INTRODUCTION
In women with breasts that are considered “dense” for assessment via mammogram, the sensitivity of mammography is reported to be between 24% and 56% in studies that evaluated mammography along with more-sensitive supplemental screening techniques [1]. These findings, coupled with implementation of Breast Density Inform Laws in 22 states, have fueled interest in offering supplemental screening to women who have dense breast tissue, even in the absence of a national consensus as to whether supplemental screening should be performed or which technique should be recommended.
Molecular breast imaging (MBI) is the most recent generation of dedicated nuclear medicine techniques being offered for supplemental screening. It represents the next evolutionary step from single-detector, breast-specific gamma imaging systems. Two manufacturers currently offer FDA-approved MBI systems that employ dual-head semiconductor-based detectors (Discovery NM750b, GE Healthcare, Milwaukee, WI; Luma-Gem, Gamma Medica, Salem, NH).
Supplemental MBI performed in women with dense breasts has been shown to improve the cancer detection rate from 3.2 cancers per 1,000 women screened with mammography alone, to 12.0 per 1,000 (P = .001); additional biopsies generated by MBI had a high positive predictive value (30.0%) [2]. MBI has a higher reported supplemental cancer detection rate compared with digital breast tomosynthesis or whole-breast screening ultrasound, a lower benign biopsy rate compared with ultrasound or breast MRI, and a reasonable cost profile. Despite these promising results, persistent concerns about radiation risk from MBI have limited its adoption as a screening test.
Ongoing examination of potential radiation risks, especially in the screening setting, is essential to ensuring safe patient care. In addition, we appreciate that discussion of radiation risks is complex, and misunderstandings about radiation are frequent in the radiology and larger medical communities. These misunderstandings translate into recommendations against potentially beneficial imaging tests—such as MBI—on the basis of radiation concerns that are ultimately unjustified. In this article, we aim to clarify common radiation dose metrics used in breast imaging and provide practical guidelines for assessing radiation risk of tests that use ionizing radiation.
ADMINISTERED ACTIVITY
For MBI, the patient receives an intravenous injection of technetium (Tc)-99m sestamibi. The measured amount of radioactivity of the radiopharmaceutical given to the patient is referred to as “administered activity.” Activity is defined as the rate of decay of the radioisotope and is expressed in units of millicuries (mCi) or megabecquerels (MBq; 1 mCi = 37 MBq). Administered activity is sometimes interchangeably referred to as “administered dose,” but should not be confused with radiation dose, as described later.
The FDA-approved package label for Tc-99m sestamibi recommends administered activities of 740–1,100 MBq (20–30 mCi) for breast imaging, and MBI examinations traditionally have been performed using administered activities in this range. The FDA does not regulate the practice of medicine, and radiologists may legally use this drug “off-label” at lower activities if it will best serve the patient [3]. With the newer dual-head MBI systems, substantially lower activities, of 240–300 MBq (6.5–8 mCi) can be used, with no degradation in diagnostic performance. More-recent changes to injection techniques and patient preparation procedures have resulted in more-consistent breast uptake of sestamibi and should allow further reduction in the administered activities required for MBI.
RADIATION DOSE METRICS
Two metrics that are often confused with one another, and are used in considering radiation effects, are referred to as “absorbed dose” and “effective dose.” Although the absorbed dose refers to an actual quantity of radiation energy that is deposited in tissue, effective dose is a more theoretic concept that accounts for both the type of radiation delivered and the radiosensitivity of irradiated tissues in a single quantity.
Absorbed Dose
Absorbed dose refers to the quantity of radiation energy deposited per unit mass of tissue. For diagnostic imaging, absorbed dose is expressed in units of milligray (mGy) for a specific region of the body.
During mammography, only the breast tissue is exposed to x-rays. As radiation is primarily absorbed in the fibroglandular tissue, absorbed dose is referred to as mean glandular dose (MGD). The MGD for digital mammography has been reported as 1.86 mGy per view, and 4.15 mGy per patient, with a range from 0.68 to 7.41 mGy per view, with absorbed dose increasing with breast thickness.
For MBI, the administered activity is systemic, so the absorbed dose to organs throughout the body must be considered. Absorbed dose is linearly proportional to the administered activity. For 300 MBq (8 mCi) Tc-99m sestamibi, the target organs receiving the largest absorbed dose are the large intestine wall (11–15 mGy); the small intestine wall (8 mGy); and the kidneys, bladder wall, and gallbladder wall (5 mGy each). The dose to the breast from 8 mCi Tc-99m sestamibi is relatively low, at 0.5 mGy.
Effective Dose
A limitation of the absorbed dose metric is that it provides an incomplete picture of the effect of radiation on the patient. Organs vary in radiosensitivity—the brain and skin are relatively resistant to the effects of radiation, compared with tissues such as the breast, bone marrow, or colon. This variability in radiosensitivity is addressed with the metric of effective dose.
Effective dose is a method for converting a dose that was delivered to specific organs into a quantity representing a whole-body dose that would have the same radiation effect on the patient. Calculation of effective dose involves applying weighting factors to the absorbed dose of each irradiated organ, to adjust for their relative radiosensitivity. To distinguish effective dose from absorbed dose, the units are changed from mGy to milliSieverts (mSv), and they generally range from 0.5 to 10 mSv for most diagnostic imaging tests (Fig. 1).
