Abstract
Background
We evaluated the risk of incident heart failure (HF) associated with various categories of ventricular conduction defects (VCD) and examined the impact of QRS duration on the risk of HF.
Methods and Results
This analysis included 14,478 participants from the Atherosclerosis Risk in Communities (ARIC) study who were free of HF at baseline. VCDs (n=377) were categorized into right and left bundle branch blocks (RBBB and LBBB, respectively), bifascicular BBB (RBBB with fascicular block), indetermined type VCD (IVCD), and pooled-VCD group excluding lone RBBB. During an average of 18 years follow-up, 1,772 participants were hospitalized for incident HF. Compared to No-VCD, LBBB and pooled-VCD were strongly associated with increased risk of incident HF (multivariable hazard ratio 2.87 and 2.29, respectively). Compared to No-VCD with QRS duration <100 ms, HF risk was 1.17-fold for the No-VCD group with QRS duration 100–119 ms, 1.97-fold for pooled-VCD group with QRS duration 120–139 ms and 3.25-fold with QRS duration ≥140 ms. HF risk for pooled VCD group remained significant (1.74-fold for QRS duration 120–139 ms and 2.81-fold for QRS duration 140 ms or longer) in the subgroup free from cardiovascular disease at baseline. Lone RBBB was not associated with incident HF.
Conclusions
VCDs except for isolated RBBB are strong predictors of incident HF, and HF risk is further increased as the QRS duration is prolonged above 140 ms.
Keywords: heart failure, electrocardiography, bundle branch block, QRS duration
INTRODUCTION
Heart failure (HF) is known to be more common among patients with bundle branch blocks (BBB) than those with normal ventricular conduction.1 Previous reports have shown that left BBB (LBBB),2–5 but not right BBB (RBBB),5–9 is associated with excess risk of incident HF. The Framingham study in men and women with BBB found that the risk of incident HF increased progressively with increasing QRS duration.2 The objective of the present study was to compare the prognostic significance of different patterns of ventricular conduction defects (VCD) as predictors of incident. VCDs considered include the main stem BBB according to traditional definitions and indetermined type VCD (IVCD).
METHODS
Study population and design
The study population was selected from the participants in the Atherosclerosis Risk in Communities (ARIC) Study, a population-based multicenter prospective study designed to investigate the natural history and cause of atherosclerotic and cardiovascular disease. Participants (n=15,792 men and women aged 45–64 years) were recruited from 4 US communities in Forsyth County, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland. Eligible participants were interviewed at home and then invited to a baseline clinical examination between 1987 and 1989. Participants attended 3 additional clinical examinations at 3-year intervals and received a follow-up phone interview yearly. Details of the ARIC Study design, protocol sampling procedures, and selection and exclusion criteria were published elsewhere.11 The study was approved by each study site’s institutional review board. All participants provided written informed consent. A total 1,314 participants were excluded from analyses: 405 without an electrocardiogram (ECG) or completed clinical data for heart failure, 126 with inadequate quality ECG, or an external pacemaker or Wolff-Parkinson-White pattern, 44 with race other than black or white, and 739 with prevalent HF at baseline. After all exclusions, 14,478 participants, of whom 377 had VCD, remained and were included in this analysis.
