Abstract
Bone morphogenetic proteins (BMPs) and their receptors, known to be essential regulators of embryonal patterning and organogenesis, are also critical for the regulation of cardiovascular structure and function. In addition to their contributions to syndromic disorders of heart and vascular development, BMP signalling is increasingly recognized for its influence on endocrine-like functions in postnatal cardiovascular and metabolic homeostasis. In this Review, we discuss several critical and novel aspects of BMP signalling in cardiovascular health and disease, which highlight the cell- and context-specific nature of BMP signalling. Based on advancing knowledge of the physiological roles and regulation of BMP signaling, we indicate opportunities for therapeutic intervention in a range of cardiovascular conditions including atherosclerosis and pulmonary arterial hypertension, and well as for anaemia of chronic disease. Depending on the context and the repertoire of ligands and receptors involved in specific disease processes, the selective inhibition or enhancement of signaling via particular BMP ligands (such as in atherosclerosis and pulmonary arterial hypertension, respectively) might be beneficial. The development of selective small molecule antagonists of BMP receptors, and the identification of ligands selective for BMP receptor complexes expressed in the vasculature provide the most immediate opportunities for new therapies.
Introduction
The bone morphogenetic proteins (BMPs) were originally discovered because of their ability to induce the formation of bone and cartilage in ectopic sites. Marshall Urist, a pioneer in this field, first observed that demineralized bone matrix induced bone growth when implanted into rabbit muscle, suggesting that the matrix contained bone morphogenetic activity.1 Subsequent efforts to purify BMP and to elucidate its amino acid sequence revealed several closely related proteins of the transforming growth factor β (TGF-β) family of secreted ligands.2 BMPs account for 20 of the 33 known members of the TGF-β superfamily in humans. They are highly conserved throughout evolution, possessing orthologues in vertebrates and invertebrates, including Cnidaria and sponges. BMPs are usually secreted as active dimeric complexes, and some are bound to a prodomain (such as BMP9 and BMP10). BMPs communicate with neighbouring cells primarily in a paracrine or autocrine fashion, and local concentration gradients of BMPs are thus critical during early development and organogenesis. Several BMPs, including BMP6, BMP9, and BMP10 also circulate in blood,3–6 and have the potential to exert effects on distant tissues and organs. BMPs thereby function as an important endocrine regulator of cardiovascular, metabolic, and haematopoietic function (Table 1).
Table 1.
BMP ligand expression in cardiovascular biology
| Ligand | Source | Activity | Target(s) | Receptor Complementarity | Effector(s) | ||
|---|---|---|---|---|---|---|---|
| Type II | Type I |
Type III |
|||||
| BMP2/4 | EC, SMC Cardiomyocytes |
Autocrine Paracrine |
EC, SMC Cardiomyocytes |
BMPRII ACTRIIA |
ALK3 ALK2 ALK6 |
SMAD1/5/8 | |
| BMP6 | Hepatocytes | Autocrine Paracrine Endocrine |
Hepatocytes EC, SMC |
BMPRII ACTRIIA |
ALK3 ALK2 |
Hjv | SMAD1/5/8 |
| BMP9 | Hepatocytes EC |
Endocrine Autocrine |
EC EC |
BMPRII ACTRIIA |
ALK1 ALK2 |
Endoglin | SMAD1/5/8 SMAD2/3 |
| BMP10 | Cardiomyocytes | Autocrine Endocrine |
Cardiomyocytes EC |
BMPRII ACTRIIA |
ALK1 ALK2 |
Endoglin | SMAD1/5/8 SMAD2/3 |
The last 15 years has witnessed an increasing awareness of the fundamental role played by BMPs in the development and maintenance of homeostasis in the heart and circulation, and the perturbation of BMP signalling in cardiovascular disease, with evidence of increased and decreased BMP signalling activity in different disease contexts. Increased activity is associated with vascular inflammation, atherosclerosis and calcification, as well as anaemia of chronic disease. Conversely, reduced BMP signaling is associated with pulmonary arterial hypertension and hereditary hemorrhagic telangiectasia. This review is timely because strategies have now been developed to inhibit or enhance BMP signaling, which show promise in preclinical models of cardiovascular disease and anemia. Thus, these approaches hold considerable promise for the development of novel therapies for human disease.
In this article we review relevant aspects of BMP ligands, receptors and signalling pathways and their role in cardiovascular development and disease. In addition, we indicate how this knowledge might be exploited to treat specific cardiovascular diseases and anaemia.
BMPs are structurally diverse
Like other members of the TGF-β family, BMPs bind to serine-threonine kinase receptors, known as type I and type II receptors. Similar to TGF-β ligands, BMPs form heterotetrameric signalling complexes with two type I and two type II receptors, but unlike TGF-β ligands, BMPs bind with greater affinity to the type I rather than type II receptors. Based on their structural homology, BMP type I receptors can be divided into two groups: the BMP type-I A receptor (BMPR-IA, a.k.a., activing receptor-like kinase 3, or ALK3) and BMP type-I B receptor (BMPR-IB, a.k.a., ALK6) group, and the Activin Receptor Like 1 (ACVRL1, a.k.a., ALK1) and activin receptor type-1 (ACVR1, a.k.a., ALK2) group. Whereas ALK3, ALK6, and ALK2 are widely expressed by diverse cell types, ALK1 is more restricted to endothelial cells. Type II receptors of the BMP family include the BMP type II receptor type (BMPRII), and activin receptor type-2A (ACTRIIA) and activin receptor type-2B (ACTRIIB). BMPRII is selective for BMPs, whereas ACTRIIA and ACTRIIB are activated in response to activins, growth differentiation factors (GDFs), and Nodal. Both BMPRII and ACTRIIA are expressed broadly in mesenchyme-derived tissues, but only BMPRII is expressed at high levels in endothelial and endocardial tissues. Upon complex formation, the type II receptors transphosphorylate specific residues within type I receptor intracellular glycine-serine-rich juxtamembrane regions, causing type I receptor activation and phosphorylation of receptor-regulated SMAD (R-SMAD) effector proteins. BMP ligands possess the ability to activate the BMP-responsive SMADs 1, 5 and 8, yet exert diverse effects on target tissues, owing in part to their structural and receptor complementarity. BMPs form subgroups based on their affinities for specific receptors. BMP2 and BMP4 bind preferentially to BMPRII in a complex with ALK3 or ALK6, while BMP6 and BMP7 bind to ACTRIIA with ALK3, and BMP9 and BMP10 bind to BMPRII in combination with ALK1 or ALK2 (Table 1).7–10 While BMPs have been most extensively studied as homodimeric ligands, the interaction of heterodimeric ligands such as BMP2 and BMP7, and their respective heteromeric ALK2 and ALK3 receptor complexes has become an increasingly appreciated signalling motif in vertebrate development.11
H1 Regulation of BMP signalling
Since BMPs are potent modulators of cell growth and fate, each component of the BMP signalling pathway is subjected to extensive positive and negative regulation in order to maintain the integrity of tissue development and repair. The expression of BMP antagonists is especially important during development when BMP signalling gradients are established by diffusion to fine-tune the magnitude and duration of BMP signalling.12 A large number of extracellular (chordin, noggin, and gremlin) and membrane-bound antagonist molecules (BMP-binding endothelial regulator protein (BMPER), Twisted gastrulation, matrix Gla protein, and neogenin) sequester BMP ligands to reduce signalling. Noggin is the prototypical secreted antagonist, and possesses high affinity for BMP2 and BMP4, but low affinity for BMP6 and BMP9, whereas BMPER, a transmembrane protein, potently inhibits BMP9 in the context of endothelial signalling.13,14 Following signalling activation, phosphorylated R-SMADs form heteromeric complexes with a common-mediator SMAD (SMAD4) and translocate into the nucleus to directly regulate target gene expression by binding to SMAD-binding elements. This complex can also indirectly regulate gene expression by interacting with DNA-binding transcription factors, or by associating with co-activators or co-repressors, and histone-modifying factors. Targets of BMP/SMAD transcriptional activity include the inhibitory-SMADs (SMAD6 and SMAD7) and the BMP and activin membrane-bound inhibitor homolog (BAMBI). SMAD6 and SMAD7 provide feedback inhibition to antagonize BMP and TGF-β signalling by inducing degradation of their respective receptors and R-SMADs, while BAMBI sequesters ligands away from receptors, thereby inhibiting BMP signaling. In addition, several classes of BMP co-receptors that lack enzymatic activity, but modulate BMP ligand-receptor interactions, have also been identified. These co-receptors include the so-called type III receptors endoglin and betaglycan, as well as the glycosylphosphatidylinositol-anchored repulsive guidance molecule proteins, all of which potentiate ligand-specific signalling to modify the cellular consequences of signalling.15–17 These BMP co-receptors can be produced via pro-protein convertases or can be shed from the cell surface by matrix metalloproteases, resulting in soluble receptor fragments that function as BMP ligand traps.18
The repertoire of BMP receptors, co-receptors, and modulators is highly specialized in the cardiovascular system, helping to confer spatiotemporal specificity to BMP signalling, and particularly for sensitizing endothelial and endocardial tissues to BMP9 and BMP10. BMPRII, ALK1, and endoglin are abundant in vascular endothelium, as well as in embryonic ventricular and atrioventricular endocardium. In fact, the depletion of ALK119 or endoglin20,21 in endothelial cells from diverse vascular beds significantly impaired signalling via BMP9 and BMP10, and diminished BMP9-mediated modulation of angiogenic activity. While the depletion of BMPRII does not abolish BMP9 or BMP10 signalling, the loss of BMPRII in endothelium substantially impairs the downstream anti-mitotic and cytokine regulatory functions of BMP9.22 ALK2 is also expressed in the endothelium, and its signalling appears to modulate the expression of ALK1 to respond dynamically to various stimuli.23 BMPRII, ACTRIIA, ALK2, and ALK3are expressed in cardiomyocytes and vascular smooth muscle, and signalling is activated in response to their canonical ligands to modulate cell phenotype, growth, and survival.24–29 In addition, ALK2 and ALK3 contribute to the calcification of vascular smooth muscle cells.30,31 BMPER and Twisted gastrulation modulate BMP9 signalling to regulate these functions in the endothelium and thereby maintain vascular homeostasis.32 As a group, these molecules have essential roles in vasculologenesis, cardiomyogenesis, ventricular compaction, septation, valve formation, and maintenance of the integrity of the pulmonary and lymphatic circulation33.
H2 Cross-talk with other signalling pathways
BMPs and their signalling effector molecules exhibit various degrees of cross-talk with other pathways essential to vascular development and homeostasis, including vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and Notch and Wnt signalling.16,34–36 In the cardiovascular system, the recruitment or modulation of these pathways by BMP signalling can be critical for proper function; BMP signalling via SMAD1/5 activation regulates the expression of Jagged 1 in endothelial cells to transactivate Notch signaling in neighboring cells.37 BMPs also co-regulate the Notch transcriptional targets HES1 and HEY1 in mesenchymal lineages via a Notch intracellular domain–SMAD interaction to modulate cellular plasticity.38–40 Similarly, the interplay between BMP and delta-ligand 4/Notch pathways determines the identity of tip versus stalk cells during angiogenesis.41 BMP9 has been documented to suppress VEGF expression and VEGF-induced angiogenesis via ALK1 and BMPRII signalling,16,35 while in the developing outflow tract of the heart, BMP4 and BMP7 repress VEGFa expression via the miR-17-92 cluster to stimulate outflow tract cushion formation.42 Furthermore, during cardiac cushion formation, the coordination of the endothelial to mesenchymal transformation response is mediated by the interplay of Notch, BMP, and TGF-β signalling.43,44 The antagonism of FGF signalling by BMP ligands is necessary for specifying cardiomyocyte fate in the early embryo or in embryonic stem cells.45 Taken together, these findings show that the BMP pathway provides essential gating, amplifying, and damping effects on Notch, Wnt, VEGF, and FGF signalling in vascular development and homeostasis.
H2 Experimental evaluation of BMP signalling
Early BMP genetic ablation procedures that resulted in severe defects with prenatal or perinatal lethality revealed the importance of many receptors of the BMP signalling pathway for embryogenesis and organogenesis (see Table 2 for details and references). Subsequent studies to address postnatal and physiologic functions of BMP ligands have utilized Cre-Lox technology for postnatal ablation of BMP receptors, or have targeted the individual ligands themselves. (see Table 2 for details and references) Genetic and pharmacologic epistasis has been accomplished using several methods: transgenic expression or administration of recombinant BMPs or endogenous BMP signalling inhibitors (such as noggin or gremlin); antibodies directed against specific BMP ligands; and recombinant ligand traps derived from receptor extracellular domains that scavenge subsets of BMP ligands (such as ALK1-Fc, ALK3-Fc, and Hemojuvelin-Fc).31,46,47 Small molecule inhibitors of BMP type I receptors also allow temporally restricted modulation of BMP signalling (see Table 3 for details and references). Together, these experimental approaches to evaluate BMP signalling has helped to elucidate their roles in diverse biological processes such as iron metabolism and inflammation, and in cardiovascular diseases such as atherosclerosis, pulmonary hypertension, and vascular calcification (Table 3).
Table 2.
