Abstract
Purpose
To determine the role of patient/tumor characteristics, radiation dose, and fractionation using the linear-quadratic (LQ) model to predict stereotactic body radiation therapy–induced grade ≥2 chest wall pain (CWP2) in a larger series and develop clinically useful constraints for patients treated with different fraction numbers.
Methods and Materials
A total of 316 lung tumors in 295 patients were treated with stereotactic body radiation therapy in 3 to 5 fractions to 39 to 60 Gy. Absolute dose–absolute volume chest wall (CW) histograms were acquired. The raw dose-volume histograms (α/β = ∞ Gy) were converted via the LQ model to equivalent doses in 2-Gy fractions (normalized total dose, NTD) with α/β from 0 to 25 Gy in 0.1-Gy steps. The Cox proportional hazards (CPH) model was used in univariate and multivariate models to identify and assess CWP2 exposed to a given physical and NTD.
Results
The median follow-up was 15.4 months, and the median time to development of CWP2 was 7.4 months. On a univariate CPH model, prescription dose, prescription dose per fraction, number of fractions, D83cc, distance of tumor to CW, and body mass index were all statistically significant for the development of CWP2. Linear-quadratic correction improved the CPH model significance over the physical dose. The best-fit α/β was 2.1 Gy, and the physical dose (α/β = ∞ Gy) was outside the upper 95% confidence limit. With α/β = 2.1 Gy, VNTD99Gy was most significant, with median VNTD99Gy = 31.5 cm3 (hazard ratio 3.87, P<.001).
Conclusion
There were several predictive factors for the development of CWP2. The LQ-adjusted doses using the best-fit α/β = 2.1 Gy is a better predictor of CWP2 than the physical dose. To aid dosimetrists, we have calculated the physical dose equivalent corresponding to VNTD99Gy = 31.5 cm3 for the 3- to 5-fraction groups.
Introduction
Chest wall (CW) pain is among the most common late adverse effects of stereotactic body radiation therapy (SBRT) and has been reported with an incidence between 10% and 40% in many studies (1–8). Although transient in most patients, this toxicity can severely limit quality of life, with some patients needing chronic opioid use for analgesia for multiple months or even years. Numerous patient and tumor characteristics, such as body mass index (BMI), diabetes mellitus, and tumor distance to CW, have been described as predictors of CW pain, although there is some disagreement among the studies (1–8). There are various dose-volume–based models of CW toxicity, including a modified equivalent uniform dose to adjust for radiobiological effects (8).
The aim of this study was to build on our previous report by Mutter et al. (5). Our initial experience on CW pain identified a clinically useful guideline (V30Gy = 70 cm3) to minimize this adverse effect. However, we continued to observe clinically significant CW pain after implementation of this guideline. From a scientific perspective, the model at the time lacked sufficient power to study the effect of prescription dose and fraction number. Here we report an update of our experience, with a significantly larger patient population, and also develop clinically useful constraints to prevent CW pain for patients treated with different fraction numbers.
Methods and Materials
Study design and patients
All patients with node-negative non-small cell lung cancer and oligometastatic tumors in the thorax treated with 3 to 5 fractions of SBRT at Memorial Sloan Kettering Cancer Center (MSKCC) between May 2006 and October 2012 were identified in our institutional database, according to number of fractions and site of disease. Exclusion criteria included history of prior thoracic radiation or radiofrequency ablation. Patients with SBRT to more than 1 lesion were included if they were located in distinct anatomic areas and the CW contours did not overlap. A total of 316 lung tumors in 295 patients were analyzed. Fifteen patients with 2 lesions were treated synchronously. All synchronously treated lesions were clearly spatially separated either by laterality or level within the thorax, so that CWP could be independently determined for each treated site. Six patients with 2 metachronously treated lesions in an area close to the initially treated lesion were censored at the time of the second treatment, to avoid any confusion regarding attribution of potentially developing CWP. The patient’s height and weight were recorded before SBRT and were available for all but 1 patient. The shortest axial tumor-to-CW distance was measured on the planning scan.