Outcome ascertainment
Incident HF occurred from baseline through December 31, 2006 was considered in the present investigation. The follow-up period was up to 20 years (mean 18 years). Incident HF was defined by International Classification of Disease (ICD) codes as the first occurrence of either a hospitalization with a HF hospital discharge diagnosis code (ICD-9th Revision, Clinical Modification, code 428), or a death certificate with any listing of a 428 ICD-9 code or code I50 ICD-10 code, without a previous record of hospitalization as code 428). Detailed definitions for incident HF classification were published previously.12–13 Cardiovascular disease (CVD) at baseline was defined as presence of ECG myocardial infarction (MI) according to the Minnesota Code (MC)14 or the NOVACODE15 criteria, or a self-reported history of a clinical diagnosis of MI, angina pectoris, coronary artery bypass surgery, coronary angioplasty, HF, or stroke at the time entered the ARIC study.16
ECG methods
Identical electrocardiographs (MAC PC, Marquette Electronics Inc., Milwaukee, Wisconsin) were used at all clinic sites, and resting, 10-second standard simultaneous 12-lead ECGs were recorded in all participants using strictly standardized procedures. All ECGs were processed in a central ECG laboratory (initially at Dalhousie University, Halifax, NS, Canada and later at the EPICARE Center, Wake Forest School of Medicine, Winston-Salem, NC), where all ECGs were visually inspected for technical errors and inadequate quality using an interactive computer graphics terminal. The ECGs were first processed by the Dalhousie ECG program and were reprocessed for the present study using the 2001 version of the GE Marquette 12-SL program (GE, Milwaukee, Wisconsin). VCD were classified according to the MC criteria,13 for complete LBBB (MC-7.1), complete RBBB (MC-7.2, QRS axis >−45 degree), indetermined type VCD (IVCD, MC-7.4), combination of RBBB and left anterior fascicular block (LAFB) (MC-7.8, RBBB and QRS axis between −45 and −120 degree), and combination of RBBB and left posterior fascicular block (LPFB) (MC-7.8, RBBB and QRS axis between 91 and 180 degree).14,17–18 Combined LBBB, IVCD, and bifascicular block was labeled pooled VCD group.
Statistical methods
Frequency distributions of ECG measurements were inspected to identify anomalies and outliers. Descriptive statistics were used to determine mean values, standard deviations, and percentile distributions for continuous variables, and frequencies and percentages for categorical variables. Cox's Proportional Hazards (CPH) analysis was used to assess the associations of BBB with the risk of HF in incremental models as follows: model-1, unadjusted; model-2 adjusted for age, sex, and race; and model-3 adjusted for age, sex, race, regional center, body mass index, systolic blood pressure, smoking status, education level, hypertension, diabetes mellitus, history of CVD status, ratio of total cholesterol/HDL, blood glucose, and serum creatinine at baseline). The effect of QRS duration on the risk of HF was compared at two dichotomized levels as suggested by a recent recommendation:19–20 at 140ms and at 150 ms as recommended also in the report of the American College of Cardiology Foundation/ American Heart Association and Heart Rhythm society for Cardiac Resynchronization Therapy.21 All analyses were performed with SAS version 9.3 (SAS Institute Inc., Cary, North Carolina).
RESULTS
Study group characteristics
The mean age at baseline was 54 years (SD 5.8), 54.6% were women, 73.4% white, and 26.3% African American. Of the study group, 32.7% had hypertension, 11.1% diabetes, and 5.2% had a history of CVD or ECG evidence of MI. Details of demographic, clinical, and ECG characteristics of the study population stratified by VCD status are summarized in Table 1. As shown, most of the demographic, clinical characteristics, and ECG measurements were different between the two groups with the VCD group having more prevalence of CVD risk factors.
Table 1.
Baseline characteristic of study participants
| Total N=14,478 Means (SD), or N (%) |
No-VCD (QRS duration <120ms) N=14,101 |
VCD (QRS duration ≥120ms) N=377 |
P*- value |
|---|---|---|---|
| Age (years) | 54 (5.7) | 57 (5.4) | <.001 |
| Body mass index (kg/m2) | 28 (5.3) | 28 (4.8) | 0.029 |
| Systolic blood pressure (mmHg) | 121 (17.7) | 124 (20.9) | <.001 |
| Women | 7780 (55.2%) | 121 (32.1%) | <.001 |
| Race/ethnicity | 0.527 | ||
| White | 10377 (73.4%) | 281 (74.5%) | |
| African-American | 3724 (26.3%) | 96 (25.5%) | |
| Education ≤ high school | 7799 (55.4%) | 233 (61.8%) | 0.002 |
| Current smoker | 3649 (25.9%) | 104 (27.6%) | 0.032 |
| History of CVD | 691 (4.9%) | 66 (17.5%) | <.001 |
| Diabetes | 1536 (11.0%) | 56 (14.9%) | 0.016 |
| Hypertension | 4583 (32.5%) | 151 (40.1%) | 0.002 |
| Antihypertensives | 3810 (27.0%) | 145 (38.5%) | <.001 |
| Hypertension/Antihypertensives | 5129 (36.4%) | 180 (47.7%) | <.001 |
| Hypertensives with ECG-LVH | 208 (4.5%) | - | <.001 |
| ECG-LVH | 261 (1.8%) | - | <.001 |
| ECG-Myocardial infarction | 469 (3.3%) | 51 (13.5%) | <.001 |
| Ratio of total cholesterol/HDL | 4.6 (1.7) | 5.1 (1.7) | <.001 |
| Blood glucose | 108 (39.4) | 110 (35.2) | 0.274 |
| Serum creatinine | 1.1 (0.4) | 1.2 (0.9) | <.001 |
| Heart rate (/min) | 66 (10.2) | 64 (10.5) | <.001 |
| QRS duration (ms) | 91 (9.5) | 136 (14.7) | <.001 |
VCD = ventricular conduction defect.
