Abstract
Introduction
Serotonin (5-HT) is an important neuromodulator, but recently has been shown to be involved in neurodevelopment. Although previous studies have demonstrated that the placenta is a major source of forebrain 5-HT during early forebrain development, the processes of how 5-HT production, metabolism, and transport from placenta to fetus are regulated are unknown. As an initial step in determining the mechanisms involved, we investigated the expression patterns of genes critical for 5-HT system function in mouse extraembryonic tissues.
Methods
Mid- through late gestation expression of 5-HT system-related enzymes, Tph1, Ddc, Maoa, and 5-HT transporters, Sert/Slc6a4, Oct3/Slc22a3, Vmat2/Slc18a2, and 5-HT in placenta and yolk sac were examined, with cell type-specific resolution, using multiplex fluorescent in situ hybridization to co-localize transcripts and immunocytochemistry to co-localize the corresponding proteins and neurotransmitter.
Results
Tph1 and Ddc are found in the syncytiotrophoblast I (SynT-I) and sinusoidal trophoblast giant cells (S-TGC), whereas Maoa is expressed in SynT-I, syncytiotrophoblast II (SynT-II) and S-TGC. Oct3 expression is observed in the SynT-II only, while Vmat2 is mainly expressed in S-TGC. Surprisingly, there were comparatively high expression of Tph1, Ddc, and Maoa in the yolk sac visceral endoderm.
Discussion
In addition to trophoblast cells, visceral endoderm cells in the yolk sac may contribute to fetal 5-HT production. The findings raise the possibility of a more complex regulation of 5-HT access to the fetus through the differential roles of trophoblasts that surround maternal and fetal blood space and of yolk sac endoderm prior to normal degeneration.
1. Introduction
Maternal-fetal interactions have long-term health consequences for the offspring[1,2], including neurodevelopmental disorders[3,4]. The placenta, central to the maternal-fetal interface, plays an important role in regulating these interactions. Recently we demonstrated that the placenta is a major site of fetal serotonin (5-hydroxytryptamine, 5-HT) production in mice and humans [5], providing the monoamine to the forebrain during early development. Although 5-HT is best known as a monoamine neurotransmitter in the adult brain, it was first discovered as intestine contractor and vasoconstrictor [6]. And 5-HT has been shown to modulate neurogenesis, axon guidance and axon pathway refinement during fetal brain development [7–9]. 5-HT is derived from tryptophan, an essential amino acid, first by tryptophan hydroxylase (TPH1 or TPH2) that converts tryptophan to 5-hydroxytryptophan (5-HTP) in a tetrahydrobiopterin (BH4)-dependent manner. This is followed by pyridoxal phosphate (PLP)-depenendent decarboxylation of 5-HTP to 5-HT via dopa decarboxylase (DDC) (Figure 1D; [6,10–13]. Serotonergic circuitry in the central nervous system originates early in embryonic development from a small number of brainstem neurons expressing both TPH2 and DDC. These neurons develop an extensive axonal network that innervate structures throughout the entire central nervous system [14]. Studies from our laboratory demonstrated that deregulation of fetal 5-HT signaling before serotonergic axons reach forebrain results in altered brain circuitry [7]. We also reported that at early developmental ages, the placenta, which expresses both TPH1 and DDC in the syncytiotrophoblasts, produces 5-HT [5]. Most importantly, 5-HT produced by the placenta reaches the embryonic circulation and is transported to the forebrain prior to brain 5-HT axons reach this region [5]. Thus, the findings connect placenta function directly to influencing brain circuitry. Nonetheless, the specific cells responsible for 5-HT synthesis, degradation, and transport in the placenta are not known.
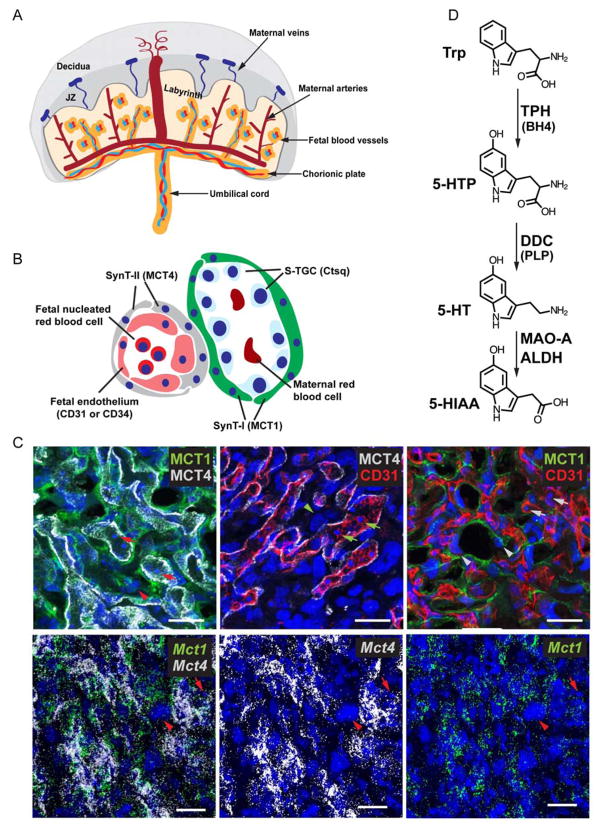
Fig 1. Different cell types and their molecular markers in the labyrinth.
(A) Diagram showing the general structure of the murine placenta. (B) Schematic drawing depicting the cell types and cell organizations in the labyrinth. Molecular markers used to label specific cell type are shown in parentheses. SynT-I, syncytiotrophoblast I; SynT-II, syncytiotrophoblast II; S-TGC, sinusoidal trophoblast giant cell. (C) Top panels: Co-IF used to detect SynT-I, SynT-II, and fetal endothelial cells using antibodies against MCT1, MCT4, and CD31, respectively. Bottom panels: Multiplex fluorescent ISH used to detect Mct1 and Mct4 mRNA. Arrowheads point to S-TGC. Arrows point to fetal nucleated red blood cell. Scale bar = 30 μm. (D) Diagram of the serotonin metabolic pathway. Serotonin (5-hydroxytryptamine, 5-HT) synthesis begins with hydroxylation of tryptophan, an essential amino acid, by tryptophan hydroxylase (TPH1 or TPH2), in a tetrahydrobiopterin (BH4)-dependent manner. This reaction is followed by a pyridoxal phosphate (PLP)-dependent decarboxylation by dopa decarboxylase (DDC, also called aromatic L- amino acid decarboxylase). 5-HT is cleared from cells after being converted to 5-HIAA by MAO-A and ALDH. Trp, tryptophan; 5-HTP, 5-hydroxytryptophan; DDC, dopa decarboxylase; PLP, pyridoxal phosphate; MAO-A, monoamine oxidase A; ALDH, aldehyde dehydrogenase; 5-HIAA, 5-hydroxyindoleacetic acid.
