Abstract
Study Objective
To investigate the outcomes of adolescent pregnancy.
Design
Retrospective cohort study from the Consortium on Safe Labor between 2002 and 2008.
Setting
12 clinical centers with 19 hospitals in the United States.
Participants
43,537 nulliparous women <25 years, including 1,189 younger adolescents (age ≤15.9), 14,703 older adolescents (age 16–19.9)], and 27,645 young adults (age 20–24.9).
Interventions
aOR with 95%CI were calculated, controlling for maternal characteristics and pregnancy complications (young adults as a reference group).
Main outcome Measure
Maternal, neonatal outcomes, cesarean indications, and length of labor.
Results
Younger adolescents had increased risk of maternal anemia (aOR=1.25; 95%CI=1.07–1.45), preterm delivery <37 weeks of gestation (aOR=1.36; 95%CI=1.14–1.62), postpartum hemorrhage (aOR=1.46; 95%CI=1.10–1.95), preeclampsia/HELLP syndrome (aOR=1.44; 95%CI= 1.17–1.77) but had decreased risk of cesarean delivery (aOR=0.49; 95%CI= 0.42–0.59), chorioamnionitis (aOR=0.63; 95%CI=0.47–0.84), and neonatal intensive care unit (NICU) admission (aOR=0.80; 95%CI=0.65–0.98). Older adolescents had increased risk of maternal anemia (aOR=1.15; 95%CI= 1.09–1.22), preterm delivery at <37 weeks of gestation (aOR=1.16; 95%CI=1.08–1.25), and blood transfusion (aOR=1.21; 95%CI=1.02–1.43), but had decreased risk of cesarean delivery (aOR=0.75; 95%CI= 0.71–0.79), chorioamnionitis (aOR=0.83; 95%CI=0.75–0.91), major perineal laceration (aOR=0.82; 95%CI= 0.71–0.95), and NICU admission (aOR=0.89; 95%CI=0.83–0.96). Older adolescents were less likely to have cesarean for failure to progress or cephalopelvic disproportion (aOR=0.89; 95%CI 0.81–0.98). For adolescents who entered spontaneous labor, second stage of labor was shorter (P<.01).
Conclusion
Adolescents were less likely to have cesarean delivery. Failure to progress or cephalopelvic disproportion were decreased in older adolescents. Adolescents who entered spontaneous labor had shorter second stage of labor.
Keywords: Cesarean delivery, length of labor, pregnancy in adolescence, pregnancy outcomes
Introduction
Adolescent pregnancy is defined as pregnancy occuring in women between the age 10–19 years.1 The United States has a higher rate of adolescent pregnancy than other developed countries, though it has been declining.2, 3 Adolescent pregnancy and delivery are not only associated with adverse pregnancy outcomes, but also associated with low school achievement, increased health care costs, and living in poverty.4, 5, 6 Previous studies have reported that adolescent pregnacy and delivery are associated with adverse maternal and neonatal outcomes, such as low birth weight, stillbirth, preterm delivery, maternal anemia, postpartum depression, eclampsia, maternal death and postnaonatal death.5, 7, 8, 9, 10, 11, 12, 13 However, there has been conflicting findings from previous studies especially in women aged ≤15 years, primarily due to small sample size, differences in medical services, women’s social backgrounds, and homogeneous racial/ethnic populations.12, 13
It has been suggested that due to immaturity of their pelvis, adolescent pregnancy is associated with increased risk of longer labor and cesarean delivery indicated for failure to progress or descent.7 However, many recent studies have found that adolescent mothers were more likely to have vaginal delivery.7, 11, 13, 14, 15 The indication of primary cesarean delivery in adolescent pregnancy is not well studied. Also, limited data exists regarding duration of labor in adolescent pregnancy.
The aim of our study was to investigate the maternal and neonatal outcomes as well as to explore the indication of primary cesarean delivery and length of labor in adolescent pregnancy in a large contemporaneous obstetric cohort.
Materials and Methods
The Consortium on Safe Labor (CSL) was a retrospective cohort study of all women delivering at 23 weeks of gestation or greater between 2002 and 2008 in 12 clinical centers with 19 hospitals across 9 American Congress of Obstetricians and Gynecologists (ACOG) US districts.16 Institutional Review Board (IRB) approval was obtained in all participating institutions. IRB approval also was obtained for the present analysis.
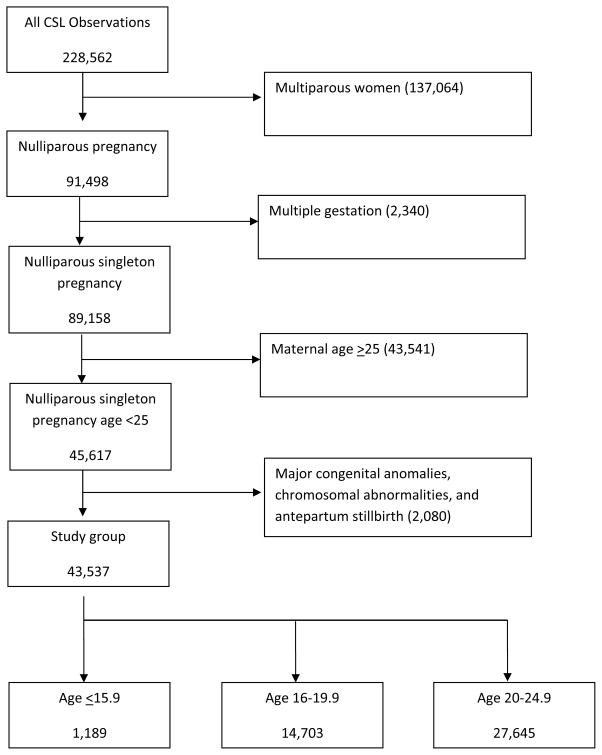
The CSL included a total of 228,562 deliveries with 233,736 newborns delivered at ≥ 23 weeks of gestation after excluding 106 deliveries due to errors in identification. Data from the electronic medical record (EMR) were abstracted and mapped to predefined categories at the data coordinating center. Although centers might have had different EMR’s, the Consortium on Safe Labor Study contracted with a data-coordinating center to review, standardize, and de-identify the data from different medical centers. The data were validated for four diagnoses including cesarean delivery for non-reassuring fetal heart rate tracing, asphyxia, and neonatal intensive care unit (NICU) admission due to a respiratory diagnosis and shoulder dystocia. The variables were highly concordant with the medical records (greater than 95% for 16 out of 20 variables and greater than or equal to 91.9% for all).16 We limited the current analysis to singleton gestations with maternal age less than 25 years old (Figure 1). Because most of the adolescents (85%) were nulliparous women14, we limited the analysis to nulliparous women. Since major fetal anomalies, chromosomal abnormalities, and antepartum stillbirth would change the labor and delivery management and tend to be associated with worse neonatal outcomes, we excluded these cases. The final analysis was limited to 43,537 deliveries (Figure 1).