P values between the groups of No-VCD and VCD.
CVD= cardiovascular disease.
ECG-LVH = ECG-Left Ventricle Hypertrophy by Cornell voltage criteria
The patterns of bundle branch blocks and incident heart failure
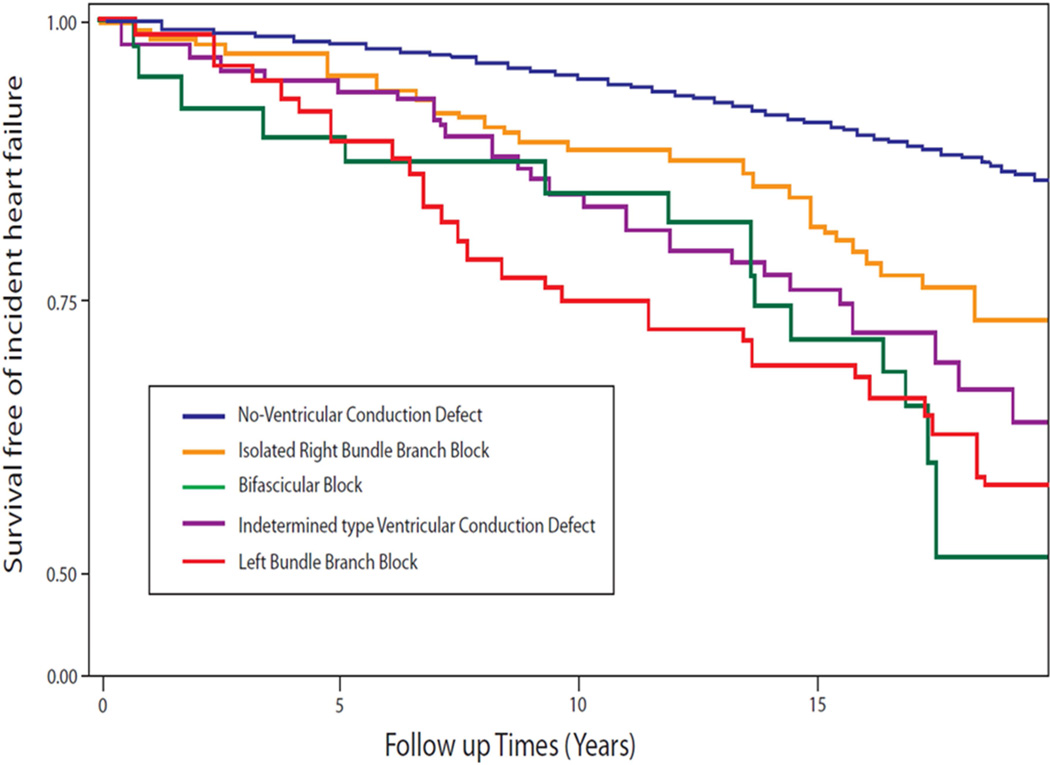
VCD was present in 2.6% (377/14,478) including 159 RBBB and 75 LBBB, and 218 in the pooled-VCD group. During an average of 18 years follow-up, 1,772 HF events occurred. The event rate was 23.7 per 1000 person-years for the participant with pooled-VCD (where LBBB, IVCD and RBBB combined with LAFB/LPFB were all strong predictors of incident HF with HRs ranging from 1.75 for IVCD to 2.87 for LBBB in Table 2), 14.7 for the participants with RBBB, and 7.2 per 1000 person-years among those with No-BBB. Compared to the No-VCD group, LBBB was a strong predictor for incident HF (HR 2.87, 95% CI 1.94–4.25, P<0.001) in multivariable adjustment models. Figure 1 shows the cumulative incidence of HF by VCD category.