In the brain, in addition to TPH and DDC, several other molecules control the availability of 5-HT. Because 5-HT is hydrophilic in nature, the neurotransmitter is packed into synaptic vesicles, via the vesicular monoamine transporter 2 (VMAT2/SLC18A2), for later release [15]. Levels of extracellular and intracellular 5-HT are regulated by monoamine transporters, including the serotonin transporter, SERT/SLC6A4 [15–17], plasma membrane monoamine transporter, PMAT/SLC29A4 [18], and extraneuronal monoamine transporter, OCT3/SLC22A3 [18]. Finally, the overall intracellular level of 5-HT is regulated by monoamine oxidase A (MAO-A), which, together with aldehyde dehydrogenase, converts 5-HT to 5-hydroxyindoleacetic acid (5-HIAA) for excretion in the urine (Fig. 1D) [16,19]. Deregulation in the function of any of these proteins disturbs 5-HT actions and has been linked to depression and other neurological dysfunction [9,20]. Although much is known about the regulation of 5-HT production, transport and metabolism in the adult brain and peripheral organs such as the enteric nervous system, little is known about these parameters in the fetus. Using an in vitro TPH assay, we showed that both mouse (embryonic day 14.5 and 18.5) and human placenta (gestation week 11) synthesize 5-HTP from tryptophan [5]. We also demonstrated that 5-HT produced in the placenta can be transported to fetal forebrain by ex vivo perfusion of mouse placenta and by in vivo injection of a TPH inhibitor directly to the labyrinth to block 5-HT synthesis.
The fetus is provided with nutrients from the maternal environment via the yolk sac visceral endoderm and the placenta [21]. In the murine placenta, there are three layers of trophoblast cells and a layer of fetal endothelial cells separating the maternal from the fetal blood spaces [22,23]. The trilaminar trophoblast cells in the mouse placental labyrinth include mononuclear sinusoidal trophoblast giant cells (S-TGCs) that line the maternal blood sinusoids [24–26], and two layers of thin, multinucleated syncytiotrophoblast cells, SynT-I apposed to maternal vascular endothelial cells, and SynT-II apposed to fetal capillary endothelial cells [22,23,25,27,28]. Each placental labyrinth cell type express unique molecular properties that provide unique identifiers [26,29]. We took advantage of this feature to investigate the expression patterns of genes critical for 5-HT system function during embryogenesis in extraembryonic tissues in pregnant mice. The expression patterns of serotonergic pathway-related genes in extraembryonic tissues were determined at the cellular resolution using co-immunofluorescence (IF) staining and multiplex fluorescence in situ hybridization (MF-ISH) by double and triple labeling with cell type-specific markers. The data indicate that it is likely that a more complex regulation of 5-HT in extraembryonic tissues exist than previously appreciated.
2. Materials and methods
2.1 Animals
Time pregnant ICR/CD-1 mice were purchased from Harlan Laboratories, Inc. Mice were maintained on a 12-hour light /12-hour dark cycle with free access to food and water. The day when a vaginal plug was observed is considered to be day 0.5 of gestation (E0.5). Pregnant females were euthanized with isoflurane vapors followed by cervical dislocation prior to cesarean section to harvest embryonic tissues. All experimental procedures using animals were approved by the Institutional Animal Care and Use Committee of Children’s Hospital Los Angeles and conformed to US National Institutes of Health guidelines.
2.2 Immunofluorescence (IF) staining
Placenta tissues with yolk sac attached were harvested at E12.5, E14.5, and E17.5 and fixed at 4°C in 4% paraformaldehyde in PBS (pH7.4) overnight, followed by cryoprotection with 10, 20, and 30% sucrose in PBS overnight at 4°C. Tissues were then embedded in tissue freezing medium (Triangle Biomedical Sciences, Inc.) over liquid nitrogen vapors and stored at −80°C. Tissues were cryosectioned at 16- μm thickness and stored at −80°C until processing. All primary and secondary antibodies are listed in Supplemental Table 1. Except for TPH1 and SERT, immunostaining was performed as follows: sections were permeabilized and blocked with 5% normal donkey serum (Jackson ImmunoResearch Laboratories) in PBS with 0.1% Triton X-100 and incubated overnight at room temperature with primary antibodies in a humidified chamber. Slides were then incubated at room temperature for 1 hour in secondary antibodies diluted in blocking solution. Slides were incubated with 950 nM of DAPI (Life Technology) for 5 minutes and mounted with Prolong Gold antifade mounting agent (Life Technology). For Co-IF with anti-TPH1 or anti-SERT, sections were treated with 3% hydrogen peroxide in methanol for 15 minutes at room temperature followed by blocking and incubation with primary antibodies cocktails as described above. Horseradish peroxidase conjugated secondary antibodies were used for detection. TSA plus-Cy3 amplification was performed according to the manufacturer’s recommendation (Perkin Elmer NEL744001KT).
2.3 Multiplex Fluorescence In Situ Hybridization (MF-ISH)
Placenta with yolk sac attached were immediately frozen in ice-cold isopentane for 15 to 25 seconds and then stored at −80°C. Tissues were cryosectioned at 16 μm thickness, and stored at −80°C. Commercially available RNAscope Multiplex Flu orescent reagent kits and RNAscope probes were used for transcript detection (Advanced Cell Diagnostics, Hayward, CA). Supplemental Table 2 has the complete probe list. Each set of probes contained a tag that enables the target transcript to be visualized in a specific color channel (C): C1, Alexa488; C2, Atto555; and C3, Atto647. MF-ISH was performed according to the manufacturer’s protocol. A multiplex positive control probe mix consisting of probes directed against 3 different housekeeping genes, Polr2a (C1), Ppib (C2), and Ubc (C3), and negative control probes directed against a bacterial gene, DapB (all 3 channels), were purchased from Advanced Cell Diagnostics and were used as references for the signal intensity and background level in each channel.