Figure 1.
Women were categorized based on maternal age at the time of delivery (younger adolescent [age ≤15.9], older adolescent [age 16–19.9], and young adult [age 20–24.9, referent group]).
Maternal outcomes included mode of delivery (non-operative vaginal delivery, operative vaginal delivery, and cesarean delivery), maternal anemia, preterm premature rupture of membrane (PPROM), preterm delivery <37 weeks of gestation, <34 weeks of gestation, <28 weeks of gestation, placental abruption, chorioamnionitis, endometritis, postpartum hemorrhage, blood transfusion, preeclampsia/HELLP (hemolysis, elevated liver enzyme levels, and low platelet) syndrome, eclampsia, deep venous thrombosis/pulmonary embolism (DVT/PE), major perineal laceration, maternal intensive care unit (ICU) admission, and maternal death. PPROM was defined as premature rupture of the membranes at less than 37 weeks of gestation. Postpartum hemorrhage was defined as recorded in the medical record, as well as estimated blood loss greater than 500 mL for vaginal delivery and greater than 1,000 mL for cesarean delivery and supplemented with ICD-9 code. Major perineal laceration was defined as third or fourth degree perineal laceration. For analysis of major perineal lacerations, women who underwent cesarean delivery were excluded.
Neonatal outcomes included low birth weight (<2500 g), very low birth weight (<1500 g), birth trauma, shoulder dystocia, Apgar score at 5 min <7, neonatal intensive care unit (NICU) admission, NICU length of stay, intraventricular hemorrhage (IVH)/periventricular hemorrhage (PVH), asphyxia, hypoxic-ischemic encephalopathy (HIE), respiratory distress syndrome (RDS), neonatal sepsis, meningitis, pneumonia, neonatal seizure, and perinatal death. Perinatal death included intrapartum stillbirth and neonatal death.
Indications for cesarean delivery included failure to progress or cephalopelvic dispropotion, malpresentation, nonreassuring fetal heart tracing, elective, hypertensive disease, macrosomia, fetal indication or anomaly, failed induction, placenta previa or vasa previa, chorioamnionitis, human immunodeficiency virus (HIV) or active herpes simplex virus (HSV) lesions, placental abruption, and failed trial of forceps or vacuum. Duration of first stage of labor was defined as the time from admission to cervical dilation of 10 cm for women with a vaginal delivery. Duration of second stage of labor was defined as the time from cervical dilation of 10 cm to delivery. Total length of labor was defined as the time from admission to delivery.
Descriptive statistics were calculated for all study variables. Chi-square test, Fisher’s exact test, or Wilcoxon rank-sum test were performed to determine associations between outcomes and maternal age. P-value <.05 was considered significant. Binomial and multinomial logistic regression models were developed to investigate the associations between age group and maternal and neonatal outcomes, adjusted for race/ethnicity, marital status, insurance type, substance abuse, body mass index (BMI) at admission, hospital type, gestational age at delivery, and any diabetes (pregestational or gestational). Due to the retrospective design, some sites were missing data for specific variables; therefore, for logistic regression analysis, only sites reporting on the specific variable were included in the model. In a sub-group analysis of women with cesarean delivery, associations between age groups and indications of cesarean delivery were calculated. In a sub-group analysis of nulliparous women with vaginal delivery after spontaneous labor or induction of labor, duration of first stage of labor, second stage of labor, and total length of labor were evaluated by age group. For evaluation of length of labor the models were further adjusted for epidural and initial cervical exam. Adjusted odds ratios(aORs) with corresponding 95% confidence intervals (CIs) were generated based on Wald test with an alpha level of 0.05. All statistical analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC) and R 3.1.
Results
Maternal demographics and medical comorbidities of women are shown in Table 1. There were 1,189 younger adolescents, 14,703 older adolescents, and 27,645 young adults. Younger and older adolescents were more likely to be non-Hispanic black or Hispanic, single, of normal weight, and to have public insurance or self pay (P<.01). Older adolescents were more likely to smoke and use recreational drugs (P<.01). Young adults were more likely to have pre-existing and gestational diabetes (P<.01).
Table 1.
