Abstract
Root respiration is a major contributor to terrestrial carbon flux. Many studies have shown root respiration to increase with an increase in root tissue nitrogen (N) concentration across species and study sites. Studies have also shown that both root respiration and root N concentration typically decrease with root age. The effects of added N may directly increase respiration of existing roots or may affect respiration by shifting the age structure of a root population by stimulating growth. To the best of our knowledge, no study has ever examined the effect of added N as a function of root age on root respiration. In this study, root respiration of 13-year-old Populus tremuloides Michx. trees grown in the field and 1-year-old P. tremuloides seedlings grown in containers was analyzed for the relative influence of root age and root N concentration independent of root age on root respiration. Field roots were first tracked using root windows and then sampled at known age. Nitrogen was either applied or not to small patches beneath the windows. In a pot experiment, each plant was grown with its root system split between two separate pots and N was applied at three different levels, either at the same or at different rates between pots. Root N concentration ranged between 1.4 and 1.7% in the field experiment and 1.8 and 2.6% in the seedling experiment. We found that addition of N increased root N concentration of only older roots in the field but of roots of all ages in the potted seedlings. In both experiments, the age-dependent decline in root respiration was largely consistent, and could be explained by a negative power function. Respiration decreased ∼50% by 3 weeks of age. Although root age was the dominant factor affecting respiration in both experiments, in the field experiment, root N also contributed to root respiration independent of root age. These results add further insight into respiratory responses of roots to N addition and mechanisms underlying the tissue N–respiration relationship.
Keywords: patchy N availability, root N concentration
Introduction
Soil respiration accounts for the majority of ecosystem respiration in temperate forests (Valentini et al. 2000, Law et al. 2002). Root respiration and heterotrophic soil respiration contribute approximately equally to soil CO2 efflux (Hanson et al. 2000, Högberg et al. 2001, Fahey et al. 2005, Ceccon et al. 2011). Higher nitrogen (N) deposition (Vitousek 1994, Sala et al. 2000) and higher soil-temperature-driven rates of soil organic matter mineralization (Melillo et al. 2002) may lead to higher N availability in soil. However, the results of N availability on soil respiration have been mixed. Increases in soil respiration following N addition are commonly observed in young plantations, where increasing N availability enhances photosynthesis and carbon allocation to belowground organs (Janssens et al. 2010). In contrast, long-term studies conducted in mature stands indicate an overall reduction in CO2 efflux with an increase of soil N availability (Burton et al. 2004, Janssens et al. 2010). These contrasting responses may be the result of a differential response of soil microbial respiration (heterotrophic) and root respiration to increasing soil N availability.
To better predict the influence of N addition on root respiration, a deeper understanding of the physiological responses of roots to increased N supply is needed. Increased N availability may increase tissue N, resulting in more actively growing meristems and more active synthesis and maintenance of storage and enzymatic proteins that stimulate respiration (Bloom et al. 1992, Bouma et al. 1996, Reich et al. 1998, 2008, Oren et al. 2001, Throop et al. 2004). Indeed, the strong influence of N supply on root respiration has led some researchers to partition the respiration of nongrowing roots into a maintenance and ion-uptake component (Veen 1980, Bouma et al. 1996). The tissue N–respiration relationship, however, may be potentially uncoupled under conditions of N saturation, where at least the woody roots of trees at high N supply have similar respiration but higher N concentrations than those under ambient supply (Burton et al. 2012).
We hypothesize that the influence of increased soil N availability on root respiration will depend on whether the whole root system or only a small portion of the root system is exposed. An increase of N to the whole plant typically reduces belowground biomass partitioning (Reich 2002), while localized N addition often stimulates root growth in the fertile patch (Hodge 2004). In addition, root proliferation responses are often influenced by the nutrient status of the plant, with plants being more responsive to a fertile patch if they are more nutrient deficient (Friend et al. 1990). Similar to root growth, responses of root respiration may be influenced both by the nutrient status of the plant and the spatial pattern of N addition.
When interpreting the relations of root respiration with root N concentration, variation in the fine root system should also be considered (McCormack et al. 2015). First-order roots (i.e., the most distal roots or root tips) have greater tissue N concentrations and faster respiration rates than higher order roots (Burton et al. 2012, Jia et al. 2013). Among the first-order fibrous roots, respiration and N concentration both can decline steeply with root age (Volder et al. 2005, 2009). Because localized N addition can stimulate root initiation and growth, roots of a younger age may be the direct cause of N-stimulated respiration, rather than a change in N concentration of the root tissues.
In this study, we used both 13-year-old trees growing in the field and 1-year-old potted seedlings of Populus tremuloides Michx. to examine the influence of exogenous N supply on the relationship between root tissue N and root respiration. Specifically, we tested (i) the relative role of root age and root N concentration as determinants of root respiration and (ii) whether a localized increase in N availability increases the respiration per unit root N concentration more than a whole root system increase in N supply. Collectively, this research was intended to help unravel the complex patterns of root respiratory responses to N addition.
Materials and methods
Field experiment
The effect of localized N availability on fine root respiration was studied in a common garden at the Russell E. Larson Experimental Station (PA, USA; 40°40′N, 78°02′W; 350 m above sea level) from June to September 2009. The garden contained 16 tree species in a completely randomized design with 8 blocks (McCormack et al. 2012, Adams et al. 2013). Soils were relatively fertile Hagerstown silt loam, well-drained, with a pH ranging from 6.1 to 6.5. Background soil solution N (ammonium + nitrate) in naturally occurring high N patches (highest 10% of samples) was ∼0.81 mM N (Adams et al. 2013). Each species was planted in groups of six trees in a double row of three trees each with a spacing of 3 m between trees within the row, 3 m between the double rows and 5 m spacing between the six tree plots. Populus tremuloides was chosen for this study because of its rapid growth and high root density. Trees were planted in 1996 and were 13 years old at the time of this study. On average, trees had a diameter at breast height of 20.5 cm (±4.7 cm, SD). Eight root boxes, one per block, were installed between two adjacent poplar trees, 90 cm from each trunk in the spring of 2009 (Comas et al. 2000, Zadworny and Eissenstat 2011, Adams and Eissenstat 2014). Each box had two windows on two opposite sides that allowed root samplings during the experiment. Windows were made of a thin transparent acetate film, which allowed for observing and tracking root growth and root age prior to collection. When not in use, the windows were kept covered with foam insulation panels (3 cm thick) to avoid light penetration and to minimize temperature fluctuations. In addition, the boxes were covered and kept closed by a wood panel. From the end of June until mid-September, new and existing roots were traced every week on the acetate using paint pens of different colors (Marvy DecoColor pens, Uchida of America Corp., Torrance, CA, USA) on different tracing dates, in order to keep track of root age. On 27 July and 16 September 2009, fine first- and second-order roots were sampled, by cutting the acetate film with a small pair of scissors. A total of 51 and 113 samples were collected on the first and second date, respectively.