Cardiovascular phenotypes of BMP mutant models
| Gene/Protein | Mutation | Tissue | Phenotype | Analogous human syndrome |
|---|---|---|---|---|
|
Bmpr2/ BMPRII |
Null | Global | Embryonal lethality due to gastrulation defect189 |
|
| Heterozygous | Global | Mild susceptibility to PAH 190 |
Pre-clinical HPAH |
|
| N-terminal exon 2 deletion |
Global | Outflow tract and septation defects191 and susceptibility to hypoxic PAH192 |
Congenital heart disease with PAH193 |
|
| Dominant negative transgene |
Smooth muscle |
PAH and pulmonary vascular remodelling130 |
HPAH121,194 | |
| R899X (premature truncation) |
Smooth muscle or global |
PAH and pulmonary vascular lesions195,196 |
HPAH121,194 | |
|
Acvrl1/ Alk1 |
Null | Global | Midgestational lethality due to cavernous vessel defects, arterial dilation, smooth muscle recruitment defects 155,156 |
|
| Heterozygous | Global | Arteriovenous malformations with HHT- like features155,197 |
HHT-2198 with or without PAH 199 |
|
| Endoglin | Null | Global | Embryonal lethality at E10.5 due to yolk-sac angiogenesis defect154,200 |
|
| Heterozygous | Global | Protection against RV hypertrophy in RV pressure overload178,179 |
HHT-1201 | |
| BMP9 | Null | Global | Viable with abnormal lymphatic development and drainage202 |
HHT-5160 |
| BMP10 | Null | Global | Embryonal lethality with diminished cardiomyocyte proliferation96 |
|
| BMP6 | Null | Global | Massive iron overload due to defective hepcidin expression203 |
Hemochromatosis due to mutations at other loci |
| BMP2 | Null | Global | Embryonic lethality with defects in extra-embryonic and cardiac development204 |
|
| BMP4 | Null | Myocardium | Defects in atrioventricular septation205 |
Atrioventricular canal defect |
| Alk2 | Null | Endocardial cells | Defects in atrioventricular septa and valves95 |
Congenital septal and valvular defects |
|
Bmpr1a/ Alk3 |
Null | Global | Early embryonal lethality (E7.5)206 |
|
| Null | Smooth muscle |
Cardiac structural and vasculogenesis defect207 |
||
| Heterozygous or Null |
Patchy smooth muscle |
Decreased susceptibility to hypoxic PAH and increased proximal pulmonary arterial stiffness208,209 |
Familial juvenile polyposis210,211 |
|
|
Bmpr1b/ Alk6 |
Null | Global | Chondrogenesis defects212 | Associated mutations in pediatric PAH 213 |
Table 3.
Cardiovascular disease models probed with BMP inhibitors
| Inhibitor | Model | Route | Impact | Application |
|---|---|---|---|---|
| Dorsomorphin | TNF-α induced vascular inflammation in mice214 |
Systemic | Inhibited leukocyte adhesion following challenge with TNF-α |
Vascular inflammation |
| Dorsomorphinand LDN- 193189 |
Zebrafish vasculogenesis215 |
Embryonal (24 hpf) |
Dorsalization without intersomitic vessel disruption using selective inhibitor LDN-193189 |
|
| LDN- 193189 |
Subtotal nephrectomy model in mice216 |
Systemic | Attenuation of chronic kidney disease induced endothelial dysfunction and smooth muscle osteogenic differentiation |
Chronic kidney disease and associated vascular calcification |
| LDN- 193189 and ALK3-Fc |
LDLr- deficient hyperlipidemic mice31 |
Systemic | Attenuation of vascular calcification, vascular inflammation, and atherosclerosis |
Atherosclerosis and vascular calcification |
| LDN- 193189 and ALK3-Fc |
MGP- deficient mice30 |
Systemic | Attenuated vascular calcification and improved survival |
Vascular medial (Monckeberg’s) calcification |
| LDN- 193189 |
ApoE- deficient hyperlipidemic mice173 |
Systemic | Reduced foam cell formation, atherosclerotic plaque formation, and vascular inflammation via inhibition of hepcidin to reduce macrophage iron stores |
Atherosclerosis and vascular inflammation |
| Anti-ALK1 |
In vitro endothelial cell sprouting217 |
In vitro | Inhibited in vitro angiogenic activity in a VEGF-independent manner |
Solid tumour angiogenesis |
| sEng-Fc | Angiogenesis and tumour xenograft vascularization180 |
Systemic | Inhibited tumor xenograft vascularization in a BMP9 and BMP10-dependent manner |
Solid tumour angiogenesis |
| ALK1-Fc | Angiogenesis and tumour xenograft vascularization56 |
Systemic | Inhibited tumour xenograft vascularization |
Solid tumour angiogenesis |
| LDN- 193189 and HJV-Fc |
Rats with anemia of chronic disease187 |
Systemic | Blockade of BMP signalling mobilized iron from reticuloendothelial cells and reversed anemia |
Anaemia of chronic disease |
| LDN- 193189 and ALK3-Fc |
Mice with anemia of chronic disease186 |
Systemic | Blockade of BMP signalling restores iron bioavailability, decreases hepcidin, prevents development of anemia, and reverses established anemia due to chronic inflammation |
Anemia of chronic disease |
| LDN- 193189 |
Wild-type mice188 |
Oral | Oral administration of BMP inhibitor prevents anaemia associated with inflammation |
Anemia of chronic disease |
A small molecule inhibitor of BMP signalling, dorsomorphin, was identified by screening a library of small molecules for their ability to induce dorsalization in embryonic zebrafish.48 This compound mimicked a mild form of dorsalization seen in zebrafish deficient in BMP type I receptor orthologues, or fish overexpressing noggin or chordin.49 Dorsomorphin inhibited the expression of hepcidin, a master iron regulatory hormone, in zebrafish and mice via the antagonism of BMP signalling in the liver. Since dorsomorphin is a broad acting multi-kinase inhibitor identified originally for its ability to inhibit AMP-activated protein kinase (compound C), further potent and selective inhibitors of BMP type I receptors were generated, including pyrazolopyrimidine analogues,50 and other scaffolds such as 2-aminopyridine compound K02288 and its derivatives.51,52 These small molecules have been utilized extensively to study the role of BMP signalling in vitro, as well as the postnatal roles of BMP signalling in vivo.
H1 BMPs and the cardiovascular system
H2 BMP signalling in the vasculature
The critical role of BMP signalling in the vasculature is evident from both clinical and experimental observations. Genetic lesions in this pathway result in the development of congenital vascular syndromes, and corresponding phenotypes are also observed in loss-of-function animal models (Table 2). Consistent with these roles in disease, BMPs exert potent effects on all vascular cell lineages, impacting the ability of endothelial cells to migrate, proliferate, and form basic tubular structures, and establishing the fates of surrounding pericytes, vascular smooth muscle, and adventitial cells that contribute to the structural integrity and function of the vessel.53 Similar to other developmental processes, BMP signalling is tightly regulated in a spatiotemporal manner, and is thus lineage-, tissue-, and context-sensitive (Figure 1).
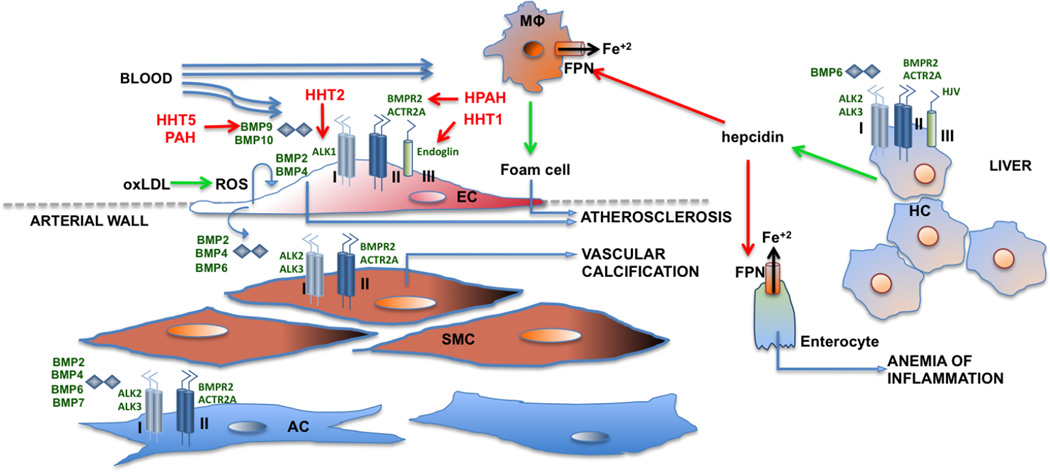
Figure 1. The repertoire of receptor complexes and ligands involved in cardiovascular and related metabolic responses to BMP signalling.
A variety of BMP ligands (BMP6, BMP9, and BMP10) are present in the circulation at biologically active concentrations. Endothelial cells (EC), vascular smooth muscle cells (SMC), and adventitial myofibroblast cells (AC) express receptors, co-receptors, and antagonists in a tissue-specific manner to permit complementarity and precise regulation of signalling. Congenital vascular syndromes are shown in red to depict the loss-of-function of several of these signalling molecules, resulting in human hereditary telangiectasia (HHT) syndromes HHT1, HHT2, HHT5, and heritable pulmonary arterial hypertension (HPAH). Positive regulatory effects downstream of BMP signalling are depicted by green arrows, whereas negative effects are depicted with red arrows. Hepatocytes respond to BMP6 signaling to express hepcidin, which subsequently downregulates expression of ferroportin (FPN) to increase the retention of Fe+2 in macrophages (MΦ) and duodenal enterocytes, consequently enhancing macrophage activation and reducing serum iron. This activity contributes to iron-deficiency anaemia associated with chronic inflammatory states, and potentiates the activity of macrophages and foam cells in atherosclerosis. While BMP9 signalling appears to be protective in endothelial cells, signalling by BMP2, BMP4, and BMP6 appear to be osteogenic and pro-atherogenic in SMC and EC, acting downstream of atherogenic stimuli including oxidized LDL (oxLDL) via the induction of reactive oxygen species to promote atherosclerosis and vascular calcification. Abbreviations:
BMPs can function in a pro-angiogenic or anti-angiogenic manner, depending on its ligand, concentration, cellular target, and the context.54 Although a number of BMPs have been reported to modulate angiogenesis, BMP9 is the most important BMP ligand in the vasculature owing to its presence in the circulation and the high expression of its cognate receptors BMPRII, ALK1, and endoglin in endothelial and smooth muscle cells. In human pulmonary artery endothelial cells, BMP9 signals through distinct type II receptors to stimulate SMAD1 and SMAD2 phosphorylation and to induce distinct target genes.22 Under normal conditions, BMP9 primarily has an angiostatic effect. BMP9 inhibits the formation of vessel-like tubular networks in vitro, which requires the function of BMPRII and ALK1. In addition, BMP9 inhibits VEGF and FGF-induced angiogenic responses. Furthermore, BMP9 has been shown to inhibit lymphangiogenesis.33 Interestingly, low BMP9 concentrations were reported to have pro-angiogenic properties55 and inhibiting BMP9 levels using an ALK1-Fc ligand trap was shown to inhibit tumour angiogenesis.56,57 In addition, other BMPs, including BMP2, BMP4, BMP6, and BMP7, have been shown to induce endothelial cell proliferation, migration, and formation of tube-like structures, and to promote angiogenesis in vitro and in vivo in mouse models.58 The induction of inhibitor of DNA binding protein (Id) expression by BMP contributes to its pro-angiogenic response.59,60 The indirect induction of alpha-crystallin B chain signalling via Id1 by BMP also contributes to the survival of pulmonary microvascular endothelial cells.61 Id proteins also play an important effector role in the regulation of smooth muscle cell function by BMPs. Id1 and Id3 are potently regulated by BMP signalling in pulmonary arterial smooth muscle cells in vitro,62 are jointly upregulated by hypoxia in pulmonary vascular smooth muscle in vivo,63 and might play a complementary and partially redundant role in regulating cell cycling in vascular and other tissues.64 BMPs also function to maintain the contractile phenotype of vascular smooth muscle cells by inducing the expression of microRNA-21,65 either through suppression of microRNA-96,66 or via the induction of myocardin-related transcription factors.67
Finally, physiological stimuli including shear stress can influence endothelial cell behaviour in a BMP-dependent manner. For example, in response to oscillatory shear stress, BMPRII mediates a physical association between BMPR1B and integrin αVβ3 in endothelial cells, thereby inducing endothelial proliferation.68 The mechanism of BMP receptor activation under conditions of shear might involve the cyclic AMP-dependent transcription factor ATF-2 pathway, which can be activated by fluid shear stress, and can stimulate the secretion of BMP ligands during early haematopoietic stem cell differentiation.69 In zebrafish, BMP10 induces vascular quiescence via shear stress in an ALK1-dependent manner.5,70 Thus, whether BMP has positive or negative effects on the vasculature is highly dependent on the BMP isoform, concentration, and cellular context.71
BMP signalling in cardiovascular development
A critical event during embryonic development is the establishment of a vascular network and a beating heart. The growth of the heart is initiated by specification of the cardiac mesoderm, bilaterally distributed on both sides of the primitive streak.72,73 These mesodermal patches migrate to the midline, fuse, and form the cardiac crescent. On day 7 of murine embryonic development, the cardiac crescent develops into the primitive heart tube with an inner endocardial layer and an outer myocardial layer, separated by a layer of extracellular matrix, known as the cardiac jelly.74,75 The heart then undergoes “looping” to form the atrioventricular canal, the hallmark of the chambered heart. In the atrioventricular canal, the cardiac cushions form the valve primordia. The endocardial layer at the site of the atrioventricular canal undergoes endocardial to mesenchymal transition (EMT) and migrates as mesenchymal cells to populate the cardiac jelly.76
The differentiation of pluripotent stem cells and embryonic stem cells into the cardiac lineage is partly regulated by BMP signalling in a tightly controlled spatiotemporal sequence, as demonstrated by in vivo studies,77 as well as in vitro studies78,79. BMP4 cooperates with FGF2 via the activation of ERK signaling to induce cardiac mesoderm formation by increasing expression of Brachyury protein, homeobox protein CDX-2, and homeobox protein NANOG80 in a SMAD1-dependent manner,81 while inhibiting endoderm differentiation. BMP4 signaling again plays a critical role in the terminal differentiation of cardiomyocyte progenitors into cardiomyocytes, but its effect at commitment stages is dependent on a precise balance with activin A, Nodal, and WNT signals in combinations that are specific not only to lineages, but specific also to progenitor clones.82–84 Importantly, if BMP signalling persists for 3 days beyond when cardiac mesoderm is formed, cardiac progenitor cells differentiate into epicardial lineages instead of cardiomyocycytes.85,86 The epicardium has been shown to serve as an important reservoir of cardiac fibroblasts and vascular smooth muscle progenitors in the developing heart, and plays an important role in cardiac repair after injury87. BMP signal transduction has been demonstrated in the atrioventricular canal,88,89 and to a lesser extent in the cardiac crescent88–90 using BMP reporter mice, which harbour a BMP responsive element that regulates the reporter genes lacZ or GFP. BMP2 and BMPR-1A are both expressed in the cardiac crescent; deletion of BMPR-1A in the cardiac mesoderm results in embryos that lack the cardiac crescent and cardiomyocytes, suggesting a role for BMP in early cardiomyocyte formation.91,92 BMPs 2, 4, 5, 6, and 7, as well as ALK2 and BMPR-1A, are expressed in the atriocentricular canal.76 Studies using mouse and chicken models have shown that only BMP2 is required for EMT and cushion formation in the atrioventricular canal. Furthermore, endocardial-specific deletion studies involving ALK2 and BMPR-1A have demonstrated that they are both involved in EMT, albeit for different processes.93–95 While deletion of ALK2 and BMPR-1A both led to a reduction in SMAD1/5/8 phosphorylation, deletion of ALK2 also diminished phosphorylation of SMAD2/3. Only myocardial-specific deletion of BMPR-1A resulted in cardiac defects, namely reduced atrioventricular cushion size and myocardial thinning.93–95
BMP10 is transiently expressed in the ventricular trabeculae during midgestation when the ventricles grow and mature.96 BMP10 levels are tightly controlled because any fluctuations in expression can result in abnormalities in trabecular development and maturation of the ventricular wall. Myocardin, a muscle specific transcription factor, induces the expression of BMP10 by binding to its promoter.97 In the adult heart, expression of BMP10 is restricted to the right atrium.