2.4 Imaging and fluorescent signal intensity measurement
Images were acquired as Z stacks using a Zeiss Axio Observer Inverted microscope fitted with a LSM700 confocal scanner (Cellular Imaging Core at the Saban Research Institute at Children’s Hospital Los Angeles) controlled by Zeiss Zen 2009 program. Figures were prepared digitally using Adobe Photoshop CS5.1 and Adobe Illustrator CS5.1 (Adobe Systems In., San Jose, CA). To compare the expression differences between yolk sac and labyrinth, fluorescent intensities from Maoa, Tph1, or Ddc were quantified using FIJI ImageJ software (NIH) [30]. The mean intensities from 3 random areas of the same size (45.19 μm x 34.92 μm) in the labyrinth or yolk sac were measured for each probe.
2.5 In vitro TPH enzymatic assay and HPLC analysis
In vitro TPH enzymatic assay was performed as previously described [5] with the following modifications. Five to eight E12.5 yolk sacs from a pregnant CD-1 dam were collected and frozen immediately with liquid nitrogen vapor. The collected tissues were homogenized in 400 μl of 0.05M Tris buffer, pH7.5, containing 1mM dithiothreitol and 1mM EGTA. Homogenates were centrifuged at 29,000g for 15 min at 4°C and the supernatants were transferred to a fresh tube on ice. A 20 μl aliquot of the supernatants were incubated with 0.05 mg/mL catalase, 200 μM L-tryptophan, 100 μM ferrous ammonium sulfate, and 50 μM BH4 for 30min at 37°C and stopped by flash freezing in liquid nitrogen. An equivalent amount of supernatant was used in parallel without addition of tryptophan and BH4 as a negative control. 5-HTP was measured using HPLC as described below. Yolk sacs from 3 different dams were collected separately and used as 3 independent samples.
The HPLC analysis of 5-HTP was performed on an Eicom 700 system (Eicom Corporation) consisting of an ECD-700 electrochemical detector, an Eicom 700 Insight autosampler, and the Envision integration software. Eicompak SC-3ODS 3 μm C18 reversed-phase column (3.0 x 100 mm I.D.) analytical column was used for separation. 100 μl of enzymatic assay samples were extracted with equal volume of 0.2 M perchloric acid with 100 μM EDTA and incubated at 4°C for 15 min. Extracted samples were centrifuged at 21,000g for 15 min at 4°C, and a volume of 10 μL was injected into the column. The mobile phase used for separation consisted of 0.1M citric acid, 0.2M sodium phosphate dibasic, 7% methanol (JT Baker), and 5 mg EDTA in ultrapure water at a flow rate of 400 μL/minute.
3. Results
Supplemental Table 3 summarizes expression patterns of the transcripts and proteins investigated in the present study.
Expression of Tph1 and Ddc in the labyrinth by syncytiotrophoblast I and sinusoidal trophoblast giant cells
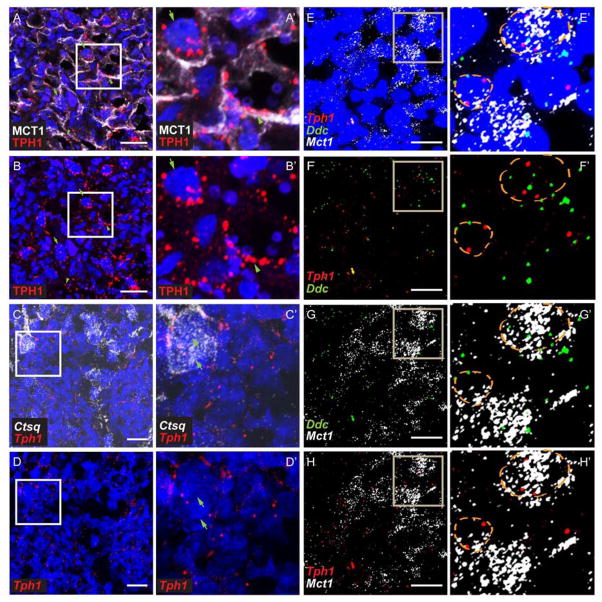
Serotonin can only be synthesized from tryptophan by TPH and DDC [31,32]. Thus, cells expressing TPH and DDC make 5-HT. There are two TPH isoforms: TPH1 is expressed in non-neuronal cells including placenta and TPH2 is only expressed in the brainstem and a small number of gut enteric neurons [32,33]. We previously reported that TPH1 and DDC are expressed in the placental labyrinth at E14.5 [5]. The specific cell types and the dynamic range of expression during development however were not determined. In the mouse placenta, three layers of trophoblast cells (SynT-I, SynT-II, and S-TGC) and a layer of fetal endothelial cells separate the maternal blood space from fetal blood space (Fig.1A and B). Genes and proteins specifically expressed by these different types of cells in the labyrinth have been identified and were used as markers [26,29]. Monocarboxylic acid transporter 1, MCT1 (solute carrier family 16 member 1, SLC16A1), is a SynT-I apical protein; Monocarboxylic acid transporter 4, MCT4 (solute carrier family 16 member 3, SLC16A3) is expressed in SynT-II; cathepsin Q mRNA (Ctsq) is present exclusively in S-TGC; CD31 is expressed by endothelial cells (Fig. 1B–C) [26,29]. MCT1 delineates a cell layer apposed to maternal red blood cells and S-TGC is identified by their large nucleus (Fig. 1C, arrowheads). MCT4 marks SynT-II cells, which surround CD31+ fetal vascular endothelial cells (Fig. 1C) and fetal nucleated red blood cells (Fig. 1C, arrows). Using MF-ISH, expression of Mct1 and Mct4 mRNA exhibited identical expression patterns (Fig. 1C bottom panels). Co-IF revealed that TPH1 was co-localized with MCT1+ SynT-I cells and some MCT1-negative cells with large nuclei, most likely S-TGCs (Fig. 2A, A’, B, and B’, arrows). By MF-ISH, Ctsq and Tph1 mRNAs were co-localized in the labyrinth from E12.5 onward (Fig. 2C, C’, D, and D’; Supplemental Table 3). These results confirm the expression of Tph1 in the S-TGC cells. Similar to Tph1, Ddc mRNA is expressed by labyrinth cells. Co-localization of Ddc and Tph1 was observed at all prenatal ages examined (Fig. 2E, E’, F, F’, G, G’, H, and H’; Supplemental Table 3). Collectively, the data show that TPH1/Tph1 and Ddc colocalize in the SynT-I and S-TGC, but not in SynT-II cells.