From June on, ammonium nitrate (NH4NO3) was applied with a 2-week interval in a small soil area (0.12 m2, 60 × 20 cm, later referred to as N+) behind one window of each root box, distributing 10 l of a 2.15 mM NH4NO3 solution (1.72 g NH4NO3 dissolved in 10 l water), which represents ∼2.7-fold higher available N than naturally occurring high-N patches (see above). The other window acted as control (later referred to as N−) and received 10 l water. The N treatment was applied six times over a 12-week period.
On the first harvest date, after the N treatments had been applied three times, we sampled and measured respiration on combined first- and second-order roots of five age classes such as <2, 3–9, 10–16, 17–23 and 24–33 days using similar methods to those reported previously in grape (Comas et al. 2000). For simplicity of illustration, the age of roots for each age class is plotted using the midpoint of each class in the figures. On the second date, after N treatments had been applied six times, root respiration rate was measured on combined first- and second-order roots belonging to 12 age classes: 1–7, 8–14, 15–22, 23–29, 30–36, 37–42, 43–48, 49–50, 51–57, 58–64, 65–71 and 72–81 days. Each time, fine roots of the same age class, grown on the same window, were pooled together. The number of sampled roots in each age class ranged from 1 to 5. On average, samples were 3.4 mg (±3.1 mg) dry weight (n = 324). Once collected, they were immediately immersed in a buffer solution (1 mM CaSO4 + 5 mM MES buffer, pH 5.5 using KOH) and transported to the laboratory, where respiration was determined within 2 h from collection by oxygen (O2) consumption (nmol O2 g dry weight−1 s−1) at 20 °C, using a Clark-type O2 electrode (Hansatech Oxygraph, King’s Lynn, UK). Roots were then oven dried at 65 °C and their dry weight determined. Root N concentration was then determined with an elemental analyzer (Flash-EA 2000, Thermo Fisher Scientific, Waltham, MA, USA).
Greenhouse experiment
To assess how different levels of N, supplied either in a uniform or localized spatial pattern, affected fine root respiration, 1-year-old poplar seedlings (P. tremuloides) were grown in containers in a greenhouse from July until mid-October in Pennsylvania State University (University Park, PA, USA). Temperatures were controlled with evaporative cooling to not exceed 27 °C. No artificial lighting was used. Sixty-five plants were first grown for 1 month in small pots (1 l volume), then 25 uniform plants were selected and transplanted to larger containers (5.5 l) where the root system was split between two containers (Figure 1). In total, each plant had 11 l of substrate for root growth. The substrate used for the experiment was one-third (by volume) sieved soil (collected at the common garden, where the field experiment was conducted) and two-thirds fine sand. The plastic 5.5 l pots had a window covered by an acetate sheet, to allow tracking of fine root growth every 2 weeks. When roots were not tracked, windows were covered to prevent light penetration.
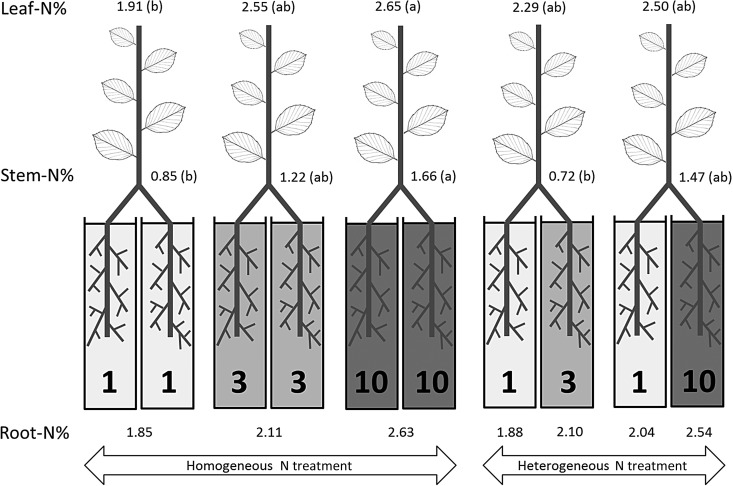
Figure 1.
Nitrogen concentration (% DW) in leaves, stems and roots as affected by N supply in the greenhouse experiment (the numbers 1, 3 and 10 refer to the three different levels of N supply). For leaf or stem N concentrations, different letters (in brackets) indicate significantly different values according to Tukey’s test (P = 0.05). Nutrient addition significantly affected root N concentration among the 1, 3 and 10 homogeneous treatments (P < 0.05) and in the heterogeneous treatments, 1 vs 10 (P < 0.05).
Three different levels of N, to cover a broad range of N-availability situations, were applied to the soil at weekly intervals at rates of 21, 63 or 210 mg N l−1, later referred to as N1, N3 and N10, respectively. These concentrations of N represent levels that we expected to represent mildly deficient to saturating conditions (i.e., 10–100% of standard Hoagland’s N). Nitrogen was applied as NH4NO3 dissolved in a solution containing all other macro- and micronutrients. Nitrogen was applied weekly either in a homogeneous way (both pots of each plant received the same N treatment) or in a heterogeneous way (different N levels were distributed between the two adjacent pots of each plant). In total, the experiment encompassed five treatments, three homogeneous (N1, N3 and N10) and two heterogeneous (N1¦N3 and N1¦N10), each replicated five times. Each time a plant received fertilization, enough solution was distributed to completely saturate the soil. During the remaining part of the week, plants received only water through drip irrigation.