In addition to their role in cardiac development, BMPs are also involved in the development of the vascular network that is crucial for the embryo once the heart begins to beat. Vascularization is a two-step process: firstly, vasculogenesis—the de novo formation of vessels from angioblasts—takes place, followed by angiogenesis, which involves the sprouting of new vessels from pre-existing vessels.98 During vasculogenesis, a new vessel forms when endothelial cells start to proliferate, migrate, and form a primitive tube. The primitive tube becomes stabilized by the deposition of extracellular matrix proteins and coverage by mural cells (pericytes or smooth muscle cells).99 BMP signalling is required for both endothelial cell and mural cell development and function. BMPs 2, 4, 6, and 7 stimulate the growth of endothelial cells and induce their migration.59,100 BMP9 has a dual effect on angiogenesis, depending partly on its concentration. BMP9 and ALK1 signalling at low concentrations induces proliferation and migration of endothelial cells,55 whereas complete inhibition of BMP9 and ALK1 signalling using a recombinant ligand trap (ALK1-Fc) prevents tumour angiogenesis.56 However, other studies showed that BMP9 and ALK1 signalling inhibited VEGF- and FGF-induced angiogenesis3,16 in cooperation with Notch signalling,41 thereby inhibiting the activation phase of angiogenesis. BMP9 and ALK1 signalling can also inhibit lymphangiogenesis.33
During vascular development, BMP signalling can also influence the differentiation of smooth muscle cells. BMP2 and BMP7 inhibit the growth and migration of smooth muscle cells, and promote their differentiation into a contractile phenotype.101–104 Mutations in ALK1 were associated with reduced mural cell coverage in brain vessels105. Interestingly, TGF-β was shown to reduce BMP4 signalling in smooth muscle cells, suggesting a cross-talk between the two signalling pathways.106 Therefore, the effect of BMP signalling on angiogenesis is context-dependent; proper signalling in both endothelial cells and mural cells is necessary for normal development of the vasculature.
BMP signalling and cardiovascular disease
The essential role of BMP signalling in cardiovascular homeostasis and function is underscored by numerous genetic studies in humans. Mutations in the genes that encode transducers of the BMP signalling pathway have been associated with cardiovascular diseases such as pulmonary arterial hypertension and hereditary haemorrhagic telangiectasia. Other vascular abnormalities, such as those contributing to Marfan syndrome and Loeys-Dietz syndrome have been linked to exaggerated TGF-β activity and reduced responsiveness to BMPs.107,108 BMPs have also been implicated in the promotion of vascular calcification and tumour angiogenesis. Furthermore, studies conducted in the past 5 years support a protective role of BMP signalling during post-infarction remodelling of the heart,109–112, and patients with coronary artery disease also exhibit aberrant expression of BMP signal transducers.113,114 Finally, the level BMP receptor expression correlates with anemia and the degree of iron deficiency, which is prevalent in patients with heart failure.
Pulmonary arterial hypertension
Pulmonary arterial hypertension (PAH) is defined as an increase in mean pulmonary artery pressure greater than 25 mmHg at rest in the setting of normal left atrial pressure and increased pulmonary vascular resistance.115 The increase in pulmonary vascular resistance is attributable to a reduction in the cross-sectional area of the pulmonary vascular bed, which occurs as a result of obliteration of the vascular lumen in small pulmonary arterioles.116 Affected individuals present with dyspnoea on exertion and are at increased risk of death from right heart failure.117 Although a number of systemic conditions are associated with PAH,118 such as congenital heart disease, liver cirrhosis, connective tissue diseases, and HIV infection, a rare, idiopathic form of PAH has been observed, with an annual incidence of 1–2 cases per million people.117 Approximately 6–10% of patients with PAH has more than one affected family member, and has inherited the disease via an autosomal dominant pattern of inheritance (though with reduced penetrance of approximately 20–30%).119 Data from two studies published in 2000 established that this familial form of PAH was caused by heterozygous germline mutations in BMPR2.120,121 Subsequent studies have shown that more than 70% of heritable cases of PAH are caused by mutations in BMPR2,122 and approximately 20% of apparently sporadic cases of idiopathic PAH are in fact also caused by BMPR-II mutations, either as a result of de novo mutations or owing to the combination of reduced disease penetrance and small family size.123 Approximately 70% of mutations in BMPR2 result in premature termination codons; the remaining 30% are missense mutations that might lead to loss of function by a variety of mechanisms, including loss of kinase activity and aberrant trafficking of misfolded BMPR2 in the endoplasmic reticulum.122
Although our understanding of the pathogenesis of PAH has progressed since the seminal papers published in 2000, major questions still remain. For example, it remains unclear how loss of function mutations in BMPR2 cause a disease that appears to be confined to the pulmonary circulation, as are the mechanisms that influence variable penetrance. Notably, BMPR2 has a very long C-terminal extension and might also regulate and modify intracellular signalling by recruitment of factors other than SMADs, such as protein kinase G, in the vasculature.25,124,125 The high prevalence of asymptomatic carriers of disease-associated mutations in families with PAH suggest that additional factors are required to trigger the onset and progression of the disease. Mice that are heterozygous for BMPR2 deficiency develop minimal signs of pulmonary hypertension in the absence of additional stimuli.126 However, mice with a heterozygous knock-in of a human disease-causing mutation in BMPR2 (R899X) do show susceptibility to spontaneous pulmonary hypertension by 6 months of age,127 highlighting potential important differences between heterozygous null and knock-in mouse models. Additional genetic factors, including polymorphisms in the CYP1B1 gene (linked to estrogen metabolite levels) and cerebellin-2128 have been suggested to have a role in the development of PAH, but these require confirmation in prospective cohorts. Environmental factors, particularly inflammation, have also been shown to increase disease penetrance in BMPR2 heterozygous mice.129 Interestingly, overexpression of dominant negative kinase deficient BMPR2 in pulmonary artery smooth muscle cells is sufficient to promote pulmonary hypertension in transgenic mice.130 These observations suggest that a critical reduction in the expression or function of BMPR2 is required to trigger the development of PAH.
In patients with PAH and BMPR2 mutations, pulmonary arterial smooth muscle cells exhibit a loss of the normal BMP/SMAD-mediated growth suppression,131,132 and demonstrate heightened proliferation to growth factors such as platelet-derived growth factor, TGF-β, and serotonin.126 Therefore, loss-of-function mutations of BMPR2 promotes pulmonary arterial smooth muscle cell proliferation and resistance to apoptosis, key factors in the occlusion of small pulmonary arteries. Downstream of BMP/SMAD signalling, the induction of Id genes plays a critical role in smooth muscle growth suppression.133 Interestingly, approaches currently employed to treat PAH, such as phosphodiesterase type 5 inhibition and prostanoids, have been shown to partly rescue SMAD/Id gene signalling in vitro and in vivo via mechanisms involving cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP).134,135
Studies from the past 8 years have demonstrated that BMPR2 forms a signalling complex with ALK1, which is almost exclusively expressed in endothelial cells, and signals specifically in response to BMP9 and BMP10 activation.22,136 Although mutations in ALK1 usually cause hereditary haemorrhagic telangiectasia (HHT), there are well-documented cases of severe PAH in families affected by HHT that are indistinguishable from idiopathic PAH.137 Therefore, BMP9 and BMP10 signalling via BMPR2/ALK1 is likely to play a central role in the pathobiology of PAH as well as HHT, and implicates the endothelial cell as an important cell type involved in the initiation of both diseases. Indeed, endothelial selective deletion of BMPR2138 or ALK1 deficiency139 can also promote the development of pulmonary hypertension in mice.
Loss of BMPR2 in endothelial cells promotes endothelial apoptosis and increases endothelial permeability in vitro and in vivo,140,141 whereas BMP9 protects endothelial cells from apoptosis and reduces agonist-induced endothelial permeability.141 Loss of BMPR2 function, therefore, leads to changes in the function of vascular wall cells that promote the development of PAH, particularly in the presence of an injurious trigger. An increased understanding of the role of BMPR2 in these processes suggests that approaches to restore BMPR2 expression, function, or signalling might have a role in disease treatment or prevention. Proof-of-concept studies in vitro have shown the potential for rescue of translational read through of mutations, which result in premature termination codons by small molecules,142 and for the use of chemical chaperones to rescue misfolded BMPR2 mutants.143 In addition, targeted delivery of BMPR2 gene therapy to the pulmonary vascular endothelium has been shown to prevent and reverse PAH in the monocrotaline rat model.144
Another potential approach to the treatment and prevention of PAH stems from the identification of the rapid turnover of cell surface BMPR2 and its subsequent degradation by the lysosome.145 This mechanism appears to involve an E3 ubiquitin protein ligase called Itch. Inhibition of the lysosomal degradation of BMPR2 by chloroquine prolongs the half-life of BMPR2 at the cell surface and restores signalling in pulmonary artery smooth muscle cells and endothelial cells that harbour haploinsufficiency-inducing mutations in BMPR2.146 In nongenetic models of pulmonary hypertension, such as those caused by the plant alkaloid toxin, monocrotaline, protein levels of BMPR2 are reduced. In these rodent models of pulmonary hypertension the 4-aminoquinoline, chloroquine and the better tolerated hydroxychloroquine have shown preclinical efficacy in preventing the development and progression of pulmonary hypertension, and can be shown to restore BMPR2 protein levels. In addition to lowering pulmonary blood pressure, chloroquine also inhibits autophagy, which promotes apoptosis of pulmonary artery smooth muscle cells.146–148 Thus, as the use of chloroquine and hydroxychloroquine has already been well-established for the treatment of malaria, sarcoidosis, rheumatoid arthritis and systemic lupus erythematosus, repurposing these agents for the treatment of PAH might prove a novel and readily testable approach. While the mechanism of action of these drugs should aid in the normalization of BMPR2 expression levels in mutation-carrying individuals with PAH, they might also augment BMPR2 expression and signalling and possibly benefit non-mutation-carrying individuals.
