Cytokines are small secreted proteins that bind to specific receptors and act in a primary autocrine or paracrine manner. Cytokines play important roles in the immune response, inflammation, and carcinogenesis. More importantly, many cytokines and their related products have been developed into biomedical drugs [1]. Therefore, the identification and characterization of novel potential cytokines is of great value for both basic research and clinical applications. To isolate novel potential cytokines, a platform based on immunogenomics has been established [2], and several secreted proteins with important functions, including CYTL1, have been found.
CYTL1 was first identified in CD34+ cells derived from bone marrow and cord blood [3]. It is a classical secreted protein of small molecular size. Previous studies have identified the close relationships between CYTL1 and inflammation as well as tumors [4]. At present, the functions of CYTL1 as a novel potential cytokine in the immune response, inflammation, and carcinogenesis remain largely unknown.
To analyze the expression of CYTL1, polyclonal antibodies against CYTL1 were generated by immunizing rabbits with three CYTL1 peptides (P1, P2, and P3, as shown in Supplementary Fig. S1A). To confirm the specificity of the three antibodies, the expression level of CYTL1 protein produced by human embryonic kidney 293T cells (HEK293T cells) was detected. Results showed that the antibodies against P1 and P3 could detect CYTL1 at a concentration of 10 μg/ml, and the affinity of the antibody against P1 was higher than that against P3 (Supplementary Fig. S1B). Furthermore, Supplementary Fig. S1C showed that the antibody against P1 was able to detect CYTL1 at a lower concentration of ∼5 μg/ml. We also compared this antibody with anti-His antibody to confirm the specificity of anti-CYTL1 antibodies and obtained the same specific band. CYTL1 protein has also been expressed and purified in a eukaryotic expression system for functional studies. Our results showed that CYTL1 with its native signal peptide was secreted at low levels in HEK293T cells (data not shown). Therefore, the expression and secretion levels of CYTL1 need to be increased for its function study.
Three main elements that can affect the expression and secretion of secreted proteins are the signal peptide, the expression vectors, and the host cells. First, a suitable signal peptide is one of the most important elements for the efficient expression and secretion of secreted proteins. Currently, many types of signal peptides are used to express recombinant protein, such as the mouse IgGκ signal peptide (IgGκSP), α-factor signal peptide, and erythropoietin signal peptide. Specifically, IgGκSP has been used in several eukaryotic expression vectors and adenoviral vectors [5].
CYTL1 contains a signal peptide with a cleavage site between amino acids 22 and 23, as shown in Supplementary Fig. S1A. So, the signal peptide of CYTL1 was replaced to increase the level of secreted CYTL1. IgGκSP (METDTLLLWVLLLWVPGSTG) was ligated to the N terminus of the cDNA fragment encoding mature CYTL1 using three-step polymerase chain reaction (PCR). In Step 1, the template vector pcDNA3.1/CYTL1-Myc-His(−)B was constructed in our laboratory. Forward primer FP1 and reverse primer RP1 were used for PCR with the following cycling conditions: 95°C for 2 min, followed by 30 cycles of denaturing at 95°C for 30 s, primer annealing at 55°C for 30 s, extension at 72°C for 1 min, and a final extension at 72°C for 10 min. DNA fragment F1 was obtained. Step 2 was similar to Step 1 but used the amplified DNA fragment F1 as a template, FP2 as the forward primer, and RP1 as the reverse primer. DNA fragment F2 was obtained. Step 3 was also similar to Step 1, but used the amplified DNA fragment F2 as a template, FP3 as the forward primer, and RP1 as the reverse primer. This fused fragment was subcloned into the pcDNA3.1-Myc-His(−)B vector (named pcDB-IgGκSP-CYTL1-6 × His). The expression vector pcDB-IgGκSP-CYTL1-6 × His was successfully constructed, as verified by DNA sequencing. The expression vector pcDNA3.1/CYTL1-His(−)B (named pcDB-CYTL1-6 × His) was also constructed using the vector pcDNA3.1/CYTL1-Myc-His(−)B as a template, forward primer FP4, reverse primer RP1, and the above-described methods. It differed from pcDB-IgGκ-CYTL1-6 × His only in the signal peptide (primers used in PCR were shown in Supplementary Table S1).
