Abstract
Objective
Patients with osteoarthritis have increased bone mass, but no decrease in fractures. We studied the association between self-reported osteoarthritis and incident falls and fractures in postmenopausal women.
Methods
GLOW is a prospective, multinational cohort of 60 393 non-institutionalised women aged ≥55 years who had visited primary care practices within the previous 2 years. Questionnaires were mailed at yearly intervals. Patients were classified as osteoarthritic if they answered yes to the question “Has a doctor or other health provider ever said that you had osteoarthritis or degenerative joint disease?”, and this was validated against primary care records. Information on incident falls, fractures, and covariates was self-reported. Cox and Poisson models were used for incident fractures and number of falls, respectively, to compute hazard ratios (HRs) and rate ratios (RRs) for baseline osteoarthritis status.
Results
Of 51 386 women followed for a median of 2.9 (interquartile range 2.1 to 3.0) years, 20 409 (40%) reported osteoarthritis. The adjusted HR for osteoarthritis predicting fracture was 1.21 (95% CI 1.13 to 1.30; p<0.0001) and the adjusted RR for falls was 1.24 (95% CI 1.22 to 1.26; p<0.0001). However, the association between osteoarthritis and fracture was not significant after adjustment for incident falls: HR 1.06 (95% CI 0.98 to 1.15; p=0.13).
Conclusion
Postmenopausal women with self-reported osteoarthritis have a 20% increased risk of fracture and experience 25% more falls than osteoarthritis-free peers. Our data suggest that increased falls are the causal pathway of the association between osteoarthritis and fractures.
Keywords: Osteoporosis, Osteoarthritis, Fractures, Bone, Accidental Falls, Epidemiology
INTRODUCTION
Osteoarthritis and osteoporosis are both common conditions among the elderly, and are associated with significant morbidity and healthcare costs. The residual lifetime risk of any fracture among women aged over 60 years has been estimated to be 44% in an international cohort study.[1] Osteoarthritis is the most prevalent joint disease, with radiographic knee and hip osteoarthritis present in 33% and 27% in people aged over 60 years, respectively.[2] The lifetime risks of symptomatic knee and hip osteoarthritis are 45% and 25%, respectively.[3, 4] In terms of direct costs for the healthcare system, it has been shown that more than 60% of the patients with osteoarthritis are offered drug treatments by their GP, and 47% are referred to a specialist.[5]
A possible association between osteoarthritis and osteoporosis (and fragility fractures) has long been studied, with discordant results. First observations[6] suggested a protective effect of osteoarthritis for osteoporosis and subsequent fractures. Furthermore, several studies demonstrated an increased bone mineral density (BMD) in patients with osteoarthritis. This association appeared to be stronger for knee and hip osteoarthritis than for generalised osteoarthritis or osteoarthritis at other sites.[7, 8] However, case-control and prospective cohort studies later showed either no relationship between osteoarthritis and osteoporosis,[9, 10] or, more recently, an increased risk of fracture in patients with osteoarthritis.[11, 12] Different aetiologies for this association have been suggested, including increased body sway in patients with knee or hip osteoarthritis,[10] more high-impact falls in the context of osteoarthritis,[13] and higher severity of falls sustained.[14] However, none of these has been proven to be the causal pathway of the observed increase in fracture rates among osteoarthritis patients. Hence, we aimed to assess the existing association between self-reported osteoarthritis and incident falls and fractures. In particular, we wished to study if, and to what extent, falls contribute to the association.
METHODS
Study design
GLOW is an observational follow-up study designed to improve the understanding of international patterns of susceptibility, recognition, management, and outcomes of care in women aged 55 years and older at risk of fragility fractures. The study methods have been described previously[15] and are briefly outlined herein.
Participants and recruitment
GLOW was conducted at 723 physician practices in 17 study sites in 10 countries in Europe, North America, and Australia. A scientific advisory board, consisting of investigators at each of the 17 sites, was constituted to provide scientific oversight and study management. Practices typical of each region were recruited through primary care networks, or by identifying all physicians in a geographic area. Enrolment occurred between December 2007 and March 2009. Each primary care practice provided a list of the names and addresses of women aged 55 years and older who had consulted their physician in the past 24 months. These lists comprised the sampling frame. Sampling was stratified by age to ensure that two thirds of the women surveyed were aged 65 years and older. Patients were excluded from GLOW if they were unable to complete the study survey due to cognitive impairment, language barriers, or institutionalisation, or because they were too ill. In addition, women with missing baseline osteoarthritis or fracture information, and those with coeliac disease or rheumatoid arthritis were excluded from the current analysis.
Source of information
Questionnaires were designed to be self-administered and covered several health-related domains. Where possible, items from published validated instruments were used, including the National Health and Nutrition Examination Survey (NHANES), EuroQol (EQ-5D), and short-form 36 (SF-36). Questions that had not been used previously were tested cognitively in the context of the complete questionnaire in a sample of women the same age as those in the study. The complete baseline questionnaire was also pilot-tested before being finalised to gauge subject comprehension and completion time. Baseline questionnaires, along with invitations to participate in the study signed by the local principal investigator, were mailed to all potential participants. Women who responded were surveyed annually for the next 3 years. The process for entering, verifying, and managing survey data was uniform across all study sites, and was carried out in the central coordinating centre.
Definition and validation of osteoarthritis status
Participants were categorised as having “osteoarthritis” or “no osteoarthritis” based on their baseline response to “has a doctor or other health provider ever said that you had osteoarthritis or degenerative joint disease?”.
In order to validate self-reported OA status within the GLOW registry, we linked baseline data for the 3,043 participants recruited in one of the enrolment sites (Barcelona, Spain) to a primary care electronic records database (www.sidiap.org), which includes clinical information coded using ICD-10 codes for >85% of the local population. We then identified among the linked participants those with an ICD code for Osteoarthritis (M15 to M19) at the time when they returned the filled in baseline GLOW questionnaire. Finally, we calculated concordance rates (true positives + true negatives over total number of patients assessed) and Sensitivity and Specificity using standard methods.