A further approach to restoring BMP signalling was identified by screening 3,756 FDA-approved drugs and bioactive compounds in a transcriptional high-throughput luciferase reporter assay.149 The best response was achieved with the drug tacrolimus, which although is recognized as a calcineurin inhibitor, also binds to the peptidyl-prolyl cis-trans isomerase FKBP12, a repressor of BMP signalling. Tacrolimus releases FKBP12 from type I receptors ALK1, ALK2, and ALK3, which are activated downstream of SMAD1/5 and mitogen-activated protein kinase signalling and ID1 gene regulation. In pulmonary artery endothelial cells from patients with idiopathic PAH, low-dose tacrolimus therapy was able to reverse the reduction in BMP signalling.149 In vivo, low-dose tacrolimus therapy reversed severe monocrotaline-induced PAH in rats with medial hypertrophy and in rats with neointima formation caused by VEGF receptor blockade and chronic hypoxia.149 A clinical trial examining low-dose tacrolimus for the treatment of PAH is currently underway (https://clinicaltrials.gov/ct2/show/NCT01647945)150
In a study published in 2015, systemic administration of BMP9 was shown to prevent and reverse PAH in various genetic and nongenetic preclinical models.141 BMP9 is highly selective for endothelial cells, owing to its specificity for the BMPR2/ALK1 receptor complex. The growth of angioproliferative lesions observed in rats exposed to chronic hypoxia and a VEGF receptor 2 antagonist, which are thought to closely resemble the lesions observed in patients with severe PAH, was suppressed by systemic administration of BMP9.127 BMP9 administration also increased the expression of BMPR2 in these models in a SMAD-dependent manner. Although BMP9 has been shown to be a potent stimulus of ossification at high concentrations, no ossification was observed in animals treated with BMP9 daily for 4 weeks at the site of injection, either via the peritoneal or the intramuscular route.141 These studies raise the possibility that BMP9, BMP10, or their analogues might be promising candidates for novel therapies in patients with PAH. Potential approaches to restoring BMPR2 expression or function in PAH are summarized in Figure 2.
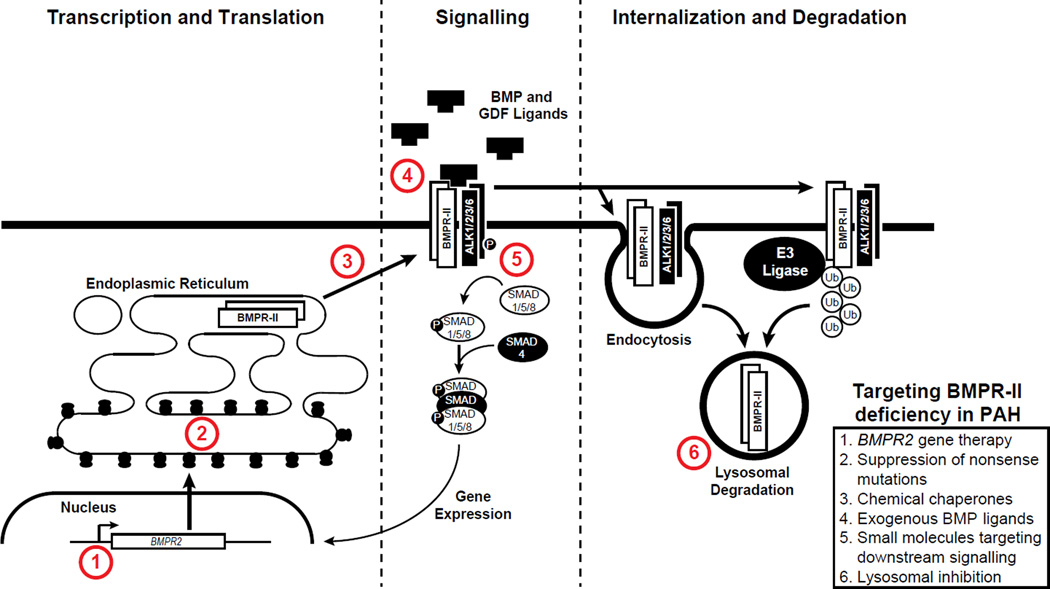
Figure 2. Potential approaches to target and enhance signalling or expression of BMPRII.
BMPRII cell surface expression can be increased by endothelial gene therapy targeted to the pulmonary circulation. This increase in BMPRII can be achieved using small molecules that increase translational read through of BMPRII mutations encoding premature termination codons, and chemical chaperones that increase trafficking of BMPRII misfolded mutations that are held up within the endoplasmic reticulum. In addition, BMPRII signalling can be restored by ligands (BMP9 and BMP10) that target the BMPRII/ALK1 receptor complex on endothelial cells, and agents that enhance downstream SMAD phosphorylation, such as tacrolimus, sildenafil, and prostanoids. Finally, inhibition of BMPRII turnover by the lysosome, using agents such as hydroxychloroquine, can enhance cell surface expression of BMPRII protein. Abbreviations:
BMP signalling in HHT
HHT is a genetic autosomal dominant vascular disorder, with a prevalence of 1 in 5,000–10,000 people worldwide.56,151 Clinical manifestations of HHT include spontaneous and recurrent epistaxis, telangiectasias of the lips, hands, and nasal and oral mucosa, and the development of arteriovenous malfomations in visceral organs, in particular the lung, brain, and liver.152 These presentations suggest that HHT predominantly involves vascular cells, although a contribution of dysfunctional mononuclear cells cannot be excluded. Different subtypes of HHT have been recognized based on their underlying mutation. HHT-1 and HHT-2 are caused by mutations in ENG153,154 and ACVRL1,155–157 respectively, and together represent 80% of all cases of HHT. Juvenile polyposis-HTT (JP-HHT) refers to a group of patients with HHT displaying a mutation in SMAD4 and features of JP, such as the development of benign polyps.158,159 Recently, mutations in BMP9 have been associated with a syndrome resembling HHT. These patients develop noose bleads and dermal telangiactesia although not limited to the hands, face, and mouth, typically found in HHT.160 Furthermore, impaired BMP signaling is also central in PPH and several families have been identified have both pathologies.158 The tortuous and fragile HHT vessels, caused by abnormal endothelial cell proliferation and aberrant recruitment of perivascular cells, can be linked to impaired BMP9 and BMP10 signalling.3,16 Since BMP9 and BMP10 are present in the circulation, bind to ALK1 and endoglin with high affinity, and are highly expressed in endothelial cells, dysfunctional BMP signalling is likely to be central to HHT pathology.
Given that the BMP9/ALK1/endoglin pathway suppresses VEGF expression and inhibits angiogenesis, patients with HHT exhibit elevated levels of VEGF and dysregulated angiogenesis. Accordingly, inhibition of VEGF with anti-VEGF-A antibody attenuated the vascular dysplasia in a heterozygous ACVRL1+/− mouse model of HHT2.19,161,162 Furthermore, treatment of HHT1 mice with thalidomide163 and HHT2 mice with an anti-VEGF-A antibody164 stabilized vascular sprouts and reduced arteriovenous malformation expansion, respectively. Given these positive in vivo findings, anti-angiogenic agents are currently being tested in clinical trials as therapy for HHT.165–169 The first reports show that treating HHT patients with severe liver vascular malformations with the anti-VEGF antibody Bevacizumab decreased recurrent noose bleedings and cardiac index.169,170
Park and colleagues171 demonstrated that a homozygous deletion of both ACVRL1 alleles was by itself insufficient to recapitulate the formation of arteriovenous malformations in a mouse model for HHT2, but required an additional local wound injury. The formation of the telangiectasia and arteriovenous malformations in this mouse model also required the complete loss of ACVRL1 expression in endothelial cells. Depletion of ALK1 expression in endothelial cells results in increased angiogenesis, which is characterized by faster migration, increased sprouting, and resistance to BMP9-induced inhibition of angiogenesis.19 To date, loss of heterozygosity or epigenetic modification of endoglin have not been reported in HHT1, suggesting that the additional insult required for the disease phenotype to manifest is not a second genetic event at this locus.
Atherosclerosis and vascular calcification
BMP ligands have been detected in arteries from patients with advanced atherosclerosis associated with vascular calcification.172 The role of BMP signalling in atherogenesis has been explored using a variety of BMP signalling inhibitors. Saeed and colleagues showed that administration of LDN-193189 could reduce vascular inflammation and inhibit the development of atherosclerosis in APOE−/− mice.173 Subsequently, Derwall and colleagues demonstrated that both LDN-193189 and ALK3-Fc could inhibit atherogenesis in LDL receptor-deficient (LDLr−/−) mice fed a Western diet.31 Moreover, they found that LDN-193189 reduces the vascular calcification seen in LDLr−/− mice with advanced atherosclerosis.
In vitro and in vivo studies suggest that there are many potential mechanisms by which BMP signalling can promote atherogenesis and, perhaps, secondary vascular calcification. These mechanisms include BMP-mediated endothelial cell activation174 and reactive oxygen species generation31. A compelling potential anti-inflammatory mechanism for the anti-atherogenic effects of LDN-193189 was proposed by Saeed and coworkers,173 following observations that BMP inhibition modified iron-loading in macrophages.175 They also reported that LDN-193189 reduced hepatic hepcidin expression, increased ferroportin expression in peritoneal macrophages, and reduced macrophage iron levels and hydrogen peroxide production. Furthermore, peritoneal macrophages from mice treated with LDN-193189 showed enhanced gene expression of ABCA1 and ABCG1, two cholesterol efflux transporters, associated with an increased ability to export cholesterol. All the effects of in vivo treatment with LDN-193189 on peritoneal macrophage function could be reversed by ex vivo administration with hepcidin to induce ferroportin degradation.173 Together, these observations suggest that inhibiting BMP signalling can modulate macrophage phenotypes by altering hepcidin–ferroportin signalling and intracellular iron levels. Whether BMP inhibition modulates macrophage function in atherosclerotic vessels via hepcidin–ferroportin signaling remains to be determined.
BMP signalling in myocardial remodelling
BMP4 expression can be acutely or chronically elevated in pressure overload-induced pathogical hypertrophy; importantly, an increase in BMP4 expression is not observed in exercise-induced physiologic hypertrophy.29,176,177 BMP4 expression is similarly upregulated in acute infarction and ischaemia-reperfusion injury, as well as in chronic ischaemic heart disease28,29. BMP4-induced hypertrophy in isolated cardiomyocytes can be inhibited upon treatment with BMP inhibitors noggin, dorsomorphin, or DMH1,28,177 or interestingly, antagonized by the co-administration of BMP2.110 The direct effect of BMP4 signalling on the development of cardiac hypertrophy was demonstrated in in vivo studies showing that pressure overload-induced left ventricular hypertrophy177 and cardiac ischemia-reperfusion injury,28 were attenuated using BMP inhibitors or in BMP4-deficient animals, respectively.
The expression of endoglin is elevated in both failing human hearts and in mice with pressure overload-induced heart failure.178 Both endoglin heterozygosity as well as the expression of a soluble form of endoglin limited cardiac fibrosis and improved survival in an experimental model of left ventricular pressure overload.178 In follow-up studies, haploinsufficiency of endoglin, and inhibition of endoglin in the myocardium using a specific neutralizing antibody limited calcineurin activation in pressure overload-induced right heart failure, limited myofibroblast transformation and cardiac fibrosis, and improved survival.179 These findings were attributed to the ability of endoglin to modulate TGF-β signalling. Since soluble endoglin selectively binds to and inhibits BMP9 and BMP10 to exert antiangiogenic effects in vivo,180 the reduction in cardiac fibrosis as a result of treatment with soluble endoglin or endoglin-neutralizing antibody might also result from attenuated signalling of those ligands or their downstream effects. However, BMP10 has also been reported to reduce cardiac fibrosis and improve cardiac function following experimental myocardial infarction in mice.111
Anaemia of Inflammation
Anaemia of chronic disease, or anaemia of inflammation, deserves special consideration in this discussion of cardiovascular disease for several reasons. The reduction in oxygen-carrying capacity associated with anaemia is an important exacerbating factor in cardiovascular disease, aggravating symptoms of hypoxic lung and pulmonary vascular disease, heart failure, as well as coronary and systemic atherosclerotic disease. In moderate to severe states, all of these conditions can be linked to a clinically relevant degree of anemia that is caused by a disease-associated systemic inflammatory state, through a mechanism that is BMP-dependent. BMP signal transduction regulates the expression of hepcidin, the central hormonal regulator of iron metabolism.181 Ferroportin, is the sole molecule responsible for exporting iron from intracellular stores within intestinal epithelial cells, hepatocytes, and macrophages into the circulation.182 Ferroportin is targeted for internalization and degradation by hepcidin to reduce circulating iron levels. Hepcidin synthesis in the liver is regulated by BMP6, numerous inflammatory stimuli including IL-6, as well as increased iron levels themselves (Figure 1).