Treatment
As previously described (5, 9), patients were immobilized in a patient-specific alpha cradle or the MSKCC stereotactic body frame (9). A computed tomography (CT) simulation was performed, which included a free-breathing planning scan and a respiratory-correlated CT scan. Patients were typically treated with 3 to 7 coplanar 6-MV beams using intensity modulated radiation therapy and were planned with the MSKCC in-house treatment planning system using tissue inhomogeneity correction (10, 11). The gross tumor volume was modified according to the respiratory-correlated CT scan to generate an internal target volume (ITV) that accounted for respiratory motion. The clinical target volume included the ITV plus a 2- to 3-mm margin for microscopic tumor extension; the planning target volume (PTV) included the clinical target volume plus a 5-mm margin in all directions for setup error. Image-guided treatments were delivered on linear accelerators; the tumor visualized on cone beam CT was registered within the ITV contour from simulation. The PTV was treated to doses of 39 to 60 Gy in 3 to 5 fractions, prescribed to the 100% isodose line. The goal was to cover 95% of the PTV with the prescription dose (no minimum dose requirement was used). The selection of a fractionation regimen was guided by tumor location. Typically, peripheral lesions were treated to 18 to 20 Gy × 3, large tumors (>3 cm) and those near the CW were treated to 12 Gy × 4, and central tumors were treated to 9 to 10 Gy × 5 fractions. The CW was contoured as a 2-cm expansion from the ipsilateral lung/CW interface and vertebral body, with contours extended 4 slices (1.0 cm) above and below the PTV, as previously described by Mutter et al. (5). All CW contours were reviewed for adherence to these definitions. For all patients with 2 lesions, the CW was individually contoured for each lesion. These were spatially separate, thus ensuring that there was no overlap between CW contours and no dose contribution from the other treatment. In September 2010 we implemented a dosimetric CW guideline to limit the volume of CW receiving ≥30 Gy (V30Gy) to <70 cm3, on the basis of our initial analysis. In patients treated since September 2010, a shift toward lower CW volumes exposed to 30 Gy occurred. The median V30 of the complete data set shifted from approximately 70 cm3 to approximately 50 cm3, which gave us higher statistical power to detect complications arising from these lower exposures. Tumor coverage was a priority, and underdosing of the tumor was not permitted, to meet the CW guideline.
Follow-up
Patients were typically followed at 3-month intervals for 2 years. Subsequently, patients were seen for follow-up at 6-month intervals until 4 years from SBRT and annually thereafter. A chest CT scan was performed at every follow-up. Toxicities were scored according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0, with added specifications. Grade 1 CW pain was defined as mild pain, not interfering with function. Grade 2 was defined as moderate pain, interfering with function but not activities of daily living, requiring acetaminophen or nonsteroidal anti-inflammatory medications. Grade 3 was defined as severe pain interfering with activities of daily living, requiring narcotics, or needing interventions (eg nerve block). The date of onset of CW pain was recorded.
Analysis
The CW absolute-dose absolute-volume histograms were obtained from the treatment planning software. The Kaplan-Meier method was used to analyze the cumulative incidence of grade ≥2 CW pain (CWP2). The Cox proportional hazards (CPH) model was used in univariate and multivariate analyses to identify and assess predictive factors of CW pain. In addition, log-rank tests were performed to assess the clinical utility of candidate thresholds. All variables with a P value <.1 and clinically indicated variables were considered for inclusion in the multivariate model; variables considered were BMI, Karnofsky performance status, age, gender, total prescription dose, prescription dose per fraction, number of fractions, distance to CW, and the absolute volume of CW exposed to a range of physical doses (VD) and of linear-quadratic (LQ)-adjusted doses (from here on referred to as normalized total dose [VNTD]). Models containing variables with P values <.05 were selected.
Normalized total dose was used to account for the various dose-fractionation schemes in the cohort. Chest wall DVH dose bins were corrected to LQ equivalent doses in 2-Gy fractions according to Equation 1:
| (1) |
Here, Di is the physical dose in the given volume, and n is the number of fractions. In this analysis the α/β ratio was taken as a free parameter and varied from 0 to 25 Gy in 0.1-Gy steps. At each α/β value the best VNTD Cox model was determined; the likelihood for this model was recorded. The best-fit α/β ratio and associated confidence intervals (CIs) were determined using the likelihood profile method and yielded a CPH model based on the resulting VNTD of the CW. A log-rank test based on a split at the corresponding median VNTD was assessed. We determined physical dose-volume guidelines for treatment planning purposes as VD3, VD4, and VD5, where V is the median VNTD, and D3, D4, and D5 are the physical doses corresponding to that NTD when treating with 3, 4, and 5 fractions, respectively.
Dose-volume atlases
To facilitate future analyses of these data (12, 13), dose-volume atlases of the incidence of CWP2 (14, 15) based on physical dose are provided in Excel files in Electronic Appendix 1 (available online at www.redjournal.org). Atlases are provided for each fraction number for clinically obese (BMI ≥30 kg/m2) and nonobese (BMI <30 kg/m2) patients at the time of treatment. The format and usage of these files is described in Electronic Appendix 2 (available online at www.redjournal.org).