Definition of outcomes
The primary outcome of our study was time to first fracture. This was defined according to each woman’s response to the questions “In the last 12 months, how many times did you break or fracture a bone?” and “In what month and year did it happen?”, which appeared repeatedly in the follow-up questionnaires for years 1, 2, and 3. Further, site of fracture (“Upper arm”, “Collar bone or clavicle”, “Wrist”, “Spine”, “Rib”, “Pelvis”, “Hip”, “Ankle”, “Upper leg”, “Lower leg”, and “Other”) was ascertained accordingly. In the present study, in addition to analysing time to any/overall fracture, we studied the four most frequent fracture locations separately: hip, clinical spine, wrist/forearm, and upper arm.
Our secondary outcome was number of incident falls. This was defined based on the participant response to “In the last 12 months, how many times have you fallen?”, with the corresponding possibilities of “None”, “Once”, “Two times or more”. This question was repeated in the three follow-up surveys.
Statistical methods
Cumulative fracture incidence was calculated by the Kaplan-Meier method, using the counting process approach to accommodate gaps in women’s follow-up.[16] Cox models for incident fracture were used to compute unadjusted hazard ratios (HRs) for baseline osteoarthritis status. A multivariable Cox model for incident fracture was fitted using backwards selection, beginning with all variables with univariate p-values <0.20. Variables that remained significant (p<0.05) in the multivariable setting were retained for the final model: potential confounders adjusted for were age, body mass index (BMI), anti-osteoporosis medication use, chronic obstructive pulmonary disease or emphysema, Parkinson’s disease, fracture history, parental hip fracture history, baseline oral corticosteroid use, and secondary osteoporosis (as defined by use of aromatase inhibitors, diagnosis of inflammatory bowel disease, type 1 diabetes, and menopause before age 45 years). A Kaplan-Meier curve showing fracture by baseline osteoarthritis diagnosis was computed using the subset of women with complete follow-up (baseline, year 1, year 2, and year 3). Also using this subset of complete data, univariate and zero-inflated multivariable Poisson regression models were fitted to estimate rate ratios (RRs) for incident falls for osteoarthritis versus non-osteoarthritis participants. These models were fitted using similar stepwise backwards selection methods. Robust standard errors were used in order to account for the fact that falls are not independent events. All analyses were performed using SAS version 9.2 and Stata version 10.0.
RESULTS
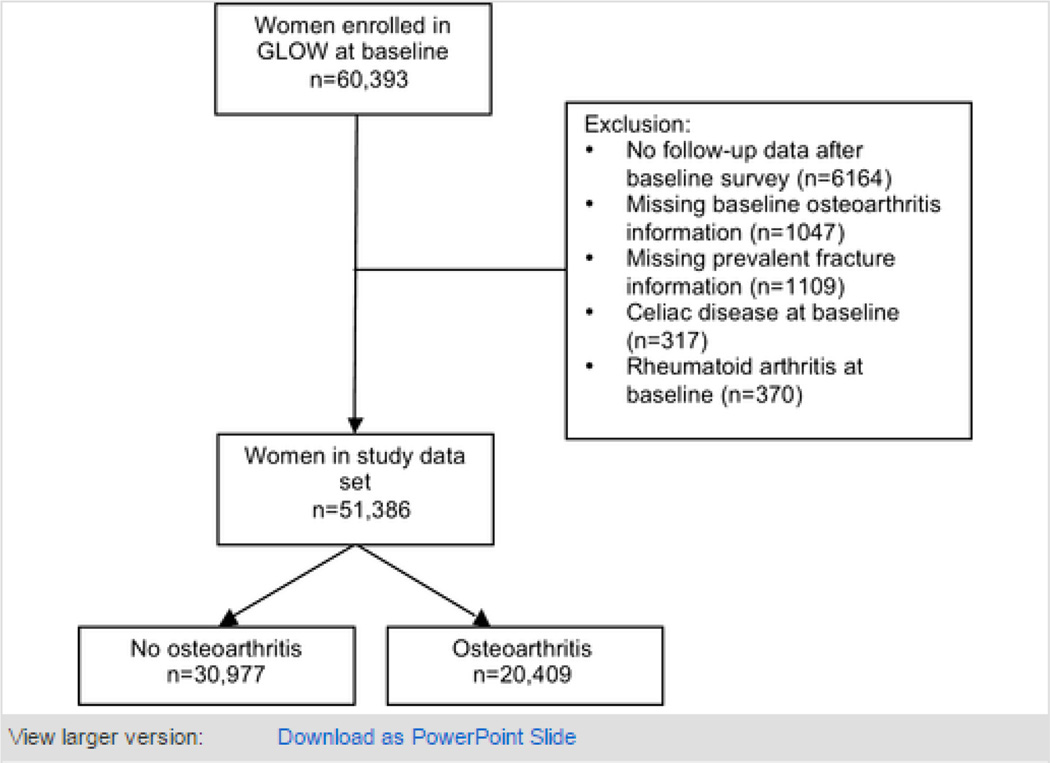
Of the 60 393 women enrolled in GLOW, 51 386 (85%) were included in the current analysis, and followed up for a median (interquartile range) of 2.9 (2.1 to 3.0) years. Among them, 20 409 (40%) reported a physician diagnosis of osteoarthritis at baseline (see population flow-chart in figure 1). In the subsample of 3 043 participants recruited in Barcelona (Spain), 2 757(90.6%) were linked to primary care electronic medical records, and 2 555(92.7%) out of these had information on baseline OA status. Among these, concordance between self-reported OA and GP records was 79.5%, with corresponding sensitivity 94% (95CI 92% to 95%) and specificity 71% (69% to 73%). Osteoarthritis patients were significantly older, had a higher BMI, and were more likely to have a diagnosis of asthma, chronic obstructive pulmonary disease, stroke, inflammatory bowel disease, Parkinson’s disease, and cancer. They also reported more fractures at baseline. Baseline characteristics for the whole and for both the osteoarthritis and non-osteoarthritis participants are shown in table 1. Potential follow-up bias was analysed by comparing baseline characteristics for the population who completed the 3-year follow-up questionnaire with those for women who were lost to follow-up. Lost patients were older, and had a higher prevalence of several co-morbid conditions and risk factors for fracture, including a history of previous fractures (data not shown).
