Abstract
In both demographic and clinical studies, frailty is understood as a multidimensional state of increased vulnerability compared with the status of others of the same age. Of the many theoretical definitions of frailty, two are commonly employed: the physical frailty/phenotypic approach and the deficit accumulation approach. The purpose of this chapter is to discuss how frailty is conceptualized and operationalized based on these two approaches.
The term ‘frailty’ has been used scientifically since at least 1979, when Vaupel et al. [1] employed it to describe variability in life expectancy (hidden heterogeneity). They borrowed the idea of an individual, unobserved susceptibility to death from the actuarial literature to explain why some individuals tend to have a long life. In geriatric medicine, we are more inclined to see frailty as a nonconstant factor that increases with age. It is worth recalling that the notion of frailty in geriatric medicine arose during the era of the controlled clinical trial, when comprehensive geriatric assessment was shown to be most effective when targeting vulnerable older adults. At one point, such people were called the ‘targeted elderly’, whereas now, these vulnerable older adults are understood as frail. As the concept of frailty has become more readily accepted, a variety of definitions of frailty have emerged; these definitions are currently the focus of debate. Two of the most commonly used approaches to conceptualize and define frailty are the phenotypic approach and the deficit accumulation approach. The phenotypic definition operationalizes frailty as a biological syndrome, whereas the deficit accumulation approach sees frailty as a multidimensional risk state. Various sets of criteria have been proposed to operationalize frailty by evaluating specific physiological changes and deficits; however, currently, none of the proposed operational definitions of frailty provide a definitive diagnosis [2, 3]. Most operational definitions of frailty specify impairments in mobility, balance, muscle strength, motor processing, physical function, disability, cognition, nutrition, endurance, and physical activity [2]. Those impairments most commonly specified are physical function, mobility, disability, and cognition [2].
The Deficit Accumulation, or Frailty Index, Approach
This approach sees frailty as a multidimensional risk state that can be measured by the quantity rather than by the nature of health problems. Frailty reflects a stochastic dynamic process in a system with high redundancy of multiple interdependent items. On average, this system accumulates deficits that impair the ability of the system to repair damage that arises either externally or as the byproduct of internal processes (e.g. metabolism, respiration, and inflammation), including genetically induced damage. Even though some events can accelerate the development of frailty, typically frailty develops slowly, even insidiously, and this process can vary in important ways between individuals. The deficit accumulation/frailty-as-a-state approach proposes that frail older adults have many things wrong with them; the more things that they have wrong, the higher the likelihood that they will be frail and the greater their risk of adverse health outcomes.
The origin of deficit accumulation can generally be understood from a stochastic point of view. Accordingly, there is a simple relationship between the average number of deficits (N) present in an individual of a certain age, the intensity of the stream of environmental stresses (λ) and the average recovery time (R) [4], which is written as N = λR, known as Little’s Law in the operation research area [5]. During the individual’s life course, both environmental stresses and the recovery time are clearly stochastic (as evidenced by the generally irregular individual trajectories of the frailty index) [6]. In contrast, the population-based trajectories of frailty are clearly regular, showing an acceleration in deficit accumulation that is well fitted by an exponential curve with an exponent of about 0.03. Because the frailty index increases by 10-fold on average between 20 and 90 years of age and because environmental intensity remains on average unchanged, we can conclude from Little’s Law that the recovery time is what changes over the life course, explaining the increases in the frailty index value [4].
Operationalizing the Deficit Accumulation Approach
The application of the deficit accumulation approach is the frailty index [7]. This index can include deficits such as symptoms, signs, diseases, disabilities, and laboratory abnormalities. These deficits should be age-related, should be associated with adverse outcomes, and, when combined, should cover several organ systems. Five or 10 specific deficits might not capture all aspects of frailty, which has hindered agreement between investigators on one frailty scale that includes specific deficits. Older adults are very heterogeneous and become frail through different pathways, and any scale that includes enough items could be used as an indicator of frailty, especially if the items are integrated variables such as mobility and physical activity. Prior studies suggest that at least 20 deficits should be considered; 30 or more is preferred to achieve stable estimates [8]. An individual’s frailty index score is calculated based on the number of deficits a person has in relation to the total number of measures included in the index (e.g. someone with 10 deficits out of 40 counted has a frailty index of 10/40 = 0.25). In this way, the frailty index score is continuous (0–1); the higher the score, the more likely that the individual is vulnerable to adverse health outcomes.