Table 2.
Hazard ratios with 95% confidence intervals for incident heart failure associated with various ventricular conduction defects
|
N=1,772/14,478 |
Event Rate (n/N) |
Events/1000 Person yrs. |
Hazard Ratio (95% CI) | ||
|---|---|---|---|---|---|
| Model 1a | Model 2b | Model 3c | |||
| No VCD | 1667/14101 | 7.2 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| RBBB | 35/159 | 14.7 | 1.74 (1.25–2.44)‡ | 1.50 (1.07–2.12)† | 0.89 (0.62–1.28) |
| Bifascicular-block* | 16/40 | 27.5 | 3.16 (1.93–5.18)§ | 2.93 (1.79–4.79)§ | 2.81 (1.71–4.62)§ |
| IVCD | 28/103 | 20.3 | 2.53 (1.74–3.67)§ | 2.07 (1.42–3.01)§ | 1.75 (1.19–2.57)‡ |
| LBBB | 26/75 | 26.4 | 2.77 (1.88–4.07)§ | 2.96 (2.00–4.36)§ | 2.87 (1.94–4.25)§ |
| Pooled-VCD‖ | 70/218 | 23.7 | 2.74 (2.16–3.48)§ | 2.52 (1.98–3.21)§ | 2.29 (1.80–2.92)§ |
Denotes P<0.05;
P<0.01;
P<0.001 for P values of hazard ratios.
RBBB= right bundle branch block, LAFB=left anterior fascicular block, LPFB=left posterior fascicular block, IVCD= indetermined type ventricular conduction defect, and LBBB= left bundle branch block.
Bifascicular-block =RBBB with LAFB or RBBB with LPFB.
Pooled-VCD Group =Combined LBBB, IVCD, and Bifascicular block.
Model 1: Unadjusted model.
Model 2: Adjusted for age, sex and race;
Model 3: Adjusted for key demographic and clinical variables of age, sex, race, region of residence, body mass index, systolic blood pressure, smoking status, education level, hypertension, diabetes mellitus, cardiovascular disease status, ratio of total cholesterol/HDL, blood glucose, and serum creatinine at baseline.
Figure 1.
Survival free of incident heart failure during an average 18 years follow-up by ventricular conduction defect category
No-Ventricular Conduction Defect= QRS duration <120 ms; Bifascicular Block= Right bundle branch block with left anterior fascicular block, or with left posterior fascicular block.
The QRS prolongation and incident heart failure
Using the No-VCD participants with QRS duration <100 ms as the reference group, the group with QRS duration 100–119 ms had a 1.17-fold (p<0.01) risk of incident HF (Table 3). When stratified by the level of QRS duration 140 ms as a cut point, the risk of incident HF in the pooled-VCD group was 1.97-fold (p<0.001) for QRS duration range 120–139 ms and 3.25-fold (p<0.001) for QRS duration ≥140 ms. Survival free of incident heart failure graphs for HF by VCD category and QRS duration by 140ms cutoff are shown in Figure 2. The results were essentially the same when QRS duration 150ms was used as the cutoff point (not shown). Excluding participants CVD at baseline, a significant HF risk was retained in the pooled-VCD group with 1.74-fold (HR 1.74, 95% CI 1.16–2.62, P<0.01) increased risk in the fully adjusted model for QRS duration 120–139 ms and 2.81-fold (HR 2.81, 95% CI 1.80–4.39, P<0.001) increased risk and for QRS duration >140 ms (Table 4).
Table 3.
Hazard ratios with 95% confidence intervals for incident heart failure by QRS duration and bundle branch block categories
| N=1,772/14,478 | Events/1000 Person years |
Hazard Ratio (95% CI) | |||
|---|---|---|---|---|---|
| Model 1a | Model 2b | Model 3c | |||
| No-VCD Group | |||||
| QRS duration | <100ms | 6.7 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| QRS duration | 100–119ms | 9.5 | 1.42 (1.27–1.59)§ | 1.29 (1.14–1.44)§ | 1.17 (1.04–1.32)‡ |
| Lone RBBB‖ | |||||
| QRS duration | 120–139ms | 14.3 | 1.81 (1.16–2.81)‡ | 1.64 (1.05–2.55)† | 1.06 (0.68–1.65) |
| QRS duration | ≥140ms | 15.4 | 2.00 (1.20–3.33)§ | 1.58 (0.93–2.68) | 0.78 (0.43–1.43) |
| Pooled-VCD Group# | |||||
| QRS duration | 120–139ms | 20.2 | 2.66 (1.93–3.66)§ | 2.36 (1.71–3.25)§ | 1.97 (1.42–2.73)§ |
| QRS duration | ≥140ms | 30.5 | 3.46 (2.42–4.94)§ | 3.29 (2.30–4.71)§ | 3.25 (2.27–4.67)§ |
VCD = ventricular conduction defect.
