Abstract
Objective
To evaluate 52-week clinical outcomes of children with co-occurring attention-deficit/hyperactivity disorder (ADHD), disruptive behavior disorder, and serious physical aggression who participated in a prospective, longitudinal study that began with a controlled, 9-week clinical trial comparing the relative efficacy of parent training + stimulant medication + placebo (Basic; n=84) versus parent training + stimulant + risperidone (Augmented; n=84).
Method
Almost two-thirds (n=108; 64%) of families in the 9-week study participated in Week 52 follow-ups (Basic, n=55; Augmented, n=53), and they were representative of the initial study sample. The assessment battery included caregiver and clinician ratings and laboratory tests.
Results
Only 43% of Augmented and 36% of Basic still adhered to their assigned regimen (not significant [ns]); 23% of Augmented and 11% of Basic were taking no medication (ns). Both randomized groups improved baseline to follow-up, but the three primary parent-reported behavioral outcomes showed no significant between-group differences. Exploratory analyses indicated Augmented (65%) was more likely (p=.02) to have a Clinical Global Impressions (CGI) severity score of 1-3 (normal to mildly ill) at follow-up than Basic (42%). Parents rated 45% of children as impaired often or very often from ADHD, noncompliant, or aggressive behavior. Augmented had elevated prolactin levels, and Basic decreased in weight over time. Findings were generally similar whether groups were defined by randomized assignment or follow-up treatment status.
Conclusion
Both treatment strategies were associated with clinical improvement at follow-up, and primary behavioral outcomes did not differ significantly. Many children evidenced lingering mental health concerns, suggesting the need for additional research into more effective interventions.
Keywords: ADHD, oppositional defiant disorder, conduct disorder, risperidone, methylphenidate
INTRODUCTION
Although the concurrent use of multiple medications to treat specific disorders is commonplace in some areas of medicine, this practice has often generated skepticism and concern in psychiatry, particularly for pediatric patients. This may be attributed in part to the significant role that environmental factors play in child behavior, responsiveness of child syndromes to behavioral intervention, and the general absence of sound experimental evidence supporting the safety and efficacy of medication combinations.1-3 Aggressive, noncompliant behaviors have always been a leading cause of referral to child mental health professionals,4 and their treatment requires special consideration for many reasons.5 In order to determine the relative benefits of combining evidence-based interventions, we conducted a prospective, multi-site study that compared mono-versus multiple-drug therapy for child aggression.6,7 A total of 168 children with attention-deficit/hyperactivity disorder (ADHD) and co-occurring oppositional defiant disorder (ODD) or conduct disorder (CD) and whose parents reported serious physical aggression participated in a controlled, 9-week clinical trial (Treatment of Severe Childhood Aggression or TOSCA). The study began with 3 weeks of parent training in child behavior management techniques plus stimulant medication, after which participants received an additional randomly assigned medication, either placebo (“Basic”) or risperidone (“Augmented”). Children who responded favorably to their assigned treatment had the option of participating in a 3-month, double-blind treatment extension. Approximately 52 weeks after their initial baseline evaluation, all children were eligible to participate in a follow-up evaluation, which is the focus of the present paper.
In our initial report of results for the 9-week acute clinical trial, Aman et al.6 found that Augmented provided moderate improvement over Basic in severity of disruptive behaviors as measured by the primary outcome, the parent-completed Nisonger Child Behavior Rating Form-Typical IQ Disruptive Total score (NCBRF D-total),8 which was also an inclusion criterion. Moreover, there was little evidence of increased risk of adverse events with Augmented. We subsequently reported that Augmented resulted in a greater reduction in parents’ ratings of ODD severity and peer aggression and less ODD symptom-induced impairment than Basic, and a greater reduction in teachers’ ratings of ADHD severity.9 There was also evidence that Augmented was associated with improvement in a range of non-targeted symptoms, including teacher-rated anxiety and social avoidance.10 Both interventions were associated with marked symptom reduction, and satisfaction with participation in the study was high;11 effect sizes for treatment group differences generally ranged from small to moderate for the primary outcome.6,9,10 However, a relatively large percentage of children remained impaired at the end of the acute trial.9
The present article examines the clinical status of TOSCA participants approximately 12 months after their initial baseline evaluation. This outcome was of considerable interest because few prospective longitudinal studies of rigorously diagnosed children with serious physical aggression and co-occurring ADHD have been conducted. Even fewer studies have examined multiple- versus mono-drug therapy. We assessed changes to the treatment regimen, differential behavioral outcomes for treatment groups, and adverse events. In addressing these topics, we sought to determine if children who were randomly assigned to Augmented, previously found to be more effective than Basic, had more favorable outcomes. Although many variables influence clinical course, it seemed reasonable to hypothesize that a better start to treatment may have longer-term consequences, recognizing that treatment regimens often change. We also examined clinical status based on the treatment regimens children were receiving at the time of follow-up because they would presumably have more immediate relevance for at least some outcome variables. Exploratory effectiveness analyses were undertaken to contrast outcomes for children who were receiving their respective, randomly assigned interventions at follow-up.
METHOD
Participants
The initial study sample was 168 children (6-12 years old) recruited at four sites (Columbus, OH; Cleveland, OH; Pittsburgh, PA; Stony Brook, NY). Participants were primarily boys of average IQ and White/Caucasian/European geographic ancestry living with working parents who had at least some college education and family incomes ≤ $40,000 per year. Inclusion criteria were (1) blinded clinician rating of Level 3 or greater for an adapted version of the Overt Aggression Scale–M,12 (2) parent rating of severe disruptive behavior (≥ 90th percentile NCBRF D-Total), (3) ADHD plus ODD or ODD+CD, and (4) score ≥4 Clinical Global Impression-Severity (CGI-S) for aggression.13 Exclusion criteria included full-scale IQ<70; pregnancy; medical consideration (seizures, abnormal liver function, first degree family history of Type II Diabetes); current bipolar disorder or major depressive disorder; lifetime history of pervasive developmental, psychotic, eating, or substance use disorder; attempted suicide; or evidence of child abuse. Participants needed to be free of psychotropic medicines for 2 or 4 weeks for short- and long-acting drugs, respectively. The study was approved by the institutional review board of each site; parents/guardians signed permission forms; and child participants provided assent before enrollment.