Results
Patients
Patient, tumor, and treatment characteristics are described in Table 1. The median patient age was 77 years, with a median follow-up of 15.4 months. A majority of the tumors were within 1 cm of the CW (218; 69%), and 91 (28.8%) of the tumors were in patients with a BMI ≥30 kg/m2.
Table 1.
Patient, tumor, and treatment characteristics
| Characteristic | n | % |
|---|---|---|
| Age (y), median (range) | 77 (49–95) | |
| KPS, median (range) | 70 (50–100) | |
| Gender | ||
| Male | 138 | 46.8 |
| Female | 157 | 53.2 |
| BMI (kg/m2)* | ||
| <30 | 225 | 71.2 |
| ≥30 (obesity) | 91 | 28.8 |
| Tumor | ||
| Primary NSCLC | 285 | 90.2 |
| Oligometastatic | 13 | 4.1 |
| Recurrent | 18 | 5.7 |
| Tumor distance to CW (cm) | ||
| <1 | 218 | 69.0 |
| ≥1 | 98 | 31.0 |
| Prescription doses | ||
| 18–20 Gy × 3 | 113 | 35.8 |
| 12 Gy × 4 | 114 | 36.1 |
| 9–10 Gy × 5 | 62 | 19.6 |
| Other | 27 | 8.5 |
Abbreviations: BMI = body mass index; CW = chest wall; KPS = Karnofsky performance status; NSCLC = non-small cell lung cancer.
Body mass index for patients with multiple treatments was calculated separately before each treatment.
CW toxicity
A total of 62 lesions (19.6%) were associated with the development of CWP2, with a median onset of 7.2 months. The 2-year actuarial incidence for CWP2 was 28.4%. There were a total of 26 lesions associated with grade 2 and 36 with grade 3 CW pain, with 1 patient experiencing both left- and right-sided CWP2 from 2 separate SBRT treatments.
Models of CWP2 based on physical dose
In our previously described analysis of a subset of these data (5), CPH modeling found a broad region of physical dose and volume that significantly predicted the development of CWP2. In our current dataset, a broader range of VD (physical dose) correlated with CWP2 (P<.05 for D < 60 Gy), and the best-fit variable was V39Gy. However, V30Gy (P<.001) was used as the threshold in multivariate analysis because of its familiarity from prior publications (4) and ease of applicability. The sensitivities and specificities for V30Gy thresholds of 30 cm3, 50 cm3, and 70 cm3 were 0.891 and 0.294, 0.828 and 0.524, and 0.656 and 0.726, respectively.
Other patient and tumor characteristics that reached significance on univariate analysis were total prescription dose (P<.001), prescription per fraction (P<.001), dose to hottest 83 cm3 of CW (D83cc) (P<.001), number of fractions (P=.0075), tumor distance to CW (P=.0014), and BMI (P=.031). V30Gy (P<.001) ranged from 0 to 246.2 cm3, with a median value of 49.3 cm3, and was more significant than the preceding variables for the development of CW pain (Electronic Appendix 3; available online at www.redjournal.org). Karnofsky performance status, gender, and age did not reach significance as predictors in a univariate CPH model (Table 2). On multivariate analysis, several factors reached significance, and the best combination of variables was V30Gy + BMI + prescription dose. The log-rank split at the median value of the multivariate metric (V30Gy × βV30Gy + BMI × βBMI + Total Dose × βTotal Dose = 4.36, where β is the CPH coeffecient value [Table 2]) was significant (P<.001).
Table 2.
Patient and tumor characteristics predicting chest wall pain
| Characteristic | CPH model HR |
CPH model HR 95% confidence interval |
P |
|---|---|---|---|
| Univariate variable | |||
| V30 Gy (cm3) | 1.013 | 1.009–1.017 | <.001 |
| D83cc (Gy) | 1.080 | 1.051–1.109 | <.001 |
| Prescription dose (Gy) |
1.085 | 1.042–1.130 | <.001 |
| Prescription dose (Gy)/fraction |
1.105 | 1.042–1.172 | <.001 |
| No. of fractions | 0.625 | 0.439–0.889 | .0075 |
| Tumor distance to CW (cm) |
0.595 | 0.418–0.846 | .0014 |
| BMI (kg/m2) | 1.041 | 1.001–1.082 | .031 |
| KPS | 0.980 | 0.961–1.000 | .25 |
| Gender | 0.835 | 0.502–1.390 | .48 |
| Age (y) | 0.990 | 0.971–1.010 | .83 |
| Multivariate variables | |||
| Trivariate model | |||
| V30 Gy (cm3) | 1.127 | 1.123–1.132 | <.001 |
| BMI (kg/m2) | 1.041 | 1.001–1.082 | .028 |
| Prescription dose (Gy) |
1.053 | 1.011–1.098 | .010 |
| Bivariate model | |||
| VNTD99Gy (cm3) | 1.018 | 1.012–1.023 | <.001 |
| BMI (kg/m2) | 1.042 | 1.003–1.082 | .036 |
Abbreviations: CPH = Cox proportional hazards; HR = hazard ratio.