Fine root sampling was performed on 14 October 2009 by collecting first- and second-order fine roots of the same age from each pot. Roots were pooled into eight root age classes: <4, 5–12, 13–19, 20–25, 26–56, 57–72, 73–89 and 90–100 days after first appearance on the window. Respiration of 161 samples was determined using the same methodology as in the field experiment. After root respiration was determined, root dry weight and N concentration were determined as previously described. After roots of known age were harvested from the windows, plants were destructively harvested and divided into shoots (including leaves) and roots, and each organ was oven dried and weighed and analyzed for N by elemental analysis (see above).
Statistical analyses
The effects of N supply on total N concentration and biomass of plant organs and on fine root respiration were assessed by one-way analysis of variance (Statgraphics Centurion XV, Warrenton, VA, USA) according to a randomized block design or a completely randomized design for field and greenhouse experiments, respectively. Mean separation was performed according to Tukey’s test at P = 0.05.
To find the best equations relating root N concentration or root respiration rate (as dependent variables) with root age (as the independent variable), separate data from the field and the greenhouse experiments (both variables or just the dependent variable) showed a linear relationship when plotted on a log scale, suggesting possibly a power function (y = a × xb) or an exponential function (y = a × ebx). For the root respiration vs root age data, a power function fit the data best (as determined by R2). For root N concentration vs root age for both field and greenhouse data, an exponential function fit the data best. To test whether N supply had an effect on the relationships between root N concentration with root age, or between root respiration data with root age, the 95% confidence intervals of the coefficients ‘a’ and ‘b’ of the power or exponential equations run for each experiment and each N supply level were analyzed (see Table S1 available as Supplementary Data at Tree Physiology Online). The analyses were carried out using the function nls of the stats package of the R language version 3.2.1 (R Development Core Team 2015). For each age class, the midpoint of each age range was calculated and used in the regression analysis.
Unlike the relationship of respiration or root N concentration with root age, the relationship of root respiration with root N concentration approximated a linear relationship; thus, a simple linear regression analysis was applied for each level of N supply, separately for field and greenhouse experiments. The 95% confidence intervals of slope and intercept values of linear regressions were first compared across different levels of N supply and type of experiment (field vs greenhouse) (data not shown), then, the entire dataset was used to assess the relationship between root N concentration and root respiration by simple linear regression analysis.
In order to test the relative role of root age and root N concentration as determinants of root respiration, linear models with root respiration (first linearized with log transformation) as the response variable, and root age (log transformed) and root N concentration as the continuous predictor variables, were built separately for field (second harvest) and greenhouse experiments and for the entire dataset. We then built multiple linear regression models including root age and root N concentration as well as their interaction. The procedure used, starting from the full model (two variables and their interaction), identifies the minimum adequate model by backward deletion procedure (discarding variables with a P > 0.05). For all simple and multiple regression analyses, we used the ‘lm’ function from the R statistical computing environment (R Development Core Team 2015). The relative importance of the significant terms was obtained applying the function ‘calc.relimp’ of the R package ‘relaimpo’ (Groemping 2006) using the default options.
Results
Effects of N supply and age on tissue N concentration
In the field experiment, where only a small portion of the root system was exposed to increased N supply, root N concentration was similar in the N− and N+ plots in the first sampling, but higher in the N+ treatment by the second sampling (Table 1). In the greenhouse experiment, when N was homogeneously supplied to the entire root system in the two adjacent pots, root N concentration progressively increased with an increase in N supplied in the fertilizing solution (Figure 1). Leaf and stem N concentrations were also affected by the level of N supply (Figure 1). When N was supplied at different levels to the two adjacent pots so that only a portion of the root system was exposed to elevated N supply (heterogeneous supply), the N concentration of roots exposed to the lowest N level (N1) was significantly (P < 0.05) lower than that of the highest N treatment (N10). However, the N1 treatment under heterogeneous supply was not significantly different from the N1 uniform.
Table 1.
Effects of the N supply on respiration and N concentration of first- and second-order roots of 13-year-old P. tremuloides in the field experiment (SE, standard error; n.s., not significant; ***P < 0.001).
| Treatment | First sampling date |
Second sampling date |
||||
|---|---|---|---|---|---|---|
| N− | N+ | P | N− | N+ | P | |
| Root age (days) | ||||||
| Mean | 15 | 17 | 45 | 46 | ||