Denotes P<0.05;
P<0.01;
P<0.001 for P values of hazard ratios.
Lone RBBB= isolated right bundle branch block with no other VCD.
Pooled-VCD Group= combined LBBB, IVCD, and Bifascicular block (RBBB with LPFB, or RBBB with LAFB).
Model 1: Unadjusted model.
Model 2: Adjusted for age, sex and race;
Model 3: Adjusted for age, sex, race, region of residence, body mass index, systolic blood pressure, smoking status, education level, hypertension, diabetes mellitus, cardiovascular disease status, ratio of total cholesterol/HDL, blood glucose, and serum creatinine at baseline.
Figure 2.
Survival free of incident heart failure during an average 18 years follow-up by QRS duration and ventricular conduction defect category
No-VCD= No ventricular conduction defect;
Lone RBBB= Isolated right bundle branch block;
Pooled-VCD Group= combined left bundle branch block, indetermined type VCD, and bifascicular block (right bundle branch block with left anterior fascicular block, or with left posterior fascicular block).
Table 4.
Hazard ratios with 95% confidence intervals for incident heart failure in the group with No-VCD and the pooled-VCD group by QRS duration in the subgroup free from cardiovascular disease at baseline.
| N=1,488/13,721 | Events/1000 Person years |
Hazard Ratio (95% CI) | |||
|---|---|---|---|---|---|
| Model 1a | Model 2b | Model 3c | |||
| No VCD Group | |||||
| QRS duration | <100ms | 6.1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| QRS duration | 100–119ms | 7.7 | 1.25 (1.11–1.42)§ | 1.18 (1.04–1.35)‡ | 0.92 (0.73–1.16) |
| Pooled-VCD Group‖ | |||||
| QRS duration | 120–139ms | 14.8 | 2.07 (1.38–3.11)§ | 1.86 (1.24–2.80)‡ | 1.74 (1.16–2.62)‡ |
| QRS duration | ≥140ms | 24.2 | 2.94 (1.92–4.48)§ | 2.86 (1.87–4.37)§ | 2.81 (1.80–4.39)§ |
VCD = ventricular conduction defect.
Denotes P<0.05;
P<0.01;
P<0.001 for P values for hazard ratios.
Pooled-VCD Group= combined LBBB, IVCD, and Bifascicular block (RBBB with LPFB, or RBBB with LAFB).
Model 1: Unadjusted model.
Model 2: Adjusted for age, sex and race;
Model 3: Adjusted for age, sex, race, region of residence, body mass index, systolic blood pressure, smoking status, education level, hypertension, diabetes mellitus, ratio of total cholesterol/HDL, blood glucose, and serum creatinine at baseline.
DISCUSSION
The present study is a comprehensive analysis to evaluate the risk of incident HF in subgroups stratified by specific BBB category, QRS duration, sex, race, and age. The key findings in the fully-adjusted risk model: 1) Compared to No-VCD with QRS duration <120ms, LBBB, IVCD and RBBB combined with LAFB or LPFB (bifascicular block) were all strong predictors of incident HF; 2) Lone RBBB was not a significant predictor of incident HF; 3) Using No-VCD with QRS duration <100 ms as the reference group, incident HF risk was nearly 2-fold for pooled-VCD group with QRS duration 120–139 ms and over 3-fold for QRS duration ≥140 ms; 4) Excluding participants with CVD at baseline, a significant HF risk was retained in the pooled-VCD group.