Other abbreviations as in Table 1.
Models of CWP2 based on LQ-adjusted dose-volume parameters
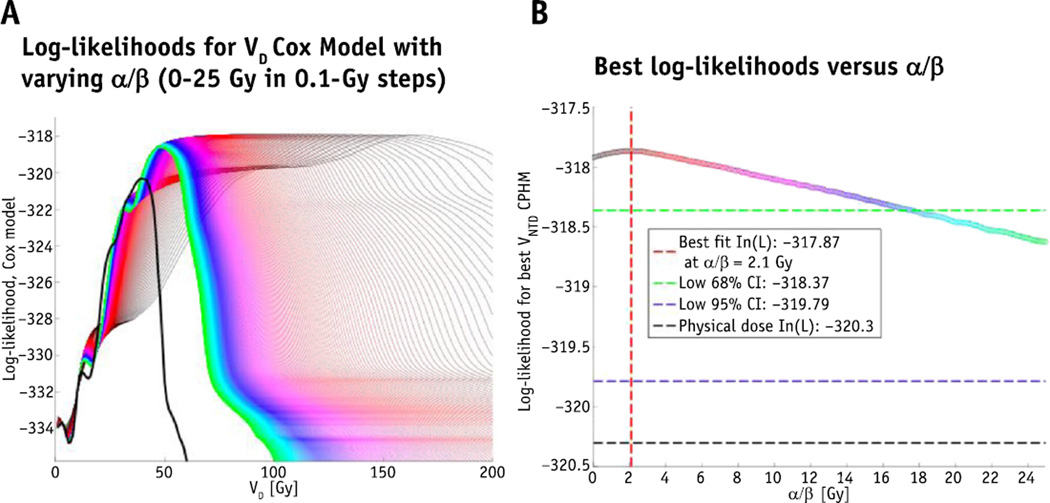
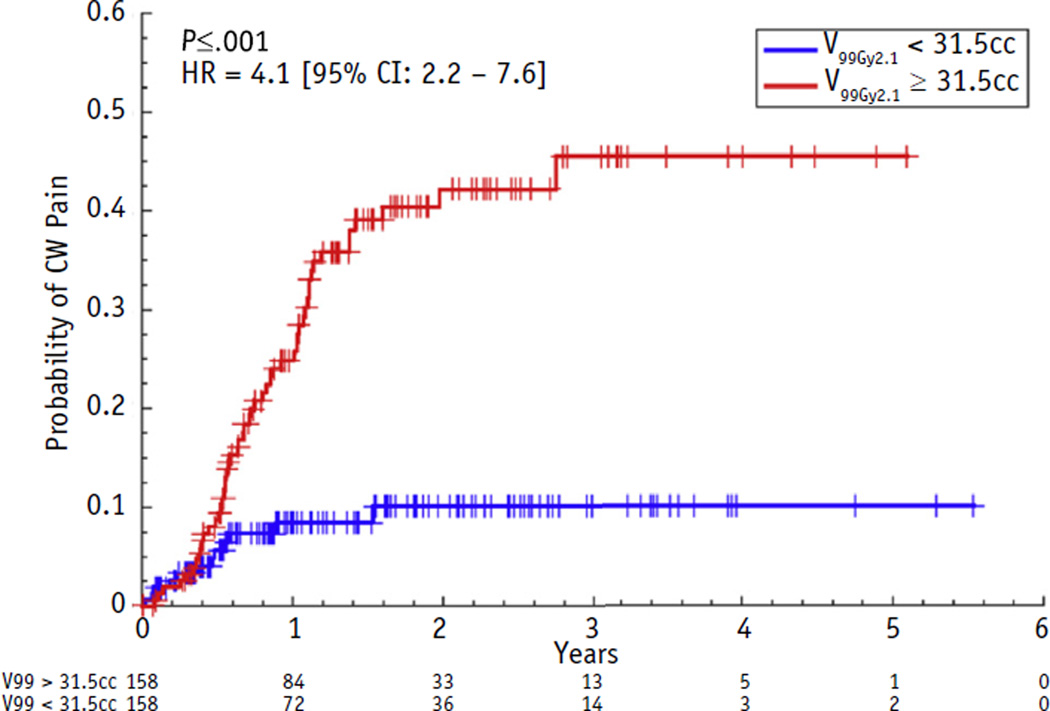
Replacing dose by the LQ-corrected doses significantly improved the CPH models. As shown in Figure 1, the maximum log-likelihood CPH model was observed at α/β = 2.1 Gy (68% CI 0–17.7 Gy). Physical dose (α/β = ∞ Gy) was outside the 95% CI. With α/β = 2.1 Gy, the maximum log-likelihood CW model was based on VNTD99Gy. The log-rank test of VNTD99Gy split at the median value of 31.5 cm3 demonstrated a hazard ratio of 4.1 (P<.001) (Fig. 2).