Demographic data by maternal age.
| Younger Adolescents | Older Adolescents | Young Adults | P-valuee | |
|---|---|---|---|---|
| Age ≤ 15.9 (n= 1,189) | Age 16–19.9 (n= 14,703) | Age 20–24.9 (n= 27,645) | ||
| Race/ethnicity | <.01 | |||
| Non-Hispanic white | 185 (15.56) | 4,225 (28.74) | 13,648 (49.37) | |
| Non-Hispanic black | 600 (50.46) | 5,819 (39.58) | 6,552 (23.70) | |
| Hispanic | 314 (26.41) | 3,448 (23.45) | 4,599 (16.64) | |
| Asian/Pacific islander | 4 (0.34) | 141 (0.96) | 839 (3.03) | |
| Other, multiracial, unknown | 86 (7.23) | 1,070 (7.28) | 2,007 (7.26) | |
| Marital status | <.01 | |||
| Married | 25 (2.10) | 1,482 (10.08) | 12,555 (45.42) | |
| Not married (divorced/widowed) | 1 (0.08) | 24 (0.16) | 156 (0.56) | |
| Not married (single) | 1,132 (95.21) | 12,743 (86.67) | 14,211 (51.41) | |
| Unknown | 31 (2.61) | 454 (3.09) | 723 (2.62) | |
| BMI (kg/m2) at admissiona | <.01 | |||
| Underweight | 83 (6.98) | 837 (5.69) | 1,340 (4.85) | |
| Normal | 474 (39.87) | 5,630 (38.29) | 10,897 (39.42) | |
| Overweight >=25, <30 | 144 (12.11) | 1,891 (12.86) | 3,757 (13.59) | |
| Obese >=30 | 55 (4.63) | 1,128 (7.67) | 2,854 (10.32) | |
| Unknown/missing | 433 (36.42) | 5,217 (35.48) | 8,797 (31.82) | |
| Health insurance | <.01 | |||
| Private | 304 (25.57) | 4,541 (30.88) | 14,037 (50.78) | |
| Public or self-pay | 746 (62.74) | 8,704 (59.20) | 11,237 (40.65) | |
| Other, unknown | 139 (11.69) | 1,458 (9.92) | 2,371 (8.58) | |
| Smoking | 50 (4.21) | 1,157 (7.87) | 1,837 (6.64) | <.01 |
| Alcohol | 12 (1.01) | 267 (1.82) | 491 (1.78) | 0.13 |
| Recreational drug use | 19 (1.80) | 370 (2.85) | 500 (1.98) | <.01 |
| Any maternal diseaseb | 186 (15.64) | 2,276 (15.48) | 23,223 (16.00) | 0.38 |
| Any Diabetes c | 12 (1.01) | 331 (2.25) | 986 (3.57) | <.01 |
| Preexisting diabetes | 4 (0.34) | 138 (0.94) | 315 (1.14) | <.01 |
| Gestational diabetes | 8 (0.67) | 193 (1.31) | 671 (2.43) | <.01 |
| Any hypertensive diseased | 168 (14.13) | 1,940 (13.19) | 3,512 (12.70) | 0.16 |
| Gestational hypertension | 73 (6.14) | 809 (5.50) | 1,501 (5.43) | 0.56 |
| Chronic hypertension | 25 (2.10) | 367 (2.50) | 612 (2.21) | 0.16 |
Numbers are n (%).
Maternal BMI (body mass index) was categorized as underweight for BMI <18.5 kg/m2, normal weight for BMI 18.5 to 24.9 kg/m2, overweight for BMI 25.0 to 29.9 kg/m2, obese for BMI 30.0 to 34.9 kg/m2, and morbidly obese for BMI >=35.0 kg/m2
Any maternal disease included any diabetes, any hypertensive disease, heart and renal disease.
Any diabetes included preexisting diabetes and gestational diabetes
Any hypertensive disease included chronic hypertension, gestational hypertension, preeclampsia, HELLP syndromeeclampsia, or unspecified hypertension.
P-value from Chi-square test
Maternal outcomes of women are shown in Table 2. Younger adolescents had decreased risks of cesarean delivery (aOR=0.49; 95%CI=0.42–0.59) and chorioamnionitis (aOR=0.63; 95%CI=0.47–0.84) and increased risks of maternal anemia (aOR=1.25; 95%CI=1.07–1.45), preterm delivery at <37 weeks of gestation (aOR=1.36; 95%CI=1.14–1.62), postpartum hemorrhage (aOR=1.46; 95%CI=1.10–1.95), and preeclampsia/HELLP syndrome (aOR=1.44; 95%CI= 1.17–1.77). Older adolescents had decreased risks of cesarean delivery (aOR=0.75; 95%CI=0.71–0.79), chorioamnionitis (aOR=0.83; 95%CI=0.75–0.91), and major perineal laceration (aOR=0.82; 95%CI=0.71–0.95) and increased risks of maternal anemia (aOR=1.15; 95%CI=1.09–1.22), preterm delivery at <37 weeks of gestation (aOR=1.16; 95%CI=1.08–1.25), and blood transfusion (aOR=1.21; 95%CI=1.02–1.43).
Table 2.
Maternal outcomes by maternal age.