Fig 2. Expression of Tph1 and Ddc in the SynTI and S-TGC.
(A and B) Co-IF staining with anti-MCT1 and anti-TPH1 were performed on E12.5 placenta sections. (A’ and B’) are higher magnification images of the boxed area in (A and B). Arrowheads point to TPH1 immunoreactivity in a MCT1+ cell; Arrows point to TPH1 immunoreactivity next to a trophoblast giant cell. (C and D) Multiplex fluorescent ISH was performed on E12.5 placenta sections using RNAscope probes directed against Ctsq and Tph1. (C’ and D’) higher magnification images of the boxed area in (C and D). Arrows point to several Ctsq+ S-TGCs that are Tph1 +. (E–H) Multiplex fluorescent ISH was performed on E14.5 placenta sections, using RNAscope probes directed against Mct1, Ddc, and Tph1. (E’– H’) are higher magnification images of the boxed area in (E–H). Orange dotted circles indicate area of a nucleus revealed by DAPI staining. Scale bar = 30 μm.
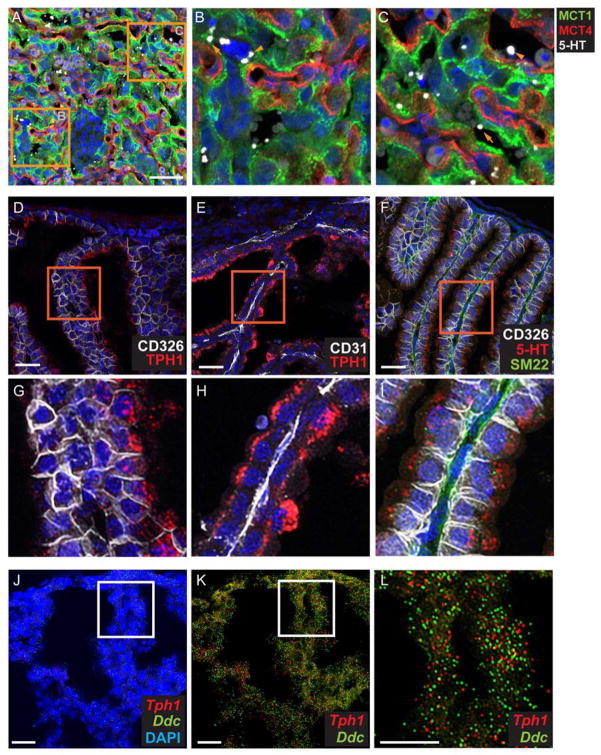
Given the distribution of the synthetic enzymes in SynT-I and S-TGC, we next examined the patterns of accumulation of 5-HT in the placenta. Using co-IF, we detected 5-HT in the labyrinth, consistent with previous reports of the synthetic capacity of the placenta to produce 5-HT [5]. At all prenatal ages, 5-HT immunoreactivity is observed in S-TGC as well as in the SynT-I, SynT-II, and fetal blood space close to the endothelial cells (Fig. 3A–C). Surprisingly, 5-HT appeared as large profiles that were approximately 1 to 3 μm in diameter, possibly in extracellular vesicles.
Fig 3. TPH1 and 5-HT are present in the visceral endoderm yolk sac.
(A–C) 5-HT is present in all cell types in the labyrinth, as well as in the fetal blood space. Images of CoIF staining with anti-5HT, anti-MCT1, anti-MCT4 performed on E12.5 placenta. (B and C), higher magnification images of the boxed area in (A). In (B), arrowhead points to a S-TGC apposed to SynT-I. In (C), arrowhead points to fetal blood space and arrow points to MCT1+ SynT-I. Scale bar in A = 50 μm. (D–I) Co-IF staining was performed with anti-5HT and yolk sac cell type specific molecular markers, CD326, visceral endoderm marker, CD31, endothelial cell marker, and SM22, mesoderm marker. (G–I), higher magnification of boxed area of (D–H) respectively. Scale bar in D to F = 30 μm. (J–L) Multiplex fluorescent ISH using RNAscope probes directed against Tph1 and Ddc performed on the same section. (L), higher magnification of boxed area in J and K. Blue: DAPI. Scale bar = 30 μm.
Expression of Tph1 and Ddc in the yolk sac visceral endoderm
Unexpectedly, we observe dense TPH1 labeling of cells in the yolk sac at E12.5 and onward. Co-IF analysis shows the presence of TPH1 restricted to the cells expressing CD326 (Fig. 3D and G), a membrane proteins expressed in the yolk sac visceral endoderm (VE) [34]. The expression in VE was specific, as TPH1 labeling was absent from SM22alpha+ mesoderm cells [35], and CD31+ endothelium cells (Fig. 3E and H and data not shown). The cellular specificity of the immunolocalized proteins was validated by MF-ISH for Tph1 and Ddc mRNAs (Fig. 3J–L). Differences in labeling intensity between syncytiotrophoblasts and endoderm were very evident (compare Fig. 2A and 3D), with the most intense staining in the yolk sac cells. The presence of both Tph1 and Ddc in the yolk sac suggests a capacity for 5-HT synthesis. Consistent with this finding, 5-HT also was detected on the apical side of the VE, appearing in large vacuoles similar in size to the 5-HT+ profiles visualized in the placental labyrinth (Fig. 3J). In vitro TPH assay also confirms the presence of TPH activity in the yolk sac. An approximately 194 fold increased of 5-HTP was detected in the presence of Trp and BH4 (3.1±0.35 ng/20 μl yolk sac homogenates) compared with the negative controls (0.017±0.0036 ng /20 μl yolk sac homogenates). Together these data show that yolk sac visceral endoderm cells can synthesize 5-HT.