Figure 1.
Population flow-chart.
Table 1.
Baseline characteristics for the study population and by baseline osteoarthritis status
| Total population (n=51 386) |
Non-OA participants (n=30 977) |
OA participants (n=20 409) |
p Value | |
|---|---|---|---|---|
| Age (years), mean (SD) | 68.2 (8.6) | 67.5 (8.7) | 69.1 (8.6) | <0.0001 |
| BMI (kg/m2), mean (SD) | 26.9 (5.9) | 26.6 (5.7) | 27.4 (6.2) | <0.0001 |
| Current or past AOM, excluding oestrogen, n (%) |
13 817 (28) | 7427 (25) | 6390 (32) | <0.0001 |
| Current or past oestrogen or hormone replacement, n (%) |
21 929 (43) | 12 609 (41) | 9320 (46) | <0.0001 |
| Region, n (%) | <0.0001 | |||
| Canada/Australia | 6066 (12) | 3851 (12) | 2215 (11) | |
| Europe | 21 390 (42) | 10 890 (35) | 10 500 (51) | |
| USA | 23 931 (47) | 16 237 (52) | 7694 (38) | |
| Falls in past 12 months, n (%) |
<0.0001 | |||
| None | 31 881 (63) | 20 264 (66) | 11 617 (57) | |
| One | 11 567 (23) | 6781 (22) | 4786 (24) | |
| Two or more | 7453 (15) | 3609 (12) | 3844 (19) | |
| Comorbidities, n (%) | ||||
| Asthma | 5763 (11) | 3015 (9.8) | 2748 (14) | <0.0001 |
| Chronic bronchitis or emphysema |
4254 (8.4) | 2048 (6.7) | 2206 (11) | <0.0001 |
| Stroke | 1926 (3.8) | 1089 (3.5) | 837 (4.2) | <0.001 |
| Ulcerative colitis or Crohn’s disease |
964 (1.9) | 483 (1.6) | 481 (2.4) | <0.0001 |
| Parkinson’s disease | 258 (0.5) | 119 (0.4) | 139 (0.7) | <0.0001 |
| Multiple sclerosis | 312 (0.6) | 196 (0.6) | 116 (0.6) | 0.39 |
| Cancer | 7212 (14) | 4252 (14) | 2960 (15) | 0.007 |
| Diabetes | 1764 (3.5) | 1064 (3.5) | 700 (3.5) | 0.99 |
| Prior fracture, n (%) | 11 903 (23) | 6307 (20) | 5596 (27) | <0.0001 |
| Risk factors for fracture, n (%) |
||||
| Current cortisone use | 1342 (2.7) | 558 (1.8) | 784 (3.9) | <0.0001 |
| Secondary osteoporosis* | 10 031 (20) | 5784 (19) | 4247 (21) | <0.0001 |
| Alcohol >20 drinks/week | 253 (0.5) | 154 (0.5) | 99 (0.5) | 0.85 |
FRAX definition: use of anastrozole, exemestane, or letrozole; diagnosis of colitis, type I diabetes, or menopause before age 45 years.
AOM, antiosteoporosis medication; BMI, body mass index; OA, osteoarthritis; SD, standard deviation.
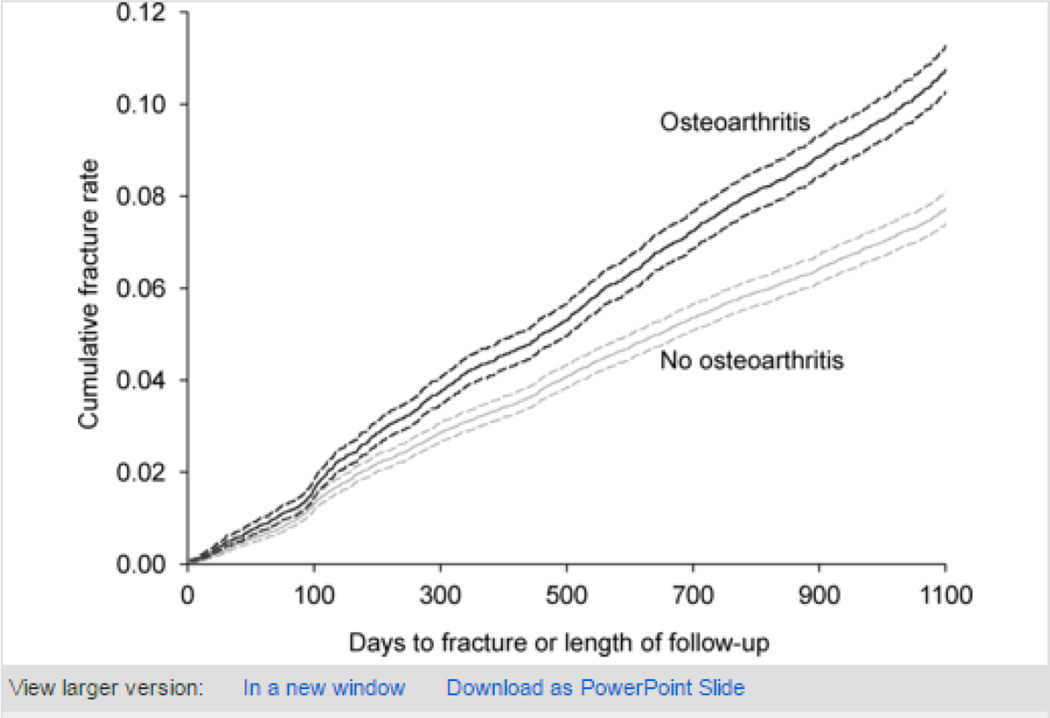
Overall fracture prevalence was 23.2% (95% confidence interval (CI) 22.8 to 23.5%) for the whole population, 27.4% (95% CI 26.8 to 28.0%) for osteoarthritis women and 20.4% (95% CI 19.9 to 20.8%) for non-osteoarthritis participants. Corresponding cumulative incidence rates at 3 years were 10.0% (95% CI 9.7 to 10.3%), 12.1% (95% CI 11.6 to 12.6%) and 8.7% (95% CI 8.3 to 9.0%) respectively (figure 2; table 2)
Figure 2.