| Young Adults (Referent) | Younger Adolescents | Adjusted OR (95%CI) | Older Adolescents | Adjusted OR (95%CI) | |
|---|---|---|---|---|---|
| Age 20–24.9 | Age ≤ 15.9 | Age 16–19.9 | |||
| n (%) | n (%) | n (%) | |||
| Non-operative vaginal delivery | 18,599 (67.30) | 925 (77.8) | -- | 10,618 (72.2) | -- |
| Operative vaginal deliverya | 2,498 (9.04) | 86 (7.23) | 1.05 (0.84, 1.33) | 1,038 (7.06) | 0.99 (0.91, 1.08) |
| Cesarean deliverya | 6,548 (23.69) | 178 (14.97) | 0.49 (0.42, 0.59) | 3,047 (20.72) | 0.75 (0.71, 0.79) |
| Maternal anemiaa | 4,035 (14.60) | 231 (19.43) | 1.25 (1.07, 1.45) | 2,614 (17.78) | 1.15 (1.09, 1.22) |
| PPROMa, b | 618 (2.24) | 35 (2.94) | 0.85 (0.57, 1.28) | 397 (2.70) | 0.93 (0.80, 1.09) |
| Preterm delivery <37 wkc | 2,622 (9.48) | 173 (14.55) | 1.36 (1.14, 1.62) | 1,779 (12.10) | 1.16 (1.08, 1.25) |
| Preterm delivery <34 wkc | 784 (2.84) | 54 (4.54) | 1.25 (0.94, 1.68) | 561 (3.82) | 1.13 (1.00, 1.27) |
| Preterm delivery <28 wkc | 159 (0.58) | 8 (0.67) | 0.78 (0.38, 1.60) | 107 (0.73) | 0.94 (0.73, 1.22) |
| Placental abruptiona | 262 (0.95) | 12 (1.01) | 0.97 (0.53, 1.79) | 148 (1.01) | 1.02 (0.82, 1.28) |
| Chorioamnionitisa | 1,324 (4.79) | 49 (4.12) | 0.63 (0.47, 0.84) | 727 (4.94) | 0.83 (0.75, 0.91) |
| Endometritisa | 300 (1.09) | 20 (1.68) | 1.08 (0.68, 1.72) | 226 (1.54) | 1.11 (0.92, 1.33) |
| Postpartum hemorrhagea | 898 (3.25) | 55 (4.63) | 1.46 (1.10, 1.95) | 524 (3.56) | 1.10 (0.98, 1.24) |
| Blood transfusiona | 753 (4.58) | 19 (2.71) | 1.45 (0.89, 2.35) | 253 (3.07) | 1.21 (1.02, 1.43) |
| Preeclampsia/HELLPd | 2,065 (7.47) | 115 (9.67) | 1.44 (1.17, 1.77) | 1,127 (7.67) | 1.06 (0.98, 1.16) |
| Eclampsiaa | 45 (0.16) | 6 (0.50) | 1.81 (0.75, 4.37) | 32 (0.22) | 0.94 (0.59, 1.51) |
| DVT or PE e | 57 (0.21) | 4 (0.34) | 1.38 (0.49, 3.90) | 23 (0.16) | 0.64 (0.40, 1.09) |
| Major perineal laceration (VD only)a | 956 (4.53) | 30 (2.97) | 1.00 (0.68, 1.46) | 325 (2.79) | 0.82 (0.71, 0.95) |
| Maternal intensive care unit admissiona | 106 (0.50) | 8 (0.80) | 1.21 (0.55, 2.66) | 95 (0.81) | 1.31 (0.96, 1.78) |
| Maternal deatha | 1 (<0.01) | 0 (0) | -- | 1 (0.01) | 2.18 (0.13, 35.74) |
Reference group: Maternal age 20–24.9 years.
Adjusted for maternal race/ethnicity, marital status, insurance type, substance abuse, BMI at admission, hospital type, gestational age at delivery, any diabetes, and any hypertensive disease.
PPROM, Preterm premature rupture of membrane: rupture of membranes before 37 weeks of gestation
Adjusted for maternal race/ethnicity, marital status, insurance type, substance abuse, BMI at admission, hospital type, any diabetes, and any hypertensive disease.
HELLP syndrome (hemolysis, elevated liver enzyme levels, and low platelet). Adjusted for maternal race/ethnicity, marital status, insurance type, substance abuse, BMI at admission, hospital type, gestational age at delivery, any diabetes, chronic hypertension, and gestational hypertension.
Deep venous thrombosis or pulmonary embolism
Neonatal outcomes of women are shown in Table 3. Younger adolescents and older adolescents had decreased a risk of NICU admission (aOR=0.80; 95%CI=0.65–0.98 and aOR=0.89; 95%CI=0.83–0.96, respectively).
Table 3.
Neonatal outcomes by maternal age.
| Young Adults (Referent) | Younger Adolescents | Adjusted OR (95%CI)a | Older Adolescents | Adjusted OR (95%CI)a | |
|---|---|---|---|---|---|
| Age 20–24.9 | Age ≤ 15.9 | Age 16–19.9 | |||
| n (%) | n (%) | n (%) | |||
| Low birth weightb | 2,074 (7.60) | 131 (11.10) | 0.82 (0.63, 1.08) | 1,507 (10.34) | 1.04 (0.94, 1.15) |
| Very low birth weightc | 335 (1.23) | 14 (1.19) | 0.46 (0.18, 1.16) | 241 (1.65) | 1.28 (0.92, 1.78) |
| Birth trauma | 722 (2.61) | 33 (2.78) | 1.10 (0.77, 1.58) | 422 (2.87) | 1.08 (0.94, 1.23) |
| Shoulder dystocia | 374 (1.35) | 13 (1.09) | 0.76 (0.43, 1.33) | 237 (1.61) | 1.07 (0.90, 1.28) |
| Apgar score at 5 min < 7 | 374 (1.36) | 20 (1.70) | 0.92 (0.57, 1.50) | 230 (1.57) | 0.96 (0.80, 1.15) |
| NICU d admission | 3,104 (11.23) | 144 (12.11) | 0.80 (0.65, 0.98) | 1,754 (11.93) | 0.89 (0.83, 0.96) |
| NICU length of stay (d), median (10th, 90th) | 5.92 (1, 34.77) | 7 (1, 39) | -- | 6 (1, 38.86) | -- |
| IVH/PVHe | 83 (0.30) | 4 (0.34) | 0.91 (0.29, 2.91) | 39 (0.27) | 0.83 (0.53, 1.30) |
| Asphyxia | 46 (0.17) | 2 (0.17) | 1.06 (0.25, 4.52) | 26 (0.18) | 1.09 (0.64, 1.83) |
| Hypoxic-ischemic encephalopathy | 1 (<0.01) | 0 (0) | -- | 1 (0.01) | 3.09 (0.13, 71.61) |
| Respiratory distress syndrome | 504 (1.82) | 23 (1.93) | 0.92 (0.54, 1.58) | 267 (1.82) | 0.90 (0.73, 1.09) |
| Neonatal sepsis | 561 (2.03) | 34 (2.86) | 1.04 (0.72, 1.52) | 363 (2.47) | 0.02 (0.88, 1.18) |
| Meningitis | 3 (0.01) | 0 (0) | -- | 2 (0.01) | 1.80 (0.24, 13.29) |
| Pneumonia | 154 (0.56) | 9 (0.76) | 1.17 (0.57, 2.37) | 64 (0.44) | 0.73 (0.54, 1.01) |
| Neonatal seizure | 24 (0.09) | 1 (0.08) | 0.98 (0.13, 7.53) | 16 (0.11) | 1.26 (0.64, 2.50) |
| Perinatal deathf | 63 (0.23) | 5 (0.42) | 1.42 (0.50, 1.30) | 36 (0.24) | 0.80 (0.50, 1.30) |
Reference group: Maternal age 20–24.