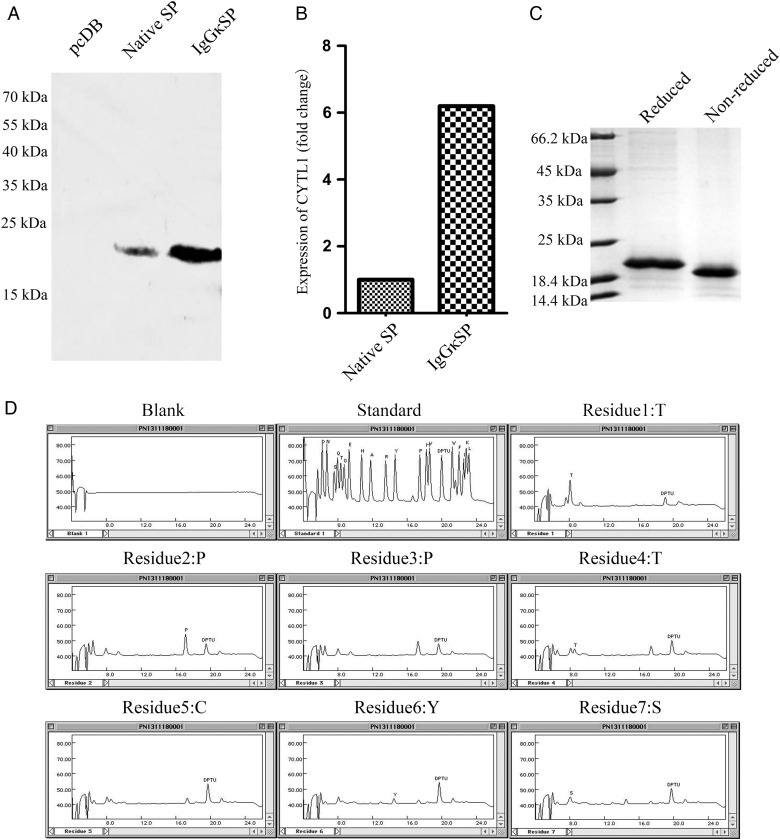
The expression vectors pcDB-CYTL1-6 × His and pcDB-IgGκSP-CYTL1-6 × His were individually transfected into HEK293T cells to evaluate the expression of CYTL1. As shown in Fig. 1A,B, CYTL1 containing its native signal peptide was secreted at low levels, whereas the expression of CYTL1 was increased by 6 folds in the pcDB-IgGκSP-CYTL1-6 × His group compared with the pcDB-CYTL1-6 × His group, as detected by western blot analysis using the antibody against CYTL1 P1. Approximately 0.25 mg of recombinant human CYTL1 protein with a His tag at the C-terminus was obtained from 1 l of supernatant from HEK293T cells transiently transfected with pcDB-IgGκSP-CYTL1-6 × His (Fig. 1C). Subsequently, the amino acid sequence of the N terminus was determined to confirm that IgGκSP was cleaved at the expected site. The N-terminus sequence of the purified CYTL1 protein was Thr-Pro-Pro-Thr-Cys-Tyr-Ser, which matched the mature CYTL1 amino acid sequence (Fig. 1D). The replacement of the signal peptide did not influence the cleavage site of the signal peptide in CYTL1.
Figure 1.
Replacement of the signal peptide increased the expression level of CYTL1 (A) HEK293T cells were transfected with pcDB-CYTL1-6 × His or pcDB-IgGκSP-CYTL1-6 × His using VigoFect. The supernatant was harvested 48 h later. The concentration of antibody against CYTL1 P1 used in the western blot analysis was 5 μg/ml. (B) The fold change in expression between pcDB-CYTL1-6 × His group and pcDB-IgGκSP-CYTL1-6 × His group in HEK293T cells. (C) Identification of the purified CYTL1 protein in the transient HEK293T expression system by SDS-PAGE. (D) The first seven amino acids of mature CYTL1 protein according to the amino acid sequencing.