| Root N concentration (%) | ||||||
| Mean | 1.66 | 1.71 | n.s. | 1.37 | 1.64 | *** |
| SE | 0.06 | 0.07 | 0.04 | 0.03 | ||
| Respiration per unit biomass (nmol O2 g−1 DW s−1) | ||||||
| Mean | 11.20 | 9.13 | n.s. | 5.70 | 5.92 | n.s. |
| SE | 1.08 | 1.10 | 0.50 | 0.48 | ||
| Respiration per unit N (nmol O2 g−1 N s−1) | ||||||
| Mean | 683.5 | 533.0 | n.s. | 410.2 | 351.3 | n.s. |
| SE | 65.5 | 66.8 | 34.3 | 32.8 | ||
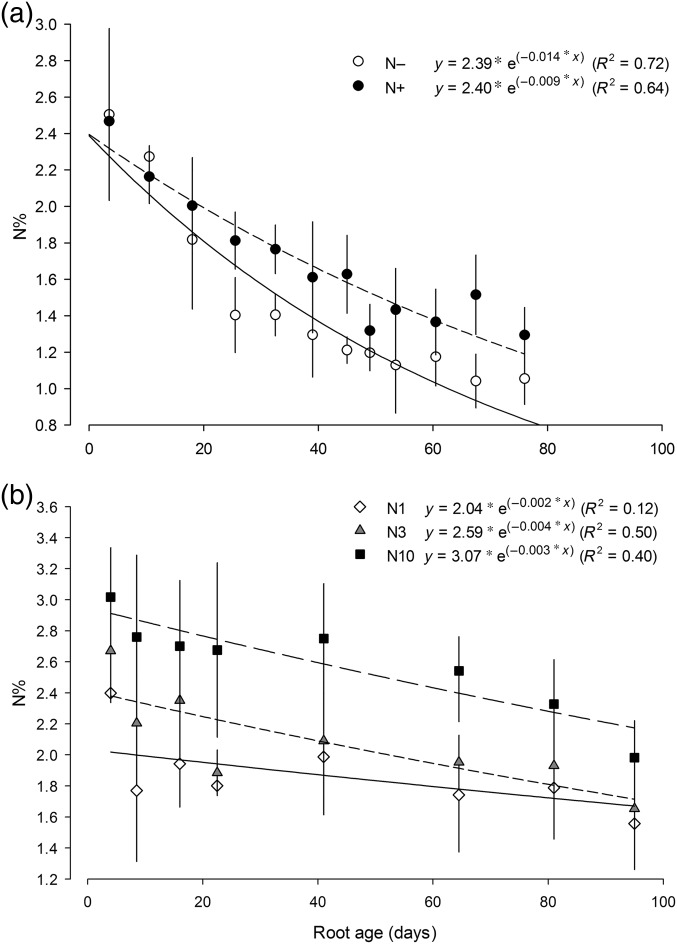
In both the greenhouse and field experiments, the N concentration decreased with root age following a trend that was best explained by a negative exponential function (Figure 2). In the field experiment, the influence of N supply on root N concentration was affected by root age, as indicated by a significant influence of N supply on the ‘b’ parameters of the exponential equations (Figure 2a; see Table S1 available as Supplementary Data at Tree Physiology Online). The ‘a’ parameter, which indicates an overall effect of N supply on root N concentration independent of root age (i.e., the Y-intercept of a log plot), was not significantly affected by N supply. In the field experiment, the youngest fine roots (<3 weeks old) had similar N concentration regardless of N supply, but N concentration of older roots was increased by N supply, so that roots that were >10 weeks old in the N fertilized treatment had ∼40% higher N concentration than unfertilized roots (Figure 2a).
Figure 2.
Relationships between N concentration and root age in P. tremuloides as affected by the N supply level. (a) Roots from the second sampling in the field experiment. (b) The greenhouse experiment when N was homogeneously supplied between the two adjacent pots. Bars represent ±1 standard errors. Points on the X-axis represent the middle age of each age class. Statistical results of the effects of N supply on the relationships between root N concentration and root age are reported in Table S1 available as Supplementary Data at Tree Physiology Online.
In the greenhouse experiment, the ‘a’ parameter of the exponential equations relating the influence of root age on root N concentration was significantly different among N1, N3 and N10 treatments, while the ‘b’ parameter did not differ among the N fertilization levels (Figure 2b; see Table S1 available as Supplementary Data at Tree Physiology Online). In general, roots of similar age had higher N concentration in the N10 pot than those in either of the two lower N pots (Figure 2b). In contrast to the field experiment where N fertilization only affected older roots, in the greenhouse experiment, increasing rates of N supply increased the N concentration of roots of all ages (Figure 2b).
Effect of N supply and age on root respiration
In the greenhouse experiment, there was no evidence to support our hypothesis that localized N supply stimulated respiration per unit N or per unit dry weight more than uniformly supplied N (Table 2). Indeed, roots exposed to the lowest N level (N1) tended to have the fastest respiration per unit N regardless of the spatial pattern of nutrient supply (P = 0.06; Table 2). Moreover, root growth also was not stimulated by localized N supply nor was whole plant or shoot biomass stimulated by increased N supply (Table 2).
Table 2.
Effects of the homogeneous or heterogeneous N supply on shoot and root biomass and respiration of fine roots of P. tremuloides measured in the greenhouse experiment (n.s., not significant; P > 0.05). See Figure 1 for a graphical explanation of treatment application.
| Treatment | Homogeneous N supply |
Heterogeneous N supply |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N1 | N3 | N10 | P | N1 (N3) | N3 (N1) | P | N1 (N10) | N10 (N1) | P | |
| Shoot DW (g plant−1) | ||||||||||
| Mean | 8.78 | 14.93 | 14.09 | n.s. | 16.49 | 10.02 | n.s. | |||
| SE | 4.94 | 5.48 | 7.68 | 3.67 | 5.34 | |||||
| Root DW (g plant−1) | ||||||||||
| Mean | 13.33 | 12.58 | 14.30 | n.s. | 5.74 | 6.58 | n.s. | 4.51 | 6.77 | n.s. |
| SE | 7.65 | 3.54 | 6.04 | 3.22 | 3.81 | 2.24 | 4.42 | |||
| Fine root age (days) | ||||||||||
| Mean | 63 | 53 | 54 | 59 | 60 | 50 | 48 | |||
| Respiration per unit biomass (nmol O2 g−1 DW s−1) | ||||||||||
| Mean | 6.70 | 6.83 | 6.72 | n.s. | 5.45 | 6.52 | n.s. | 7.30 | 7.42 | n.s. |
| SE | 0.78 | 0.77 | 0.75 | 1.08 | 1.02 | 1.13 | 1.18 | |||
| Respiration per unit N (nmol O2 g−1 N s−1) | ||||||||||
| Mean | 354.8 | 308.0 | 244.3 | n.s. (0.06) | 298.0 | 316.5 | n.s. | 347.2 | 293.7 | n.s. (0.06) |
| SE | 33.2 | 32.5 | 32.0 | 52.0 | 48.8 | 50.7 | 52.7 | |||
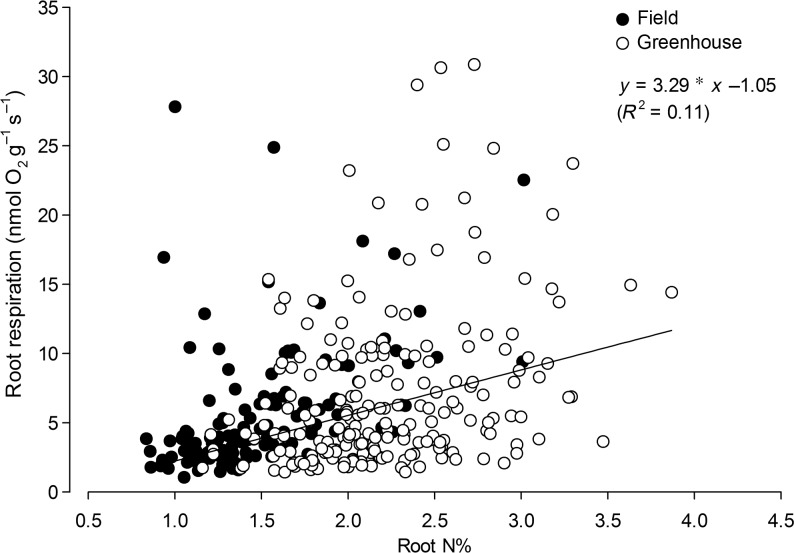
Fine root respiration per unit dry mass (nmol O2 g−1 DW s−1) was also not significantly affected by level of N supply in either the field (Table 1) or greenhouse (Table 2) experiments. Although N supply did not affect root respiration in the greenhouse experiment, we found a positive linear relationship of root respiration with root N concentration for each level of N supply. A similar relationship was observed in field roots. Consistent with the analyses in Tables 1 and 2, there were no significant differences among N treatments or between greenhouse and field experiments in either slopes or intercepts obtained in the linear equations used to fit the data to each level of N supply (see Table S1 available as Supplementary Data at Tree Physiology Online). Therefore, we pooled the data from both experiments and all N supply levels to illustrate the relationship of root respiration with root N concentration (Figure 3).