The important role of BMP signaling in the regulation of hepcidin expression was identified by Babitt and colleagues, who observed that haemojuvelin, a protein defective in severe forms of haemochromatosis, is a BMP co-receptor and that soluble haemojuvelin could inhibit the ability of BMP ligands to induce hepatocyte hepcidin gene expression.181 Moreover, these investigators observed that soluble haemojuvelin prevents the induction of hepcidin gene expression by IL-6, suggesting that inhibition of BMP signalling could be used to treat anaemia of inflammation. The role of BMP signal transduction in the regulation of iron transport and potentially anaemia of chronic disease was subsequently confirmed by the use of BMP inhibitor dorsomorphin to reduce hepcidin gene expression and increase serum iron levels in zebrafish and mice.48
Anaemia of inflammation is attributable to a reduction in red blood cell production by the bone marrow owing to reduced systemic iron bioavailability, yet frequently occurs in healthy individuals with an iron replete diet. Several factors contribute to reduced red blood cell production including: sequestration of iron in macrophages leading to a reduction in plasma iron levels; reduced absorption of iron from the duodenum; impaired erythropoiesis despite an increase in erythropoietin levels; and in some cases, a reduction of red blood cell life-span.183,184 Anaemia of inflammation is associated with a wide variety of disorders including infection, chronic kidney disease, cancer, connective tissue diseases such as rheumatoid arthritis and systemic lupus erythematosus, inflammatory bowel disease, and congestive heart failure. All of these disorders share acute or chronic immune activation pathways leading to the production of inflammatory cytokines, including IL-6. Although haemoglobin levels are generally only moderately reduced in anaemia of chronic disease, the decreased oxygen delivery associated with this condition is sufficient to exacerbate symptoms in patients with cardiovascular comorbidities.185 Treatment in these individuals is directed towards alleviating symptoms such as angina, fatigue, or functional limitation.
Steinbicker and colleagues assessed whether inhibiting BMP signalling could prevent the induction of hepatic hepcidin gene expression by IL-6.186 Intraperitoneal injection of LDN-193189 or a soluble ALK3 receptor inhibited IL-6’s ability to increase hepatic hepcidin mRNA levels. This group and others subsequently showed that administration of LDN-193189, or a BMP ligand trap using recombinant haemojuvelin expressed as an IgG-Fc domain fusion protein could prevent and reverse established anaemia in several rodent models of anaemia of chronic disease.186,187 Mayeur and colleagues subsequently showed that oral administration of LDN-193189 was sufficient to prevent anaemia in this model of chronic inflammation.188 Taken together, these observations suggest that inhibiting BMP signalling might represent a novel and practical therapeutic target for treating anemia of chronic disease.
Conclusion
Human genetic studies have revealed major, previously unsuspected roles for the BMP family of ligands and receptors in cardiovascular disease and iron homeostasis. The large BMP family of ligands, receptors, ligand traps, and cell surface modifiers of signalling suggests a finely regulated pathway with complex interactions. Nevertheless, meticulous dissection of the BMP pathway over the last 15 years has led to a greater understanding of the cell-specific nature of signalling and has revealed potential opportunities for therapeutic intervention. In particular, restoration of BMPRII receptor expression or function, and the use of selective ligands such as BMP9 and BMP10 have shown promise for PAH. By contrast, small molecule inhibition of other BMPs and their receptors might provide novel opportunities in conditions as diverse as atherosclerosis and anaemia. The next few years will determine whether this intriguing family of bone forming proteins can be exploited safely for the treatment of cardiovascular disease.
Key Points.
Bone morphogenetic proteins (BMPs) play important roles in cardiovascular growth, homeostasis, and disease development
The wide repertoire of BMP ligands and receptors, coupled with numerous modifiers of BMP signaling, confer marked tissue- and cell-specific responses to BMPs
Understanding the context-specific nature of BMP signalling can help guide the development of novel therapeutics to treatment cardiovascular disease and anaemia
Small molecule inhibition of BMP signalling is efficacious in preclinical models of atherosclerosis, vascular calcification, and anaemia
Enhancement of BMP receptor-II (BMPR-II) signalling by increasing cell surface levels of BMPR-II or by endothelial-selective BMPR-II agonists such as BMP9 show promise in preclinical models of pulmonary arterial hypertension
Footnotes
Competing interests
No competing interests
References
- 1.Urist MR, Jurist JM, Jr, Dubuc FL, Strates BS. Quantitation of new bone formation in intramuscular implants of bone matrix in rabbits. Clinical orthopaedics and related research. 1970;68:279–293. [PubMed] [Google Scholar]
- 2.Rosen V, et al. Purification and molecular cloning of a novel group of BMPs and localization of BMP mRNA in developing bone. Connect Tissue Res. 1989;20:313–319. doi: 10.3109/03008208909023902. [DOI] [PubMed] [Google Scholar]
- 3.David L, et al. Bone morphogenetic protein-9 is a circulating vascular quiescence factor. Circ Res. 2008;102:914–922. doi: 10.1161/CIRCRESAHA.107.165530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vukicevic S, Grgurevic L. BMP-6 and mesenchymal stem cell differentiation. Cytokine Growth Factor Rev. 2009;20:441–448. doi: 10.1016/j.cytogfr.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 5.Laux DW, et al. Circulating Bmp10 acts through endothelial Alk1 to mediate flow-dependent arterial quiescence. Development. 2013;140:3403–3412. doi: 10.1242/dev.095307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herrera B, Inman GJ. A rapid and sensitive bioassay for the simultaneous measurement of multiple bone morphogenetic proteins. Identification and quantification of BMP4, BMP6 and BMP9 in bovine and human serum. BMC Cell Biol. 2009;10:20. doi: 10.1186/1471-2121-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ten Dijke P, et al. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J Biol Chem. 1994;269:16985–16988. [PubMed] [Google Scholar]
- 8.Nohno T, et al. Identification of a human type II receptor for bone morphogenetic protein-4 that forms differential heteromeric complexes with bone morphogenetic protein type I receptors. J Biol Chem. 1995;270:22522–22526. doi: 10.1074/jbc.270.38.22522. [DOI] [PubMed] [Google Scholar]
- 9.Rosenzweig BL, et al. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc Natl Acad Sci U S A. 1995;92:7632–7636. doi: 10.1073/pnas.92.17.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) endothelial cells. Blood. 2006 doi: 10.1182/blood-2006-07-034124. [DOI] [PubMed] [Google Scholar]
- 11.Little SC, Mullins MC. Bone morphogenetic protein heterodimers assemble heteromeric type I receptor complexes to pattern the dorsoventral axis. Nat Cell Biol. 2009;11:637–643. doi: 10.1038/ncb1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones CM, Smith JC. Establishment of a BMP-4 morphogen gradient by long-range inhibition. Developmental biology. 1998;194:12–17. doi: 10.1006/dbio.1997.8752. [DOI] [PubMed] [Google Scholar]
- 13.Song K, et al. Identification of a key residue mediating bone morphogenetic protein (BMP)-6 resistance to noggin inhibition allows for engineered BMPs with superior agonist activity. The Journal of biological chemistry. 2010;285:12169–12180. doi: 10.1074/jbc.M109.087197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao Y, et al. Crossveinless 2 regulates bone morphogenetic protein 9 in human and mouse vascular endothelium. Blood. 2012;119:5037–5047. doi: 10.1182/blood-2011-10-385906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirkbride KC, Townsend TA, Bruinsma MW, Barnett JV, Blobe GC. Bone morphogenetic proteins signal through the transforming growth factor-beta type III receptor. The Journal of biological chemistry. 2008;283:7628–7637. doi: 10.1074/jbc.M704883200. [DOI] [PubMed] [Google Scholar]
- 16.Scharpfenecker M, et al. BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J Cell Sci. 2007;120:964–972. doi: 10.1242/jcs.002949. [DOI] [PubMed] [Google Scholar]
- 17.Corradini E, Babitt JL, Lin HY. The RGM/DRAGON family of BMP co-receptors. Cytokine & growth factor reviews. 2009;20:389–398. doi: 10.1016/j.cytogfr.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venkatesha S, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nature medicine. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 19.Choi EJ, et al. Enhanced responses to angiogenic cues underlie the pathogenesis of hereditary hemorrhagic telangiectasia 2. PLoS One. 2013;8:e63138. doi: 10.1371/journal.pone.0063138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi EJ, et al. Minimal homozygous endothelial deletion of Eng with VEGF stimulation is sufficient to cause cerebrovascular dysplasia in the adult mouse. Cerebrovasc Dis. 2012;33:540–547. doi: 10.1159/000337762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nolan-Stevaux O, et al. Endoglin requirement for BMP9 signaling in endothelial cells reveals new mechanism of action for selective anti-endoglin antibodies. PLoS One. 2012;7:e50920. doi: 10.1371/journal.pone.0050920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Upton PD, Davies RJ, Trembath RC, Morrell NW. Bone morphogenetic protein (BMP) and activin type II receptors balance BMP9 signals mediated by activin receptor-like kinase-1 in human pulmonary artery endothelial cells. J Biol Chem. 2009;284:15794–15804. doi: 10.1074/jbc.M109.002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao Y, et al. High-density lipoproteins affect endothelial BMP-signaling by modulating expression of the activin-like kinase receptor 1 and 2. Arterioscler Thromb Vasc Biol. 2008;28:2266–2274. doi: 10.1161/ATVBAHA.108.176958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Yang HY, Giachelli CM. BMP-2 promotes phosphate uptake, phenotypic modulation, and calcification of human vascular smooth muscle cells. Atherosclerosis. 2008;199:271–277. doi: 10.1016/j.atherosclerosis.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan MC, et al. A novel regulatory mechanism of the bone morphogenetic protein (BMP) signaling pathway involving the carboxyl-terminal tail domain of BMP type II receptor. Molecular and cellular biology. 2007;27:5776–5789. doi: 10.1128/MCB.00218-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu PB, et al. Bone Morphogenetic Protein (BMP) Type II Receptor Is Required for BMP-mediated Growth Arrest and Differentiation in Pulmonary Artery Smooth Muscle Cells. J Biol Chem. 2008;283:3877–3888. doi: 10.1074/jbc.M706797200. [DOI] [PubMed] [Google Scholar]
- 27.Yu PB, Beppu H, Kawai N, Li E, Bloch KD. Bone morphogenetic protein (BMP) type II receptor deletion reveals BMP ligand-specific gain of signaling in pulmonary artery smooth muscle cells. J Biol Chem. 2005;280:24443–24450. doi: 10.1074/jbc.M502825200. [DOI] [PubMed] [Google Scholar]
- 28.Pachori AS, et al. Bone morphogenetic protein 4 mediates myocardial ischemic injury through JNK-dependent signaling pathway. J Mol Cell Cardiol. 2010;48:1255–1265. doi: 10.1016/j.yjmcc.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Wu X, et al. Expression of bone morphogenetic protein 4 and its receptors in the remodeling heart. Life Sci. 2014;97:145–154. doi: 10.1016/j.lfs.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 30.Malhotra R, et al. Inhibition of bone morphogenetic protein signal transduction prevents the medial vascular calcification associated with matrix Gla protein deficiency. PLoS One. 2015;10:e0117098. doi: 10.1371/journal.pone.0117098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derwall M, et al. Inhibition of bone morphogenetic protein signaling reduces vascular calcification and atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:613–622. doi: 10.1161/ATVBAHA.111.242594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heinke J, et al. Antagonism and synergy between extracellular BMP modulators Tsg and BMPER balance blood vessel formation. Journal of cell science. 2013;126:3082–3094. doi: 10.1242/jcs.122333. [DOI] [PubMed] [Google Scholar]
- 33.Yoshimatsu Y, et al. Bone morphogenetic protein-9 inhibits lymphatic vessel formation via activin receptor-like kinase 1 during development and cancer progression. Proc Natl Acad Sci U S A. 2013;110:18940–18945. doi: 10.1073/pnas.1310479110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minina E, Kreschel C, Naski MC, Ornitz DM, Vortkamp A. Interaction of FGF, Ihh/Pthlh, and BMP signaling integrates chondrocyte proliferation and hypertrophic differentiation. Developmental cell. 2002;3:439–449. doi: 10.1016/s1534-5807(02)00261-7. [DOI] [PubMed] [Google Scholar]
- 35.Shao ES, Lin L, Yao Y, Bostrom KI. Expression of vascular endothelial growth factor is coordinately regulated by the activin-like kinase receptors 1 and 5 in endothelial cells. Blood. 2009;114:2197–2206. doi: 10.1182/blood-2009-01-199166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tokuda H, et al. p38 MAP kinase regulates BMP-4-stimulated VEGF synthesis via p70 S6 kinase in osteoblasts. Am J Physiol Endocrinol Metab. 2003;284:E1202–E1209. doi: 10.1152/ajpendo.00300.2002. [DOI] [PubMed] [Google Scholar]