Measures
Behavioral assessments
Because the TOSCA assessment battery is described in detail in several publications,6,9,10 only a brief overview appears here. A more detailed description is available in Farmer et al.7 (see Supplement 1, available online). The three primary behavioral outcomes were the same measures that revealed treatment effects in the acute trial:6,10 NCBRF–D Total score and Positive Social subscale and the Reactive (Instrumental) Aggression subscale of the Antisocial Behavior Scale (ABS).14 Secondary/exploratory outcomes were the remaining subscales of the NCBRF and ABS, the Child and Adolescent Symptom Inventory-4R (CASI-4R),15 and the Improvement (I) and Severity (S) subscales of the CGI.
Safety assessments
Adverse events (AEs) assessments included extrapyramidal symptoms, Barnes Akathisia Scale,16 Abnormal Involuntary Movement Scale (AIMS),17 Simpson-Angus;18 vital signs (heart rate, systolic and diastolic blood pressure, height-for-age z-score, weight-for-age z-score); and laboratory measures (prolactin levels).
Treatment history
At each visit, caregivers reported the medication(s) their children were currently receiving (name of medication, dose, frequency, route, start date and end date, and the reason for which it was prescribed, which is referred to here as “indication”) and changes to treatment since their last visit.
Procedure
Randomization was determined at baseline (n=84 for each condition), stratified by site, and balanced by ODD versus CD.6,7 Following the baseline assessment, families began a 9-week intervention (acute trial) that started with the primary caregiver participating in parent training, and all children receiving an open trial of stimulant monotherapy. During the first 3 weeks, the primary clinician adjusted stimulant dose to achieve an optimal therapeutic response defined as a CGI-I score of 1 by a blinded clinician and a parent-rated NCBRF D-Total score <15. If participants did not show a sufficient clinical response at Week 3, or if they showed deterioration at Week 4 through Week 6 (i.e., dropped below a blinded CGI-I of 1 or had a NCBRF D-Total >15), the second agent (risperidone or placebo) was added to the treatment package. This meant that some children having an excellent response to parent training (PT) and stimulant alone would not receive a placebo or risperidone augmentation. As described in prior publications,6,9 14 children (Basic=3; Augmented=11) dropped out before completing Week 3 and therefore did not receive the second medication. An additional 8 children were clinical responders by the end of Week 3 (Basic=3; Augmented=5) and did not take the second medication. Treatment dropouts were largely lost to further follow-up. More details regarding the background, methods, design, patient recruitment and retention, adverse events, responder criteria, and data analysis models are provided elsewhere.6,7,9
Sixty percent of the original sample (103 children) were favorable responders and agreed to enter double-blind treatment extension, and 88 (50% of the original sample) were completers (Week 21). Participation in the extension required monthly visits for 3 months, and parents were offered two more parent training sessions. Non-responders in the acute trial, acute-trial dropouts, and those who declined did not enter the extension. At the end of the extension, the blind was broken, and study staff tried to assist families in locating follow-up care.
For children who continued to experience difficulties during or at the end of the acute trial or extension, the clinician usually recommended an open trial of another medication or therapy. In the case of children randomized to Basic, parents had the option of trying Augmented. For the acute and extensions phases of the drug study, clinic visits, procedures, tests, and medication were provided free of charge.
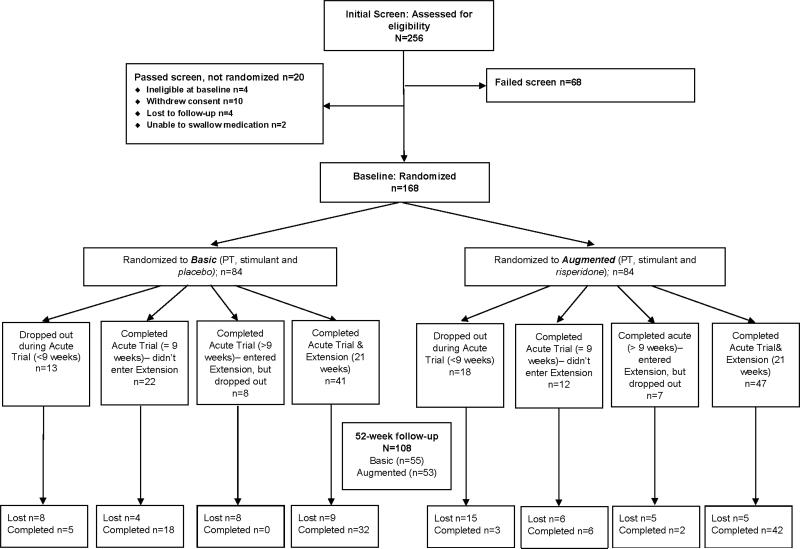
Excluding participants who indicated a desire for no further involvement during the acute trial or the extension, all families were contacted about participating in the Week 52 follow-up evaluation (Figure 1).
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram showing participant allocation and attrition. Note: PT = parent training.
Statistical Analyses
For behavioral and safety outcome measures, a mixed effects model was used to assess within- and between-group changes for the two treatment groups (Basic, Augmented) based on randomized assignment, regardless of Week 52 follow-up treatment status. Fixed effects included those for time, treatment group, treatment group-by-time interaction (randomized group assignment effect), site, and co-occurring ODD/CD. An unstructured variance covariance matrix was assumed for the correlated measures within each participant and empirical-based sandwich estimators were obtained to assess the group differences at follow-up. These models included only baseline and follow-up data, due to issues with model convergence when including all available time points. The Bonferroni-corrected alpha for the three primary behavioral outcomes was p<.017. Owing to concerns about Type 2 error, we report effect sizes (Cohen's d) for marginally significant (.017>p<.10) group assignment effects for primary outcomes.
The exploratory effectiveness analyses were conducted by comparing children who were still receiving their randomly assigned treatment at the end of the acute trial (Week 9) and follow-up using mixed models, similarly as described above, with effects for treatment group, time, and treatment group-by-time interaction (randomized group assignment effect). Site and ODD/CD were not included as covariates in these models due to small sample size concerns. Models included only baseline and follow-up data.