The population-based studies that have used the frailty index approach (but differing frailty indices, depending on the data available) give robust results: people accumulate an average of 0.03 deficits per year after the age of 70; the frailty index has a strong association with adverse health outcomes; women accumulate more deficits than men of the same age; and the frailty index has maximal limit of approximately 0.7 [8]. These studies, including studies in North America [9], Europe [10], and Australia [11], have all identified nonlinear increases with age. While the relationship between age and frailty index scores generally was best fit with an exponential function (accelerating with age), the relationship between the frailty index score and mortality rate best fit with a sigmoidal (dose-response) function. The sigmoidal relationship between age and mortality in cross-sectional data, which is characterized by an initial acceleration in mortality risk followed by a deceleration at older ages, has been well described in the literature, representing a fundamental observation in the reliability theory of aging.
The existence of a health-survival paradox between genders, in which two people of the same frailty score but different genders demonstrate differing vulnerability (described in detail in Chapter 4), underscores the notion that frailty scales are imperfect in their ability to measure variable risk of death. Other factors beyond gender have been found to influence the relationship between a score on a frailty scale and the risk for mortality, including individual-level factors like social vulnerability, exercise, and tobacco use as well as environmental factors including country of residence. Differing vulnerability at the same level of identified frailty is important consideration because the concept of frailty was initially developed in order to grade this vulnerability itself. This discrepancy suggests that some factors that modify the effect of frailty or that are unmeasured sources of differential vulnerability among people of the same frailty score need to be considered when examining frailty.
The Frailty Phenotype, or Syndromic, Approach
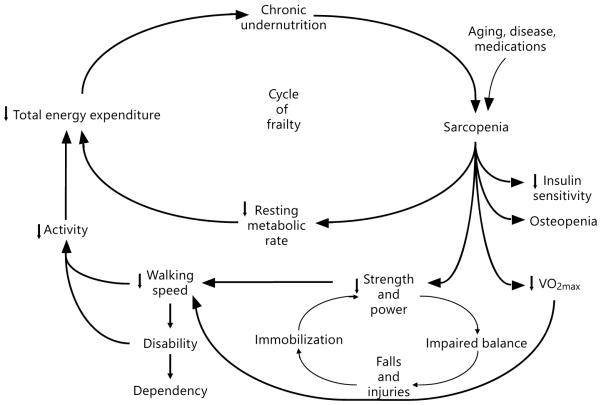
This approach is based on a cluster of signs and symptoms that commonly occur in vulnerable older adults, including weight loss, weakness, fatigue, slowness in walking, and low levels of physical activity. Aggregations of common signs and symptoms have long been used in early stages of disease characterization to conceptualize and define medical conditions including type 2 diabetes mellitus, hyperlipidemia, and rheumatological conditions such as lupus. Hence, the conceptualization of frailty as a syndrome with an underlying age-related biological basis was further developed by Fried and Walston [12]. The Cycle of Frailty, first published in 1998, facilitated the conceptualization of frailty as a deeply biological entity that largely drives the accumulation of associated adverse outcomes. It also provided an important framework that helped in the development of testable biological hypotheses related to syndromic frailty. This model highlights individual components of a cycle of decline that are associated in a step-wise fashion with other declines, providing the biological basis for the development of vulnerability to functional decline, disease states, and, ultimately, mortality. This model connects the underlying physiology of low energy expenditure, low physical activity, nutritional deficits, and loss of skeletal muscle (sarcopenia) into a cycle of decline (fig. 1). These interconnected domains reinforce each other, and in turn, this interaction influences other crucial physiological systems, including insulin sensitivity, VO2max, muscle strength, and power. These changes then contribute to a subcycle of disability, functional decline, and reduced activity levels that further reinforces the physiological decline. Importantly, this model also suggests multiple possible entry points into an underlying biological cycle of decline and illustrates how specific illnesses, injuries, or medications can trigger and/or accelerate this biological decline. As discussed in Chapter 1, the notion of frailty arising in an interconnected web of deficits can also be seen as consistent with how deficit accumulation accelerates. As noted in that chapter, in a constant environment, the accumulation of deficits is seen to reflect a prolongation of the recovery time.