Figure 3.
Relationship of fine root respiration with root N concentration. Data from the two experiments are pooled together. Statistical analysis of the effects of N supply or experimental setting (field vs greenhouse) on the relationships of root respiration with root N concentration are reported in Table S1 available as Supplementary Data at Tree Physiology Online.
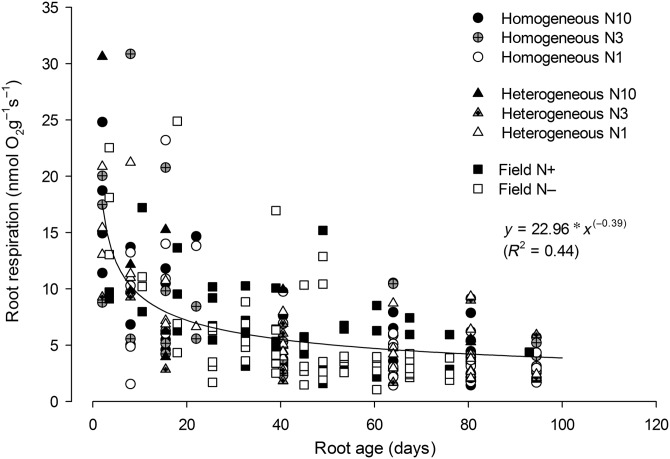
The relationship of root respiration with root age was best explained in both experiments by a negative power equation (Figure 4). The relationship was unaffected by N supply, and similarly, the ‘a’ and ‘b’ parameters were not significantly different between field and greenhouse data (see Table S1 available as Supplementary Data at Tree Physiology Online). Consequently, we pooled data for both experiments and all N supply levels to illustrate the overall relationship of root respiration with root age (Figure 4).
Figure 4.
Relationship of root respiration with root age. All original data from the two experiments are fitted to a single power equation. Points on the X-axis represent the middle age of each age class. There was no significant (P > 0.05) effect of N supply or experimental condition (greenhouse vs field) on the relationships of root respiration with root age (see Table S1 available as Supplementary Data at Tree Physiology Online).
Relative influence of root N concentration and root age on root respiration
When the relative influence of root N concentration and root age on root respiration was examined, we found different patterns depending on whether the experiment was conducted in the greenhouse or in the field. In the greenhouse study, root age was the only predictor of respiration, whereas in the field experiment, both root age and root N concentration were important predictors of root respiration (Table 3). When the field and greenhouse data were combined, root age was the dominant predictor of root respiration (Table 3), although both factors together explained only 43% of the variation in the data.
Table 3.
Significance of the minimum adequate models to explain the relationship of root respiration (log transformed) as a function of root age (days log transformed), root N concentration (root N, N%) and their interaction. Relative importance of parameters, where total equals 1, was determined using the R package ‘relaimpo’. ***Significant at P < 0.001.
| Dataset | n | R2 | Relative importance of terms in model |
Minimum adequate model | |
|---|---|---|---|---|---|
| Field experiment | 163 | 0.43*** | Log root age (x1) Root N (x2) |
0.57 0.43 |
y = 1.736 − 0.258x1 + 0.522x2 |
| Greenhouse experiment | 161 | 0.46*** | Log root age (x1) | 1.00 | y = 3.138 − 0.427x1 |
| Field and greenhouse experiment combined | 324 | 0.43*** | Log root age (x1) Root N (x2) |
0.85 0.15 |
y = 2.546 − 0.362x1 + 0.185x2 |
Discussion
Remarkably, Populus root respiration exhibited a similar decline with root age that was largely independent of whether the first- and second-order roots were collected from trees in the field or seedlings in the greenhouse or of whether the roots were exposed to low or high N supply levels (Figure 4). The best fit model of all the data (n = 324) explained 43% of the total variability, but root age had a higher predictive power (85%) than root N concentration (15%) (Table 3). At least for roots that were <3 weeks old, age has a strong and consistent effect on respiration over a wide range of environmental conditions. Similar declines with root age have been observed previously in apple, citrus (Bouma et al. 2001) and grape (Comas et al. 2000, Volder et al. 2005, 2009), but, to our knowledge, no one previously had examined whether N supply, either uniformly or locally distributed, would affect this relationship in plants that were not strongly N deficient.