Owing to the controversy surrounding the augmentation of stimulant drug regimens with atypical antipsychotics and thus the need to thoroughly explore alternative design considerations, we also parsed participants according to their follow-up treatment status (i.e., regardless of their randomized group assignment). This also had the added benefit of accomodating changes in the drug regminen that likely impacted, and were impacted by, clinical status at time of follow-up. To do this, we used three additional strategies: the number of concurrent medications, drug class, or the reason (indication) for which medication was prescribed. Analyses for these strategies included follow-up data only. Groups based on number of medications (none, one, two or three) were compared on quantitative outcomes with analysis of variance (ANOVA) or Kruskal-Wallis depending on distribution of the data, and on categorical coutcomes with Fisher's exact test. For outcomes where significant omnibus tests were found, pairwise comparisons (e.g., none vs. one, none vs. multiple, one vs. multiple) were explored with Bonferroni-adjusted p-values. For drug class and indication parsing strategies, groups were compared via two-sample t-test or Mann-Whitney U test, depending on distribution of the data, for quantitative outcomes or Fisher's exact test for categorical outcomes.
Model assumptions for longitudinal data were assessed by examination of residuals. Some variables were square-root transformed to accommodate the assumption of normality. All analyses were conducted in SAS, version 9.3 (SAS Institute, Cary, NC).
RESULTS
Participants
Almost two-thirds of families (n=108; 65%) enrolled in the acute trial also participated in the Week 52 follow-up, and the two treatment groups were equally represented: Basic (n=55), Augmented (n=53) (Table 1). Average time to follow-up for Basic and Augmented was 12.7±1.6 months (range 10-18) and 13.5±4.1 months (range 9-35), respectively. Of families that completed the acute trial, 73% participated in the follow-up compared with 26% for the non-completers (Chi-Square, p<.0001). Figure 1 is the Consolidated Standards of Reporting Trials diagram showing the disposition of participants.
Table 1.
Characteristics of Week 52 Follow-up Participants as Assessed at Screen or Baselinea
| Characteristic | Basic (n=55) | Augmented (n=53) | Overall (n=108) |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 42 (76.4) | 41 (77.4) | 83 (76.9) |
| Female | 13 (23.6) | 12 (22.6) | 25 (23.1) |
| Disorder, n (%) | |||
| CD | 15 (27.3) | 13 (24.5) | 28 (25.9) |
| ODD | 40 (72.7) | 40 (75.5) | 80 (74.1) |
| Age (y) at screening, mean (SD) | 8.9 (2.0) | 8.8 (2.0) | 8.8 (2.0) |
| Age (y) at Week 52, mean (SD) | 10.0 (2.0) | 10.0 (2.0) | 10.0 (2.0) |
| IQ at screening, m (SD) | 99.5 (14.1) | 98.3 (14.4) | 98.9 (14.2) |
| Race, n (%) | |||
| White | 28 (50.9) | 33 (62.3) | 61 (56.5) |
| Black | 17 (30.9) | 15 (28.3) | 32 (29.6) |
| Asian | 1 (18) | 0 (0.0) | 1 (0.9) |
| American Indian or Alaskan Native | 1 (18) | 0 (0.0) | 1 (0.9) |
| Multiracial | 8 (14.6) | 5 (9.4) | 13 (12.0) |
| Ethnicity, n (%) | |||
| Hispanic origin | 3 (5.5) | 2 (3.8) | 5 (4.6) |
| Non-Hispanic origin | 52 (94.6) | 50 (94.3) | 102 (94.4) |
| Child's type of school, n (%) | |||
| Other | 7 (12.7) | 6 (11.3) | 13 (12.0) |
| Regular public (or parochial) | 48 (87.3) | 47 (88.7) | 95 (88.0) |
| Mother's employment, n (%) | |||
| Keeping house | 8 (14.5) | 9 (17.0) | 17 (15.7) |
| Other | 16 (29.1) | 16 (30.2) | 32 (29.6) |
| Working full-/part-time | 31 (56.4) | 28 (52.8) | 59 (54.6) |
| Father's employment, n (%) | |||
| Other | 24 (43.6) | 21 (39.6) | 45 (41.7) |
| Working full-/part-time | 30 (54.5) | 32 (60.4) | 62 (57.4) |
| Unknown | 1 (18) | 0 (0.0) | 1 (0.9) |
| Mother's education, n (%) | |||
| Some high school or less | 1 (1.8) | 5 (9.4) | 6 (5.6) |
| High school graduate or GED | 9 (16.4) | 17 (32.1) | 26 (24.1) |
| Some college or more | 44 (80.0) | 31 (58.5) | 75 (69.4) |
| Not in household or unknown | 1 (18) | 0 (0.0) | 1 (0.9) |
| Father's education, n (%) | |||
| Some high school or less | 1 (18) | 2 (3.8) | 3 (2.8) |
| High school graduate or GED | 13 (23.6) | 15 (28.3) | 28 (25.9) |
| Some college or more | 23 (41.8) | 22 (41.5) | 45 (41.7) |
| Not in household or unknown | 18 (32.7) | 14 (26.4) | 32 (29.6) |
| Income, n (%) | |||
| Less than $20,000 | 19 (34.5) | 17 (32.1) | 36 (33.3) |
| $20,001-$40,000 | 11 (20.0) | 9 (17.0) | 20 (18.5) |
| $40,001-$60,000 | 4 (7.3) | 10 (18.9) | 14(13.0) |
| $60,001-$90,000 | 10 (18.2) | 6 (11.3) | 16 (14.8) |
| More than $90,000 | 8 (14.5) | 9 (17.0) | 17 (15.7) |
| Unknown | 3 (5.5) | 2 (3.8) | 5 (4.6) |
| NCBRF, D-Total | |||
| Mean (SD) | 45.8 (11.7) | 45.7 (10.2) | 45.8 (10.9) |
| CGI-S, n (%) | |||
| 4 | 7 (12.7) | 5 (9.4) | 12 (11.1) |
| 5 | 35 (63.6) | 36 (67.9) | 71 (65.7) |
| 6 | 13 (23.6) | 12 (22.6) | 25 (23.1) |
| OAS-M (parent rated)b, n (%) | |||
| Assault against objects | 41 (74.5) | 41 (77.4) | 82 (75.9) |
| Assault against others | 51 (92.7) | 51 (96.2) | 102 (94.4) |
| Assault against self | 6 (10.9) | 3 (5.7) | 9 (8.3) |
Note: CD = conduct disorder; CGI=Clinical Global Impressions; GED = general educational development; NCBRF=Nisonger Child Behavior Rating Form; OAS-M=Overt Aggression Scale-Modified; ODD = oppositional defiant disorder.
No statistically significant differences between treatment groups except for mother's education (Chi-Square, p=.027).
Number and percent for those with scores of 3 or higher.