Fig. 1.
The cycle of frailty.
For example, it is clear that many common chronic disease states, including metastatic or advanced cancers, chronic congestive heart failure, chronic obstructive pulmonary disease, and inflammatory conditions such as rheumatoid arthritis, can help trigger or accelerate the biological cycle of decline that underlies syndromic frailty. However, frailty often exists independently of identifiable disease states. This can be read as further supporting the concept of an independent underlying pathophysiological etiology that is driving syndromic frailty and related outcomes [13]. This pathophysiological change may in part be driven by specific cellular aging processes such as mitochondrial decline and cellular senescence, which contribute to adverse health outcomes in this and the deficit accumulation conceptualizations of frailty.
Operationalizing Phenotypic Frailty
Building on clinical observations of vulnerable older adults, multiple syndromic models of frailty have been developed using data derived from large longitudinal population-based studies of older adults that most often include measures related to clinical observations of vulnerable older adults including weight decline, muscle strength, walking speed and subjective measures of energy levels and physical activity. Early focused efforts in this area include a study by Chin et al. [14] that compared three previously developed working definitions of frailty, namely inactivity combined with (1) low energy intake, (2) weight loss, or (3) low body mass index. The combination of inactivity with weight loss was found to be most strongly associated with reductions in subjective health and performance measures and with increased disease and disability. In addition, the 3-year relative risk for mortality was substantially higher in this group compared to others in the study cohort (odds ratio 4.1, 95% confidence interval 1.8–9.4) [14]. Fried et al. [13] utilized a syndromic approach and developed and operationalized a frailty phenotype based on common physiological signs and symptoms characteristic of frail, older adults. This tool consists of five items, including muscle strength (lowest quartile as determined by dynamometric measurement of grip strength), weight loss (more than 10 pounds of unintended weight loss in the previous year), walking speed (lowest quartile of performance on a timed 15 meter walk), low levels of physical activity as measured by the Minnesota Leisure Time Activities questionnaire, and fatigue (measured by questions about energy levels from a depression survey). It was first operationalized in the Cardiovascular Health Study (CHS), an epidemiological study of over 5,000 community-dwelling adults over the age of 65, who were followed for 9 years in order to better characterize cardiovascular disease and functional decline late in life. The participants were deemed frail if they met three of the five criteria, intermediate, or ‘pre-frail’, if they met one or two of the criteria, and robust, or not frail, if they met none of the criteria. Seven percent of these CHS participants met the criteria to be considered as frail at their baseline exam. A significant overlap between disability, chronic illness, and frailty was observed, although there were many frail participants who were not disabled and who did not have medical illnesses, supporting the previously mentioned hypothesis that syndromic or physical frailty has an underlying etiology that is likely independent of disease and disability [13]. Predictive validity analyses were also performed, revealing that those who were frail were significantly more likely to fall, enter a nursing home, be hospitalized, and suffer mortality over 7 years of follow-up. These results have been confirmed in many large cohort studies, including the Women’s Health and Aging Study, which demonstrated an even stronger association between frailty and the 3-year mortality rates [15]. This syndromic tool to measure frailty has gone on to become among the most commonly cited frailty measurement tools in the medical literature and has been utilized extensively to assess clinical risk in a variety of settings and to study the biological basis of frailty, chronic disease states, and late-life vulnerability. This syndromic approach to frailty measurement has also been adapted and altered by many investigators in order to more feasibly measure frailty in populations of older adults and in clinical practice. Published validity data for many of these adaptations suggests that these adapted tools also predict adverse outcome in older adults relatively well [16–19]. Future studies in this area are needed to more fully explore the validity of these tools.
To date, the majority of biological studies of frailty have taken place using the syndromic definition of frailty. These studies, which have been carried out as large epidemiological data studies or smaller scale clinical observational studies [20–23], have enabled the identification of core biological changes that are highly related to and perhaps drive the development of frailty and late-life vulnerability to adverse health outcomes observed among frail individuals. Important findings include significant relationships between chronic activation of inflammation as measured by serum cytokine levels, increased hypothalamic-pituitary-adrenal axis activation as measured by salivary cortisol levels, altered glucose metabolism, and decreased mitochondrial mass as key correlates and perhaps drivers of syndromic frailty and its incumbent risk [20–23].