- 37.Morikawa M, et al. ChIP-seq reveals cell type-specific binding patterns of BMP-specific Smads and a novel binding motif. Nucleic acids research. 2011;39:8712–8727. doi: 10.1093/nar/gkr572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dahlqvist C, et al. Functional Notch signaling is required for BMP4-induced inhibition of myogenic differentiation. Development. 2003;130:6089–6099. doi: 10.1242/dev.00834. [DOI] [PubMed] [Google Scholar]
- 39.de Jong DS, et al. Regulation of Notch signaling genes during BMP2-induced differentiation of osteoblast precursor cells. Biochem Biophys Res Commun. 2004;320:100–107. doi: 10.1016/j.bbrc.2004.05.150. [DOI] [PubMed] [Google Scholar]
- 40.Itoh F, et al. Synergy and antagonism between Notch and BMP receptor signaling pathways in endothelial cells. The EMBO journal. 2004;23:541–551. doi: 10.1038/sj.emboj.7600065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moya IM, et al. Stalk cell phenotype depends on integration of Notch and Smad1/5 signaling cascades. Developmental cell. 2012;22:501–514. doi: 10.1016/j.devcel.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bai Y, et al. Bmp signaling represses Vegfa to promote outflow tract cushion development. Development. 2013;140:3395–3402. doi: 10.1242/dev.097360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garside VC, Chang AC, Karsan A, Hoodless PA. Co-ordinating Notch, BMP, andTGF-beta signaling during heart valve development. Cellular and molecular life sciences : CMLS. 2013;70:2899–2917. doi: 10.1007/s00018-012-1197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mustonen T, et al. Lunatic fringe, FGF, and BMP regulate the Notch pathway during epithelial morphogenesis of teeth. Developmental biology. 2002;248:281–293. doi: 10.1006/dbio.2002.0734. [DOI] [PubMed] [Google Scholar]
- 45.Tirosh-Finkel L, et al. BMP-mediated inhibition of FGF signaling promotes cardiomyocyte differentiation of anterior heart field progenitors. Development. 2010;137:2989–3000. doi: 10.1242/dev.051649. [DOI] [PubMed] [Google Scholar]
- 46.Mitchell D, et al. ALK1-Fc inhibits multiple mediators of angiogenesis and suppresses tumor growth. Mol Cancer Ther. 2010;9:379–388. doi: 10.1158/1535-7163.MCT-09-0650. [DOI] [PubMed] [Google Scholar]
- 47.Babitt JL, et al. Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. The Journal of clinical investigation. 2007;117:1933–1939. doi: 10.1172/JCI31342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu PB, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mintzer KA, et al. Lost-a-fin encodes a type I BMP receptor, Alk8, acting maternally and zygotically in dorsoventral pattern formation. Development. 2001;128:859–869. doi: 10.1242/dev.128.6.859. [DOI] [PubMed] [Google Scholar]
- 50.Cuny GD, et al. Structure-activity relationship study of bone morphogenetic protein (BMP) signaling inhibitors. Bioorg Med Chem Lett. 2008;18:4388–4392. doi: 10.1016/j.bmcl.2008.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohedas AH, et al. Structure-Activity Relationship of 3,5-Diaryl-2-aminopyridine ALK2 Inhibitors Reveals Unaltered Binding Affinity for Fibrodysplasia Ossificans Progressiva Causing Mutants. Journal of medicinal chemistry. 2014;57:7900–7915. doi: 10.1021/jm501177w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanvitale CE, et al. A new class of small molecule inhibitor of BMP signaling. PLoS One. 2013;8:e62721. doi: 10.1371/journal.pone.0062721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cai J, Pardali E, Sanchez-Duffhues G, ten Dijke P. BMP signaling in vascular diseases. FEBS Lett. 2012;586:1993–2002. doi: 10.1016/j.febslet.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 54.Pardali E, Ten Dijke P. TGFbeta signaling and cardiovascular diseases. Int J Biol Sci. 2012;8:195–213. doi: 10.7150/ijbs.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suzuki Y, et al. BMP-9 induces proliferation of multiple types of endothelial cells in vitro and in vivo. J Cell Sci. 2010;123:1684–1692. doi: 10.1242/jcs.061556. [DOI] [PubMed] [Google Scholar]
- 56.Cunha SI, et al. Genetic and pharmacological targeting of activin receptor-like kinase 1 impairs tumor growth and angiogenesis. J Exp Med. 2010;207:85–100. doi: 10.1084/jem.20091309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hawinkels LJ, Garcia de Vinuesa A, Ten Dijke P. Activin receptor-like kinase 1 as a target for anti-angiogenesis therapy. Expert Opin Investig Drugs. 2013;22:1371–1383. doi: 10.1517/13543784.2013.837884. [DOI] [PubMed] [Google Scholar]
- 58.ten Dijke P, Goumans MJ, Pardali E. Endoglin in angiogenesis and vascular diseases. Angiogenesis. 2008;11:79–89. doi: 10.1007/s10456-008-9101-9. [DOI] [PubMed] [Google Scholar]
- 59.Valdimarsdottir G, et al. Stimulation of Id1 expression by bone morphogenetic protein is sufficient and necessary for bone morphogenetic protein-induced activation of endothelial cells. Circulation. 2002;106:2263–2270. doi: 10.1161/01.cir.0000033830.36431.46. [DOI] [PubMed] [Google Scholar]
- 60.Benezra R, Rafii S, Lyden D. The Id proteins and angiogenesis. Oncogene. 2001;20:8334–8341. doi: 10.1038/sj.onc.1205160. [DOI] [PubMed] [Google Scholar]
- 61.Ciumas M, et al. Bone morphogenetic proteins protect pulmonary microvascular endothelial cells from apoptosis by upregulating alpha-B-crystallin. Arterioscler Thromb Vasc Biol. 2013;33:2577–2584. doi: 10.1161/ATVBAHA.113.301976. [DOI] [PubMed] [Google Scholar]
- 62.Yang J, et al. Id proteins are critical downstream effectors of BMP signaling in human pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2013;305:L312–L321. doi: 10.1152/ajplung.00054.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lowery JW, et al. ID family protein expression and regulation in hypoxic pulmonary hypertension. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1463–R1477. doi: 10.1152/ajpregu.00866.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Asirvatham AJ, Schmidt MA, Chaudhary J. Non-redundant inhibitor of differentiation (Id) gene expression and function in human prostate epithelial cells. Prostate. 2006;66:921–935. doi: 10.1002/pros.20366. [DOI] [PubMed] [Google Scholar]
- 65.Kang H, et al. Bone morphogenetic protein 4 promotes vascular smooth muscle contractility by activating microRNA-21 (miR-21), which down-regulates expression of family of dedicator of cytokinesis (DOCK) proteins. J Biol Chem. 2012;287:3976–3986. doi: 10.1074/jbc.M111.303156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim S, Hata A, Kang H. Down-regulation of miR-96 by bone morphogenetic protein signaling is critical for vascular smooth muscle cell phenotype modulation. J Cell Biochem. 2014;115:889–895. doi: 10.1002/jcb.24730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lagna G, et al. Control of phenotypic plasticity of smooth muscle cells by bone morphogenetic protein signaling through the myocardin-related transcription factors. J Biol Chem. 2007;282:37244–37255. doi: 10.1074/jbc.M708137200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou J, et al. BMP receptor-integrin interaction mediates responses of vascular endothelial Smad1/5 and proliferation to disturbed flow. J Thromb Haemost. 2013;11:741–755. doi: 10.1111/jth.12159. [DOI] [PubMed] [Google Scholar]
- 69.Kim PG, et al. Flow-induced protein kinase A-CREB pathway acts via BMP signaling to promote HSC emergence. J Exp Med. 2015;212:633–648. doi: 10.1084/jem.20141514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Corti P, et al. Interaction between alk1 and blood flow in the development of arteriovenous malformations. Development. 2011;138:1573–1582. doi: 10.1242/dev.060467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miquerol L, Kelly RG. Organogenesis of the vertebrate heart. Wiley Interdiscip Rev Dev Biol. 2013;2:17–29. doi: 10.1002/wdev.68. [DOI] [PubMed] [Google Scholar]
- 73.Tam PP, Parameswaran M, Kinder SJ, Weinberger RP. The allocation of epiblast cells to the embryonic heart and other mesodermal lineages: the role of ingression and tissue movement during gastrulation. Development. 1997;124:1631–1642. doi: 10.1242/dev.124.9.1631. [DOI] [PubMed] [Google Scholar]
- 74.Wessels A, Markwald R. Cardiac morphogenesis and dysmorphogenesis. I. Normal development. Methods Mol Biol. 2000;136:239–259. doi: 10.1385/1-59259-065-9:239. [DOI] [PubMed] [Google Scholar]
- 75.Srivastava D, Olson EN. A genetic blueprint for cardiac development. Nature. 2000;407:221–226. doi: 10.1038/35025190. [DOI] [PubMed] [Google Scholar]
- 76.Kruithof BP, Duim SN, Moerkamp AT, Goumans MJ. TGFbeta and BMP signaling in cardiac cushion formation: lessons from mice and chicken. Differentiation. 2012;84:89–102. doi: 10.1016/j.diff.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 77.van Wijk B, Moorman AF, van den Hoff MJ. Role of bone morphogenetic proteins in cardiac differentiation. Cardiovascular research. 2007;74:244–255. doi: 10.1016/j.cardiores.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 78.Hao J, et al. Dorsomorphin, a selective small molecule inhibitor of BMP signaling, promotes cardiomyogenesis in embryonic stem cells. PLoS One. 2008;3:e2904. doi: 10.1371/journal.pone.0002904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yuasa S, et al. Transient inhibition of BMP signaling by Noggin induces cardiomyocyte differentiation of mouse embryonic stem cells. Nat Biotechnol. 2005;23:607–611. doi: 10.1038/nbt1093. [DOI] [PubMed] [Google Scholar]
- 80.Bernardo AS, et al. BRACHYURY and CDX2 mediate BMP-induced differentiation of human and mouse pluripotent stem cells into embryonic and extraembryonic lineages. Cell Stem Cell. 2011;9:144–155. doi: 10.1016/j.stem.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Faial T, et al. Brachyury and SMAD signalling collaboratively orchestrate distinct mesoderm and endoderm gene regulatory networks in differentiating human embryonic stem cells. Development. 2015;142:2121–2135. doi: 10.1242/dev.117838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Noseda M, Peterkin T, Simoes FC, Patient R, Schneider MD. Cardiopoietic factors: extracellular signals for cardiac lineage commitment. Circ Res. 2011;108:129–152. doi: 10.1161/CIRCRESAHA.110.223792. [DOI] [PubMed] [Google Scholar]
- 83.Cai W, et al. Coordinate Nodal and BMP inhibition directs Baf60c-dependent cardiomyocyte commitment. Genes & development. 2013;27:2332–2344. doi: 10.1101/gad.225144.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kattman SJ, et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 85.Witty AD, et al. Generation of the epicardial lineage from human pluripotent stem cells. Nat Biotechnol. 2014;32:1026–1035. doi: 10.1038/nbt.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Iyer D, et al. Robust derivation of epicardium and its differentiated smooth muscle cell progeny from human pluripotent stem cells. Development. 2015;142:1528–1541. doi: 10.1242/dev.119271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gittenberger-de Groot AC, et al. The arterial and cardiac epicardium in development, disease and repair. Differentiation; research in biological diversity. 2012;84:41–53. doi: 10.1016/j.diff.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 88.Javier AL, et al. Bmp indicator mice reveal dynamic regulation of transcriptional response. PLoS One. 2012;7:e42566. doi: 10.1371/journal.pone.0042566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Monteiro RM, de Sousa Lopes SM, Korchynskyi O, ten Dijke P, Mummery CL. Spatio-temporal activation of Smad1 and Smad5 in vivo: monitoring transcriptional activity of Smad proteins. J Cell Sci. 2004;117:4653–4663. doi: 10.1242/jcs.01337. [DOI] [PubMed] [Google Scholar]
- 90.Monteiro RM, et al. Real time monitoring of BMP Smads transcriptional activity during mouse development. Genesis. 2008;46:335–346. doi: 10.1002/dvg.20402. [DOI] [PubMed] [Google Scholar]
- 91.Klaus A, Saga Y, Taketo MM, Tzahor E, Birchmeier W. Distinct roles of Wnt/beta-catenin and Bmp signaling during early cardiogenesis. Proc Natl Acad Sci U S A. 2007;104:18531–18536. doi: 10.1073/pnas.0703113104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gaussin V, et al. Endocardial cushion and myocardial defects after cardiac myocyte-specific conditional deletion of the bone morphogenetic protein receptor ALK3. Proc Natl Acad Sci U S A. 2002;99:2878–2883. doi: 10.1073/pnas.042390499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ma L, Lu MF, Schwartz RJ, Martin JF. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development. 2005;132:5601–5611. doi: 10.1242/dev.02156. [DOI] [PubMed] [Google Scholar]
- 94.Wang Y, et al. Endocardial to myocardial notch-wnt-bmp axis regulates early heart valve development. PLoS One. 2013;8:e60244. doi: 10.1371/journal.pone.0060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang J, et al. Atrioventricular cushion transformation is mediated by ALK2 in the developing mouse heart. Developmental biology. 2005;286:299–310. doi: 10.1016/j.ydbio.2005.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen H, et al. BMP10 is essential for maintaining cardiac growth during murine cardiogenesis. Development. 2004;131:2219–2231. doi: 10.1242/dev.01094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang J, et al. Myocardin regulates BMP10 expression and is required for heart development. The Journal of clinical investigation. 2012;122:3678–3691. doi: 10.1172/JCI63635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Geudens I, Gerhardt H. Coordinating cell behaviour during blood vessel formation. Development. 2011;138:4569–4583. doi: 10.1242/dev.062323. [DOI] [PubMed] [Google Scholar]
