaria. Ann. Soc. Belges Med. Trop. Parasitol. Mycol. 48, 535–540 (1968). [PubMed] [Google Scholar]
- Garnham P. C. C., Landau I. & Killick-Kendrick R. Primary exo-erythrocytic schizonts of three rodent malaria parasites. Trans. R. Soc. Trop. Med. Hyg. 60, 3–4 (1966). [Google Scholar]
- Spring M. et al. First-in-human evaluation of genetically attenuated Plasmodium falciparum sporozoites administered by bite of Anopheles mosquitoes to adult volunteers. Vaccine 31, 4975–4983 (2013). [DOI] [PubMed] [Google Scholar]
- Matuschewski K. & Mueller A. K. Vaccines against malaria-an update. FEBS J. 274, 4680–4687 (2007). [DOI] [PubMed] [Google Scholar]
- Van Dijk M. R. et al. Genetically attenuated, P36p-deficient malarial sporozoites induce protective immunity and apoptosis of infected liver cells. Proc. Natl. Acad. Sci. USA 102, 12194–12199 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly A. S. et al. Targeted deletion of SAP1 abolishes the expression of infectivity factors necessary for successful malaria parasite liver infection. Mol. Microbiol. 69, 152–163 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvie O., Goetz K. & Matuschewski K. A sporozoite asparagine-rich protein controls initiation of Plasmodium liver stage development. PLoS Pathog. 4, e1000086 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe O. et al. Genetically attenuated Plasmodium berghei liver stages induce sterile protracted protection that is mediated by major histocompatibility complex class I-dependent interferon-γ-producing CD8 + T cells. J. Infect. Dis. 196, 599–607 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M. et al. The fatty acid biosynthesis enzyme FabI plays a key role in the development of liver-stage malarial parasites. Cell Host Microbe 4, 567–578 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel A. et al. A new approach to generate a safe double-attenuated Plasmodium liver stage vaccine. Int. J. Parasitol. 43, 503–514 (2013). [DOI] [PubMed] [Google Scholar]
- Singer M. et al. Zinc finger nuclease-based double-strand breaks attenuate malaria parasites and reveal rare microhomology-mediated end joining. Genome Biol. 16, 249 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janse C. J., Ramesar J. & Waters A. P. High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei. Nat. Protoc. 11, 346–356 (2006). [DOI] [PubMed] [Google Scholar]
- Hegge S., Münter S., Steinbüchel M., Heiss K., Engel U., Matuschewski K., & Frischknecht F. Multi-step adhesion of Plasmodium sporozoites. FASEB J. 24, 2222–2234 (2010). [DOI] [PubMed] [Google Scholar]
- Tsuji M., Mattei D., Nussenzweig R. S., Eichinger D. & Zavala F. Demonstration of heat-shock protein 70 in the sporozoite stage of malaria parasites. Parasitol. Res. 80, 16–21 (1994). [DOI] [PubMed] [Google Scholar]
- Burda P. C. et al. A Plasmodium phospholipase is involved in disruption of the liver stage parasitophorous vacuole membrane. PLoS Pathog. 11, e1004760 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.