Kaplan-Meier curve predicting fracture by year 3, by baseline osteoarthritis status, with 95% confidence intervals (women with all 4 years of survey data only; n=40 132). Log-rank test for equality over strata yields p<0.0001. Red, osteoarthritis; blue, no osteoarthritis.
Table 2.
Fracture prevalence (at baseline) and cumulative incidence rates in years 1, 2, and 3 for the study population, and for the osteoarthritis and non-osteoarthritis participants
| Total population (n=51 386) |
Non-OA participants (n=30 978) |
OA participants (n=20 409) |
|
|---|---|---|---|
| Baseline survey, fracture prevalence (95% CI) |
23.2 (22.8 to 23.5) | 20.4 (19.9 to 20.8) | 27.4 (26.8 to 28.0) |
| Year 1, cumulative fracture incidence (95% CI) |
3.5 (3.4 to 3.7) | 3.1 (2.9 to 3.3) | 4.2 (3.9 to 4.4) |
| Year 2, cumulative fracture incidence (95% CI) |
6.6 (6.4 to 6.9) | 5.8 (5.5 to 6.1) | 7.9 (7.5 to 8.3) |
| Year 3, cumulative fracture incidence (95% CI) |
10.0 (9.7 to 10.3) | 8.7 (8.3 to 9.0) | 12.1 (11.6 to 12.6) |
CI, confidence interval; OA, osteoarthritis.
Site-specific fracture prevalence, incidence rates, and unadjusted HRs are shown in table 3. This increase in fracture rates remained significant after adjusting for potential confounders: multivariable adjusted HR 1.21 (95% CI 1.13 to 1.30; p<0.0001). Further adjustment for falls history (as reported in the baseline questionnaire) slightly attenuated the risk estimate: HR 1.16 (95% CI 1.08 to 1.25; p<0.0001). When we studied each of the fracture sites separately (hip, clinical spine, wrist/forearm, upper arm, and ankle/lower leg), both prevalence and cumulative incidence rates were significantly higher in the osteoarthritis subjects (table 3). Corresponding unadjusted HRs and 95% CIs are shown in table 3.
Table 3.
Fracture prevalence at baseline and incidence over 3 years, by fracture site, in osteoarthritis and non-osteoarthritis participants
| Non-OA participants (n=30 978) | OA participants (n=20 409) | Unadjusted HR | |||
|---|---|---|---|---|---|
| Fracture prevalence (95% CI) |
Overall fracture incidence rate at 3 years (95% CI) |
Fracture prevalence (95% CI) |
Overall fracture incidence rate at 3 years (95% CI) |
(95% CI) | |
| Any fracture site | 20.4 (19.9 to 20.8) | 8.7 (8.3 to 9.0) | 27.4 (26.8 to 28.0) | 12.1 (11.6 to 12.6) | 1.40 (1.32 to 1.48) |
| Hip | 1.3 (1.2 to 1.5) | 0.66 (0.57 to 0.77) | 2.2 (2.0 to 2.4) | 0.93 (0.80 to 1.1) | 1.46 (1.17 to 1.81) |
| Clinical spine | 1.4 (1.3 to 1.5) | 0.84 (0.73 to 0.96) | 3.4 (3.1 to 3.6) | 1.5 (1.3 to 1.7) | 1.80 (1.50 to 2.17) |
| Wrist/forearm | 7.4 (7.1 to 7.7) | 2.0 (1.8 to 2.2) | 10.2 (9.8 to 10.6) | 2.8 (2.6 to 3.1) | 1.38 (1.22 to 1.57) |
| Upper arm | 2.5 (2.3 to 2.7) | 0.76 (0.66 to 0.88) | 3.5 (3.2 to 3.7) | 1.1 (0.96 to 1.3) | 1.38 (1.13 to 1.69) |
| Lower leg/ankle | 2.2 (2.0 to 2.3) | 0.54 (0.46 to 0.63) | 2.9 (2.7 to 3.2) | 0.74 (0.62 to 0.89) | 1.34 (1.05 to 1.70) |
CI, confidence interval; HR, hazard ratio; OA, osteoarthritis.
Separate multivariable analyses for different fracture sites showed a significant increase in spine and wrist/forearm fractures among the osteoarthritis participants (adjusted HRs of 1.27 (95% CI 1.02 to 1.58; p=0.032) and 1.24 (1.07 to 1.44; p=0.004), respectively), but not in hip, upper arm, or lower leg/ankle fracture rates (table 4). After adjusting for baseline falls, the increase in spine fractures was attenuated (HR 1.23; 95% CI 0.99 to 1.54; p=0.061) and no longer statistically significant, but wrist/forearm fractures remained significantly higher in the osteoarthritis group (HR 1.21; 95% CI1.04 to 1.40; p=0.014).
Table 4.