- 100.Rothhammer T, Bataille F, Spruss T, Eissner G, Bosserhoff AK. Functional implication of BMP4 expression on angiogenesis in malignant melanoma. Oncogene. 2007;26:4158–4170. doi: 10.1038/sj.onc.1210182. [DOI] [PubMed] [Google Scholar]
- 101.Nakaoka T, et al. Inhibition of rat vascular smooth muscle proliferation in vitro and in vivo by bone morphogenetic protein-2. The Journal of clinical investigation. 1997;100:2824–2832. doi: 10.1172/JCI119830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Willette RN, et al. BMP-2 gene expression and effects on human vascular smooth muscle cells. J Vasc Res. 1999;36:120–125. doi: 10.1159/000025634. [DOI] [PubMed] [Google Scholar]
- 103.Dorai H, Vukicevic S, Sampath TK. Bone morphogenetic protein-7 (osteogenic protein-1) inhibits smooth muscle cell proliferation and stimulates the expression of markers that are characteristic of SMC phenotype in vitro. J Cell Physiol. 2000;184:37–45. doi: 10.1002/(SICI)1097-4652(200007)184:1<37::AID-JCP4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 104.Dorai H, Sampath TK. Bone morphogenetic protein-7 modulates genes that maintain the vascular smooth muscle cell phenotype in culture. J Bone Joint Surg Am. 2001;83-A(Suppl 1):S70–S78. [PubMed] [Google Scholar]
- 105.Chen W, et al. Reduced mural cell coverage and impaired vessel integrity after angiogenic stimulation in the Alk1-deficient brain. Arterioscler Thromb Vasc Biol. 2013;33:305–310. doi: 10.1161/ATVBAHA.112.300485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Upton PD, Davies RJ, Tajsic T, Morrell NW. Transforming growth factor-beta(1) represses bone morphogenetic protein-mediated Smad signaling in pulmonary artery smooth muscle cells via Smad3. Am J Respir Cell Mol Biol. 2013;49:1135–1145. doi: 10.1165/rcmb.2012-0470OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Loeys BL, Mortier G, Dietz HC. Bone lessons from Marfan syndrome and related disorders: fibrillin, TGF-B and BMP at the balance of too long and too short. Pediatric endocrinology reviews : PER. 2013;10(Suppl 2):417–423. [PubMed] [Google Scholar]
- 108.Quarto N, Li S, Renda A, Longaker MT. Exogenous activation of BMP-2 signaling overcomes TGFbeta-mediated inhibition of osteogenesis in Marfan embryonic stem cells and Marfan patient-specific induced pluripotent stem cells. Stem Cells. 2012;30:2709–2719. doi: 10.1002/stem.1250. [DOI] [PubMed] [Google Scholar]
- 109.Guo WT, Dong DL. Bone morphogenetic protein-4: a novel therapeutic target for pathological cardiac hypertrophy/heart failure. Heart failure reviews. 2014;19:781–788. doi: 10.1007/s10741-014-9429-8. [DOI] [PubMed] [Google Scholar]
- 110.Lu J, et al. Bone morphogenetic protein-2 antagonizes bone morphogenetic protein-4 induced cardiomyocyte hypertrophy and apoptosis. J Cell Physiol. 2014;229:1503–1510. doi: 10.1002/jcp.24592. [DOI] [PubMed] [Google Scholar]
- 111.Sun L, et al. Bone morphogenetic protein-10 induces cardiomyocyte proliferation and improves cardiac function after myocardial infarction. J Cell Biochem. 2014;115:1868–1876. doi: 10.1002/jcb.24856. [DOI] [PubMed] [Google Scholar]
- 112.Ebelt H, et al. Treatment with bone morphogenetic protein 2 limits infarct size after myocardial infarction in mice. Shock. 2013;39:353–360. doi: 10.1097/SHK.0b013e318289728a. [DOI] [PubMed] [Google Scholar]
- 113.Son JW, et al. Serum BMP-4 levels in relation to arterial stiffness and carotid atherosclerosis in patients with Type 2 diabetes. Biomark Med. 2011;5:827–835. doi: 10.2217/bmm.11.81. [DOI] [PubMed] [Google Scholar]
- 114.Stahls PF, 3rd, Lightell DJ, Jr, Moss SC, Goldman CK, Woods TC. Elevated serum bone morphogenetic protein 4 in patients with chronic kidney disease and coronary artery disease. J Cardiovasc Transl Res. 2013;6:232–238. doi: 10.1007/s12265-012-9429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hoeper MM, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D42–D50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 116.Tuder RM, et al. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D4–D12. doi: 10.1016/j.jacc.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation. 2006;114:1417–1431. doi: 10.1161/CIRCULATIONAHA.104.503540. [DOI] [PubMed] [Google Scholar]
- 118.Simonneau G, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 119.Loyd JE, Primm RK, Newman JH. Familial primary pulmonary hypertension: clinical patterns. Am Rev Respir Dis. 1984;129:194–197. doi: 10.1164/arrd.1984.129.1.194. [DOI] [PubMed] [Google Scholar]
- 120.International, P.P.H.C., et al. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000;26:81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 121.Deng Z, et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet. 2000;67:737–744. doi: 10.1086/303059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Machado RD, et al. Mutations of the TGF-beta type II receptor BMPR2 in pulmonary arterial hypertension. Human mutation. 2006;27:121–132. doi: 10.1002/humu.20285. [DOI] [PubMed] [Google Scholar]
- 123.Thomson JR, et al. Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II, a receptor member of the TGF-beta family. J Med Genet. 2000;37:741–745. doi: 10.1136/jmg.37.10.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hassel S, et al. Proteins associated with type II bone morphogenetic protein receptor (BMPR-II) and identified by two-dimensional gel electrophoresis and mass spectrometry. Proteomics. 2004;4:1346–1358. doi: 10.1002/pmic.200300770. [DOI] [PubMed] [Google Scholar]
- 125.Schwappacher R, et al. Novel crosstalk to BMP signalling: cGMP-dependent kinase I modulates BMP receptor and Smad activity. The EMBO journal. 2009;28:1537–1550. doi: 10.1038/emboj.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Long L, et al. Serotonin increases susceptibility to pulmonary hypertension in BMPR2-deficient mice. Circ Res. 2006;98:818–827. doi: 10.1161/01.RES.0000215809.47923.fd. [DOI] [PubMed] [Google Scholar]
- 127.Long L, et al. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nature medicine. 2015;21:777–785. doi: 10.1038/nm.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Austin ED, Loyd JE. The genetics of pulmonary arterial hypertension. Circ Res. 2014;115:189–202. doi: 10.1161/CIRCRESAHA.115.303404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Song Y, et al. Increased susceptibility to pulmonary hypertension in heterozygous BMPR2-mutant mice. Circulation. 2005;112:553–562. doi: 10.1161/CIRCULATIONAHA.104.492488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.West J, et al. Pulmonary hypertension in transgenic mice expressing a dominant-negative BMPRII gene in smooth muscle. Circ Res. 2004;94:1109–1114. doi: 10.1161/01.RES.0000126047.82846.20. [DOI] [PubMed] [Google Scholar]
- 131.Yang X, et al. Dysfunctional Smad Signaling Contributes to Abnormal Smooth Muscle Cell Proliferation in Familial Pulmonary Arterial Hypertension. Circ Res. 2005 doi: 10.1161/01.RES.0000166926.54293.68. [DOI] [PubMed] [Google Scholar]
- 132.Morrell NW, et al. Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-beta(1) and bone morphogenetic proteins. Circulation. 2001;104:790–795. doi: 10.1161/hc3201.094152. [DOI] [PubMed] [Google Scholar]
- 133.Yang J, et al. Mutations in bone morphogenetic protein type II receptor cause dysregulation of Id gene expression in pulmonary artery smooth muscle cells: implications for familial pulmonary arterial hypertension. Circ Res. 2008;102:1212–1221. doi: 10.1161/CIRCRESAHA.108.173567. [DOI] [PubMed] [Google Scholar]
- 134.Yang J, et al. Smad-dependent and smad-independent induction of id1 by prostacyclin analogues inhibits proliferation of pulmonary artery smooth muscle cells in vitro and in vivo. Circ Res. 2010;107:252–262. doi: 10.1161/CIRCRESAHA.109.209940. [DOI] [PubMed] [Google Scholar]
- 135.Yang J, et al. Sildenafil potentiates bone morphogenetic protein signaling in pulmonary arterial smooth muscle cells and in experimental pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2013;33:34–42. doi: 10.1161/ATVBAHA.112.300121. [DOI] [PubMed] [Google Scholar]
- 136.David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood. 2007;109:1953–1961. doi: 10.1182/blood-2006-07-034124. [DOI] [PubMed] [Google Scholar]
- 137.Trembath RC, et al. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N Engl J Med. 2001;345:325–334. doi: 10.1056/NEJM200108023450503. [DOI] [PubMed] [Google Scholar]
- 138.Hong KH, et al. Genetic ablation of the BMPR2 gene in pulmonary endothelium is sufficient to predispose to pulmonary arterial hypertension. Circulation. 2008;118:722–730. doi: 10.1161/CIRCULATIONAHA.107.736801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jerkic M, et al. Pulmonary hypertension in adult Alk1 heterozygous mice due to oxidative stress. Cardiovasc Res. 2011;92:375–384. doi: 10.1093/cvr/cvr232. [DOI] [PubMed] [Google Scholar]
- 140.Teichert-Kuliszewska K, et al. Bone morphogenetic protein receptor-2 signaling promotes pulmonary arterial endothelial cell survival: implications for loss-of-function mutations in the pathogenesis of pulmonary hypertension. Circ Res. 2006;98:209–217. doi: 10.1161/01.RES.0000200180.01710.e6. [DOI] [PubMed] [Google Scholar]
- 141.Long L, et al. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat Med. 2015 doi: 10.1038/nm.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Drake KM, Dunmore BJ, McNelly LN, Morrell NW, Aldred MA. Correction of nonsense BMPR2 and SMAD9 mutations by ataluren in pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2013;49:403–409. doi: 10.1165/rcmb.2013-0100OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sobolewski A, et al. Failure of bone morphogenetic protein receptor trafficking in pulmonary arterial hypertension: potential for rescue. Hum Mol Genet. 2008;17:3180–3190. doi: 10.1093/hmg/ddn214. [DOI] [PubMed] [Google Scholar]
- 144.Reynolds AM, Holmes MD, Danilov SM, Reynolds PN. Targeted gene delivery of BMPR2 attenuates pulmonary hypertension. Eur Respir J. 2012;39:329–343. doi: 10.1183/09031936.00187310. [DOI] [PubMed] [Google Scholar]
- 145.Durrington HJ, et al. Identification of a lysosomal pathway regulating degradation of the bone morphogenetic protein receptor type II. J Biol Chem. 2010;285:37641–37649. doi: 10.1074/jbc.M110.132415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dunmore BJ, et al. The lysosomal inhibitor, chloroquine, increases cell surface BMPR-II levels and restores BMP9 signalling in endothelial cells harbouring BMPR-II mutations. Hum Mol Genet. 2013;22:3667–3679. doi: 10.1093/hmg/ddt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Long L, et al. Chloroquine prevents progression of experimental pulmonary hypertension via inhibition of autophagy and lysosomal bone morphogenetic protein type II receptor degradation. Circ Res. 2013;112:1159–1170. doi: 10.1161/CIRCRESAHA.111.300483. [DOI] [PubMed] [Google Scholar]
- 148.Ryan JJ. Chloroquine in pulmonary arterial hypertension: a new role for an old drug? Circ Cardiovasc Genet. 2013;6:310–311. doi: 10.1161/CIRCGENETICS.113.000214. [DOI] [PubMed] [Google Scholar]
- 149.Spiekerkoetter E, et al. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. The Journal of clinical investigation. 2013;123:3600–3613. doi: 10.1172/JCI65592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Spiekerkoetter E, et al. Low-Dose FK506 (Tacrolimus) in End-Stage Pulmonary Arterial Hypertension. American journal of respiratory and critical care medicine. 2015;192:254–257. doi: 10.1164/rccm.201411-2061LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shovlin CL, Letarte M. Hereditary haemorrhagic telangiectasia and pulmonary arteriovenous malformations: issues in clinical management and review of pathogenic mechanisms. Thorax. 1999;54:714–729. doi: 10.1136/thx.54.8.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.McDonald J, Bayrak-Toydemir P, Pyeritz RE. Hereditary hemorrhagic telangiectasia: an overview of diagnosis, management, and pathogenesis. Genet Med. 2011;13:607–616. doi: 10.1097/GIM.0b013e3182136d32. [DOI] [PubMed] [Google Scholar]
- 153.Sorensen LK, Brooke BS, Li DY, Urness LD. Loss of distinct arterial and venous boundaries in mice lacking endoglin, a vascular-specific TGFbeta coreceptor. Developmental biology. 2003;261:235–250. doi: 10.1016/s0012-1606(03)00158-1. [DOI] [PubMed] [Google Scholar]
- 154.Arthur HM, et al. Endoglin, an ancillary TGFbeta receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Developmental biology. 2000;217:42–53. doi: 10.1006/dbio.1999.9534. [DOI] [PubMed] [Google Scholar]
- 155.Urness LD, Sorensen LK, Li DY. Arteriovenous malformations in mice lacking activin receptor-like kinase-1. Nat Genet. 2000;26:328–331. doi: 10.1038/81634. [DOI] [PubMed] [Google Scholar]
- 156.Oh SP, et al. Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci U S A. 2000;97:2626–2631. doi: 10.1073/pnas.97.6.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Johnson DW, et al. A second locus for hereditary hemorrhagic telangiectasia maps to chromosome 12. Genome Res. 1995;5:21–28. doi: 10.1101/gr.5.1.21. [DOI] [PubMed] [Google Scholar]
- 158.Abdalla SA, et al. Primary pulmonary hypertension in families with hereditary haemorrhagic telangiectasia. Eur Respir J. 2004;23:373–377. doi: 10.1183/09031936.04.00085504. [DOI] [PubMed] [Google Scholar]