Root respiration is strongly affected by root age. In both experiments in our study, root respiration at 20 °C ranged from 1 to 33 nmol O2 g−1 DW s−1, with an average of 7.0 nmol O2 g−1 DW s−1 (n = 324), which is very close to the mean value for poplar trees reported by Burton et al. (2002). Respiration sharply decreased by 50–55% within the first 3 weeks after roots appeared on the windows. After roots were older than 3–4 weeks, respiration declined little with age. The steepness of the respiratory decline with age can vary among species. In one study in grape, respiration rates declined by half within 2 days after root emergence (Volder et al. 2005), whereas in this study, it took ∼21 days for Populus root respiration to decline from 16 nmol O2 g−1 DW s−1 for roots <4 days old to 8 nmol O2 g−1 DW s−1 (Figure 4). While all species seem to show quite steep declines in respiratory activity with root age, the degree of decline may depend on the amount of energy being used for root construction after the root is no longer elongating, and for ion-uptake and maintenance processes, as well as the temporal resolution of the experimental observations. The intensive respiration of young roots most likely reflects the initial growth (Eissenstat and Volder 2005) and respiratory costs associated with maintenance of abundant carrier enzymes as well as the considerable costs of nitrate reduction and other costs associated with nutrient absorption, which may account for up to 70% of total root respiratory costs (Poorter et al. 1991). It should be noted that we measured only the first- and second-order roots of the root module. They are not undergoing secondary thickening and are not becoming woody but are typically accumulating tannins and other secondary phenolics that may reflect a slower metabolism with less energy devoted to nutrient-uptake carrier proteins as they age (Volder et al. 2005). Fine root N- and P-uptake rates are highest during the first days of a root’s life and typically sharply decline with age (Bouma et al. 2001, Volder et al. 2005, 2009). Thus, the respiratory declines with root age likely are related to the declines in uptake activity with root age.
We found that increases in N supply increased the total root N concentration but, at least in the greenhouse experiment, had little or no effect on root respiration (Tables 1 and 2). Increased root N concentration was observed in older first- and second-order roots of field-grown trees and root of all ages in greenhouse-grown seedlings. Our results are not in line with the assumption of a positive correlation of root respiration with N tissue concentration, based on data from different species and different sites (Burton et al. 2002, Reich et al. 2008, Chen et al. 2010). Root N concentration does not always correlate positively with root respiration, as shown in a study of Eucalyptus (Thongo M’Bou et al. 2004), where root maintenance respiration, which accounted for >90% of total root respiration, decreased with increasing root N concentration, although possible differences in average root age between N treatments may also have contributed to these differences. Nitrogen addition to saturating levels may shift the tissue N relationship with respiration (Ryan et al. 1996, Desrochers et al. 2002, Wang et al. 2010, Burton et al. 2012). Indeed, maintenance respiration may poorly track N concentration if the excess N is no longer associated with enzymatic activity such as protein turnover or enzymes associated with maintaining membrane potentials. One possibility is that this nonmetabolic N is associated with storage proteins, which should require little maintenance energy. At multiple Acer saccharum-dominated sites, Burton et al. (2012) found that compared with ambient plots, long-term N addition (15 years or longer) increased N concentration in the coarser and deeper woody roots where storage proteins might be more typically found. The form of the excess N of limited metabolic activity in the roots receiving the highest N supply is largely unknown and needs to be further explored. The answer may lie in the soluble protein fraction and in N chemically bound to more recalcitrant compounds (e.g., lignins and tannins: acid insoluble fractions of root tissue), which may be in excess of 50% of total root N (Xiong et al. 2013).
While broadly similar, there were some important differences in the effects of N addition on the tissue N–respiration relationship in the greenhouse seedling study compared with the field experiment. It was evident that the seedlings were not N limited as we observed no above- or belowground growth stimulation by the N addition, in contrast to many previous studies (reviewed by Hodge 2004). If the added N does not stimulate growth, it may accumulate in metabolically inactive forms, even in young roots. In contrast to the saturating N conditions in the greenhouse experiment, the field experiment likely was not N saturated. Although we did not measure total root length on the root boxes, previous research did show stimulation of root growth as a consequence of N addition in P. tremuloides and other species at this common garden (Adams et al. 2013). In the field experiment, the relatively large trees and the small portion of the root system that was exposed to N addition likely eliminated any potential for N saturation in this experiment. Differences in N saturation between the greenhouse and field experiments could explain the lack of an N-addition effect on respiration independent of root age in the greenhouse experiment and the fact that some influence of root N concentration independent of age was present in the field experiment. Interestingly, the N-induced respiratory increase was more evident in the older field roots than in the younger field roots (Figure 4). Previous work has shown that N addition can extend root longevity of P. tremuloides in N-enriched patches (Adams et al. 2013). Based on a cost–benefit analysis (Eissenstat and Yanai 1997), longevity should be extended to those roots that are providing the most N benefit to the plant (i.e., those in N-rich patches), which might entail active synthesis of mobile defenses and maintenance of more N-carrier enzymes, which would lead to higher respiratory activity in older roots. Thus, patchy N addition may modestly stimulate root respiration in older roots compared with similar age roots in unenriched patches.
In the greenhouse experiment, when plants received a heterogeneous N supply, we did not observe faster respiration per unit N in the roots receiving localized N compared with those receiving uniform N (Table 2). However, we also did not observe stimulated root growth in response to localized N supply either, unlike many previous studies (reviewed by Hodge 2004). Thus, the experimental system may not have been ideal to test whether spatial patterns of N supply can affect the respiration–N relationship.
Conclusions
Our results showed that first- and second-order Populus roots exhibited markedly slower respiration for roots of the same root module as the roots aged. These declines were especially marked within the first 3 weeks of life of the roots and were surprisingly robust, largely independent of tree age, site conditions (field vs greenhouse) and N supply. Previously reported effects of N supply on root respiration may have been partially mediated through shifts in the age of the root population studied. Increased N supply can increase root tissue concentrations without a concomitant increase in respiration, especially if constrained by root age and if plants are N saturated. Thus, the strong correlations of root tissue N with respiration across species and sites should not necessarily be realized with N addition or N deposition, especially where N no longer limits productivity.
Supplementary data
Supplementary data for this article are available at Tree Physiology Online.
Conflict of interest
None declared.