At follow-up, randomized treatment groups did not differ significantly in any child or family characteristics or baseline inclusion criteria with the exception of maternal education (Chi-Square, p=.03). More mothers of children in the Basic group (80%) reported having at least some college compared with the Augmented group (59%). The 60 children who were lost to follow-up were not significantly different from the 108 follow-up participants in any child or family characteristics or inclusion criteria with the exception of child's IQ (two-sample t-test, p=.02): follow-up participants M=98.9±14.2; lost to follow-up M=93.8±13.5 (see Table S1, available online).
Description of Treatment at Week 52
At follow-up 17% of the children (Basic, 11%; Augmented, 23%) were receiving no psychotropic medication (Table 2). The remaining children were prescribed one (30%), two (43%), or three (10%) drugs. In these and subsequent analyses, stimulant (or other ADHD-exclusive) medications were counted only once if prescribed in combination and for ADHD. The percentage of children receiving multiple medications at follow-up was similar for children randomized to Basic and Augmented, 51% and 57%, respectively (p=.08). Comparing only those with single- versus multiple-drug regimens (excluding those not taking medication), this relationship was still not statistically significant (p=0.11); thus groups did not differ for multiple medication use at follow-up.
Table 2.
Week 52 Drug Regimen by Type of Treatment (Basic, Augmented) and Definition of Treatment Terminology
| Medication Classification | Overall Frequency and % | Treatment Definitionsb | |||
|---|---|---|---|---|---|
| Augmenteda n (%) | Basica n (%) | Drug class | Indication | Consistent treatment | |
| Antiepileptic | 1 (19) | 0 (0) | |||
| Augmented (stimulant and atypical antipsychotic) | 23 (43.4) | 12 (21.8) | ✓ | ✓ | ✓ |
| Augmented and alpha-agonist | 2 (3.8) | 4 (7.3) | ✓ | ||
| Augmented and SSRI | 1 (19) | 2 (3.6) | ✓ | ||
| Augmented and antiepileptic | 1 (19) | 0 (0) | ✓ | ||
| Basic (stimulant only) | 9 (17.0) | 20 (36.4) | ✓ | ✓ | ✓ |
| Basic and alpha-agonist | 1 (19) | 7 (12.7) | ✓ | ||
| Basic and SSRI | 1 (19) | 2 (3.6) | ✓ | ||
| Mono alpha-agonist | 1 (19) | 0 (0) | |||
| Atypical antipsychotic | 1 (19) | 1 (18) | |||
| None | 12 (22.6) | 6 (10.9) | |||
| SSRI and alpha-agonist | 0 (0) | 1 (18) | |||
Note: SSRI = selective serotonin reuptake inhibitor.
Randomized group assignment: Augmented (stimulant+risperidone [n=53]), Basic (stimulant [n=55]).
Drug class=Basic (stimulant) and Augmented (stimulant, atypical antipsychotic) based on type of medication; Indication=Basic Indications and Augmented Indications, based on the reason (indication) for which medication was prescribed; and Consistent treatment=original random assignment to Basic (stimulant) or Augmented (stimulant+risperidone) at baseline and Week 52.
Outcome Analyses Based on Randomized Treatment Status
Behavioral measures
NCBRF D-total scores (primary outcome) declined in both groups from baseline to follow-up (p<.0001). Although Augmented obtained a lower score at follow-up than Basic (p=.03), the randomized group assignment by time effect failed to reach significance (p=.08; Cohen's d=0.34) (see Table S2, available online). There was a marginally significant finding for the Proactive (Instrumental) Aggression scale (primary outcome) of the ABS (p=.09; Cohen's d=0.35) also favoring Augmented.
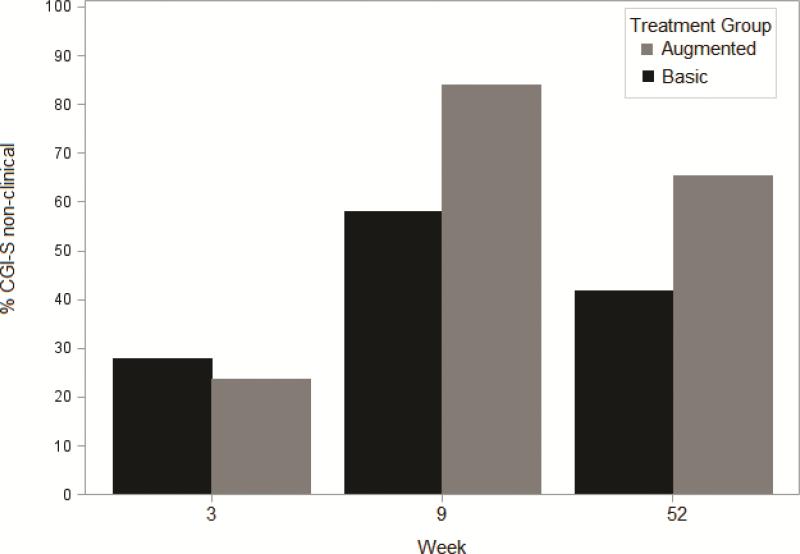
CGI-S scores (secondary outcome) were categorized as non-clinical (1-3 normal to mildly ill) versus clinical 4-7 (moderately ill to among the most ill). There was clear evidence of marked improvement in clinical status from baseline (non-clinical, 0%, both groups) to Week 3 (non-clinical, Basic=28%; Augmented=24%) and from Week 3 to the end of the acute trial at Week 9 (non-clinical, Basic=58%; Augmented=84%) (Figure 2). Compared with Week 9, fewer children were rated in the non-clinical range at Week 52 follow-up (Basic=42%; Augmented=65%); the Augmented group was rated superior (Fisher's exact test, p=.02).
Figure 2.