Fig. 1.
(A) Curve demonstrates log-likelihoods for VD Cox model with varying α/β (0–25 Gy in 0.1-Gy steps). The black curve is the physical dose, the green is α/β of 25 Gy, with values decreasing toward the red curves. (B) Curve plots the best log-likelihoods for each α/β; the red vertical line is the best fit (2.1 Gy), and the green horizontal line is the 68% confidence interval (CI) (0–17.7 Gy). The physical dose (α/β = ∞ Gy) is outside of the 95% confidence interval. A color version of this figure is available at www.redjournal.org.
Fig. 2.
Cumulative incidence curves for grade ≥2 chest wall (CW) pain using the best-fit α/β (2.1 Gy) and with volume of chest wall receiving a normalized total dose of 99 Gy split at the median 31.5 cm3. Numbers at risk in the 2 categories are given at yearly intervals below the time axis. The hazard ratio (HR) and 95% confidence limits are calculated with a Cox proportional hazards model on dichotomized data (patients with VNTD99Gy <31.5 cm3 or ≥31.5 cm3 are coded as 0 or 1, respectively).
For each fractionation group, the corresponding physical VD was split at the median 31.5 cm3 using Equation 1. For example, for patients treated with 3 fractions, the corresponding physical dose was 31.9 Gy; the V31.9Gy split at the median CW volume (31.5 cm3) demonstrated a hazard ratio of 3.05 for the prediction of CW pain (Table 3). The significance of this split demonstrated a trend (P=.052) for 3 fractions, was significant (P=.0016) for 4 fractions, and did not reach significance for 5 fractions, possibly because this group contained fewer patients.
Table 3.
Corresponding physical doses for a 3-, 4-, or 5-fraction regimen
| Fractions | Corresponding physical dose (Gy) |
HR split at median 31.5 cm3, (95% CI) |
P |
|---|---|---|---|
| 3 | 31.9 | 3.05 (0.94–9.9) | .052 |
| 4 | 36.3 | 4.36 (1.6–11.8) | .0016 |
| 5 | 40.1 | 2.79 (0.69–10.7) | .14 |
Abbreviations as in Table 2.
Using LQ-adjusted doses in multivariate CPH models of the best combination of factors was VNTD99Gy + BMI (P<.001). This bivariate model with LQ-adjusted dose has Akaiake information criterion (AIC) = 637.4, whereas the AIC for the best model using physical dose (V30Gy + BMI + total radiation dose) is 639.7. This difference of 2.3, with the bivariate model being lower, exceeds the standard threshold for determining the statistical superiority of the model with lower AIC.
Clinical application
The CPH model indicated that the LQ-adjusted doses were more significant predictors of CW pain than physical dose. Therefore, for treatment planning purposes, we determined the physical doses D3, D4, and D5 corresponding to NTD = 99 Gy with α/β = 2.1 Gy, when treating with 3, 4, and 5 fractions, respectively. These are D3 = 31.9 Gy, D4 = 36.3 Gy, and D5 = 40.1 Gy (Table 3). These constraints apply to the patient population as a whole.