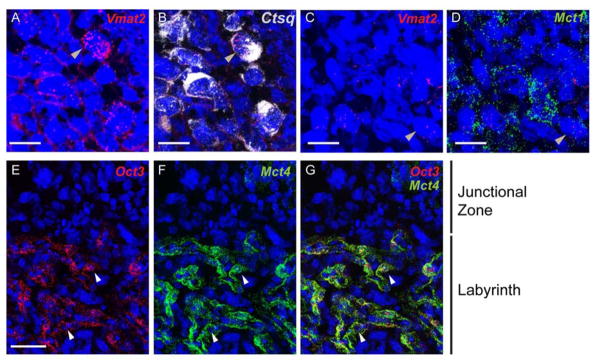
Finally, MF-ISH localization of Vmat2 revealed low levels of Vmat2 mRNA in Ctsq+ S-TGC and in some Mct1+ SynT-I (Figure 4A–D). No Vmat2 mRNA was observed in the yolk sac at any stage examined (data not shown). These data are consistent with a limited capacity of SynTs and VE to store 5-HT in classic vesicles as observed in the brain.
Fig 4. Expression of Vmat2 and Oct3 in the labyrinth.
(A and B), Multiplex fluorescent ISH using RNAscope probes directed against Vmat2 (red) and Ctsq (White) performed on the same section. (C and D), multiplex fluorescent ISH for Vmat2 (red) and Mct1 (White) on same section. Blue: DAPI. Scale bar for A to D = 30 μm. (E–G) Multiplex fluorescent ISH using RNAscope probes directed against Mct4, and Oct3 on E12.5 placenta sections. Scale bar for E to G = 50 μm.
Maoa and Oct3 expression in labyrinth
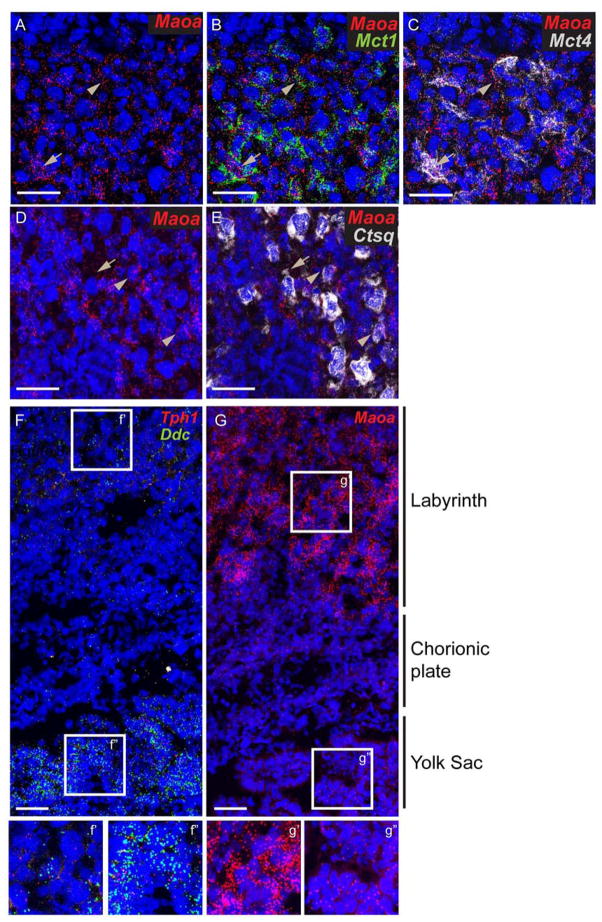
In the brain, both extra- and intracellular concentration of 5-HT is highly regulated to ensure proper physiological activity. Deficits of the major degradation enzyme, MAO-A and the transporters, SERT and OCT3, lead to dysfunction of the serotoninergic systems in the brain, resulting in aberrant behavioral outcomes [9]. A prior study reported that Maoa and Oct3 are expressed in the labyrinth from E12.5 onward, whereas Sert expression in the same placental region was minimal [36]. To examine this more precisely across prenatal development and with cellular resolution, we used the multiplex fluorescent ISH labeling strategy. The majority of Maoa mRNA was co-expressed with Mct1 and Mct4 (Fig. 5A–C), markers of SynT-I (maternal lining) and SynT-II (fetal lining) whereas most of the Oct3 mRNA was co-localized to Mct4+ cells only (Fig. 4E–G). Very low levels of Maoa were co-localized with Ctsq (Fig. 5D and E). As noted previously, we found that Sert mRNA expression was undetectable in the labyrinth until E17.5. The data are consistent with cellular segregation of synthetic and metabolic capacities to regulate 5-HT.
Fig 5. Expression of Maoa in the labyrinth and yolk sac.
Multiplex fluorescent ISH using RNAscope probes directly against (A–C) Maoa, Mct1, and Mct4 on the same section, (D–E) Maoa and Ctsq on the same section, (F–G) Maoa, or Ddc, and Tph1 (on the same section) on E14.5 placenta sections. (f’, f’’, g’, and g’’) magnify view of boxed region in F and G. Blue: DAPI. Scale bar = 50 μm.
Maoa in the visceral endoderm yolk sac
Because gene expression analysis suggests that 5-HT synthesis can occur in the yolk sac VE, we also assessed the presence of Maoa, Sert, and Oct3. Only Maoa is expressed in VE (Fig. 5G and data not shown), with levels appearing much lower than in the labyrinth. To confirm this finding, we measured the mean fluorescent intensity (FI; expressed as arbitrary unit) for Maoa in the labyrinth and yolk sac. The FI is 3.2 fold higher in the labyrinth (62.90±2.64 a. u.) than in the yolk sac (19.49±0.80 a. u.). We also measured the FI of Tph1 and Ddc. Confirming our qualitative observations, these transcripts are expressed at lower levels in the labyrinth than in the yolk sac (7.51±1.77 vs 13.52±0.59 respectively for Tph1 and 9.09±5.46 vs 50.66±3.96 respectively for Ddc). Thus, expression levels of Maoa appear to be inversely correlated to that of Tph1 and Ddc, with the level of Tph1 and Ddc mRNA in the yolk sac much greater than in the labyrinth, and the reciprocal relation for Maoa (yolk sac vs labyrinth: 1.8 fold for Tph1, 5.57 fold for Ddc, and 0.3 fold for Maoa) (Fig. 5F and G).