Numbers shown as n (%) unless otherwise specified.
Adjusted for maternal race/ethnicity, marital status, insurance type, substance abuse, BMI at admission, hospital type, gestational age at delivery, any diabetes, and any hypertensive disease.
Low birth weight defined as birth weight less than 2500 g
Very low birth weight defined as birth weight less than 1500 g.
Neonatal Intensive Care Unit (NICU)
Intraventricular hemorrhage (IVH)/periventricular hemorrhage (PVH)
Perinatal death included intrapartum stillbirth and neonatal death
Indications for cesarean delivery are shown in Table 4. The indications for cesarean delivery between younger adolescents and young adults were not statistically different, although there was a trend for fewer cesarean deliveries for failure to progress or cephalopelvic disproportion. Older adolescents had fewer cesarean deliveries performed for failure to progress or cephalopelvic disproportion (aOR=0.89; 95%CI=0.81–0.98) or placenta previa or vasa previa (aOR=0.39; 95%CI=0.11–0.80), but were more likely to undergo cesarean delivery for hypertensive disease (aOR=1.34; 95%CI=1.02–1.77).
Table 4.
Indications of cesarean delivery by maternal age.
| Young Adults (Referent) | Younger Adolescents | Adjusted OR (95%CI)a | Older Adolescents | Adjusted OR (95%CI)a | |
|---|---|---|---|---|---|
| Age 20–24.9 | Age ≤ 15.9 | Age 16–19.9 | |||
| n (%) | n (%) | n (%) | |||
| Failure to progress or cephalopelvic disproportion | 3,210 (49.02) | 67 (37.64) | 0.73 (0.53, 1.01) | 1,342 (44.04) | 0.89 (0.81, 0.98) |
| Malpresentation | 1,480 (22.60) | 39 (21.91) | 1.13 (0.78, 1.65) | 653 (21.43) | 1.06 (0.94, 1.18) |
| Nonreassuring fetal heart tracing | 1,721 (26.28) | 64 (35.96) | 1.24 (0.90, 1.71) | 915 (30.03) | 1.04 (0.94, 1.15) |
| Elective | 365 (5.57) | 16 (8.99) | 1.42 (0.82, 2.46) | 169 (5.55) | 0.883(0.722, 1.079) |
| Hypertensive disease | 187 (2.86) | 6 (3.37) | 1.25 (0.51, 3.07) | 108 (3.54) | 1.34 (1.02, 1.77) |
| Macrosomia | 136 (2.08) | 0 (0) | -- | 46 (1.51) | 0.82 (0.58, 1.18) |
| Fetal indication or anomaly | 83 (1.27) | 3 (1.69) | 1.53 (0.47, 4.98) | 40 (1.31) | 1.13 (0.76, 1.70) |
| Failed induction | 165 (2.52) | 3 (1.69) | 0.59 (0.19, 1.90) | 71 (2.33) | 0.79 (0.59, 1.06) |
| Placenta previa or vasa previa | 31 (0.47) | 0 (0) | -- | 5 (0.16) | 0.30 (0.11, 0.80) |
| Chorioamnionitis | 85 (1.30) | 0 (0) | -- | 37 (1.21) | 0.77 (0.52, 1.15) |
| HIVb, active herpes simplex virus lesions | 56 (0.86) | 3 (1.69) | 1.13 (0.35, 3.71) | 45 (1.48) | 1.17 (0.78, 1.75) |
| Placental abruption | 32 (0.49) | 2 (1.12) | 1.16 (0.25, 5.28) | 27 (0.89) | 1.36 (0.77, 2.41) |
| Failed trial of forceps or vacuum | 22 (0.34) | 0 (0) | -- | 14 (0.46) | 1.58 (0.76, 3.27) |
Because more than one indication could have been listed, indications may add up to more than 100%.
Adjusted for maternal race/ethnicity, marital status, insurance type, substance abuse, BMI at admission, hospital type, gestational age at delivery, and any diabetes.
Human immunodeficiency virus (HIV)
Vaginal birth after cesarean delivery
Table 5 details the length of labor in nulliparous women who underwent vaginal delivery. Younger adolescents with spontaneous onset of labor had shorter durations for the first and second stages of labor (P<.01). After adjusting for confounders, only the duration of the second stage of labor was shorter in younger and older adolescents. For women who underwent labor induction, a shorter first stage and total length of labor were noted in young adults and a shorter second stage of labor was observed among younger adolescents (P<.01). None of these differences were statistically significant after adjusting for confounders (P>.05).
Table 5.