Poisson regression for number of falls and Cox regression for time to first fracture in osteoarthritis versus non-osteoarthritis participants
| Unadjusted HR/RR (95% CI; p Value) |
Multivariable adjusted HR/RR (95% CI; p Value) |
HR/RR, further adjusted for baseline falls (95% CI; p Value) |
HR, further adjusted for incident falls‡ (95% CI; p value) |
|
|---|---|---|---|---|
| Falls* | 1.26 (1.24 to 1.28; p<0.0001) |
1.24 (1.22 to 1.26; p<0.0001) |
1.14 (1.12 to 1.16; p<0.0001) |
-- |
| Overall fracture† | 1.40 (1.32 to 1.48; p<0.0001) |
1.21 (1.13 to 1.30; p<0.0001) |
1.16 (1.08 to 1.25; p<0.0001) |
1.06 (0.98 to 1.15; p=0.128) |
| Hip fracture† | 1.46 (1.17 to 1.81; p<0.0001) |
1.22 (0.94 to 1.59; p=0.131) |
1.19 (0.91 to 1.55; p=0.206) | 1.08 (0.79 to 1.48; p=0.636) |
| Clinical spine fracture† |
1.80 (1.50 to 2.17; p<0.0001) |
1.27 (1.02 to 1.58; p=0.032) |
1.23 (0.99 to 1.54; p=0.061) | 1.23 (0.96 to 1.59; p=0.104) |
| Wrist/forearm fracture† |
1.38 (1.22 to 1.57; p<0.0001) |
1.24 (1.07 to 1.44; p=0.004) |
1.21 (1.04 to 1.40; p=0.014) | 1.07 (0.91 to 1.26; p- 0.424) |
| Upper arm fracture† |
1.38 (1.13 to 1.69; p<0.0001) |
1.21 (0.96 to 1.54; p=0.112) |
1.17 (0.92 to 1.48; p=0.208) | 1.00 (0.77 to 1.30; p=0.995) |
| Lower leg/ankle | 1.34 (1.05 to 1.70; p=0.018) |
1.00 (0.74 to 1.35; p=0.994) |
0.96 (0.71 to 1.30; p=0.790) | 0.89 (0.63 to 1.25; p=0.500) |
Multivariable models for number of falls (Poisson regression) are adjusted for: age, body mass index, current or past hormone replacement therapy, antiosteoporosis medication use, baseline oral corticosteroid use, region of origin (USA/Canada/Australia/Europe), asthma, chronic obstructive pulmonary disease or emphysema, stroke, Parkinson’s disease, cancer, prior fracture, and smoking status.
Multivariable models are adjusted for: age, body mass index, antiosteoporosis medication use, chronic obstructive pulmonary disease or emphysema, Parkinson’s disease, fracture history, parental hip fracture history, baseline oral corticosteroid use, and secondary osteoporosis (as defined by use of aromatase inhibitors, diagnosis of inflammatory bowel disease, type 1 diabetes, and menopause before age 45 years).
Model includes only those women with complete survey follow-up
CI, confidence interval; HR, hazard ratio; RR, rate ratio.
Regarding falls, whilst 10,390 (33.9%) non-osteoarthritis subjects had one or more falls, 8,630 (42.6%) of the osteoarthritis participants reported at least one fall in the year before enrolment. After 3 years of follow-up, 21,839 (70.5%) of the non-osteoarthritis patients had fallen at least once, vs 16,089 (78.8%) among the osteoarthritis women. We thus observed an increased rate of falls in osteoarthritis vs non-osteoarthritis patients: fall incidence rates were 23/100 person-years (22 to 23) and 20/100 person-years (19 to 20) respectively. The corresponding multivariable adjusted RR was 1.24 (1.22 to 1.26; p<0.0001), which remained significant after adjusting for baseline falls history (RR 1.14 [1.12 to 1.16]; p<0.0001).
As the observed increase in falls in osteoarthritis subjects might explain the higher risk of fracture in these patients, we further adjusted our multivariable Cox models of osteoarthritis predicting fracture for incident falls on or before fracture (yes/no), after which the resulting HR was no longer significant (HR 1.06 [0.98 to 1.15]; p=0.13).
DISCUSSION
Key results
We found that self-reported physician diagnosis of osteoarthritis was associated with a significant increase in fracture risk of up to about 20%, even after multivariable adjustment for potential confounders. The effect size observed was very similar for each of the fracture sites assessed separately, but the rate increase was only significant for wrist/forearm fractures, possibly due to sample size. Secondly, we have demonstrated that postmenopausal women reporting osteoarthritis are at an increased risk of falls of about 25%, again after multivariable adjustment, with only small reduction in the effect size observed even after further adjustment for baseline fall status. Finally, our results show for the first time that the increase in falls observed in osteoarthritis patients is a key determinant of the higher fracture rate in this population: when we adjusted the multivariable survival model for osteoarthritis predicting fracture for incident falls on or before fracture, the association became weaker with an important reduction in the adjusted excess risk, and was no longer significant. These findings suggest that the increase in fall rate in osteoarthritis patients explains most of the observed increase in fractures in this population.
Interpretation
The association between osteoarthritis and fractures has been controversial, with some authors suggesting a protective effect and increased BMD in osteoarthritis-affected subjects,[6– 9] but others showing an increase in fractures among people with osteoarthritis. While some studies have reported a joint-specific association, suggesting an increased risk of fractures in hip osteoarthritis patients, but not for spine, knee, or hand osteoarthritis,[11] others have suggested that such an association also exists between knee osteoarthritis and non-vertebral fractures.[14] In addition, some reports have described a time-varying association, with an increased risk in the first years after the diagnosis of osteoarthritis,[17] and a subsequent decline over time.[18]
The most widely accepted possible explanations for the increased risk of fracture in patients with lower limb (hip or knee) osteoarthritis include: an increase in the rate of bone loss in patients with radiographic osteoarthritis;[13, 19, 20] and a higher number and/or severity of falls in patients with either knee pain[13] or self-reported clinical diagnosis of osteoarthritis.[14, 21] Our data are consistent with the second hypothesis and support the theory that osteoarthritis symptoms such as joint pain and stiffness (recently defined as illness osteoarthritis[22]) lead to increased body sway,[23] and a higher propensity to trip on an obstacle,[24] which finally translate into higher rates of falls and fractures. An alternative or additional cause for increased falls and fractures in this population is the existing association between OA and vitamin D deficiency [1–3].