4. Discussion
The expression studies presented here reveal more complex patterns of expression and parcellation of 5-HT synthetic, transport, and metabolic capacities than previously recognized. While descriptive in nature, the localization at cellular resolution provides a basis for determining cell type-specific roles for 5-HT regulation in extraembryonic tissues. Studies have shown that the maternal-fetal interface plays an important role in brain development [4,3], but the mechanisms that underlie this influence are not well understood. At a molecular level, the placenta appears to be quite complex [37], and here we show that even for a single metabolic pathway that synthesizes and regulates levels of 5-HT, expression patterns are more intricate than previously appreciated. These are likely to reflect regulatory precision of 5-HT production and transport to the fetus, which can influence fetal brain development [7,9].
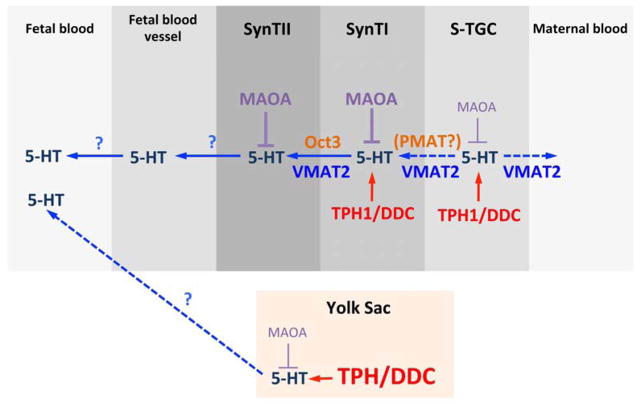
Previous studies revealed the presence of metabolic enzymes TPH1, DDC, and MAOA, and the low affinity 5-HT transporter, Oct3, in the labyrinth of the placenta [5,36], but did not provide cellular resolution. The results presented here offer a more complete view of the organization of the synthesis, metabolism, and transport of 5-HT from the placenta, which is summarized in Figure 11. New discovery is that the VE of the yolk sac is an additional source of 5-HT, which, based on intensity of labeling under identical experimental conditions, may have more synthetic capacity than the placenta proper. In the labyrinth, TPH1 is expressed by SynT-I, which is situated between the maternal blood space and SynT-II. Interestingly, although the selective 5-HT transporter, SERT, is not detected in the trophoblast cells, the low affinity 5-HT transporter OCT3 is expressed highly in SynT-II. It is possible that 5-HT is produced in SynT-I and transported to SynT-II via OCT3 for access to the fetal capillary bed. The data also reveal that in addition to SynT-I, S-TGC is a source to have 5-HT synthesis capacity. This is consistent with our detection of accumulated 5-HT in S-TGC. Interestingly, Vmat2, a membrane protein that transports 5-HT from the cytosol to synaptic vesicles in the brain, was expressed in S-TGCs. This suggests that S-TGCs have the capacity to secrete 5-HT into the maternal blood space. The function of this transport, if it occurs, is unknown. Since S-TGCs are adjacent to SynT-I, it is not clear whether 5-HT released from S-TGCs can be taken up by SynT-I. Our present results suggest it is unlikely, because neither Sert nor Oct3 is expressed in SynT-I. We are currently examining the possibility that other 5-HT membrane transporters may be expressed by SynT-I, such as the plasma membrane monoamine transporter, PMAT (Slc29a4) [18].
The data also show that the main 5-HT degradation enzyme in the placenta, Maoa, is expressed in SynT-I, SynT-II, and S-TGC, consistent with the concept that the level of either free 5-HT available in the maternal blood space or of locally synthesized 5-HT for potential transport across SynT-II into the fetal blood space is under tight regulation. Intriguingly, 5-HT immunostaining labeled large profiles in the labyrinth, both intra- and extracellularly. Although it could result from 5-HT packaging into large vesicles, only a very low level of Vmat2 expression was observed in the placenta and co-localized with Ctsq. Protein and miRNA packed exosomes and microvesicles have been shown to be released from placenta into the maternal blood [38,39]. The results suggest that 5-HT may also be packed into placental microvesicles, possibly to facilitate transport to the fetus. However, whether the placenta can release exosomes and/or microvesicles into the fetal blood is not known.
Unexpectedly, we also discovered 5-HT metabolic enzymes expression, as well as 5-HT itself in the yolk sac VE (Figure 3D–I). The visceral yolk sac endoderm plays a role in transferring essential nutrients to the fetus via the vitelline vein [21,40]. These results suggest that 5-HT arising from the endodermal cells, which secrete directly into fetal capillaries, could reach the fetus through this route as well. Interestingly, Vmat2 mRNA was not detected in the yolk sac VE. Therefore, alternative 5-HT storage and release mechanisms, that are different from those in the brain, must be present in these cells. The yolk sac also is known to be the initial site for hematopoiesis during mammalian development. Although Tph1 knockout mice have decreased number of red blood cells and reduced red blood cell survival [41,42], it has yet to be determined whether yolk sac 5-HT plays a supporting role in maintaining the survival of red blood cells or their precursors during development.
Several lines of evidence, both from clinical and animal studies, indicate that maternal stress, inflammation, and obesity can influence the development of neural circuitry in their offspring [1,4,43,44]. A potential role for the placenta and yolk sac in brain development, particularly in the first trimester, is not well understood. Recent finding of a placental source of 5-HT, which can be delivered to the fetus and modulate brain circuitry formation, provides a possible link between placenta and neurodevelopment. In addition, our present yolk sac data suggest that both extraembryonic tissues may be influential. This raises the possibility that genetic factors and the maternal environment can directly affect 5-HT metabolism and transport, which in turn may influence development of brain circuitry. It is worth noting that several 5-HT receptors are expressed in the developing forebrain prior to 5-HT axon innervation, suggesting that the extraembryonic sources of 5-HT can act upon these regions and modulate their development [7,45]. Currently, we are studying how mild maternal inflammation affects brain development in embryos with placental-specific perturbation of 5-HT pathway genes.