Possible mechanisms accounting for increased HF risk in bundle branch blocks
On body surface ECG, QRS complex represents ventricular electrical depolarization that initiates ventricular contraction, whereas the T wave represents ventricular electrical repolarization that is associated with ventricular relaxation. Delayed left ventricular excitation and contraction in VCD may lead into dyssynchrony of ventricular contraction, and also into delayed left ventricular repolarization and relaxation. Reduced cardiac function in VCD reflects a possible cause-and-effect relation between VCD and HF, and it has become an important consideration in resynchronizing therapy for HF patients.20–26 In normal ventricular depolarization and repolarization, the direction of repolarization in the left lateral wall is predominantly reversed with respect to the direction of depolarization. In BBB particularly with more pronounced QRS prolongation and delayed excitation times, the repolarization sequence becomes predominantly concordant with respect to the direction of depolarization. Prolonged left ventricular depolarization prolongs and alters the spatial direction of the repolarization sequence which in turn is thought to be associated with impaired diastolic function.26 Our results demonstrated that VCD with more pronounced prolonged QRS (≥140 ms) was associated with a substantial additional increase of the risk for HF.
Our results in relation to work done by other investigators
LBBB has been associated with excess risk of incident HF in many studies,2–5 but not RBBB.5–7 In a report from the Heart Outcomes Prevention Evaluation (HOPE) trial,3 baseline LBBB was an independent predictor of HF, sudden death, CVD death, and all cause death, but baseline RBBB was not associated with increased CVD risk. Zhang5 et al. evaluated the risk of incident HF for BBBs with over 14 years of follow-up for 65,975 participants in the Women’s Health Initiative study, and found that LBBB, IVCD, and RBBB combined with LAFB were strong predictors of incident HF in multivariable adjusted risk models, but RBBB was not a significant predictor. The study also showed that LBBB in women with QRS duration ≥140 ms had a much greater predictive value for incident HF than LBBB with QRS duration 120–139 ms. In the predominantly white Framingham population, the impact of QRS duration on HF risk was evaluated among subject with and without BBB, with baseline log-QRS duration modeled as a continuous variable.2 the incidence of HF increased 1.2-fold for 1 SD increase in QRS duration (HR 1.23; 95% CI 1.08–1.38; P<0.001) in multivariable model. And the highest rates of HF were for LBBB (HR 4.45, P=0.0001) and for IVCD (HR 2.18, P=0.02), but the risk was not significantly increased for RBBB. Our results in BBB categories are consistent with the above results from the Framingham study. MESA study evaluated in their multiethnic population with normal ventricular conduction the impact of QRS duration >100 ms on incident HF.10. In multivariable model adjusted for age, sex and race, the risk for incident HF was increased over 2-fold (HR 2.10, 95% CI 1.29–3.42, p<0.01) during the mean follow-up 7.1 years. Aro27 et al. in their study population of 2,049 men found that a QRS duration threshold of 110 ms was an optimal cut point to define a prolonged QRS duration as a risk factor. The findings in that study and those in MESA study are consistent with those in our study group without VCD.
Clinical implications
VCDs are manifestations of a gradually evolving generalized degenerative process involving not only bundle branches but also structural changes in working myocardium. This suggests that early primary prevention of CHD can be expected to have a more significant overall impact. More prolonged QRS duration may indicate a more pronounced risk of HF before and also after VCD develops. Once clinical signs of HF develop, secondary prevention efforts involve management of HF including consideration for resynchronization therapy.
Study limitations
Similar to other observational studies, residual confounding remains a possibility despite adjusting for several potential confounders. Also, our results may not be generalizable to race/ethnicities other than whites and blacks. Finally, new-onset HF was defined by hospitalizations for HF or death certificate identifying HF as the cause of death without prior hospitalization. This means that our results pertain mainly to the risk of the HF requiring hospitalization. Despite these limitations, this is the first comprehensive analysis of the impact of QRS morphology (i.e. VCD patterns) and duration (i.e. prolongation) on the risk of HF. Further, all data used in the analysis including ECG data were carefully ascertained and/or read at central core labs.
Conclusion
VCDs except for isolated RBBB are strong predictors of incident HF, and HF risk is further increased as the QRS duration is prolonged above 140ms.
Highlight.
Heart failure (HF) risk was assessed for ventricular conduction defects (VCD).
VCDs except for isolated RBBB were strong predictors of new-onset HF.
HF risk was further increased as QRS duration was prolonged above 140 ms.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Funding
The ARIC Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: None declared.