Percent of children randomized to basic or augmented with Clinical Global Impression–Severity (CGI-S) scores of 1-3 (normal to mildly ill, non-clinical) and who participated in Week 52 follow-up
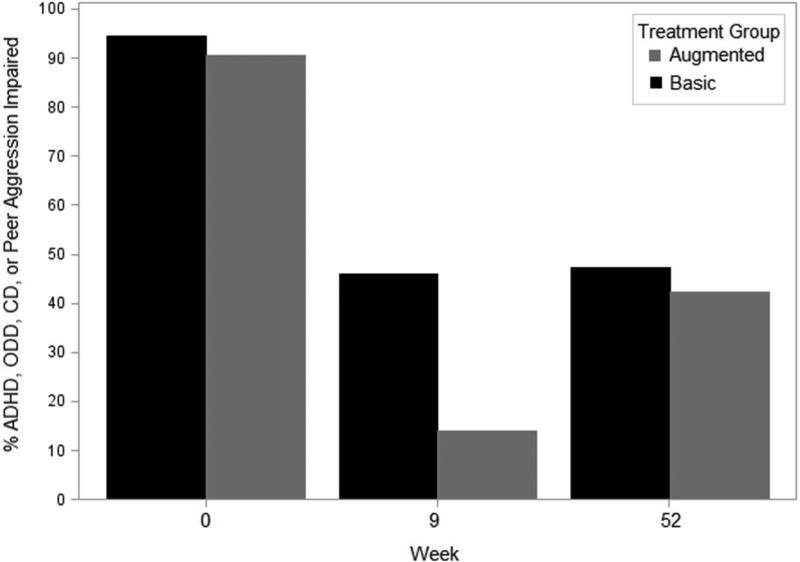
Almost half (45%) of all children met the CASI-4R Impairment cutoff criterion for ADHD, ODD, CD, or peer aggression at follow-up, and rates were comparable between randomized groups (Figure 3). Notably, impairment rates did not change appreciably from Week 9 to Week 52 follow-up for Basic (46% to 47%), whereas Augmented deteriorated from 14% at Week 9 to 42% at follow-up (Figure 3).
Figure 3.
Percent of children randomized to basic or augmented who met parent-rated Child and Adolescent Symptom Inventory-4R (CASI-4R) impairment cutoff for either attention-deficit/hyperactivity disorder (ADHD), oppositional defiant disorder (ODD), conduct disorder (CD), or peer aggression and who participated in Week 52 follow-up.
Safety measures
There were two safety measures that indicated randomized group assignment effects. Although groups did not differ in weight (weight-for-age z-score) or height (height-for-age z-score) at follow-up, Basic experienced a decrease in weight (p=.0007), whereas the Augmented did not (p=.94); the group assignment effect was significant (p=.01).
At follow-up, groups differed in prolactin levels (p=.003). Both Augmented and Basic levels increased over time (p<.0001 and .004, respectively); the group assignment effect was significant (p=.004). There were significant group differences in follow-up elevated prolactin levels: Basic (15%) versus Augmented (36%) (Fisher's exact test, p=.03). (At screen, only one participant from the Augmented group had a prolactin level above threshold.) Of the eight children randomly assigned to Basic with elevated prolactin levels at follow-up, seven were actually receiving risperidone at follow-up.
Outcome Analyses for Consistent Treatment
A subgroup of children were still receiving their randomized treatment at follow-up: Basic (n=20) and Augmented (n=23). Of these children, 93% completed the acute trial, and 85% qualified for the treatment extension. At follow-up, the Augmented group obtained lower NCBRF D-total scores, and the randomized group assignment effect was marginally significant (p=.06; Cohen's d=0.33) (see Table S3, available online). Two of the secondary outcomes also indicated group assignment effects: NCBRF-ADHD (p= .02) and CASI-4R Other Anxiety (p= .04), in both cases favoring Augmented over Basic.
Weight
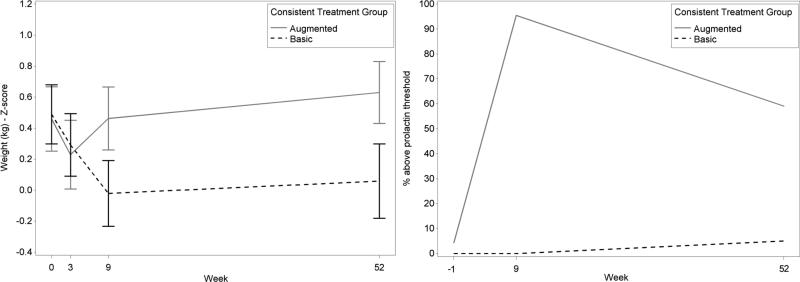
The change in weight (weight-for-age z-score) from baseline to follow-up evidenced a randomized group assignment effect (p=.0005) with Basic experiencing a greater decrease (Figure 4). Descriptively, both groups experienced a decrease in weight from baseline to Week 3. From Week 4 to Week 9, whereas Basic continued to decrease, Augmented increased to higher than baseline levels and continued to increase from Week 9 to follow-up. Basic, however, increased only slightly from Week 9 to follow-up not attaining baseline levels.
Figure 4.
Weight-by-age adjusted z scores (A) and prolactin levels (B) for consistent basic and augmented treatment.
Prolactin
Prolactin changes from screen to follow-up differed between groups (p<.0001) (Figure 4). Augmented was associated with increase from screen to follow-up. For prolactin threshold (boys, >18ng/mL; girls, >30ng/mL) at follow-up, Augmented (59%) had a greater proportion of children who were above this threshold than Basic (5%).
Outcome Analyses Based on Treatment Status at Week 52
Owing in part to the relatively high rate of modifications to drug regimens at time of follow-up and their likely implications for clinical status at follow-up, we examined three additional strategies for parsing participants based on medication(s) received at follow-up (Table 2). Strategy 1 was the number of medications: None (n=18), Single (n=32), and Multiple (n=58). Strategies 2 and 3 configured groups in a way that approximated the original definitions of Basic (stimulant only) and Augmented (stimulant+atypical antipsychotic). Strategy 2 excluded children receiving concomitant psychotropic medication for reasons other than ADHD/aggression: Basic (n=29; 27%) or Augmented (n=35; 32%). (Percentages were based on the entire follow-up sample.) This strategy most closely approximated the original treatment groups and is referred to here as “drug class.”
Strategy 3 disregarded concomitant psychotropic medication as long as it was prescribed for a reason (indication) other than ADHD/aggression. For example, a child receiving a stimulant for ADHD plus guanfacine for sleep problems was classified as Basic. This strategy is referred to here as “indications” (Table 2). With this strategy, two-thirds of the entire follow-up sample were either Basic Indications (n=33; 31%) or Augmented Indications (n=40; 37%). The most commonly prescribed concomitant medication to supplement what was essentially a Basic or Augmented treatment regimen was an alpha-agonist, n=8 and n=6, respectively.
Primary behavioral outcomes did not reveal group differences for Strategy 1, number of medications (see Supplement 2, available online). Similalry, there were no between-group differences in behavioral outcomes for Strategy 2, drug class, or Strategy 3, indications (see Supplement 3, Table S5A, available online).