Supplementary Material
Fig 6. Model of 5-HT production, degradation, and possible route of transport in the extraembryonic tissues.
Schematic drawing illustrates the potential routes of 5-HT production, transport, and degradation in the mouse labyrinth and yolk sac. Details are discussed in the text.
Highlights.
Tph1 and Ddc are mainly expressed by syncytiotrophoblast I and sinusoidal trophoblast giant cells
Maoa is expressed in both syncytiotrophoblast I and II, as well as in sinusoidal trophoblast giant cells
Oct3 is expressed in syncytiotrophoblast II only
Vmat2 is mainly expressed in sinusoidal trophoblast giant cells
Tph1, Ddc, and Maoa are expressed in the yolk sac visceral endoderm.
Acknowledgments
We thank Dr. Esteban Fernandez at the Cell Imaging Core of the Saban Research Institute for his excellent technical assistance, Dr. Alexandre Bonnin at Keck School of Medicine of USC for helpful comments on the manuscript, and Nick Goeden in the Bonnin lab for his excellent technical assistance with the in vitro TPH assay and HPLC measurements. This work was supported by the Vanderbilt Conte Center for Neuroscience grant NIMH 5P50MH096972 (Project 2 to PL) and the Simms/Mann Chair in Developmental Neurogenetics at CHLA. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marques AH, O’Connor TG, Roth C, Susser E, Bjørke-Monsen AL. The influence of maternal prenatal and early childhood nutrition and maternal prenatal stress on offspring immune system development and neurodevelopmental disorders. Front Neurosci. 2013;7:1–17. doi: 10.3389/fnins.2013.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moog NK, Entringer S, Heim C, Wadhwa PD, Kathmann N, Buss C. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bronson SL, Bale TL. The Placenta as a Mediator of Stress Effects on Neurodevelopmental Reprogramming. Neuropsychopharmacology. 2015:1–12. doi: 10.1038/npp.2015.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim DR, Bale TL, Epperson CN. Prenatal programming of mental illness: current understanding of relationship and mechanisms. Curr Psychiatry Rep. 2015;17:546. doi: 10.1007/s11920-014-0546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnin A, Goeden N, Chen K, Wilson ML, King J, Shih JC, et al. A transient placental source of serotonin for the fetal forebrain. Nature. 2011;472:347–350. doi: 10.1038/nature09972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mercado CP, Ziu E, Kilic F. Communication between 5-HT and small GTPases. Curr Opin Pharmacol. 2011;11:23–8. doi: 10.1016/j.coph.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonnin A, Torii M, Wang L, Rakic P, Levitt P. Serotonin modulates the response of embryonic thalamocortical axons to netrin-1. Nat Neurosci. 2007;10:588–597. doi: 10.1038/nn1896. [DOI] [PubMed] [Google Scholar]
- 8.Bonnin A, Levitt P. Placental Source for 5-HT that Tunes Fetal Brain Development. Neuropsychopharmacology. 2012;37:299–300. doi: 10.1038/npp.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Kleef ESB, Gaspar P, Bonnin A. Insights into the complex influence of 5-HT signaling on thalamocortical axonal system development. Eur J Neurosci. 2012;35:1563–72. doi: 10.1111/j.1460-9568.2012.8096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sjoerdsma a, Palfreyman MG. History of serotonin and serotinin disorders. Ann N Y Acad Sci. 1990;600:1–7. doi: 10.1111/j.1749-6632.1990.tb16869.x. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 11.Howd RA, Nelson MF, Lytle LD. L-tryptophan and rat fetal brain serotonin. Life Sci. 1975;17:803–11. doi: 10.1016/0024-3205(75)90538-x. http://www.ncbi.nlm.nih.gov/pubmed/7372670. [DOI] [PubMed] [Google Scholar]
- 12.Johansen Pa, Jennings I, Cotton RG, Kuhn DM. Tryptophan hydroxylase is phosphorylated by protein kinase A. J Neurochem. 1995;65:882–8. doi: 10.1046/j.1471-4159.1995.65020882.x. [DOI] [PubMed] [Google Scholar]
- 13.Kuhn DM, Ruskin B, Lovenberg W. Tryptophan hydroxylase. The role of oxygen, iron, and sulfhydryl groups as determinants of stability and catalytic activity. J Biol Chem. 1980;255:4137–43. http://www.ncbi.nlm.nih.gov/pubmed/7372670. [PubMed] [Google Scholar]
- 14.Aitken AR, Tork I. Early development of serotonin-containing neurons and pathways as seen in whole mount preparation of the fetal rat brain. J Comp Neurol. 1988;274:32–47. doi: 10.1002/cne.902740105. [DOI] [PubMed] [Google Scholar]
- 15.Rudnick G, Clark J. From synapse to vesicle: The reuptake and storage of biogenic amine neurotransmitters. BBA - Bioenerg. 1993;1144:249–263. doi: 10.1016/0005-2728(93)90109-S. [DOI] [PubMed] [Google Scholar]
- 16.PLETSCHER A. Metabolism, Transfer and Storage of 5-Hydroxytryptamine in Blood Platelets*. Br J Pharmacol Chemother. 1968;32:1–16. doi: 10.1111/j.1476-5381.1968.tb00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brenner B, Harney JT, Ahmed BA, Jeffus BC, Unal R, Mehta JL, et al. Plasma serotonin levels and the platelet serotonin transporter. J Neurochem. 2007;102:206–215. doi: 10.1111/j.1471-4159.2007.04542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duan H, Wang J. Selective transport of monoamine neurotransmitters by human plasma membrane monoamine transporter and organic cation transporter 3. J Pharmacol Exp Ther. 2010;335:743–753. doi: 10.1124/jpet.110.170142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shih JC, Chen K, Ridd MJ. Monoamine oxidase: from genes to behavior. Annu Rev Neurosci. 1999;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nordquist N, Oreland L. Serotonin, genetic variability, behaviour, and psychiatric disorders-a review. Ups J Med Sci. 2010;115:2–10. doi: 10.3109/03009730903573246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zohn IE, Sarkar Aa. The visceral yolk sac endoderm provides for absorption of nutrients to the embryo during neurulation. Birth Defects Res A Clin Mol Teratol. 2010;88:593–600. doi: 10.1002/bdra.20705. [DOI] [PubMed] [Google Scholar]