Our results have implications for the clinical management of patients with OA: they should not only be assessed for fracture risk, but also strategies to reduce falls should be evaluated and, if effective, implemented among these patients. Some interventions have been shown effective to prevent falls in the elderly, and should be evaluated in OA patients, including Tai Chi[29], multi-factorial interventions[30] and vitamin D supplementation[31].
Strengths and limitations
Our study has several limitations. One of the main potential limitations of this study is the possibility of residual confounding: due to the observational nature of this data, we cannot ensure causality in the observed associations between OA and falls and fractures. In addition, the fact that all the information collected in GLOW was self-reported, including the main exposure studied (physician diagnosis of osteoarthritis) and our primary (incident fractures) and secondary (number of incident falls) outcomes, makes misclassification errors (e.g. people reporting OA might actually suffer from other conditions associated with frailty and increased risk of falls) and unobserved confounding more likely. However, previous studies have shown information on self-reported physician diagnosis of osteoarthritis to be valid. Rasooly et al[28] showed that the sensitivity of self-reported osteoarthritis compared with a clinical diagnosis was close to 90%. Similarly, Barlow et al[32] demonstrated a concordance of self-reported physician diagnosis of osteoarthritis of almost 90% when matched with primary care medical records. Consistent with this, we have shown high concordance of almost 80% between self-reported OA and GP records in a subsample of our data. Regarding incident fractures, validity was reported within the EPOS study:[33] of those who reported a “date” of fracture on the questionnaire (which all did in our GLOW data), 91% of subjects were correct to within 1 month of the actual date of the fracture, and only 9% false negatives were detected in women. This type of error in the assessment of osteoarthritis is unlikely to be associated with incident fracture status, as the data on osteoarthritis were collected prior to the incident fracture, and therefore would lead to an underestimate of the effect of osteoarthritis on fractures.
Other limitations are the lack of information on the date of osteoarthritis diagnosis, joint pain, and on joints affected, which does not allow us to explore the potential issues on time-varying and joint-specific associations between osteoarthritis and fractures. We did not have information on the date of falls either, which limits our ability to analyse whether falls occurring in the same year as fractures did actually produce the fracture or not. Also, we do not have data on use of analgesics that could explain at least part of the observed increase in falls and fractures, such as non-steroidal anti-inflammatory drugs,[34] paracetamol,[35] or opioids.[36] Despite these limitations, the GLOW population studied herein is part of an international, community-based, cohort study and thus our results have high external validity, and can be generalised to a wide range of postmenopausal women over 55 years of age.
Conclusion
In the context of a population-based international prospective cohort, we found that postmenopausal women with osteoarthritis have a 20% increase in the risk of fracture. In addition, they experience 25% more falls than osteoarthritis-free peers. Our results suggest that increased falls are critical in the causal pathway of the association between osteoarthritis and fractures. These findings have clinical implications, as they suggest that interventions to reduce falls might be useful in preventing fractures in patients with osteoarthritis.
Acknowledgments
We thank the patients, physicians, and study coordinators participating in GLOW, and the staff at the Center for Outcomes Research, including Frederick A Anderson, Jr, Gordon FitzGerald for statistical support, Linda Chase and Sophie Rushton-Smith.
Funding Financial support for the GLOW study is provided by Warner Chilcott Company, LLC and sanofi-aventis to the Center for Outcomes Research, University of Massachusetts Medical School.
Footnotes
Competing interests DP-A, XN, MKJ, AW, NKA, and RA declare no competing interests. CC has received consulting fees from and lectured for Amgen, The Alliance for Better Bone Health (sanofi-aventis and Warner Chilcott), Lilly, Merck, Servier, Novartis, and Roche-GSK. JDA has been a consultant/speaker for Amgen, Lilly, GlaxoSmithKline, Merck, Novartis, Nycomed, Pfizer, Procter & Gamble, Roche, sanofi-aventis, Servier, Warner Chilcott and Wyeth, and has conducted clinical trials for Amgen, Lilly, GlaxoSmithKline, Merck, Novartis, Pfizer, Procter & Gamble, Roche, sanofi-aventis, Warner Chilcott, Wyeth, and Bristol-Myers Squibb. SB has received research grants from Amgen, Lilly, Novartis, Pfizer, Procter & Gamble, sanofi-aventis, Roche, and GlaxoSmithKline, and has received honoraria from, served on Speakers’ Bureaus for, and acted as a consultant/Advisory Board member for Amgen, Lilly, Merck, Novartis, Procter & Gamble, sanofi-aventis, and Servier. RC has received funding from the French Ministry of Health, the French Ministry of Research, Merck, Servier, and Lilly, has received honoraria from Amgen, Servier, Novartis, Lilly, and Roche, and has acted as a consultant/Advisory Board member for Amgen, Merck, Servier, and