REFERENCES
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. for American Heart Association Statistics Committee and Stroke Statistics Subcommittee. AHA Statistical Update-Heart Disease and Stroke Statistics–2013 Update. A report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhingra R, Pencina MJ, Wang TJ, Nam BH, Benjamin EJ, Levy D, et al. Electrocardiographic QRS duration and the risk of congestive heart failure: the Framingham Heart Study. Hypertension. 2006;47:861–867. doi: 10.1161/01.HYP.0000217141.20163.23. [DOI] [PubMed] [Google Scholar]
- 3.Haataja P, Nikus K, Kähönen M, Huhtala H, Nieminen T, Jula A, et al. Prevalence of ventricular conduction blocks in the resting electrocardiogram in a general population: The Health 2000 Survey. Int J Cardiol. 2013;167:1953–1960. doi: 10.1016/j.ijcard.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 4.Sumner G, Salehian O, Yi Q, Healey J, Mathew J, Al-Merri K, et al. for HOPE Investigators. The prognostic significance of bundle branch block in high-risk chronic stable vascular disease patients: a report from the HOPE trial. J Cardiovasc Electrophysiol. 2009;20:781–787. doi: 10.1111/j.1540-8167.2009.01440.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhang ZM, Rautaharju PM, Soliman ES, Manson JE, Martin LW, Perez M, et al. Different Patterns of Bundle Branch Blocks and the Risk of Incident Heart Failure in the Women’s Health Initiative (WHI) Study. Circ Heart Fail. 2013;6:655–661. doi: 10.1161/CIRCHEARTFAILURE.113.000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fahy GJ, Pinski SL, Miller DP, McCabe N, Pye C, Walsh MJ, et al. Natural history of isolated bundle branch block. Am J Cardiol. 1996;77:1185–1190. doi: 10.1016/s0002-9149(96)00160-9. [DOI] [PubMed] [Google Scholar]
- 7.Eriksson P, Wilhelmsen L, Rosengren A. Bundle-branch block in middle-aged men: risk of complications and death over 28 years. The Primary Prevention Study in Göteborg, Sweden. Eur Heart J. 2005;26:2300–2306. doi: 10.1093/eurheartj/ehi580. [DOI] [PubMed] [Google Scholar]
- 8.Marcus GM, Hoang KL, Hunt SA, Chun SH, Lee BK. Prevalence, patterns of development, and prognosis of right bundle branch block in heart transplant recipients. Am J Cardiol. 2006;98:1288–1290. doi: 10.1016/j.amjcard.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Bussink BE, Holst AG, Jespersen L, Bussink BE, Holst AG, Jespersen L, et al. Right bundle branch block: prevalence, risk factors, and outcome in the general population: results from the Copenhagen City Heart Study. Eur Heart J. 2013;34:138–146. doi: 10.1093/eurheartj/ehs291. [DOI] [PubMed] [Google Scholar]
- 10.Ilkhanoff L, Liu K, Ning H, Nazarian S, Bluemke DA, Soliman EZ, et al. Association of QRS duration with left ventricular structure and function and risk of heart failure in middle-aged and older adults: the Multi-Ethnic Study of Atherosclerosis (MESA) Eur J Heart Fail. 2012;14:1285–1292. doi: 10.1093/eurjhf/hfs112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 12.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study) Am J Cardiol. 2008;101:1016–1022. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 13.Rautaharju PM, Prineas RJ, Wood J, Zhang ZM, Crow R, Heiss G. Electrocardiographic predictors of new-onset heart failure in men and in women free of coronary heart disease (from the Atherosclerosis in Communities [ARIC] Study) Am J Cardiol. 2007;100:1437–1441. doi: 10.1016/j.amjcard.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 14.Prineas RJ, Crow RS, Zhang ZM. The Minnesota code manual of electrocardiographic findings. Second. Springer-London; 2010. pp. 16–166. Published by. [Google Scholar]
- 15.Rautaharju PM, Park LP, Chaitman BR, Rautaharju F, Zhang ZM. The Novacode criteria for classification of ECG abnormalities and their clinically significant progression and regression. J Electrocardiol. 1998;31:157–187. [PubMed] [Google Scholar]