Length of labor for women with a vaginal delivery by maternal age.
| Age ≤ 15.9 | Age 16–19.9 | Age 20–24.9 | P-value | |
|---|---|---|---|---|
| Spontaneous labor (n=21,656) | ||||
| Duration of first stage of labor (hrs), median (10%, 90%) | 6.81 (2.25, 16.25) | 7.42 (2.59, 15.66) | 7.21 (2.55, 14.97) | <.01a |
| Less than 5 hrs, n (%) | 180 (32.26) | 1,718 (27.80) | 3,313 (29.28) | 0.30b |
| 5 to 12 hrs, n (%) | 267 (47.85) | 3,146 (50.91) | 5,887 (52.02) | |
| More than 12 hrs, n (%) | 111 (19.89) | 1,316 (21.29) | 2,116 (18.70) | |
| Duration of second stage of labor (min), median (10%, 90%) | 41 (11, 150) | 45 (12, 140) | 55 (15, 154) | <.01a |
| Less than 60 min, n (%) | 370 (64.35) | 3,853 (60.55) | 6,167 (53.32) | <.01b |
| 60 to 119 min, n (%) | 113 (19.56) | 1,614 (25.37) | 3,307 (28.59) | |
| 120 to 179 min, n (%) | 57 (9.91) | 547 (8.60) | 1,337 (11.56) | |
| 180 or more, n (%) | 35 (6.09) | 349 (5.48) | 754 (6.52) | |
| Total length of labor (hrs), median (10%, 90%) | 8.02 (2.58, 17.43) | 8.40 (3.08, 16.95) | 8.30 (3.03, 16.38) | 0.05a |
|
| ||||
| Induction of labor (n=12,108) | ||||
| Duration of first stage of labor (hrs), median (10%, 90%) | 12.97 (6.48, 28.20) | 13.70 (6.35, 28.70) | 12.50 (6.03, 27.38) | <.01a |
| Less than 5 hrs, n (%) | 11 (4.42) | 152 (4.70) | 335 (5.27) | 0.31b |
| 5 to 12 hrs, n (%) | 103 (41.37) | 1,197 (37.05) | 2,669 (41.98) | |
| More than 12 hrs, n (%) | 135 (54.22) | 1,882 (58.25) | 3,354 (52.75) | |
| Duration of second stage of labor (min), median (10%, 90%) | 43.5 (13, 145) | 46 (13, 141) | 53 (14, 151) | <.01a |
| Less than 60 min, n (%) | 158 (62.70) | 1,979 (60.26) | 3,503 (54.56) | 0.83b |
| 60 to 119 min, n (%) | 55 (21.83) | 832 (25.33) | 1,851 (28.83) | |
| 120 to 179 min, n (%) | 22 (8.73) | 291 (8.86) | 671 (10.45) | |
| 180 or more, n (%) | 17 (6.75) | 182 (5.54) | 396 (6.17) | |
| Total length of labor (hrs), median (10%, 90%) | 14.37 (7.52, 29.15) | 14.80 (7.30, 29.28) | 13.98 (7.13, 28.4) | <.01a |
Duration of first stage of labor was defined as the time from admission to cervical dilation 10 cm.
Duration of second stage of labor was defined as the time from cervical dilation 10 cm to delivery.
Total length of labor was defined as the time from admission to delivery.
Kruskal-Wallis Test
Wald Chi-Square test from multinomial logistic regression model (P-value adjusted by maternal race/ethnicity, marital status, insurance type, substance abuse, BMI at admission, hospital type, gestational age at delivery, epidural, any diabetes, any hypertensive disease, and initial cervical exam).
Discussion
As reported previously, we found that younger and older adolescents had higher risks for preterm delivery at <37 weeks of gestation and maternal anemia, but lower risk for cesarean delivery. Furthermore, younger and older adolescents were more likely to be single and to have public insurance or self pay compared to young adults. Risks of adverse neonatal outcomes were not higher among younger and older adolescents. Adolescents had a lower risk of cesarean delivery for failure to progress or cephalopelvic disproportion although it was only significant in older adolescents. For adolescents who entered spontaneous labor, a shorter second stage of labor was noted. These later two findings had not been previously reported and may impact intrapartum management of the adolescent.
The findings of our study are consistent with those of previous studies describing that adolescents are more likely to deliver vaginally7, 11, 13, 14, 15 even though it has been suggested that an immature structure to the pelvis risks higher rates of failure to progress or cephalopelvic disproportion.17 In our study, adolescents had a lower risk of cesarean for failure to progress or cephalopelvic discproportion, although only reaching statistical significance for the older adolescent group. It is important to note that mode of delivery can be influenced by the practitionor policy and maternal request thus our finding might be different from older studies. In addition, even after controlling for confounders, we found that adolescents had a shorter duration of second stage of labor compared to young adults.
Published studies report contradictory risks in evaluating operative vaginal delivery in adolescents. The studies by de Vienne et al. and Torvie et al. reported lower risks for operative vaginal delivery, whereas that by Konje et al. reported a 2-fold increase in the rate of forceps delivery.13, 15, 18 Our study did not demonstrate an increased risk of operative vaginal delivery in adolescents.
Consistent with other studies, we found significantly higher risks of maternal anemia and preterm delivery before 37 weeks in both younger and older adolescents. 7, 8, 9, 11, 12, 13 Also consistent with other studies, younger adolescents had higer risk of preeclampsia/HELLP syndrome.7, 13 However, we did not find the increased risks of endometritis, and eclampsia as shown in previous studies. 7, 11, 13 In addition, we found a decreased risk of chorioamnionitis consistent with previous study.13 Published authors have suggested these adverse outcomes are the result of biologic immaturity or socioeconomic factors such as nonwhite race, public insurance or self pay, and unmarried status as well as concomitant pregnancy on nutritional demands of a still growing mother.7,14, 19, 20, 21, 22 In our study, after adjusting for race/ethnicity, marital status, and insurance type, adolescents had increased risks of adverse maternal outcomes (maternal anemia, preterm delivery <37 weeks of gestation, postpartum hemorrhage and blood transfusion) but not neonatal outcomes. The reason for these adverse outcomes remains unclear but these may be inherently due to biologic immaturity rather than socioeconomic factors as our analysis adjusted for socioeconomic factors.