For safety measures, the Multiple Medication group from Strategy 1 had higher prolactin levels than their respective comparisons (see Table S4, available online). Similarly, the Augmented groups from Strategies 2 and 3 had higher prolactin levels than their respective comparisons (see Table S5C, available online).
With regard to weight, the Single Medication group from Strategy 1 (see Table S4, available online) and Basic groups from Strategies 2 and 3 (see Table S5B, available online) had lower weight than their respective comparisons.
DISCUSSION
We examined 12-month outcomes of severely aggressive children who participated in a 9-week, randomized clinical trial. Patients were initially treated with stimulant medication for 3 weeks and then received a randomly assigned additional medication, either placebo (Basic) or risperidone (Augmented). Their parents participated in child behavior management training. Families of children who were favorable responders had the option of participating in a double-blind, 3-month treatment extension. Approximately 52 weeks after their baseline assessment, all families could participate in a follow-up evaluation. As might be expected, many children experienced changes to their treatment regimens, and the percentage receiving multiple psychotropic medications at follow-up was 51% and 57% for children randomized to Basic and Augmented, respectively (not significant [ns]). A minority, 23% of Augmented and 11% of Basic, were taking no medication at follow-up.
The three primary outcome measures did not reveal evidence of a 12-month advantage for either Basic or Augmented, and this was evident from several different strategies for conceptualizing treatment and outcome. Nevertheless, exploratory findings for secondary outcomes suggest greater benefit from Augmented. For example, compared with the end of the acute trial, more children receiving Augmented than Basic had CGI-S ratings of 1-3 (mild or better) at follow-up. Exploratory treatment effectiveness analyses for a subgroup of children who continued to receive their randomly assigned treatment at follow-up revealed a marginally significant group difference that was consistent with our 9-week, acute treatment trial (i.e., Augmented evidenced a larger decrease in NCBRF D-Total scores than Basic). 6 NCBRF ADHD and CASI Anxiety scores also favored Augmented over Basic, although these benefits were not evident in the acute trial.
At follow-up, most children were still experiencing mental health concerns. For example, 83% were receiving psychotropic medication, typically two or more drugs. Significantly more children receiving Basic (58%) than Augmented (35%) obtained CGI severity scores of ≥4 (moderately ill to among the most ill). In terms of parent-rated, symptom-induced impairment, 45% of children met the CASI-4R Impairment cutoff for ADHD, ODD, CD, or peer aggression, and this did not include school teachers’ concerns. Collectively, most (but not all) children had chronic behavior problems, and a combination of two or three evidenced-based interventions did not completely ameliorate their mental health concerns.
With regard to AEs, there were no treatment group differences for cardiovascular function or extrapyramidal symptoms regardless of participant parsing strategy. Although groups did not differ in weight at follow-up, Basic experienced a decrease whereas the Augmented did not, and the randomized group assignment effect was significant. Augmented therapy was associated with significantly larger increases in prolactin levels over time. At follow-up, the percentage above threshold (boys, >18ng/mL; girls, >30ng/mL) was 53% and 59% for the Augmented random assignment and continuous treatment groups, respectively. The rate at the end of the acute trial was 65%6 suggesting that longer-term risperidone exposure resulted in only minor change in hyperprolactinemia. Although these known risperidone AEs were not unexpected,19,20 the present study extends these observations to children receiving concurrent, chronic stimulant medication, which might be expected to suppress prolactin. Adverse events will be discussed more comprehensively in a future publication.
This study has a number of strengths, including the use of multiple sites, prospective design, rigorous assessment battery, and different strategies for parsing participants to evaluate group differences in outcomes. Nevertheless, our results are subject to several qualifications: this follow-up study was neither designed nor powered to test hypotheses about safety or efficacy; therefore, reported outcomes should not be interpreted as endorsing specific clinical recommendations. A related concern, Type 2 error (falsely concluding no differences in randomized treatment strategies) was addressed in part by reporting effect sizes for marginally significant primary outcomes. Generalization of these results to everyday clinical practice is bounded by our methodology. As we have pointed out previously,6,9 our participants were not representative of the typical ADHD clinic population. Their aggressive behaviors were severe at initial intake, and the majority was from less financially advantaged homes. All received an initial optimizing trial of stimulant medication with concurrent parent training (both of which were continued throughout the acute trial), and risperidone was randomly added only when Basic failed to achieve behavioral normalization.
It would have been optimal to have retained a larger number of the initial participants for follow-up evaluation; however, given the severity of the clinical population and level of family adversity, our participation rate was fairly good and reasonably representative of the original sample. It is not unusual for follow-up studies to report continuing behavioral improvement over time. However, in the absence of a treatment-as-usual comparison group, it is difficult to know how much is attributable to intervention. Importantly, this report is best conceptualized as a naturalistic comparison of relative outcomes for children initially assigned to a particular treatment strategy. Multiple factors occurring during and following the acute trial likely contributed to group differences at Week 52, notably, family characteristics that influenced the decision to participate in follow-up; initial response to medication; prescribing bias; discontinuation, substitution, and addition of medications; and the termination of routine parent training in behavior management at the end of the acute trial.
As previously reported,9 teachers’ ratings obtained for a subgroup of these children during the acute drug trial indicated a wider range of significant treatment effects than parents’ ratings, consistent with the results of other studies.4,21,22 It would have been optimal to include teacher input as well, but this would have also introduced source variance (different teachers at follow-up). The results of our effectiveness evaluation were, as indicated, exploratory and best conceptualized as hypothesis-generating, not hypothesis-confirming. The follow-up sample was representative of our initial sample, and group differences in treatment response for specific symptom domains were consistent with the acute trial. Variables not under our control or not assessed with our standardized measures may have explained treatment-group differences.
The clinical implications of TOSCA findings published to date suggest the following: consistent with Treatment Recommendations for the use of Antipsychotics for Aggressive Youth guidelines,5 the treatments of first choice for the management of severe aggression in children with co-occurring ADHD and ODD/CD aggression are stimulant monotherapy and parent training in behavior management techniques, either individually or in combination. In the face of intractable aggression, the addition of risperidone in combination with both stimulant medication and parent training may provide additional benefit of unknown duration for some individuals in suppressing ADHD and ODD symptoms and aggression. Short-term therapeutic effects are evident in both home and school setting. Long-term outcomes (compared with baseline) indicate continuing benefit for children who remained on medication. Exploratory analyses suggest the possibility that augmented therapy may have added modest benefit, but this remains a topic for further study. With regard to AEs, caregivers should be alerted to the likelihood of increased prolactin levels with Augmented. Basic is associated with weight loss, whereas concurrent stimulant and risperidone may attenuate weight gains associated with Augmented. As for maintenance therapy in the absence of continuing parent training, therapeutic gains stabilize or decrease over time, and the advantage of Augmented over Basic becomes less apparent, possibly as a consequence of terminating parent training or uncertain ability to afford recommended treatments. Regardless, this suggests the need periodically to evaluate the treatments being prescribed, including the necessity for continuing risperidone.