Novartis. JEC has been paid consultancy work from Servier, Shire, Nycomed, Novartis, Amgen, Procter & Gamble, Wyeth, Pfizer, The Alliance for Better Bone Health, Roche, and GlaxoSmithKline; has been a paid speaker and received reimbursement, travel and accommodation from Servier, Procter & Gamble, and Lilly; received research grants from Servier R&D and Procter & Gamble. SHG has received funding from The Alliance for Better Bone Health (sanofi-aventis and Warner Chilcott). SLG has been paid consultancy work by and is on the scientific advisory boards for Amgen, Lilly, Merck; has received research grants from the Alliance for Better Bone Health (sanofi-aventis and Proctor & Gamble) and Lilly. FHH has received funding from The Alliance for Better Bone Health (sanofi-aventis and Warner Chilcott). JCN has undertaken paid consultancy work for Roche Diagnostics, Daiichi-Sankyo, Procter & Gamble, and Nycomed, has been a paid speaker for and received reimbursement, travel and accommodation from Roche Diagnostics, Novartis, Daiichi-Sankyo, and Procter & Gamble, and has received research grants from The Alliance for Better Bone Health and Amgen. JP has received research grants from Amgen, Kyphon, Novartis, and Roche, has received other research support (equipment) from GE Lunar, has served on Speakers’ Bureaus for Amgen, sanofi-aventis, GlaxoSmithKline, Roche, Lilly Deutschland, Orion Pharma, Merck, Merckle, Nycomed, and Procter & Gamble, and has acted as an Advisory Board member for Novartis, Roche, Procter & Gamble, and Teva. MR has served on Speakers’ Bureaus for Roche, Merck Sharp & Dohme, and GlaxoSmithKline. PNS has received honoraria from and acted as a consultant/Advisory Board member for Merck, sanofi-aventis, Roche, and Servier. SS has received research grants from Wyeth, Lilly, Novartis, and Alliance, has served on Speakers’ Bureaus for Lilly, Novartis, Pfizer, and Procter & Gamble, has received honoraria from Procter & Gamble, and has acted as a consultant/Advisory Board member for Lilly, Argen, Wyeth, Merck, Roche, and Novartis. ESS has acted as a consultant for Amgen, Lilly, Novartis, and The Alliance for Better Bone Health, and has served on Speakers’ Bureaus in the past year for Amgen, and Lilly. NBW has received honoraria for lectures in the past year from Amgen, Novartis, and Warner Chilcott, has acted as a consultant in the past year for Amgen, Arena, Baxter, InteKrin, Johnson & Johnson, Lilly, Medpace, Merck, NPS, Orexigen, Pfizer/Wyeth, Takeda, Vivus, and Warner Chilcott; has received research support (through University) from Amgen, Merck, and NPS; and co-founded, has stock options in, and is a director of OsteoDynamics. AD-P has received consulting fees and lectured for Eli Lilly, Amgen, Procter & Gamble, Servier, and Daiichi-Sankyo; has been an expert witness for Merck; and is a consultant/Advisory Board member for Novartis, Eli Lilly, Amgen, and Procter & Gamble.
REFERENCES
- 1.Nguyen ND, Ahlborg HG, Center JR, et al. Residual lifetime risk of fractures in women and men. J Bone Miner Res. 2007;22:781–788. doi: 10.1359/jbmr.070315. [DOI] [PubMed] [Google Scholar]
- 2.Felson DT, Naimark A, Anderson J, et al. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1987;30:914–918. doi: 10.1002/art.1780300811. [DOI] [PubMed] [Google Scholar]
- 3.Murphy L, Schwartz TA, Helmick CG, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59:1207–1213. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy LB, Helmick CG, Schwartz TA, et al. One in four people may develop symptomatic hip osteoarthritis in his or her lifetime. Osteoarthritis Cartilage. 2010;18:1372–1379. doi: 10.1016/j.joca.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arthritis Research Campaign. Arthritis: The Big Picture. London: 2002. [accessed November 2011]. http://www.ipsos-mori.com/Assets/Docs/Archive/Polls/arthritis.pdf. [Google Scholar]
- 6.Foss MV, Byers PD. Bone density, osteoarthrosis of the hip, and fracture of the upper end of the femur. Ann Rheum Dis. 1972;31:259–264. doi: 10.1136/ard.31.4.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hannan MT, Anderson JJ, Zhang Y, et al. Bone mineral density and knee osteoarthritis in elderly men and women. The Framingham Study. Arthritis Rheum. 1993;36:1671–1680. doi: 10.1002/art.1780361205. [DOI] [PubMed] [Google Scholar]
- 8.Nevitt MC, Lane NE, Scott JC, et al. Radiographic osteoarthritis of the hip and bone mineral density. Arthritis Rheum. 1995;38:907–916. doi: 10.1002/art.1780380706. [DOI] [PubMed] [Google Scholar]
- 9.Dequeker J, Johnell O. Osteoarthritis protects against femoral neck fracture: the MEDOS study experience. Bone. 1993;14(Suppl 1):S51–S56. doi: 10.1016/8756-3282(93)90350-j. [DOI] [PubMed] [Google Scholar]
- 10.Jones G, Nguyen T, Sambrook PN, et al. A longitudinal study of the effect of spinal degenerative disease on bone density in the elderly. J Rheumatol. 1995;22:932–936. [PubMed] [Google Scholar]
- 11.Arden NK, Griffiths GO, Hart DJ, et al. The association between osteoarthritis and osteoporotic fracture: the Chingford Study. Br J Rheumatol. 1996;35:1299–1304. doi: 10.1093/rheumatology/35.12.1299. [DOI] [PubMed] [Google Scholar]
- 12.Bergink AP, van der Klift M, Hofman A, et al. Osteoarthritis of the knee is associated with vertebral and nonvertebral fractures in the elderly: the Rotterdam Study. Arthritis Rheum. 2003;49:648–657. doi: 10.1002/art.11380. [DOI] [PubMed] [Google Scholar]