Although CYTL1 was successfully expressed in HEK293T cells, the transient expression system has many disadvantages and the expression level of CYTL1 was low. To obtain a large quantity of CYTL1 protein for functional studies, the pMH3 vector, an efficient and stable expression vector, was used to stably express CYTL1 in CHO cells. CHO cells are a widely used mammalian cell expression system that is suitable for large-scale and high-density cultures. In fact, many pharmaceuticals have been produced in CHO cells [6]. Furthermore, the expression vector pMH3 is suitable to stably express proteins in CHO cells. Specifically, it contains a GC-rich noncoding DNA fragment, which is crucial for chromatin openness. This GC-rich fragment enables the exogenous gene of interest to be in a transcriptionally active state without affecting the integration of location, which sustains high-expression levels [7].
Therefore, the IgGκSP-CYTL1-6 × His fragment was subcloned into the pMH3 vector from pcDB-IgGκSP-CYTL1-6 × His to obtain the expression vector pMH3-IgGκSP-CYTL1-6 × His (pcDB-IgGκSP-CYTL1-6 × His as the template vector, FP5 and RP2 as the primers; Supplementary Table S1), which was verified by DNA sequencing.
The expression vector pMH3-IgGκ-CYTL1-6 × His was transfected into CHO cells using electroporation, and the successfully transfected cells were selected using G418 for 7 days. The cell clones were then transferred to a 96-well plate and cultured for another 7 days. After the second 7-day culture, the CYTL1 expression of each cell clone was detected using dot blot and western blot analysis. As shown in Supplementary Fig. S2A, the expression of CYTL1 was higher in the selected cell clones. The expression of CYTL1 was also detected in the supernatants of the eight cell clones using western blot analysis (Supplementary Fig. S2B). Based on these analyses, two cell clones (clone 3D and clone 5D) were selected for subsequent screening. Approximately 2.5 mg/l CYTL1 was produced from each of the two cell clones compared with the standard CYTL1 protein. These two cell clones were subject to 14-day culture just in the same manner as the first 14-day culture (7-day culture in medium with G418 and 7-day culture in a 96-well plate). After the fourth 7-day culture, the expression of CYTL1 was detected again in the same manner (Supplementary Fig. S2C,D). Here, the cell clones 2A, 3D, and 5B were selected to be individually seeded in 100 mm dishes at the same density to ensure that the expression level was not affected by other factors. A specific band in the culture supernatant of the three cell clones was detected by western blot analysis (Supplementary Fig. S2E). Finally, three cell clones (clone 2A, clone 3D, and clone 5B) that stably expressed high levels of CYTL1 were obtained after two rounds of selection.
The three high-expression clones were adapted for suspension cultures in CD fortiCHO medium to estimate the expression level to select the clone that expressed the highest levels of CYTL1 for large-scale protein expression. Results showed that cell clone 2A expressed the highest level of CYTL1, reaching 2 mg/l. Therefore, cell clone 2A was selected for large-scale cultures to purify and analyze CYTL1.