- 22.Enders AC. A comparative study of the fine structure of the trophoblast in several hemochorial placentas. Am J Anat. 1965;116:29. doi: 10.1002/aja.1001160103. &. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez-Verdun D. Morphogenesis of the syncytium in the mouse placenta. Ultrastructural study. Cell Tissue Res. 1974;148:381–96. doi: 10.1007/BF00224265. http://www.ncbi.nlm.nih.gov/pubmed/4831954. [DOI] [PubMed] [Google Scholar]
- 24.Coan PM, Ferguson-Smith aC, Burton GJ. Ultrastructural changes in the interhaemal membrane and junctional zone of the murine choriollantoic placenta across gestation. J Anat. 2005;207:783–796. doi: 10.1111/j.1469-7580.2005.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simmons DG, Cross JC. Determinants of trophoblast lineage and cell subtype specification in the mouse placenta. Dev Biol. 2005;284:12–24. doi: 10.1016/j.ydbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Simmons DG, Fortier AL, Cross JC. Diverse subtypes and developmental origins of trophoblast giant cells in the mouse placenta. Dev Biol. 2007;304:567–578. doi: 10.1016/j.ydbio.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 27.JOLLIE WP. Fine Structural Changes in the Junctional Zone of the Rat Placenta With Increasing Gestational Age. J Ultrastruct Res. 1965;12:420–438. doi: 10.1016/s0022-5320(65)80109-5. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=14328788. [DOI] [PubMed] [Google Scholar]
- 28.Watson ED. Development of Structures and Transport Functions in the Mouse Placenta. Physiology. 2005;20:180–193. doi: 10.1152/physiol.00001.2005. [DOI] [PubMed] [Google Scholar]
- 29.Nadeau V, Charron J. Essential role of the ERK/MAPK pathway in blood-placental barrier formation. Development. 2014;141:2825–37. doi: 10.1242/dev.107409. [DOI] [PubMed] [Google Scholar]
- 30.Taylor AMW, Castonguay A, Ghogha A, Vayssiere P, Pradhan AAA, Xue L, et al. Neuroimmune Regulation of GABAergic Neurons Within the Ventral Tegmental Area During Withdrawal from Chronic Morphine. Neuropsychopharmacology. 2015;41:1–24. doi: 10.1038/npp.2015.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mandell AJ, Knapp S, Hsu LL. Some factors in the regulation of central serotonergic synapses. Life Sci. 1974;14:1–17. doi: 10.1016/0024-3205(74)90241-0. [DOI] [PubMed] [Google Scholar]
- 32.Gershon MD. Enteric serotonergic neurones ... finally! J Physiol. 2009;587:507. doi: 10.1113/jphysiol.2008.167676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol. 2003;66:1673–1680. doi: 10.1016/S0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- 34.Antas VI, Brigden KWL, Prudence AJa, Fraser ST. Gastrokine-2 is transiently expressed in the endodermal and endothelial cells of the maturing mouse yolk sac. Gene Expr Patterns. 2014;16:69–74. doi: 10.1016/j.gep.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Wallingford MC, Giachelli CM. Loss of PiT-1 results in abnormal endocytosis in the yolk sac visceral endoderm. Mech Dev. 2014;133:189–202. doi: 10.1016/j.mod.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verhaagh S, Barlow DP, Zwart R. The extraneuronal monoamine transporter Slc22a3/Orct3 co-localizes with the Maoa metabolizing enzyme in mouse placenta. Mech Dev. 2001;100:127–130. doi: 10.1016/s0925-4773(00)00510-4. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T9H-41WJC5P-P&_user=145085&_coverDate=01/31/2001&_rdoc=1&_fmt=high&_orig=search&_origin=search&_sort=d&_docanchor=&view=c&_acct=C000012098&_version=1&_urlVersion=0&_userid=145085&md5=3d8635797. [DOI] [PubMed] [Google Scholar]
- 37.Mikheev AM, Nabekura T, Kaddoumi A, Bammler TK, Govindarajan R, Hebert MF, et al. Profiling gene expression in human placentae of different gestational ages: an OPRU Network and UW SCOR Study. Reprod Sci. 2008;15:866–877. doi: 10.1177/1933719108322425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mincheva-Nilsson L, Baranov V. Placenta-derived exosomes and syncytiotrophoblast microparticles and their role in human reproduction: immune modulation for pregnancy success. Am J Reprod Immunol. 2014;72:440–57. doi: 10.1111/aji.12311. [DOI] [PubMed] [Google Scholar]
- 39.Tong M, Chamley LW. Placental Extracellular Vesicles and Feto-Maternal Communication. Cold Spring Harb Perspect Med. 2015;5:a023028–a023028. doi: 10.1101/cshperspect.a023028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jollie WP. Development, Morphology, and Function of the Yolk-Sac PIacenta of Laboratory Rodents. Teratology. 1990;381:361–381. doi: 10.1002/tera.1420410403. [DOI] [PubMed] [Google Scholar]
- 41.Amireault P, Hatia S, Bayard E, Bernex F, Collet C, Callebert J, et al. Ineffective erythropoiesis with reduced red blood cell survival in serotonin-deficient mice. Proc Natl Acad Sci U S A. 2011;108:13141–6. doi: 10.1073/pnas.1103964108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amireault P, Bayard E, Launay JM, Sibon D, Le Van Kim C, Colin Y, et al. Serotonin is a key factor for mouse red blood cell survival. PLoS One. 2013;8:e83010. doi: 10.1371/journal.pone.0083010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Velasquez JC, Goeden N, Bonnin A. Placental serotonin: implications for the developmental effects of SSRIs and maternal depression. Front Cell Neurosci. 2013;7:1–7. doi: 10.3389/fncel.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li M, Fallin MD, Riley A, Landa R, Walker SO. The Association of Maternal Obesity and Diabetes With Autism and Other Developmental Disabilities. Pediatrics. 2016;137 doi: 10.1542/peds.2015-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonnin A, Peng W, Hewlett W, Levitt P. Expression mapping of 5-HT1 serotonin receptor subtypes during fetal and early postnatal mouse forebrain development. Neuroscience. 2006;141:781–794. doi: 10.1016/j.neuroscience.2006.04.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.