Discussion
Previously, Mutter et al. described a 2-year estimated actuarial incidence of ≥CWP2 of 39% and a median time to onset of 9 months. It was determined that V30Gy ≤70 cm3 for a CW defined as 2-cm expansion from the lung–CW interface was the better parameter for predicting CW pain than V30Gy ≤30 cm3 for a 3-cm contour expansion (5). Other patient parameters, such as age, gender, fraction number, dose per fraction, and total prescription dose did not reach significance, likely owing to limited statistical power from smaller patient numbers in those subsets. V30Gy ≤70 cm3 was therefore implemented in September of 2010 as our departmental guideline for CW dose. In this expanded analysis, the 2-year estimated actuarial incidence of CWP2 was 28.4%, and the median time to onset was 7.2 months. We found that a V30Gy ≤50 cm3 had greater sensitivity (0.828) than the 70-cm3 threshold (0.656), whereas V30Gy ≤30 cm3 had the highest sensitivity (0.891). In patients treated since September 2010, a shift toward lower volumes exposed to approximately 30 Gy occurred from a median V30 of approximately 70 cm3 to approximately 50 cm3. When analyzing the incidence of CWP2 in patients before and after the implementation of the new guideline, we did not see a significant change in the incidence of CWP2 (data not shown). Although this observation may have been limited by the smaller number of patients treated since 2010 (n=112), we believe that the main impact of the larger patient population including patients from both before and after 2010 gave us higher statistical power to detect complications arising from these lower exposures. Dunlap et al (4) described the V30Gy ≤30 cm3 threshold as the best predictor; however, we have a concern with this stringent dose-volume parameter because of its high false-positive rate in our data (specificity of 0.294 for 30-cm3 threshold and 0.524 for 50-cm3 threshold). Although a V30Gy ≤ 30 cm3 was similarly predictive of CW pain as V30Gy ≤50 cm3 in our study, we now have adopted physical dose V30Gy ≤50 cm3 as a clinical guideline because it is more often achievable, has a higher specificity than a V30Gy ≤30 cm3, and encourages greater protection than our previously published 70-cm3 threshold. Additionally, such a low volume constraint of 30 cm3 may not be practically achievable in many patients with larger tumors close to the CW without significant underdosing of the target volume.
Unlike our initial report, we included nondosimetric parameters reported by others: BMI and distance to CW. These and other parameters (fraction number and prescription dose) reached univariate significance in this expanded series (Table 2). The decrease in CWP2 incidence in this study may be because of the CW dose constraint implemented from our previous study and/or because of an increase in the number of patients treated with lower doses per fraction (62 patients treated with 5 fractions in the present study vs 15 previously) (5). The slight decrease in median onset time of 9 to 7.2 months is possibly related to observer bias because of our increased awareness of this toxicity during patient follow-up.
Despite the controversies and being well aware of the limitations of the LQ model in hypofractionated radiation therapy (16, 17), we sought to identify whether using the LQ-adjusted dose improved our ability to predict CW pain over the physical dose. In this large cohort, a wide range of treatment doses and fractionation schemes were analyzed, with a more complete set of clinical variables included in (multivariate) CPH analysis for predicting CW pain than previously described. In contrast to our previous study, with a larger number of tumors treated, we found that the LQ model was superior to the physical dose at predicting SBRT-induced CW pain. We analyzed a wide range of α/β (0–25 Gy in 0.1-Gy steps) and found that within 68% CI all values for α/β between 0 and 17.7 Gy were better predictors of CW pain than the physical dose (Fig. 1). All the tested values of α/β from 0 to 25 Gy were within 95% CI, but physical dose was not. Although the CI on α/β is not small, this analysis excluded the use of physical dose (outside the 95% CI). This is particularly important in the present context, where several groups have used physical dose to analyze this endpoint. We were able to estimate the value of α/β in our data set despite the fact that our cohort falls into 3 groups, ordered by prescription dose and similarly ordered by fraction size. This ordering tends to be preserved when looking at VNTD as a function of α/β. This prevented us from determining α/β in our previous publication. Higher numbers of patients proved key in this regard. Our best-fit α/β value of 2.1 Gy is consistent with an α/β of 3 Gy being commonly used for estimating late toxicity; this fits with the observation that CW pain is typically a subacute to late toxicity, with a median time to occurrence of 7.2 months. Reporting results from patients treated with 48 Gy in 4 fractions, 60 Gy in 10 fractions, and 70 Gy in 10 fractions, Nambu et al (18) tested the ability of LQ-adjusted maximum CW dose to predict rib fracture in 26 patients with and 22 randomly sampled patients without rib fracture, respectively.18 They found that an α/β value of 8 Gy gave the maximum receiver operating curve value but did not provide CIs. Because the clinical and analyzed dosimetric endpoints were different, it may not be appropriate to compare their α/β value with ours (2.1 Gy); however, their value lies within the 68% CI of ours (0–17 Gy). Eight grays is more appropriate for acute than late effects, despite their range of onset times from 4 to 58 months. In their discussion, Nambu et al (18) quote an α/β value for late bone damage of 1.8–2.8 Gy, which is more consistent with our findings, but it is unclear how this range was determined.