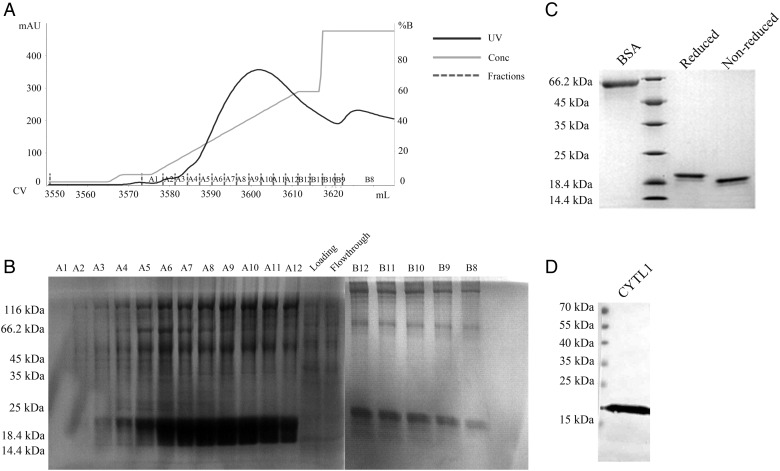
The culture of clone 2A was scaled up to a high-density culture in a 5-l flask and collect the resultant culture supernatant. The culture supernatant was dialyzed against the binding buffer and concentrated, and the protein was enriched using a Ni2+ affinity column (Fig. 2A). Fractions A3–A12 have a homogenous band at ∼19 kDa (Fig. 2B). Subsequently, the concentrated elution was desalted, and the resultant protein was further purified by gel filtration chromatography to yield ∼5 mg of CYTL1 from 5 l of culture supernatant, as indicated by bicinchoninic acid protein quantification. The expression of CYTL1 was stably increased by 4 folds in CHO cells compared with the transiently transfected HEK293T cells. The quantity and purity of protein were detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with bovine serum albumin as a standard (Fig. 2C). The polyclonal antibody against CYTL1 could recognize the band at 19 kDa; therefore, the protein was identified as CYTL1 (Fig. 2D). Furthermore, the purity exceeded 95%, as assessed by SEC-HPLC, and the endotoxin was ∼0.6 EU/mg in the purified CYTL1 protein, which met the requirements for further studies.
Figure 2.
Expression and purification of CYTL1 (A) Elution profile of CYTL1 on a Ni2+ affinity column [Milli absorbance unit (mAU) = A280, CV = column volume, %B = gradient elution]. (B) The protein was eluted by a linear gradient of 30–500 mM imidazole. Fractions (4 ml each) eluted from affinity purification were subject to 12.5% SDS-PAGE. (C) The purified CYTL1 protein exceeded 95% in purity and contained 0.6 EU/mg endotoxin. (D) The CYTL1 protein (100 ng) was separated on a 12.5% SDS-PAGE gel. The concentration of the anti-CYTL1 P1 antibody used in western blotting was 5 μg/ml.
The production of recombinant protein via genetic engineering required several steps, including the selection of signal peptides, expression vectors, and host cells, as well as the optimization of expression and purification conditions.
Most secretory proteins are initially synthesized in the cytoplasm as precursors containing 15–30 residues of additional peptides at their N terminals. These so-called signal peptides play an important role in the translocation of newly synthesized proteins across the membrane of the endoplasmic reticulum in eukaryotic cells. Specifically, signal peptides consist of three structurally and functionally distinct regions: a positively charged N-terminal region, a central hydrophobic core region, and a C-terminal region [8]. Because CYTL1 is a secretory protein, its protein expression was detected in the supernatant of HEK293T cells transiently transfected with pcDB-CYTL1-6 × His, but this level of secretion was low. Importantly, signal peptide sequences have some similarity in structure, function, and cleavage sites. However, because of a lack of common sequence information for different signal peptides, they are thought to mediate transport based on structural and physical properties, such as charge balance, hydrophobicity, and conformation, rather than strict sequence specificity. According to the signal peptide hypothesis, the transmembrane transportation of proteins mainly depends on the central hydrophobic core regions of signal peptides, which primarily consist of neutral amino acids [8,9]. Thus, the central hydrophobic core regions share common structures in spite of the differences between different secretory proteins. As such, replacing the signal peptide may increase the secretion of proteins secreted at low levels without changing the cleavage site. In this study, the signal peptide of CYTL1 was replaced with IgGκSP using three-step PCR, a process that increased the expression of CYTL1 in the supernatant of HEK293T cells by 6 folds. However, only 0.25 mg of CYTL1 protein was obtained from 1 l of supernatant from the transient HEK293T expression system, which indicated that the expression level of CYTL1 remained low in this expression system.