At follow-up, only a relatively small percentage of children had stopped medication. Compared to their randomized treatment, approximately half were receiving modified treatment regimens, and most were receiving multiple drugs. It seems plausible to infer from these events that, if these three evidenced-based and widely recommended interventions resolved the quest for normalcy with minimal risk, this would not have been the case. Furthermore, our treatment patterns appear consistent with real world practices, where clinicians respond to patient needs by trying different drug treatment strategies. Therefore, clinicians should anticipate the likelihood that the successful management of such patients will require longer-term family involvement and the possible use of drug combinations that lack adequate scientific validation, a situation that has been characterized as acting amidst ambiguity.23
Supplementary Material
Clinical Guidance.
This study evaluated 12-month outcomes of children with co-occurring ADHD, disruptive behavior disorder, and serious physical aggression initially treated (acute trial) with parent training + stimulant medication + placebo versus parent training + stimulant + risperidone.
Therapeutic gains evidenced at the conclusion of the acute trial decreased during the ensuing 10 months. Nevertheless, our participants remained less symptomatic at follow-up than baseline although treatment augmentation with risperidone appeared only marginally to influence later clinical status.
At follow-up, few children were medication free; the majority was receiving multiple psychotropic drugs; and parents rated nearly half of the children as being impaired by ADHD, aggressive, or noncompliant behavior.
The search for more effective intervention remains a societal imperative.
Acknowledgements
The authors gratefully acknowledge guidance and supervision of the Data and Safety Monitoring Board, comprising Daniel Connor, MD (University of Connecticut Medical School), Walter Meyer, III, MD (University of Texas Medical Branch, Galveston), Carson Reider, PhD (the Ohio State University), and Wesley Thompson, PhD (University of California, San Diego).
Funding:
This study was supported by grants from National Institute of Mental Health (NIMH) to The Ohio State University (R01 MH077907), Case Western Reserve University (R01 MH077750), the University of Pittsburgh (R01 MH077676), and Stony Brook University (R01MH 077997). The project was supported by a National Institutes of Health (NIH) General Clinical Research Center grant M01RR10710 (State University of New York-Stony Brook) and Clinical and Translational Science Awards from the National Center for Advancing Translational Sciences: grants 8UL1TR000090-05 (The Ohio State University) and UL1 RR024153 and UL1TR000005 (University of Pittsburgh).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the respective National Centers for Advancing Translational Sciences or the NIH.
Ms. Brown served as the statistical expert for this research.
The authors gratefully acknowledge guidance and supervision of the Data and Safety Monitoring Board, comprising Daniel Connor, MD (University of Connecticut Medical School), Walter Meyer, III, MD (University of Texas Medical Branch, Galveston), Carson Reider, PhD (the Ohio State University), and Wesley Thompson, PhD (University of California, San Diego).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical guidance is available at the end of this article.
Supplemental material cited in this article is available online.
Clinical trial registration information—Treatment of Severe Childhood Aggression (the TOSCA Study); http://clinicaltrials.gov/; NCT00796302
Disclosures:
Dr. Gadow is a shareholder in Checkmate Plus, publisher of the Child and Adolescent Symptom Inventory-4R.
Dr. Arnold has received research funding from Curemark, Forest, Eli Lilly and Co., Neuropharm, Novartis, Noven, Shire, YoungLiving, NIH, and Autism Speaks; has consulted with or been on advisory boards for Arbor, Gowlings, Ironshore, Neuropharm, Novartis, Noven, Organon, Otsuka, Pfizer, Roche, Seaside Therapeutics, Sigma Tau, Shire, Tris Pharma, and Waypoint; and has received travel support from Noven.
Dr. Bukstein has received royalties from Routledge Press. He has received research support, acted as a consultant and/or served on a speaker's bureau for Eli Lilly and Co., Shire, Novartis, Cephalon, and Johnson and Johnson.
Dr. Findling has received research support from, acted as a consultant for, and/or served on a speaker's bureau for Alcobra, the American Academy of Child and Adolescent Psychiatry, American Physician Institute, American Psychiatric Press, AstraZeneca, Bracket, Bristol-Myers Squibb, CogCubed, Cognition Group, Coronado Biosciences, Dana Foundation, Elsevier, Forest, GlaxoSmithKline, Guilford Press, Johns Hopkins University Press, Johnson and Johnson, Jubilant Clinsys, KemPharm, Eli Lilly and Co., Lundbeck, Merck, NIH, Neurim, Novartis, Noven, Otsuka, Oxford University Press, Pfizer, Physicians Postgraduate Press, Purdue, Rhodes Pharmaceuticals, Roche, Sage, Shire, Sunovion, Supernus Pharmaceuticals, Transcept Pharmaceuticals, Validus, and WebMD.
Dr. Aman has received research contracts from, consulted with, or served on advisory boards of Biomarin Pharmaceuticals, Bristol-Myers Squibb, Cog State, Inc., Confluence Pharmaceutica, Coronado Biosciences, Forest Research, Hoffmann-La Roche, Johnson and Johnson, MedAvante Inc., Novartis, Pfizer, ProPhase LLC, and Supernus Pharmaceuticals.
Drs. Butter, Farmer, Kolko, Molina, Rice, Jr., Schneider, and Mss. Brown and Buchan-Page report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Dr. Kenneth D. Gadow, Stony Brook University, Stony Brook, NY..
Ms. Nicole V. Brown, Center for Biostatistics, Ohio State University, Columbus..
Dr. L. Eugene Arnold, Nisonger Center, Ohio State University..
Ms. Kristin A. Buchan-Page, Nisonger Center, Ohio State University..
Dr. Oscar G. Bukstein, University of Pittsburgh and is now with DePelchin Children's Center, Houston..
Dr. Eric Butter, Nisonger Center, Ohio State University..
Dr. Cristan A. Farmer, Nisonger Center and is now with Pediatrics and Developmental Neuroscience Branch, National Institute of Mental Health (NIMH), Bethesda, MD..