Fig 1.
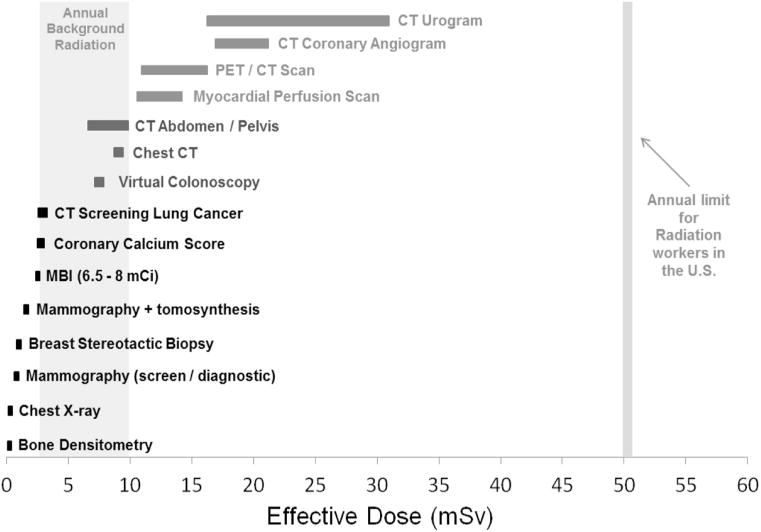
Chart showing the relative radiation (effective) dose from many of the more common diagnostic imaging procedures. Worldwide, background radiation doses range from 2.5 to 10 mSv per year. MBI = molecular breast imaging; mCi = millicuries.
In the effective dose calculation, the weighting factor for breast is 0.12. Thus, for digital mammography, the effective dose is the MGD of 4.15 mGy × 0.12 = 0.5 mSv. For MBI, the calculation is more complex, and the effective dose from 240 to 300 MBq (6.5–8 mCi) Tc-99m sestamibi is 2.0–2.5 mSv.
Effective dose is a useful metric to allow comparison of radiation dose among imaging tests that irradiate various organs, but it is not gender specific and was never intended as a tool to calculate risk to a specific patient. It was originally developed for planning and optimization in radiologic protection. However, as the only metric for comparing doses from various procedures, it has become the de facto tool for estimating the relative risk from ionizing radiation.
Risk to the Patient
Although carcinogenic effects have been documented of radiation doses at or above effective doses of 100 mSv, no prospective epidemiologic study with nonirradiated control subjects has quantitatively demonstrated adverse effects of radiation at doses <100 mSv. Hypothetic estimates of the risk at low doses are based on the 2006 Biological Effects of Ionizing Radiation (BEIR) VII report [4]. Many previous studies have used this report to calculate the lifetime attributable risk (LAR) of cancer from various breast imaging procedures by extrapolating risk levels from high-dose studies to estimate risk at low doses. The hypothetic LARs of radiation-induced cancer from a digital mammogram and MBI (8 mCi) at age 40 years are 5 per 100,000, and 10 per 100,000, respectively. By comparison, the hypothetic LAR from background radiation of 3 mSv per year is two orders of magnitude greater, at 2,200 per 100,000.
More-recent data no longer support the underlying assumptions of the BEIR report [5]. As a consequence, the major scientific organizations that oversee radiation protection have stated that speculative estimates of radiation-induced cancer or mortality at effective doses at or below worldwide background levels (2–10 mSv) represent both unsound science and inappropriate use of these risk models [5].
Any hypothetic risk of radiation at low doses must be placed in context with the potential benefits of the imaging exam. The risk of breast cancer is real: one in eight women will develop breast cancer, and this risk is greater in women with breasts that are “dense” for mammogram assessment. The risk of undetected cancer on repeat mammographic screenings, owing to breast tissue density, is real as well. Further evaluation of MBI for supplemental screening of dense breasts in multicenter trials is warranted, yet this technique is at risk of being removed from consideration, because of unnecessary concern over a very small and hypothetic risk of its low radiation dose.
SUMMARY
With modern MBI systems that have been optimized for breast imaging, the radiation dose from an MBI examination (2–2.5 mSv) is less than that received from annual background radiation and is therefore considered safe for use in routine screening.
Acknowledgments
This work was funded in part by grants from Mayo Clinic Center for Individualized Medicine and CTSA grant UL1TR000135 from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health.
C. B. Hruska and M. K. O’Connor receive royalties for licensed technologies through an agreement between the Mayo Clinic and Gamma Medica.
References
- 1.Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394–404. doi: 10.1001/jama.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hruska CB, Conners AL, Jones KN, et al. Diagnostic workup and costs of a single supplemental molecular breast imaging screen of mammographically dense breasts. AJR Am J Roentgenol. 2015;204:1345–53. doi: 10.2214/AJR.14.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck JM, Azari ED. FDA, off-label use, and informed consent: debunking myths and misconceptions. Food Drug Law J. 1998;53:71–104. [PubMed] [Google Scholar]
- 4.National Research Council. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. The National Academies Press; 2006. Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation. [PubMed] [Google Scholar]
- 5.Hendee WR, O’Connor MK. Radiation risks of medical imaging: separating fact from fantasy. Radiology. 2012;264:312–21. doi: 10.1148/radiol.12112678. [DOI] [PubMed] [Google Scholar]