- 16.Myerson M, Coady S, Taylor h, Rosamond WD, Goff DC for the ARIC investigators. Declining severity of myocardial infarction from 1987 to 2002. The atherosclerosis risk in communities (ARIC) Study. Circulation. 2009;119:503–514. doi: 10.1161/CIRCULATIONAHA.107.693879. [DOI] [PubMed] [Google Scholar]
- 17.Wong CK, Gao W, Stewart RA, van Pelt N, French JK, Aylward PE, et al. for Hirulog Early Reperfusion Occlusion (HERO-2) Investigators. Risk stratification of patients with acute anterior myocardial infarction and right bundle-branch block: importance of QRS duration and early ST-segment resolution after fibrinolytic therapy. Circulation. 2006;114:783–789. doi: 10.1161/CIRCULATIONAHA.106.639039. [DOI] [PubMed] [Google Scholar]
- 18.Willems JL, Robles de Medina EO, Bernard R, Coumel P, Fisch C, Krikler D, et al. Criteria for intraventricular conduction disturbances and pre-excitation. J Am Coll Cardiol. 1985;5:1251–1275. doi: 10.1016/s0735-1097(85)80335-1. [DOI] [PubMed] [Google Scholar]
- 19.Strauss DG, Selvester RH, Wagner GS. Defining left bundle branch block in the era of cardiac resynchronization therapy. Am J Cardiol. 2011;107:927–934. doi: 10.1016/j.amjcard.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Zusterzeel R, Selzman KA, Sanders WE, Caños DA, O’Callaghan KM, Carpenter JL, Piña IL, Strauss DG. Cardiac resynchronization therapy in women US food and drug administration meta-analysis of patient-level data. JAMA Intern Med. 2014;174:1340–1348. doi: 10.1001/jamainternmed.2014.2717. [DOI] [PubMed] [Google Scholar]
- 21.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NAM, III, Freedman RA, Gettes LS, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines and heart rhythm society. Circulation. 2013;127:e283–e352. doi: 10.1161/CIR.0b013e318276ce9b. [DOI] [PubMed] [Google Scholar]
- 22.Goldenberg I, Moss AJ, Hall WJ, Foster E, Goldberger JJ, Santucci P, et al. for MADIT-CRT Executive Committee. Predictors of response to cardiac resynchronization therapy in the multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy (MADIT-CRT) Circulation. 2011;124:1527–1536. doi: 10.1161/CIRCULATIONAHA.110.014324. [DOI] [PubMed] [Google Scholar]
- 23.Lam CS, Lyass A, Kraigher-Krainer E, Massaro JM, Lee DS, Ho JE, et al. Cardiac dysfunction and noncardiac dysfunction as precursors of heart failure with reduced and preserved ejection fraction in the community. Circulation. 2011;124:24–30. doi: 10.1161/CIRCULATIONAHA.110.979203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gold MR, Thébault C, Linde C, Abraham WT, Gerritse B, Ghio S, et al. Effect of QRS duration and morphology on cardiac resynchronization therapy outcomes in mild heart failure: results from the Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction (REVERSE) study. Circulation. 2012;126:822–829. doi: 10.1161/CIRCULATIONAHA.112.097709. [DOI] [PubMed] [Google Scholar]
- 25.Douglas RAG, Samesima N, Filho MM, Pedrosa AA, Nishioka SAD, Pastore CA. Global and regional ventricular repolarization study by body surface potential mapping in patients with left bundle-branch block and heart failure undergoing cardiac resynchronization. Ann Noninvasive Electrocardiol. 2012;2:123–129. doi: 10.1111/j.1542-474X.2012.00500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu TG, Patel C, Martin S, Quan X, Wu Y, Burke JF, et al. Ventricular transmural repolarization sequence: its relationship with ventricular relaxation and role in ventricular diastolic function. Eur Heart J. 2009;30:372–380. doi: 10.1093/eurheartj/ehn585. [DOI] [PubMed] [Google Scholar]
- 27.Aro AL, Anttonen O, Tikkanen JT, Junttila MJ, Kerola T, Rissanen HA, et al. Intraventricular conduction delay in a standard 12-lead electrocardiogram as a predictor of mortality in the general population. Circ Arrhythm Electrophysiol. 2011;4:704–710. doi: 10.1161/CIRCEP.111.963561. [DOI] [PubMed] [Google Scholar]