Our aim with this study was to produce clinically usable dosimetric guidelines for reduction of incidence of CW pain. We therefore chose to analyze dose-volume–based parameters rather than equivalent uniform doses. We analyzed Dx as well as Vx and found that Vx resulted in better parameter estimation and lower P values (data not shown). After finding the best VNTD to be 99 Gy with an α/β value of 2.1 Gy, we converted this value back to its physical dose equivalent for the 3-, 4-, and 5-fraction groups for the convenience of treatment planning (Table 3). As expected, we found that the physical tolerance dose to the CW increased as the fraction number increased and the prescription dose per fraction decreased. This suggests that treating patients with a higher number of fractions and lower prescription dose per fraction may potentially decrease the incidence of CW pain. It may also be easier for a treatment planner to achieve both target coverage and the CW dose thresholds for 5 fraction treatments, owing to the smaller difference between prescription and threshold doses. We therefore recommend considering a 4- or 5-fraction regimen in patients with targets near the CW or larger targets with a high associated dose to the CW. However, the prescription dose per fraction and VNTD have to be balanced with considerations of tumor control probability, and on the basis of current information the prescription biologically effective dose to the target should not be decreased <100 Gy, to maintain adequate tumor control probability (19–22).
Elevated BMI and obesity (BMI ≥30 kg/m2) have been significantly correlated with CW pain in multiple other studies (3, 7, 8), and in our study it was found that BMI is significantly associated with CW pain independently of dose in the multivariate models. The exact pathophysiology behind obesity and SBRT-induced CW pain is unknown; however, there are studies that suggest an association between obesity and pain (23–25). It has been reported that some hormones associated with excess fat, such as leptin, may be associated with inflammation, leading to painful conditions (26). As per Stone and Broderick in a survey of more than 1,000,000 people who were asked whether they had pain yesterday, there was a significant increase in pain for those who were overweight/obese when compared with people in the low to normal BMI range. These findings were upheld after statistically controlling for other pain-causing conditions and across gender and age (23). High-dose SBRT in patients who are at baseline more prone to pain may explain why there is an association between obesity and SBRT-induced CW pain.
In this larger series, prescription dose, dose per fraction, BMI, and tumor distance to CW were all significant predictors of CW pain on univariate CPH. On multivariate models, the best combination of variables when analyzing the physical doses was V30Gy + BMI + prescription dose. For the LQ model, the combination of VNTD99Gy + BMI reached significance and was a better predictor of CWP2 than the physical dose model. The physical dose equivalents for VNTD99Gy are 31.9 Gy in 3 fractions, 36.3 Gy in 4 fractions, and 40.1 Gy in 5 fractions. We are currently in the process of implementing the fraction number–dependent dose constraints. Meeting these tolerances, however, must be balanced against target coverage, and target coverage should generally not be compromised, to achieve CW sparing.
Supplementary Material
Summary.
Chest wall (CW) pain after stereotactic body radiation therapy is among the most common toxicities from high-dose hypofractionated radiation to thoracic tumors. We previously published our initial experience on CW pain and developed a dose constraint to minimize CW pain. However, the model lacked sufficient power for studying the impact of fractionation on radiation dose. Therefore, we reanalyzed our expanded series and developed clinically useful, fractionation-dependent constraints to prevent CW pain.
Acknowledgments
This research was supported in part by National Institutes of Health grant R01 CA129182.
Footnotes
This work was presented at the 55th Annual Meeting of the American Society for Radiation Oncology, September 22–25, 2013, Atlanta, GA.
Conflict of interest: none.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.Andolino DL, Forquer JA, Henderson MA, et al. Chest wall toxicity after stereotactic body radiotherapy for malignant lesions of the lung and liver. Int J Radiat Oncol Biol Phys. 2011;80:692–697. doi: 10.1016/j.ijrobp.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 2.Bongers EM, Haasbeek CJ, Lagerwaard FJ, et al. Incidence and risk factors for chest wall toxicity after risk-adapted stereotactic radiotherapy for early-stage lung cancer. J Thorac Oncol. 2011;6:2052–2057. doi: 10.1097/JTO.0b013e3182307e74. [DOI] [PubMed] [Google Scholar]