Previous studies have described nulliparity, episiotomy, operative delivery, Asian or Pacific Islander, birth weight >3500 g, and longer second stage of labor as risk factors for major perineal laceration.23, 24 There are conflicting data regarding the risk of major perineal lacerations in adolescents.13, 25 A previous study of 461 women aged ≤21 found an increased risk of any perineal laceration compared to women aged 31–35 and 36–40 (P<.01).25 Another study from 2007, women aged 11–14 did not demonstrate a significant difference in the incidence of third or fourth degree lacerations compared to women aged 20–24.13 In our study, older adolescents had a lower risk of major perineal laceration even after adjusting for confounders. Early pregnancy has been suggested to be the reason for obstetric fistula in developing world.26 However, since our study revealed a decreased risk of major perineal laceration in older adlescents, obstetric fistula in this setting may be due to poor access to medical care.
Limitations of our study include the retrospective nature of analysis and variability of definitions for indications of cesarean delivery across clinical sites. Also, since the CSL was conducted between 2002 and 2008, there might be some differences in medical care, access to prenatal care, and labor and delivery management. The major strength of this study is the large cohort with clinical data from a contemporary U.S. population with the ability to study wide variety of outcomes and to adjust for a number of confounding factors. Analysis of this large cohort supports the study of rare outcomes such as deep venous thrombosis/pulmonary embolism, maternal ICU admission, maternal death, neonatal seizure, and neonatal death.
In summary, younger and older adolescents were more likely to have vaginal delivery. Younger and older adolescents had higher risks of maternal anemia and preterm delivery <37 weeks of gestation, but neonatal outcomes were comparable to those of young adults. Adolescents had a lower risk of cesarean delivery for failure to progress or cephalopelvic discproportion, although only reaching statistical significance for the older adolescent group. Cesarean section in adolescencence places the patient at risk for future cesarean sections and placental complications. Thus elective cesarean delivery should be avoided in adolescents as our data demonstrates that they are more likely to have a succesful vaginal delivery despite previous concerns about an immature pelvic structure. This information is helpful when providing adolescents prenatal care and labor management to optimize both maternal and neonatal outcomes.
Acknowledgments
The Consortium on Safe Labor was funded by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, through Contract No. HHSN267200603425C. Institutions involved in the Consortium include, in alphabetical order: Baystate Medical Center, Springfield, MA; Cedars-Sinai Medical Center Burnes Allen Research Center, Los Angeles, CA; Christiana Care Health System, Newark, DE; Georgetown University Hospital, MedStar Health, Washington, DC; Indiana University Clarian Health, Indianapolis, IN; Intermountain Healthcare and the University of Utah, Salt Lake City, Utah; Maimonides Medical Center, Brooklyn, NY; MetroHealth Medical Center, Cleveland, OH.; Summa Health System, Akron City Hospital, Akron, OH; The EMMES Corporation, Rockville MD (Data Coordinating Center); University of Illinois at Chicago, Chicago, IL; University of Miami, Miami, FL; and University of Texas Health Science Center at Houston, Houston, Texas. The named authors alone are responsible for the views expressed in this manuscript, which does not necessarily represent the decisions or the stated policy of the NICHD.
Funding: This research was supported by the Intramural Research Program of the National Institutes of Health (NIH), Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The data included in this paper were obtained from the Consortium on Safe Labor, supported by the Intramural Research Program of the NIH, NICHD, through contract number HHSN267200603425C. This project was funded in part with Federal funds (Grant # UL1TR000101 previously UL1RR031975) from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH), through the Clinical and Translational Science Awards Program (CTSA), a trademark of DHHS, part of the Roadmap Initiative, “Re-Engineering the Clinical Research Enterprise.”
Footnotes
Institutions involved in the Consortium on Safe Labor are named in the Acknowledgments.
Conflict of interest statement: The authors report no conflicts of interest.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Paper presentation information
This research was presented at the NASPAG, Miami, FL (April 2012) as “Are Adolescent Pregnancies Associated with Adverse Outcomes?”.