In the present study, IgGκSP could not increase the secretion of any protein. Specifically, we replaced the signal peptide of other proteins, but this replacement actually decreased the expression and secretion of various proteins, such as LYG1 (lysozyme G-like 1) (data not shown).
The expression system also significantly influences the expression and biological activity of recombinant proteins. To increase the expression level of CYTL1, CYTL1 was stably expressed in CHO cells using the pMH3 vector.
Mammalian cell lines are important host cells for the industrial production of pharmaceutical proteins due to their capacity to correctly fold, assemble, and posttranslationally modify proteins, which is not possible in prokaryotic, yeast, and insect cells. In particular, CHO cells are the most dependable host cells for industrial production of therapeutic proteins [6]. Thus, we used pMH3, a eukaryotic expression vector containing GC-rich elements that is suitable for the CHO expression system [7], to express CYTL1 in CHO cells. Using this system, we obtained 5 mg of CYTL1 protein from 5 l of supernatant, which suggested that the expression level of CYTL1 was increased by 4 folds compared with the expression level in the transient HEK293T expression system.
In summary, when the native signal peptide of CYTL1 was replaced by IgGκSP, the expression and secretion was increased by 6 folds. And CYTL1 was stably expressed in CHO cells, its expression was increased by 4 folds. Finally, 5 mg of CYTL1 protein was obtained from 5 l of supernatant from this stable CHO cell expression system with a purity exceeding 95% and a low endotoxin level of 0.6 EU/mg. We think that this system will be useful for future functional studies and the development of clinical applications of CYTL1.
Supplementary Data
Funding
This work was supported by the grants from the Program for the Innovation of New Drugs (2013ZX09103003-023) and the Peking University–National Taiwan University Cooperation Fund, China (BMU20120316).
Supplementary Material
References
- 1.Foster D, Parrish-Novak J, Fox B, Xu W. Cytokine-receptor pairing: accelerating discovery of cytokine function. Nat Rev Drug Discov 2004, 3: 160–170. [DOI] [PubMed] [Google Scholar]
- 2.Guo X, Zhang Y, Wang P, Li T, Fu W, Mo X, Shi T et al. VSTM1-v2, a novel soluble glycoprotein, promotes the differentiation and activation of Th17 cells. Cell Immunol 2012, 278: 136–142. [DOI] [PubMed] [Google Scholar]
- 3.Liu X, Rapp N, Deans R, Cheng L. Molecular cloning and chromosomal mapping of a candidate cytokine gene selectively expressed in human CD34+ cells. Genomics 2000, 65: 283–292. [DOI] [PubMed] [Google Scholar]
- 4.Jeon J, Oh H, Lee G, Ryu JH, Rhee J, Kim JH, Chung KH et al. Cytokine-like 1 knock-out mice (Cytl1−/–) show normal cartilage and bone development but exhibit augmented osteoarthritic cartilage destruction. J Biol Chem 2011, 286: 27206–27213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu WX, Chen HP, Yu K, Shen LX, Wang CY, Su SZ, Sui WJ et al. Gene therapy of malignant solid tumors by targeting erbB2 receptors and by activating T cells. Cancer Biother Radiopharm 2012, 27: 711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Omasa T, Onitsuka M, Kim WD. Cell engineering and cultivation of Chinese hamster ovary (CHO) cells. Curr Pharm Biotechnol 2010, 11: 233–240. [DOI] [PubMed] [Google Scholar]
- 7.Xie W, Li D, Zhang J, Li Z, Acheampong DO, He Y, Wang Y et al. Generation and characterization of a novel human IgG1 antibody against vascular endothelial growth factor receptor 2. Cancer Immunol Immunother 2014, 63: 877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsuchiya Y, Morioka K, Shirai J, Yokomizo Y, Yoshida K. Gene design of signal sequence for effective secretion of protein. Nucleic Acids Res Suppl 2003, 3: 261–262. [DOI] [PubMed] [Google Scholar]
- 9.von Heijne G. Life and death of a signal peptide. Nature 1998, 396: 111, 113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.