- 159.Gallione C, et al. Overlapping spectra of SMAD4 mutations in juvenile polyposis (JP) and JP-HHT syndrome. Am J Med Genet A. 2010;152A:333–339. doi: 10.1002/ajmg.a.33206. [DOI] [PubMed] [Google Scholar]
- 160.Wooderchak-Donahue WL, et al. BMP9 mutations cause a vascular-anomaly syndrome with phenotypic overlap with hereditary hemorrhagic telangiectasia. Am J Hum Genet. 2013;93:530–537. doi: 10.1016/j.ajhg.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hao J, et al. In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem Biol. 2010;5:245–253. doi: 10.1021/cb9002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Azhar M, et al. Transforming growth factor Beta2 is required for valve remodeling during heart development. Developmental dynamics : an official publication of the American Association of Anatomists. 2011;240:2127–2141. doi: 10.1002/dvdy.22702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lebrin F, et al. Thalidomide stimulates vessel maturation and reduces epistaxis in individuals with hereditary hemorrhagic telangiectasia. Nat Med. 2010;16:420–428. doi: 10.1038/nm.2131. [DOI] [PubMed] [Google Scholar]
- 164.Han C, et al. VEGF neutralization can prevent and normalize arteriovenous malformations in an animal model for hereditary hemorrhagic telangiectasia 2. Angiogenesis. 2014;17:823–830. doi: 10.1007/s10456-014-9436-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bose P, Holter JL, Selby GB. Bevacizumab in hereditary hemorrhagic telangiectasia. N Engl J Med. 2009;360:2143–2144. doi: 10.1056/NEJMc0901421. [DOI] [PubMed] [Google Scholar]
- 166.Bauditz J, Lochs H, Voderholzer W. Macroscopic appearance of intestinal angiodysplasias under antiangiogenic treatment with thalidomide. Endoscopy. 2006;38:1036–1039. doi: 10.1055/s-2006-944829. [DOI] [PubMed] [Google Scholar]
- 167.Bauditz J, Lochs H. Angiogenesis and vascular malformations: antiangiogenic drugs for treatment of gastrointestinal bleeding. World J Gastroenterol. 2007;13:5979–5984. doi: 10.3748/wjg.v13.45.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chen W, Young WL, Su H. Induction of brain arteriovenous malformation in the adult mouse. Methods Mol Biol. 2014;1135:309–316. doi: 10.1007/978-1-4939-0320-7_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dupuis-Girod S, et al. Bevacizumab in patients with hereditary hemorrhagic telangiectasia and severe hepatic vascular malformations and high cardiac output. Jama. 2012;307:948–955. doi: 10.1001/jama.2012.250. [DOI] [PubMed] [Google Scholar]
- 170.Azzopardi N, et al. Dose - response relationship of bevacizumab in hereditary hemorrhagic telangiectasia. mAbs. 2015;7:630–637. doi: 10.1080/19420862.2015.1022693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Park SO, et al. Real-time imaging of de novo arteriovenous malformation in a mouse model of hereditary hemorrhagic telangiectasia. The Journal of clinical investigation. 2009;119:3487–3496. doi: 10.1172/JCI39482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bostrom K, et al. Bone morphogenetic protein expression in human atherosclerotic lesions. The Journal of clinical investigation. 1993;91:1800–1809. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Saeed O, et al. Pharmacological suppression of hepcidin increases macrophage cholesterol efflux and reduces foam cell formation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:299–307. doi: 10.1161/ATVBAHA.111.240101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sucosky P, Balachandran K, Elhammali A, Jo H, Yoganathan AP. Altered shear stress stimulates upregulation of endothelial VCAM-1 and ICAM-1 in a BMP-4- and TGF-beta1-dependent pathway. Arterioscler Thromb Vasc Biol. 2009;29:254–260. doi: 10.1161/ATVBAHA.108.176347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang L, et al. The bone morphogenetic protein-hepcidin axis as a therapeutic target in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:112–119. doi: 10.1002/ibd.21675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sun B, et al. Bone morphogenetic protein-4 contributes to the down-regulation of Kv4.3 K+ channels in pathological cardiac hypertrophy. Biochem Biophys Res Commun. 2013;436:591–594. doi: 10.1016/j.bbrc.2013.05.113. [DOI] [PubMed] [Google Scholar]
- 177.Sun B, et al. Bone morphogenetic protein-4 mediates cardiac hypertrophy, apoptosis, and fibrosis in experimentally pathological cardiac hypertrophy. Hypertension. 2013;61:352–360. doi: 10.1161/HYPERTENSIONAHA.111.00562. [DOI] [PubMed] [Google Scholar]
- 178.Kapur NK, et al. Reduced endoglin activity limits cardiac fibrosis and improves survival in heart failure. Circulation. 2012;125:2728–2738. doi: 10.1161/CIRCULATIONAHA.111.080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kapur NK, et al. Reducing endoglin activity limits calcineurin and TRPC-6 expression and improves survival in a mouse model of right ventricular pressure overload. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.000965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Castonguay R, et al. Soluble endoglin specifically binds bone morphogenetic proteins 9 and 10 via its orphan domain, inhibits blood vessel formation, and suppresses tumor growth. J Biol Chem. 2011;286:30034–30046. doi: 10.1074/jbc.M111.260133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Babitt JL, et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006;38:531–539. doi: 10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- 182.Nemeth E, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 183.Kim A, et al. A mouse model of anemia of inflammation: complex pathogenesis with partial dependence on hepcidin. Blood. 2014;123:1129–1136. doi: 10.1182/blood-2013-08-521419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ganz T. Hepcidin and its role in regulating systemic iron metabolism. Hematology Am Soc Hematol Educ Program. 2006:29–35. doi: 10.1182/asheducation-2006.1.29. [DOI] [PubMed] [Google Scholar]
- 185.Kaiafa G, et al. Is anemia a new cardiovascular risk factor? Int J Cardiol. 2015;186:117–124. doi: 10.1016/j.ijcard.2015.03.159. [DOI] [PubMed] [Google Scholar]
- 186.Steinbicker AU, et al. Inhibition of bone morphogenetic protein signaling attenuates anemia associated with inflammation. Blood. 2011;117:4915–4923. doi: 10.1182/blood-2010-10-313064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Theurl I, et al. Pharmacologic inhibition of hepcidin expression reverses anemia of chronic inflammation in rats. Blood. 2011;118:4977–4984. doi: 10.1182/blood-2011-03-345066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mayeur C, et al. Oral administration of a bone morphogenetic protein type I receptor inhibitor prevents the development of anemia of inflammation. Haematologica. 2015;100:e68–e71. doi: 10.3324/haematol.2014.111484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Beppu H, et al. BMP type II receptor is required for gastrulation and early development of mouse embryos. Developmental biology. 2000;221:249–258. doi: 10.1006/dbio.2000.9670. [DOI] [PubMed] [Google Scholar]
- 190.Beppu H, et al. BMPR-II heterozygous mice have mild pulmonary hypertension and an impaired pulmonary vascular remodeling response to prolonged hypoxia. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1241–L1247. doi: 10.1152/ajplung.00239.2004. [DOI] [PubMed] [Google Scholar]
- 191.Delot EC, Bahamonde ME, Zhao M, Lyons KM. BMP signaling is required for septation of the outflow tract of the mammalian heart. Development. 2003;130:209–220. doi: 10.1242/dev.00181. [DOI] [PubMed] [Google Scholar]
- 192.Frank DB, et al. Increased susceptibility to hypoxic pulmonary hypertension in Bmpr2 mutant mice is associated with endothelial dysfunction in the pulmonary vasculature. Am J Physiol Lung Cell Mol Physiol. 2008;294:L98–L109. doi: 10.1152/ajplung.00034.2007. [DOI] [PubMed] [Google Scholar]
- 193.Roberts KE, et al. BMPR2 mutations in pulmonary arterial hypertension with congenital heart disease. Eur Respir J. 2004;24:371–374. doi: 10.1183/09031936.04.00018604. [DOI] [PubMed] [Google Scholar]
- 194.Lane KB, et al. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. The International PPH Consortium. Nat Genet. 2000;26:81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 195.Johnson JA, et al. Cytoskeletal defects in Bmpr2-associated pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2012;302:L474–L484. doi: 10.1152/ajplung.00202.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.West J, et al. Mice expressing BMPR2R899X transgene in smooth muscle develop pulmonary vascular lesions. Am J Physiol Lung Cell Mol Physiol. 2008;295:L744–L755. doi: 10.1152/ajplung.90255.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Srinivasan S, et al. A mouse model for hereditary hemorrhagic telangiectasia (HHT) type 2. Hum Mol Genet. 2003;12:473–482. doi: 10.1093/hmg/ddg050. [DOI] [PubMed] [Google Scholar]
- 198.Berg JN, et al. The activin receptor-like kinase 1 gene: genomic structure and mutations in hereditary hemorrhagic telangiectasia type 2. Am J Hum Genet. 1997;61:60–67. doi: 10.1086/513903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Harrison RE, et al. Molecular and functional analysis identifies ALK-1 as the predominant cause of pulmonary hypertension related to hereditary haemorrhagic telangiectasia. J Med Genet. 2003;40:865–871. doi: 10.1136/jmg.40.12.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li DY, et al. Defective angiogenesis in mice lacking endoglin. Science. 1999;284:1534–1537. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- 201.McAllister KA, et al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet. 1994;8:345–351. doi: 10.1038/ng1294-345. [DOI] [PubMed] [Google Scholar]
- 202.Levet S, et al. Bone morphogenetic protein 9 (BMP9) controls lymphatic vessel maturation and valve formation. Blood. 2013;122:598–607. doi: 10.1182/blood-2012-12-472142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Meynard D, et al. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet. 2009;41:478–481. doi: 10.1038/ng.320. [DOI] [PubMed] [Google Scholar]
- 204.Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–2986. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- 205.Jiao K, et al. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes & development. 2003;17:2362–2367. doi: 10.1101/gad.1124803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mishina Y, Suzuki A, Ueno N, Behringer RR. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes & development. 1995;9:3027–3037. doi: 10.1101/gad.9.24.3027. [DOI] [PubMed] [Google Scholar]
- 207.El-Bizri N, et al. SM22alpha-targeted deletion of bone morphogenetic protein receptor 1A in mice impairs cardiac and vascular development, and influences organogenesis. Development. 2008;135:2981–2991. doi: 10.1242/dev.017863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.El-Bizri N, et al. Smooth muscle protein 22alpha-mediated patchy deletion of Bmpr1a impairs cardiac contractility but protects against pulmonary vascular remodeling. Circ Res. 2008;102:380–388. doi: 10.1161/CIRCRESAHA.107.161059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Vanderpool RR, El-Bizri N, Rabinovitch M, Chesler NC. Patchy deletion of Bmpr1a potentiates proximal pulmonary artery remodeling in mice exposed to chronic hypoxia. Biomech Model Mechanobiol. 2013;12:33–42. doi: 10.1007/s10237-012-0379-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Howe JR, et al. Germline mutations of the gene encoding bone morphogenetic protein receptor 1A in juvenile polyposis. Nat Genet. 2001;28:184–187. doi: 10.1038/88919. [DOI] [PubMed] [Google Scholar]
- 211.Zhou XP, et al. Germline mutations in BMPR1A/ALK3 cause a subset of cases of juvenile polyposis syndrome and of Cowden and Bannayan-Riley-Ruvalcaba syndromes. Am J Hum Genet. 2001;69:704–711. doi: 10.1086/323703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Yi SE, Daluiski A, Pederson R, Rosen V, Lyons KM. The type I BMP receptor BMPRIB is required for chondrogenesis in the mouse limb. Development. 2000;127:621–630. doi: 10.1242/dev.127.3.621. [DOI] [PubMed] [Google Scholar]
- 213.Chida A, et al. Missense mutations of the BMPR1B (ALK6) gene in childhood idiopathic pulmonary arterial hypertension. Circ J. 2012;76:1501–1508. doi: 10.1253/circj.cj-11-1281. [DOI] [PubMed] [Google Scholar]
- 214.Helbing T, et al. BMP activity controlled by BMPER regulates the proinflammatory phenotype of endothelium. Blood. 2011;118:5040–5049. doi: 10.1182/blood-2011-03-339762. [DOI] [PubMed] [Google Scholar]
- 215.Cannon JE, Upton PD, Smith JC, Morrell NW. Intersegmental vessel formation in zebrafish: requirement for VEGF but not BMP signalling revealed by selective and non-selective BMP antagonists. Br J Pharmacol. 2010;161:140–149. doi: 10.1111/j.1476-5381.2010.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kajimoto H, et al. BMP type I receptor inhibition attenuates endothelial dysfunction in mice with chronic kidney disease. Kidney Int. 2015;87:128–136. doi: 10.1038/ki.2014.223. [DOI] [PubMed] [Google Scholar]
- 217.van Meeteren LA, et al. Anti-human activin receptor-like kinase 1 (ALK1) antibody attenuates bone morphogenetic protein 9 (BMP9)-induced ALK1 signaling and interferes with endothelial cell sprouting. The Journal of biological chemistry. 2012;287:18551–18561. doi: 10.1074/jbc.M111.338103. [DOI] [PMC free article] [PubMed] [Google Scholar]