Funding
This research was partially supported by the U.S. National Science Foundation (OEI-0613832; IOS-1120482) to D.M.E. and partially funded by the Free University of Bozen - Bolzano within the project ‘Ecological and physiological consequences of soil nitrogen availability for trees and herbaceous plants’ to M.T.
Supplementary Material
Acknowledgments
This work was carried out at Pennsylvania State University, University Park, PA 16802, USA. We thank the whole Root Ecology Lab for helping make this work possible, especially M. Luke McCormack for his support in root respiration measurements and Tom Adams for general technical support. We would also like to thank the Free University of Bozen - Bolzano for analytical support and Francesca Scandellari and Damiano Zanotelli for their statistical advice. The valuable suggestions received from two anonymous reviewers and the handling editor on this manuscript are acknowledged with thanks.
References
- Adams TS, Eissenstat DM (2014) The continuous incorporation of carbon into existing Sassafras albidum roots and its implications for estimating root turnover. PLoS One 9:e95321 doi:10.1371/journal.pone.0095321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams TS, McCormack ML, Eissenstat DM (2013) Foraging strategies in trees of different root morphology: the role of root lifespan. Tree Physiol 33:940–948. doi:10.1093/treephys/tpt067 [DOI] [PubMed] [Google Scholar]
- Bloom AJ, Sukrapanna SS, Warner RL (1992) Root respiration associated with ammonium and nitrate absorption and assimilation by barley. Plant Physiol 99:1294–1301. doi:10.1104/pp.99.4.1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma TJ, Broekhuysen AGM, Veen BW (1996) Analysis of root respiration of Solanum tuberosum as related to growth, ion uptake and maintenance of biomass. Plant Physiol Biochem 34:795–806. [Google Scholar]
- Bouma TJ, Yanai RD, Elkin AD, Hartmond U, Flores-Alva DE, Eissenstat DM (2001) Estimating age-dependent costs and benefits of roots with contrasting life span: comparing apples and oranges. New Phytol 150:685–695. doi:10.1046/j.1469-8137.2001.00128.x [Google Scholar]
- Burton AJ, Pregitzer K, Ruess R, Hendrick R, Allen M (2002) Root respiration in North American forests: effects of nitrogen concentration and temperature across biomes. Oecologia 131:559–568. doi:10.1007/s00442-002-0931-7 [DOI] [PubMed] [Google Scholar]
- Burton AJ, Pregitzer KS, Crawford JN, Zogg GP, Zak DR (2004) Simulated chronic NO-3 deposition reduces soil respiration in northern hardwood forests. Glob Change Biol 10:1080–1091. doi:10.1111/j.1365-2486.2004.00737.x [Google Scholar]
- Burton AJ, Jarvey JC, Jarvi MP, Zak DR, Pregitzer KS (2012) Chronic N deposition alters root respiration-tissue N relationship in northern hardwood forests. Glob Change Biol 18:258–266. doi:10.1111/j.1365-2486.2011.02527.x [Google Scholar]
- Ceccon C, Panzacchi P, Scandellari F, Prandi L, Ventura M, Russo B, Millard P, Tagliavini M (2011) Spatial and temporal effects of soil temperature and moisture and the relation to fine root density on root and soil respiration in a mature apple orchard. Plant Soil 342:195–206. doi:10.1007/s11104-010-0684-8 [Google Scholar]
- Chen D, Zhou L, Rao X, Lin Y, Fu S (2010) Effects of root diameter and root nitrogen concentration on in situ root respiration among different seasons and tree species. Ecol Res 25:983–993. doi:10.1007/s11284-010-0722-2 [Google Scholar]
- Comas LH, Eissenstat DM, Lakso AN (2000) Assessing root death and root system dynamics in a study of grape canopy pruning. New Phytol 147:171–178. doi:10.1046/j.1469-8137.2000.00679.x [Google Scholar]
- Desrochers A, Landhäusser SM, Leiffers VJ (2002) Coarse and fine root respiration in aspen (Populus tremuloides). Tree Physiol 22:725–732. doi:10.1093/treephys/22.10.725 [DOI] [PubMed] [Google Scholar]
- Eissenstat DM, Yanai R (1997) Ecology of root lifespan. Adv Ecol Res 27:1–62. doi:10.1007/3-540-27675-0_8 [Google Scholar]
- Eissenstat DM, Volder A (2005) The efficiency of nutrient acquisition over the life of a root. Ecol Stud 81:185–220. doi:10.1007/3-540-27675-0_8 [Google Scholar]
- Fahey TJ, Siccama TG, Driscoll CT et al. (2005) The biogeochemistry of carbon at Hubbard Brook. Biogeochemistry 75:109–176. doi:10.1007/s10533-004-6321-y [Google Scholar]
- Friend AL, Eide MR, Hinckley TM (1990) Nitrogen stress alters root proliferation in Douglas-fir seedlings. Can J For Res 20:1524–1529. doi:10.1139/x90-202 [Google Scholar]
- Groemping (2006) Relative importance for linear regression in R: the package relaimpo. J Stat Softw 17:1–27. [Google Scholar]
- Hanson PJ, Edwards NT, Garten CT Jr, Andrews JA (2000) Separating root and soil microbial contributions to soil respiration: a review of methods and observations. Biogeochemistry 48:115–146. doi:10.1023/A:1006244819642 [Google Scholar]
- Hodge A. (2004) The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol 162:9–24. doi:10.1111/j.1469-8137.2004.01015.x [Google Scholar]
- Högberg P, Nordgren A, Buchmann N, Taylor AFS, Ekblad A, Högberg MN, Nyberg G, Ottosson-Löfvenius M, Read DJ (2001) Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411:789–792. doi:10.1038/35081058 [DOI] [PubMed] [Google Scholar]
- Janssens IA, Dieleman W, luyssaert S et al. (2010) Reduction of forest soil respiration in response to nitrogen deposition. Nat Geosci 3:315–322. doi:10.1038/ngeo844 [Google Scholar]