- 13.Arden NK, Nevitt MC, Lane NE, et al. Osteoarthritis and risk of falls, rates of bone loss, and osteoporotic fractures. Arthritis Rheum. 1999;42:1378–1385. doi: 10.1002/1529-0131(199907)42:7<1378::AID-ANR11>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 14.Arden NK, Crozier S, Smith H, et al. Knee pain, knee osteoarthritis, and the risk of fracture. Arthritis Rheum. 2006;55:610–615. doi: 10.1002/art.22088. [DOI] [PubMed] [Google Scholar]
- 15.Hooven FH, Adachi JD, Adami S, et al. The Global Longitudinal Study of Osteoporosis in Women (GLOW): rationale and study design. Osteoporos Int. 2009;20:1107–1116. doi: 10.1007/s00198-009-0958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosmer DW, Lemeshow S, May S. Applied Survival Analysis: Regression Modeling of Time to Event Data (Wiley Series in Probability and Statistics) 2nd. Hoboken, New Jersey: John Wiley & Sons. Inc.; 2008. [Google Scholar]
- 17.Prieto-Alhambra D, Javaid MK, Judge A, et al. Fracture risk before and after total hip replacement in patients with osteoarthritis: potential benefits of bisphosphonate use. Arthritis Rheum. 2011;63:992–1001. doi: 10.1002/art.30214. [DOI] [PubMed] [Google Scholar]
- 18.Vestergaard P, Rejnmark L, Mosekilde L. Osteoarthritis and risk of fractures. Calcif Tissue Int. 2009;84:249–256. doi: 10.1007/s00223-009-9224-z. [DOI] [PubMed] [Google Scholar]
- 19.Burger H, van Daele PL, Odding E, et al. Association of radiographically evident osteoarthritis with higher bone mineral density and increased bone loss with age. The Rotterdam Study. Arthritis Rheum. 1996;39:81–86. doi: 10.1002/art.1780390111. [DOI] [PubMed] [Google Scholar]
- 20.Sowers M, Zobel D, Weissfeld L, et al. Progression of osteoarthritis of the hand and metacarpal bone loss. A twenty-year followup of incident cases. Arthritis Rheum. 1991;34:36–42. doi: 10.1002/art.1780340106. [DOI] [PubMed] [Google Scholar]
- 21.Barrett-Connor E, Weiss TW, McHorney CA, et al. Predictors of falls among postmenopausal women: results from the National Osteoporosis Risk Assessment (NORA) Osteoporos Int. 2009;20:715–722. doi: 10.1007/s00198-008-0748-2. [DOI] [PubMed] [Google Scholar]
- 22.Lane NE, Brandt K, Hawker G, et al. OARSI-FDA initiative: defining the disease state of osteoarthritis. Osteoarthritis Cartilage. 2011;19:478–482. doi: 10.1016/j.joca.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Jones G, Nguyen T, Sambrook PN, et al. Osteoarthritis, bone density, postural stability, and osteoporotic fractures: a population based study. J Rheumatol. 1995;22:921–925. [PubMed] [Google Scholar]
- 24.Pandya NK, Draganich LF, Mauer A, et al. Osteoarthritis of the knees increases the propensity to trip on an obstacle. Clin Orthop Relat Res. 2005:150–156. doi: 10.1097/01.blo.0000150316.97009.f2. [DOI] [PubMed] [Google Scholar]
- 25.Chaganti RK, Parimi N, Cawthon P, Dam TL, Nevitt MC, Lane NE. Association of 25-hydroxyvitamin D with prevalent osteoarthritis of the hip in elderly men: the osteoporotic fractures in men study. Arthritis Rheum. 2010 Feb;62(2):511–514. doi: 10.1002/art.27241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heidari B, Heidari P, Hajian-Tilaki K. Association between serum vitamin D deficiency and knee osteoarthritis. Int Orthop. 2011 Nov;35(11):1627–1631. doi: 10.1007/s00264-010-1186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalichman L, Kobyliansky E. Association between circulatory levels of vitamin D and radiographic hand osteoarthritis. Rheumatol Int. 2012 Jan;32(1):253–257. doi: 10.1007/s00296-010-1741-6. [DOI] [PubMed] [Google Scholar]
- 28.Rasooly I, Papageorgiou AC, Badley EM. Comparison of clinical and self reported diagnosis for rheumatology outpatients. Ann Rheum Dis. 1995;54:850–852. doi: 10.1136/ard.54.10.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Church J, Goodall S, Norman R, Haas M. An economic evaluation of community and residential aged care falls prevention strategies in NSW. New South Wales Public Health Bulletin. 2012;22(4):60–68. doi: 10.1071/NB10051. [DOI] [PubMed] [Google Scholar]
- 30.Cameron ID, Murray GR, Gillespie LD, Robertson MC, Hill KD, Cumming RG, et al. Interventions for preventing falls in older people in nursing care facilities and hospitals. Cochrane Database Syst Rev. 2010;(1):CD005465. doi: 10.1002/14651858.CD005465.pub2. [DOI] [PubMed] [Google Scholar]
- 31.Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692. doi: 10.1136/bmj.b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barlow JH, Turner AP, Wright CC. Comparison of clinical and self-reported diagnoses for participants on a community-based arthritis self-management programme. Br J Rheumatol. 1998;37:985–987. doi: 10.1093/rheumatology/37.9.985. [DOI] [PubMed] [Google Scholar]
- 33.Ismail AA, O’Neill TW, Cockerill W, et al. Validity of self-report of fractures: results from a prospective study in men and women across Europe. Osteoporos Int. 2000;11:248–254. doi: 10.1007/s001980050288. [DOI] [PubMed] [Google Scholar]
- 34.Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169:1952–1960. doi: 10.1001/archinternmed.2009.357. [DOI] [PubMed] [Google Scholar]
- 35.Williams LJ, Pasco JA, Henry MJ, et al. Paracetamol (acetaminophen) use, fracture and bone mineral density. Bone. 2011;48:1277–1281. doi: 10.1016/j.bone.2011.03.435. [DOI] [PubMed] [Google Scholar]
- 36.Miller M, Stürmer T, Azrael D, et al. Opioid analgesics and the risk of fractures in older adults with arthritis. J Am Geriatr Soc. 2011;59:430–438. doi: 10.1111/j.1532-5415.2011.03318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]