Other Operational Definitions of Frailty
In addition to the frailty phenotype and the frailty index, other operational definitions of frailty include the Edmonton Frail Scale, the Groningen Frailty Indicator, the Tilburg Frailty Indicator, and the ‘FRAIL’ scale. The Edmonton Frail Scale considers 17 specific deficits, including cognition, general health status, functional independence, social support, medication use, nutrition, mood, continence, and functional performance [24]. The Groningen Frailty Indicator includes measures of physical (including mobility, physical fitness, vision, hearing, nourishment, and polypharmacy), cognitive, and psychosocial health, for a score calculated from 15 items [25]. The Tilburg Frailty Indicator scores 15 deficits in physical (weight loss, overall physical health, difficulty in walking, balance, vision problems, hearing problems, hand strength, and tiredness), psychological (cognition, depressive symptoms, anxiety, and coping), and social domains (living alone, social relations, and social support) [26]. The FRAIL scale considers five health deficits, forming its acronym: fatigue, resistance, ambulation, illness, and loss of weight; four of these components were obviously taken from the frailty phenotype [27]. New scales are also being proposed: of 27 frailty scales that have been applied to population-based studies, 14 have yet to be used by researchers beyond the group that first proposed them [28]. At the 2012 meeting of the Gerontological Society of America, 13 further frailty scales were newly introduced [29]. When eight frailty scales were recently applied to a representative sample of middle-aged and older Europeans in the Survey of Health, Ageing, and Retirement in Europe (SHARE), they all identified frailty and predicted all-cause mortality, even though they captured related but distinct components and varied in the accuracy of their mortality prediction [30]. Similarly, when three frailty scales were compared in the European Male Ageing Study, they showed differing ability to predict mortality [31]. Many of these scales have been developed to meet the needs of specific research studies and have not been operationalized in other populations. Compared with the more focused effort of the frailty phenotype, the broad heterogeneity in measurement domains and in the specific measurements contained within these domains of the frailty index can be seen as a limitation to the drawing of conclusions beyond the factors that might broadly increase risk. The vigorous debate over this point is further motivating and welcoming inquiries into how we understand the nature of frailty [32].
References
- 1.Vaupel JW, Manton KG, Stallard E. The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography. 1979;16:439–454. [PubMed] [Google Scholar]
- 2.Sternberg SA, Wershof Schwartz A, Karunananthan S, et al. The identification of frailty: a systematic literature review. J Am Geriatr Soc. 2011;59:2129–2138. doi: 10.1111/j.1532-5415.2011.03597.x. [DOI] [PubMed] [Google Scholar]
- 3.Pijpers E, Ferreiraa I, Stehouwera C, et al. The frailty dilemma: review of the predictive accuracy of major frailty scores. Eur J Intern Med. 2012;23:118–123. doi: 10.1016/j.ejim.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Mitnitski A, Song X, Rockwood K. Assessing biological aging: the origin of deficit accumulation. Biogerontology. 2013;14:709–717. doi: 10.1007/s10522-013-9446-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Little JDC. Little’s law as viewed on its 50th anniversary. Operations Research. 2011;59:536–549. [Google Scholar]
- 6.Mitnitski A, Song X, Rockwood K. Trajectories of changes over twelve years in the health status of Canadians from late middle age. Exp Gerontol. 2012;47:893–899. doi: 10.1016/j.exger.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Mitnitski A, Mogilner A, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–336. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27:17–26. doi: 10.1016/j.cger.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Kulminski A, Yashin A, Ukrainsteva S, et al. Accumulation of health disorders as a systemic measure of aging: findings from the NLTCS data. Mech Ageing Dev. 2006;127:840–848. doi: 10.1016/j.mad.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Theou O, Brothers TD, Pena F, et al. Identifying common characteristics of frailty across seven scales. J Am Geriatr Soc. 2014;62:901–906. doi: 10.1111/jgs.12773. [DOI] [PubMed] [Google Scholar]