References
- 1.World Health Organization (WHO) WHO guidelines on preventing early pregnancy and poor reproductive outcome among adolescents in developing countries. Geneva: WHO; 2011. [PubMed] [Google Scholar]
- 2.Martin JA, Hamilton EB, Osterman JK, et al. Centers for Disease Control and Prevention National Center for Health Statistics National Vital Statistics System. Births: final data for 2012. Natl Vital Stat Rep. 2013;62:1–67. [PubMed] [Google Scholar]
- 3.Darroch JE, Singh S, Frost JJ. Differences in teenage pregnancy rates among five developed countries: the roles of sexual activity and contraceptive use. Family Planning Perspectives. 2001;33(5):244–250. 281. [PubMed] [Google Scholar]
- 4.Maiden K, Gunter WD, Martin SS, et al. Teen mothers, unintended pregnancies, and costs across Delaware. Del Med J. 2014;86(4):109–16. [PubMed] [Google Scholar]
- 5.Kingston D, Heaman M, Fell D, et al. Comparison of adolescent, young adult, and adult women’s maternity expericences and practices. Pediatrics. 2012;129:e 1228–1237. doi: 10.1542/peds.2011-1447. [DOI] [PubMed] [Google Scholar]
- 6.Nord CW, Moore KA, Morrison DR, et al. Consequences of teen-age parenting. J Sch Health. 1992;62(7):310–8. doi: 10.1111/j.1746-1561.1992.tb01249.x. [DOI] [PubMed] [Google Scholar]
- 7.Ganchimeg T, Ota E, Morisaki N, et al. Pregnancy and child birth outcomes among adolescent mothers: a World Health Organization multicountry study. BJOG. 2014;121:40–48. doi: 10.1111/1471-0528.12630. [DOI] [PubMed] [Google Scholar]
- 8.Fraser AM, Brockert JE, Ward RH. Association of young maternal age with adverse reproductive outcomes. N Engl J Med. 1995;332:1113–7. doi: 10.1056/NEJM199504273321701. [DOI] [PubMed] [Google Scholar]
- 9.Olausson PO, Cnattingius S, Godenberg RL. Determinants of poor pregnancy outcomes among teenagers in Sweden. Obstet Gynecol. 1997;89:451–7. doi: 10.1016/s0029-7844(97)00009-4. [DOI] [PubMed] [Google Scholar]
- 10.Phipps MG, Blume JD, DeMonner SM. Young maternal age associated with increased risk of postneonatal death. Obstet Gynecol. 2002;100:481–6. doi: 10.1016/s0029-7844(02)02172-5. [DOI] [PubMed] [Google Scholar]
- 11.Jolly MC, Sebere MN, Harris J, et al. Obstetric risks of pregnancy in women less than 18 years old. Obstet Gynecol. 2000;96:962–6. doi: 10.1016/s0029-7844(00)01075-9. [DOI] [PubMed] [Google Scholar]
- 12.Malabarey OT, Balayla J, Klam SL, et al. Pregnancies in young adolescent mothers: a population-based study on 37 million births. J Pediatr Adolesc Gynecol. 2012;25(2):98–102. doi: 10.1016/j.jpag.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Torvie AJ, Callegari LS, Schiff MA, et al. Labor and delivery outcomes among young adolescents. Am J Obstet Gynecol. 2015;213 doi: 10.1016/j.ajog.2015.04.024. d.ed-x.ex. [DOI] [PubMed] [Google Scholar]
- 14.Timofeev J, Reddy UM, Huang CC, et al. Obstetric complications, neonatal morbidity, and indications for cesarean delivery by maternal age. Obstet Gynecol. 2013;122:1184–1195. doi: 10.1097/AOG.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Vienne CM, Creveuil C, Dreyfus M. Does young maternal age increase the risk of adverse obstetric, fetal and neonatal outcomes: a cohort study. Eur J Obstet Gynecol Reprod Biol. 2009 Dec;147(2):151–6. doi: 10.1016/j.ejogrb.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Troendle J, Reddy UM, et al. Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol. 2010;203:323. doi: 10.1016/j.ajog.2010.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moerman ML. Growth of the birth canal in adolescent girls. Am J Obstet Gynecol. 1982;143:528–32. doi: 10.1016/0002-9378(82)90542-7. [DOI] [PubMed] [Google Scholar]
- 18.Konje JC, Palmer A, Watson A, et al. Early teenage pregnancies in Hull. Br J Obstet Gynaecol. 1992;99:969–73. doi: 10.1111/j.1471-0528.1992.tb13699.x. [DOI] [PubMed] [Google Scholar]
- 19.Chen XK, Wen SW, Fleming N, et al. Teenage pregnancy and adverse birth outcomes: a large population based retrospective cohort study. Int J Epidemiol. 2007;36:368–73. doi: 10.1093/ije/dyl284. [DOI] [PubMed] [Google Scholar]
- 20.Conde-Agudelo A, Belizan JM, Lammers C. Maternal-perinatal morbidity and mortality associated with adolescent pregnancy in latin america: cross-sectional study. Am J Obstet Gynecol. 2005;192:342–9. doi: 10.1016/j.ajog.2004.10.593. [DOI] [PubMed] [Google Scholar]
- 21.Loto OM, Ezechi OC, Kalu BK, et al. Poor obstetric performance of teenagers: is it age- or quality of care-related? J Obstet Gynaecol. 2004;24(4):395–8. doi: 10.1080/01443610410001685529. [DOI] [PubMed] [Google Scholar]
- 22.Raatikainen K, Heiskanen N, Verkasalo PK, et al. Good outcome of teenage pregnancies in high-quality maternity care. Eur J Public Health. 2006;16:157–61. doi: 10.1093/eurpub/cki158. [DOI] [PubMed] [Google Scholar]
- 23.Lowder JL, Burrows LJ, Krohn MA, et al. Risk factors for primary and subsequent anal sphincter lacerations: a comparison of cohorts by parity and prior mode of delivery. Am J Obstet Gynecol. 2007;196:344 e1–5. doi: 10.1016/j.ajog.2006.10.893. [DOI] [PubMed] [Google Scholar]
- 24.Landy HJ, Laughon SK, Bailit JL, et al. Characteristics associated with severe perineal and cervical lacerations during vaginal delivery. Obstet Gynecol. 2011;117:627–35. doi: 10.1097/AOG.0b013e31820afaf2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aviram A, Raban O, Melamed N, et al. The association between young maternal age and pregnancy outcome. J Matern Fetal Neonatal Med. 2013;26:1554–8. doi: 10.3109/14767058.2013.794212. [DOI] [PubMed] [Google Scholar]
- 26.Chandra-Mouli V, Camacho AV, Michaud PA. WHO guidelines on preventing early pregnancy and poor reproductive outcomes among adolescents in developing countries. J Adolesc Health. 2013;52:517–22. doi: 10.1016/j.jadohealth.2013.03.002. [DOI] [PubMed] [Google Scholar]