- Jia S, McLaughlin NB, Gu J, Li X, Wang Z (2013) Relationships between root respiration rate and root morphology, chemistry and anatomy in Larix gmelinii and Fraxinus mandshurica. Tree Physiol 33:579–589. doi:10.1093/treephys/tpt040 [DOI] [PubMed] [Google Scholar]
- Law BE, Falge E, Gu L et al. (2002) Environmental controls over carbon dioxide and water vapor exchange of terrestrial vegetation. Agric For Meteorol 113:97–120. doi:10.1016/S0168-1923(02)00104-1 [Google Scholar]
- McCormack ML, Adams TS, Smithwick EA, Eissenstat DM (2012) Predicting fine root lifespan from plant functional traits in temperate trees. New Phytol 195:823–831. doi:10.1111/j.1469-8137.2012.04198.x [DOI] [PubMed] [Google Scholar]
- McCormack ML, Dickie IA, Eissenstat DM et al. (2015) Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol 207:505–518. doi:10.1111/nph.13363 [DOI] [PubMed] [Google Scholar]
- Melillo JM, Steudler PA, Aber JD et al. (2002) Soil warming and carbon-cycle feedbacks to the climate system. Science 298:2173–2176. doi:10.1126/science.1074153 [DOI] [PubMed] [Google Scholar]
- Oren R, Ellsworth DS, Johnsen KH et al. (2001) Soil fertility limits carbon sequestration by forest ecosystems in a CO2-enriched atmosphere. Nature 411:469–472. doi:10.1038/35078064 [DOI] [PubMed] [Google Scholar]
- Poorter H, Van der Werf A, Atkin OK, Lambers H (1991) Respiratory energy requirements of roots vary with the potential growth rate of a plant species. Physiol Plant 83:469–475. doi:10.1111/j.1399-3054.1991.tb00122.x [Google Scholar]
- R Development Core Team (2015) R: a language and environment for statistical computing. R (version) 3.2.1. R Foundation for Statistical Computing. http://www.R-project.org (19 June 2015, date last accessed). [Google Scholar]
- Reich PB. (2002) Root-shoot relations: optimality in acclimation and adaptation or the ‘Emperor’s New Clothes’. In: Waisel Y, Eshel A, Kafkafi U (eds) Plant roots: the hidden half, 3rd edn Marcel Dekker, Inc., New York, NY, pp 205–220. [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS, Vose JM, Volin JC, Gresham C, Bowman WD (1998) Relationships of leaf dark respiration to leaf nitrogen, specific leaf area and leaf life-span: a test across biomes and functional groups. Oecologia 114:471–482. doi:10.1007/s004420050471 [DOI] [PubMed] [Google Scholar]
- Reich PB, Tjoelker MG, Pregitzer KS, Wright IJ, Oleksyn J, Machado J-L (2008) Scaling of respiration to nitrogen in leaves, stems and roots of higher land plants. Ecol Lett 11:793–801. doi:10.1111/j.1461-0248.2008.01185.x [DOI] [PubMed] [Google Scholar]
- Ryan MG, Hubbard RM, Pongracic S, Raison RJ, McMurtrie RE (1996) Foliage, fine-root, woody-tissue and stand respiration in Pinus radiata in relation to nitrogen status. Tree Physiol 16:333–343. doi:10.1093/treephys/16.3.333 [DOI] [PubMed] [Google Scholar]
- Sala OE, Chapin FS, Armesto JJ et al. (2000) Global biodiversity scenarios for the year 2100. Science 287:1770–1774. doi:10.1126/science.287.5459.1770 [DOI] [PubMed] [Google Scholar]
- Thongo M’Bou A, Saint-André L, de Grandcourt A, Nouvellon Y, Jourdan C, Mialoundama F, Epron D (2004) Growth and maintenance respiration of roots of clonal Eucalyptus cuttings: scaling to stand-level. Plant Soil 332:41–53. doi:10.1007/s11104-009-0272-y [Google Scholar]
- Throop HL, Holland EA, Parton WJ, Ojima DS, Keough CA (2004) Effects of nitrogen deposition and insect herbivory on patterns of ecosystem-level carbon and nitrogen dynamics: results from the CENTURY model. Glob Change Biol 10:1092–1105. doi:10.1111/j.1529-8817.2003.00791.x [Google Scholar]
- Valentini R, Matteucci G, Dolman AJ et al. (2000) Respiration as the main determinant of carbon balance in European forests. Nature 404:861–865. doi:10.1038/35009084 [DOI] [PubMed] [Google Scholar]
- Veen BW. (1980) Energy costs of ion transport. In: Rains DW, Valentine RC, Hollaender A (eds) Genetic engineering of osmoregulation. Impact on plant productivity for food, chemicals and energy. Plenum Press, New York, NY, pp 187–195. [Google Scholar]
- Vitousek PM. (1994) Beyond global warming: ecology and global change. Ecology 75:1861–1876. doi:10.2307/1941591 [Google Scholar]
- Volder A, Smart DR, Bloom AJ, Eissenstat DM (2005) Rapid decline in nitrate uptake and respiration with age in fine lateral roots of grape: implications for root efficiency and competitive effectiveness. New Phytol 165:493–501. doi:10.1111/j.1469-8137.2004.01222.x [DOI] [PubMed] [Google Scholar]
- Volder A, Anderson LJ, Smart DR, Bloom AJ, Lakso AN, Eissenstat DM (2009) Estimating nitrogen uptake of individual roots in container- and field-grown plants using a 15N-depletion approach. Funct Plant Biol 36:621–628. doi:10.1071/FP08330 [DOI] [PubMed] [Google Scholar]
- Wang W, Peng S, Fang J (2010) Root respiration and its relation to nutrient contents in soil and root and EVI among 8 ecosystems, northern China. Plant Soil 333:391–401. doi:10.1007/s11104-010-0354-x [Google Scholar]
- Xiong Y, Fan P, Fu S, Zeng H, Guo D (2013) Slow decomposition and limited nitrogen release by lower order roots in eight Chinese temperate and subtropical trees. Plant Soil 363:19–31. doi:10.1007/s11104-012-1290-8 [Google Scholar]
- Zadworny M, Eissenstat DM (2011) Contrasting the morphology, anatomy and fungal colonization of new pioneer and fibrous roots. New Phytol 190:213–221. doi:10.1111/j.1469-8137.2010.03598.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.