- 11.Mitnitski A, Song X, Skoog I, et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53:2184–2189. doi: 10.1111/j.1532-5415.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- 12.Fried LP, Walston J. Frailty and failure to thrive. In: Hazzard W, editor. Principles of Geriatric Medicine and Gerontology. McGraw-Hill; New York: 1998. pp. 1387–1402. [Google Scholar]
- 13.Fried LP, Tangen C, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 14.Chin APM, Dekker JM, Feskens EJ, et al. How to select a frail elderly population? A comparison of three working definitions. J Clin Epidemiol. 1999;52:1015–1021. doi: 10.1016/s0895-4356(99)00077-3. [DOI] [PubMed] [Google Scholar]
- 15.Bandeen-Roche K, Xue Q, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 16.Avila-Funes JA, Amieva H, Barberger-Gateau P, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc. 2009;57:453–461. doi: 10.1111/j.1532-5415.2008.02136.x. [DOI] [PubMed] [Google Scholar]
- 17.Kiely DK, Cupples LA, Lipsitz LA. Validation and comparison of two frailty indexes: the MOBILIZE Boston Study. J Am Geriatr Soc. 2009;57:1532–1539. doi: 10.1111/j.1532-5415.2009.02394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero-Ortuno R, Walsh CD, Lawlor BA, et al. A frailty instrument for primary care: findings from the Survey of Health, Ageing and Retirement in Europe (SHARE) BMC Geriatr. 2010;10:57. doi: 10.1186/1471-2318-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ensrud KE, Ewing SK, Taylor BC, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168:382–389. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 20.Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical morbidities: Results from the Cardiovascular Health Study. Arch Intern Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 21.Leng S, Chaves P, Koenig K, et al. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: a pilot study. J Am Geriatr Soc. 2002;50:1268–1271. doi: 10.1046/j.1532-5415.2002.50315.x. [DOI] [PubMed] [Google Scholar]
- 22.Leng S, Yang H, Walston J. Decreased cell proliferation and altered cytokine production in frail older adults. Aging Clin Exp Res. 2004;16:249–252. doi: 10.1007/BF03327392. [DOI] [PubMed] [Google Scholar]
- 23.Kalyani RR, Varadhan R, Weiss CO, et al. Frailty status and altered glucose-insulin dynamics. J Gerontol A Biol Sci Med Sci. 2012;67:1300–1306. doi: 10.1093/gerona/glr141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rolfson DB, Majumdar SR, Tsuyuki RT, et al. Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006;35:526–529. doi: 10.1093/ageing/afl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuurmans H, Steverink N, Lindenberg S, et al. Old or frail: what tells us more? J Gerontol A Biol Sci Med Sci. 2004;59A:M962–M965. doi: 10.1093/gerona/59.9.m962. [DOI] [PubMed] [Google Scholar]
- 26.Gobbens RJJ, van Assen MALM, Luijkx KG, et al. The Tilburg frailty indicator: psychometric properties. J Am Med Dir Assoc. 2010;11:344–355. doi: 10.1016/j.jamda.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Abellan VKG, Rolland Y, Bergman H, et al. The I.A.N.A. task force on frailty assessment of older people in clinical practice. J Nutr Health Aging. 2008;12:29–37. doi: 10.1007/BF02982161. [DOI] [PubMed] [Google Scholar]
- 28.Bouillon K, Kivimaki M, Hamer M, et al. Measures of frailty in population-based studies: an overview. BMC Geriatr. 2013;13:64. doi: 10.1186/1471-2318-13-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.2012 GSA Annual Scientific Meeting Abstracts. The Gerontologist. 2012;52(S1):NP. [Google Scholar]
- 30.Theou O, Brothers TD, Mitnitski A, et al. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc. 2013;61:1537–1551. doi: 10.1111/jgs.12420. [DOI] [PubMed] [Google Scholar]
- 31.Ravindrarajah R, Lee DM, Pye SR, et al. The ability of three different models of frailty to predict all-cause mortality: results from the European Male Aging Study (EMAS) Arch Gerontol Geriatr. 2013;57:360–368. doi: 10.1016/j.archger.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Rodríguez-Mañas L, Féart C, Mann G, et al. Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci. 2013;68:62–67. doi: 10.1093/gerona/gls119. [DOI] [PMC free article] [PubMed] [Google Scholar]