- 3.Creach KM, El Naqa I, Bradley JD, et al. Dosimetric predictors of chest wall pain after lung stereotactic body radiotherapy. Radiother Oncol. 2012;104:23–27. doi: 10.1016/j.radonc.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Dunlap NE, Cai J, Biedermann GB, et al. Chest wall volume receiving >30 Gy predicts risk of severe pain and/or rib fracture after lung stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:796–801. doi: 10.1016/j.ijrobp.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 5.Mutter RW, Liu F, Abreu A, Yorke E, Jackson A, Rosenzweig KE. Dose-volume parameters predict for the development of chest wall pain after stereotactic body radiation for lung cancer. Int J Radiat Oncol Biol Phys. 2012;82:1783–1790. doi: 10.1016/j.ijrobp.2011.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephans KL, Djemil T, Tendulkar RD, et al. Prediction of chest wall toxicity from lung stereotactic body radiotherapy (SBRT) Int J Radiat Oncol Biol Phys. 2012;82:974–980. doi: 10.1016/j.ijrobp.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Welsh J, Thomas J, Shah D, et al. Obesity increases the risk of chest wall pain from thoracic stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2011;81:91–96. doi: 10.1016/j.ijrobp.2010.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woody NM, Videtic GM, Stephans KL, et al. Predicting chest wall pain from lung stereotactic body radiotherapy for different fractionation schemes. Int J Radiat Oncol Biol Phys. 2012;83:427–434. doi: 10.1016/j.ijrobp.2011.06.1971. [DOI] [PubMed] [Google Scholar]
- 9.Lovelock DM, Hua C, Wang P, et al. Accurate setup of paraspinal patients using a noninvasive patient immobilization cradle and portal imaging. Med Phys. 2005;32:2606–2614. doi: 10.1118/1.1951042. [DOI] [PubMed] [Google Scholar]
- 10.Mohan R, Barest G, Brewster LJ, et al. A comprehensive three-dimensional radiation treatment planning system. Int J Radiat Oncol Biol Phys. 1988;15:481–495. doi: 10.1016/s0360-3016(98)90033-5. [DOI] [PubMed] [Google Scholar]
- 11.Spirou SV, Chui CS. A gradient inverse planning algorithm with dose-volume constraints. Med Phys. 1998;25:321–333. doi: 10.1118/1.598202. [DOI] [PubMed] [Google Scholar]
- 12.Deasy JO, Bentzen SM, Jackson A, et al. Improving normal tissue complication probability models: The need to adopt a “data-pooling” culture. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S151–S154. doi: 10.1016/j.ijrobp.2009.06.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson A, Marks LB, Bentzen SM, et al. The lessons of QUANTEC: Recommendations for reporting and gathering data on dose-volume dependencies of treatment outcome. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S155–S160. doi: 10.1016/j.ijrobp.2009.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox BW, Jackson A, Hunt M, et al. Esophageal toxicity from high-dose, single-fraction paraspinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2012;83:e661–e667. doi: 10.1016/j.ijrobp.2012.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson A, Yorke ED, Rosenzweig KE. The atlas of complication incidence: A proposal for a new standard for reporting the results of radiotherapy protocols. Semin Radiat Oncol. 2006;16:260–268. doi: 10.1016/j.semradonc.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Brenner DJ. The linear-quadratic model is an appropriate methodology for determining isoeffective doses at large doses per fraction. Semin Radiat Oncol. 2008;18:234–239. doi: 10.1016/j.semradonc.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirkpatrick JP, Meyer JJ, Marks LB. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Semin Radiat Oncol. 2008;18:240–243. doi: 10.1016/j.semradonc.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Nambu A, Onishi H, Aoki S, et al. Rib fracture after stereotactic radiotherapy for primary lung cancer: Prevalence, degree of clinical symptoms, and risk factors. BMC Cancer. 2013;13:68. doi: 10.1186/1471-2407-13-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: Updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2(7 Suppl. 3):S94–S100. doi: 10.1097/JTO.0b013e318074de34. [DOI] [PubMed] [Google Scholar]
- 20.Ohri N, Werner-Wasik M, Grills IS, et al. Modeling local control after hypofractionated stereotactic body radiation therapy for stage I non-small cell lung cancer: A report from the Elekta Collaborative Lung Research Group. Int J Radiat Oncol Biol Phys. 2012;84:e379–e384. doi: 10.1016/j.ijrobp.2012.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J, Yang F, Li B, et al. Which is the optimal biologically effective dose of stereotactic body radiotherapy for Stage I non-small-cell lung cancer? A meta-analysis. Int J Radiat Oncol Biol Phys. 2011;81:e305–e316. doi: 10.1016/j.ijrobp.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 22.Park S, Urm S, Cho H. Analysis of biologically equivalent dose of stereotactic body radiotherapy for primary and metastatic lung tumors. Cancer Res Treat. 2014;46:403–410. doi: 10.4143/crt.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stone AA, Broderick JE. Obesity and pain are associated in the United States. Obesity. 2012;20:1491–1495. doi: 10.1038/oby.2011.397. [DOI] [PubMed] [Google Scholar]
- 24.Ray L, Lipton RB, Zimmerman ME, et al. Mechanisms of association between obesity and chronic pain in the elderly. Pain. 2011;152:53–59. doi: 10.1016/j.pain.2010.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright LJ, Schur E, Noonan C, et al. Chronic pain, overweight, and obesity: Findings from a community-based twin registry. J Pain. 2010;11:628–635. doi: 10.1016/j.jpain.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandell LJ. Obesity and osteoarthritis: Is leptin the link? Arthritis Rheum. 2009;60:2858–2860. doi: 10.1002/art.24862. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.