Dr. Robert L. Findling, Case Western Reserve University, Cleveland, and is now with Johns Hopkins University, Kennedy Krieger Institute, Baltimore..
Dr. David J. Kolko, University of Pittsburgh..
Dr. Brooke S.G. Molina, University of Pittsburgh..
Dr. Robert R. Rice, Jr., Nisonger Center, Ohio State University..
Dr. Jayne Schneider, Stony Brook University, Stony Brook, NY..
Dr. Michael G. Aman, Nisonger Center, Ohio State University..
References
- 1.Bussing R, Winterstein AG. Polypharmacy in attention deficit hyperactivity disorder treatment: Current status, challenges and next steps. Curr Psychiatry Rep. 2012;14:447–449. doi: 10.1007/s11920-012-0295-6. [DOI] [PubMed] [Google Scholar]
- 2.Comer JS, Olfson M, Mojtabai R. National trends in child and adolescent psychotropic polypharmacy in office-based practice, 1996-2007. J Am Acad Child Adolesc Psychiarty. 2010;49:1001–1010. doi: 10.1016/j.jaac.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duffy FF, Narrow WE, Rae DS, et al. Concomitant pharmacotherapy among youths treated in routine psychiatric practice. J Child Adolesc Psychopharmacol. 2005;15:12–25. doi: 10.1089/cap.2005.15.12. [DOI] [PubMed] [Google Scholar]
- 4.Connor DF, Glatt SJ, Lopez ID, Jackson D, Melloni RH., Jr Psychopharmacology and aggression. I: A meta-analysis of stimulant effects on overt/covert aggression-related behaviors in ADHD. J Am Acad Child Adolesc Psychiatry. 2002;41:253–261. doi: 10.1097/00004583-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Pappadopulos E, MacIntre JC, Crismon ML, et al. Treatment Recommendations for the use of Antipsychotics for Aggressive Youth (TRAAY). Part II. J Am Acad Child Adolesc Psychiatry. 2003;42:145–161. doi: 10.1097/00004583-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Aman MG, Bukstein OG, Gadow KD, et al. What does risperidone add to parent training and stimulant medication for severe aggression in child attention-deficit/hyperactivity disorder? J Am Acad Child Adolesc Psychiatry. 2014;53:47–60. doi: 10.1016/j.jaac.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farmer C, Arnold L, Bukstein O, et al. The treatment of severe child aggression (TOSCA) study: Trial design challenges. Child Adolesc Psychiatry Ment Health. 2011;5(36):1–11. doi: 10.1186/1753-2000-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aman MG, Leone S, Lecavalier L, Park L, Buican B, Coury D. The Nisonger Child Behavior Rating Form-Typical IQ version, for children with typical IQ. Int Clin Psychopharmacol. 2008;23:232–242. doi: 10.1097/YIC.0b013e3282f94ad0. [DOI] [PubMed] [Google Scholar]
- 9.Gadow KD, Arnold LE, Molina BSG, et al. Risperidone added to parent training + stimulant medication: Effects on attention-deficit/hyperactivity disorder, oppositional defiant disorder, conduct disorder, and peer aggression. J Am Acad Child Adolesc Psychiatry. 2014;53:948–959. doi: 10.1016/j.jaac.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnold L, Gadow KD, Farmer CA, et al. Comorbid anxiety and social avoidance in TOSCA: Response to adding risperidone to stimulant & parent training; Mediation of disruptive symptom response. J Child Adolesc Psychopharm. 2015;25:203–212. doi: 10.1089/cap.2014.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rundberg-Rivera EV, Townsend LD, Schneider J, et al. Participant satisfaction in a study of stimulant, parent training, and risperidone in children with severe physical aggression. J Child Adolesc Psychopharm. 2015;25:225–233. doi: 10.1089/cap.2014.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coccaro EF, Harvey PH, Kupshaw-Lawrence E, Herbert JL, Bernstein DP. Development of neuropharmacologically based behavioral assessments of impulsive aggressive behavior. J Neuropsychiatry Clin Neurosci. 1991;3(suppl 2):44–51. [PubMed] [Google Scholar]
- 13.Rapoport J, Conners CK, Reatig N. (Guest Editors). CGI (Clinical Global Impression) scale – NIMH. Psychopharmacol Bull [Special Issue] 1985;21(4):839–843. [Google Scholar]
- 14.Brown K, Atkins MS, Osborne ML, Milnamow M. A revised teacher rating scale for reactive and proactive aggression. J Abnorml Child Psychol. 1996;24:473–480. doi: 10.1007/BF01441569. [DOI] [PubMed] [Google Scholar]
- 15.Gadow KD, Sprafkin J. Child and Adolescent Symptom Inventory-4R. Checkmate Plus; Stony Brook, NY: 2005. [Google Scholar]
- 16.Barnes TRE. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–676. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- 17.Guy W: ECDEU Assessment Manual for Psychopharmacology: Revised (DHEW publication number ADM 76-338) US Department of Health, Education and Welfare, Public Health Service, Alcohol Drug Abuse and Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research Programs; Rockville, MD: 1976. pp. 534–537. [Google Scholar]
- 18.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 19.Almandil NB, Liu Y, Murray ML, et al. Weight gain and other metabolic adverse effects associated with atypical antipsychotic treatment of children and adolescents: a systematic review and meta-analysis. Pediatric Drugs. 2013;15(2):139–150. doi: 10.1007/s40272-013-0016-6. [DOI] [PubMed] [Google Scholar]
- 20.Isaac G. Risperidone use in children: Gynecomastia, hyperprolactinemia, and other controversies. Psychiatric Annals. 2015;24(4):204–211. [Google Scholar]
- 21.Gadow KD, Sverd J, Nolan EE, Sprafkin J, Schneider J. Immediate-release methylphenidate for ADHD in children with comorbid chronic multiple tic disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:840–8. doi: 10.1097/chi.0b013e31805c0860. [DOI] [PubMed] [Google Scholar]
- 22.Gadow KD, Nolan EE, Sverd J, Sprafkin J, Schneider J. Methylphenidate in children with oppositional defiant disorder and both co-morbid chronic multiple tic disorder and ADHD. J Child Neurol. 2008;23:981–990. doi: 10.1177/0883073808315412. [DOI] [PubMed] [Google Scholar]
- 23.Eisenberg L. The ethics of intervention: Acting amidst ambiguity. J Child Psychol Psychiatry. 1975;16:93–104. doi: 10.1111/j.1469-7610.1975.tb01260.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.