Abstract
PURPOSE
Previous reports have suggested that noninvasive cortical stimulation could influence speech production in patients with chronic stroke. Here, we evaluated the hypothesis that cathodal transcranial DC stimulation (ctDCS), a technique that decreases excitability of stimulated cortical sites, applied over a healthy right Broca's homologue area could improve picture naming in patients with post-stroke aphasia.
METHODS
Ten right-handed patients with post-stroke aphasia were enrolled in this double blind, counterbalanced sham-controlled, crossover study. Each patient received an intervention of ctDCS (2 mA for 20 min) and of sham tDCS (2 mA for 1 min) daily for 5 consecutive days in a randomized crossover manner with a minimum interval of one week between interventions, over a healthy right Broca's homologue area using a left supraorbital anode and simultaneous daily sessions of conventional word-retrieval training. The primary endpoint measure of this study was a standardized, validated Korean version of the Boston Naming Test, which is a measure of picture naming skills.
RESULTS
ctDCS was not found to have any adverse effects. Furthermore, significantly improved picture naming (p=0.02) was observed at 1 hour following the last (5th) ctDCS treatment session, but no changes were observed after sham tDCS.
CONCLUSION
These results demonstrate that cathodal tDCS over the right healthy Broca's homologue area with a left supraorbital anodal location can improve picture naming task performance in post-stroke aphasia.
Keywords: Aphasia, Stroke, Cortical stimulation, Transcranial direct current stimulation
Introduction
Aphasia is a devastating form of cognitive impairment and results in marked disability in 30 to 60 percent of stroke patients (Pedersen, et al., 2004; Wade, et al., 1986). It has been reported that conventional word-retrieval training effectively induces partial clinical improvements, but that it rarely leads to complete functional recovery (Meinzer, et al., 2007). Accordingly, strategies must be developed that enhance the beneficial effects of word-retrieval training protocols.
A recent open-protocol study showed that 10 day sessions of inhibitory 1 Hz rTMS over the posterior-inferior portion of the right pars triangularis significantly improve picture naming 2 months later (Naeser, et al., 2005). In the present study, we used a different noninvasive cortical stimulation technique, namely, transcranial direct current stimulation (tDCS), which can be administered simultaneously with word-retrieval training protocols, since it is barely felt on the scalp and is more easily shammed than rTMS [see Gandiga et al (2006) and Reis et al (2009) for review].
The application of inhibitory cathodal tDCS (ctDCS) to the primary motor cortex can decrease motor cortical excitability at this site (Nitsche, et al., 2003; Nitsche & Paulus, 2000; Purpura & McMurtry, 1965; Wassermann & Grafman, 2005). Furthermore, it has been demonstrated that ipsilesional facilitatory anodal tDCS or contralesional ctDCS elicits motor improvements in chronic stroke patients (Boggio, et al., 2007; Hummel, et al., 2005). In addition, tDCS is easily administered, relatively inexpensive and comfortable for patients, and it can be administered in combination with cognitive rehabilitative training (Gandiga, et al., 2006; Monti, et al., 2008).
Here, we tested the hypothesis that ctDCS applied to the right healthy Broca's homologue area simultaneously with word-retrieval training might improve picture naming task performance in patients with post-stroke aphasia. Based on previous literature, we reasoned that this paradoxical effect could be caused by the down-regulation of activity in the right healthy Broca's homologue area and secondarily by a reduction in the abnormally increased interhemispheric inhibition from the contralesional to the ipsilesional hemisphere (Fregni, et al., 2006; Mansur, et al., 2005; Naeser, et al., 2005; Oliveri, et al., 2001; Takeuchi, et al., 2005; Ward & Cohen, 2004).
The primary endpoint of this study was performance as assessed by a randomly sorted computerized Korean version of the Boston Naming Test (BNT; Kim & Na, 1997), which involves the use of 60 culturally customized picture items.
Methods
Subjects
Ten Korean patients with post-stroke aphasia due to a single left hemisphere infarction participated in this study from an outpatient clinic (Table 1). Mean patient age was 61.9 ± 2.7 years (mean ± SE; range 46–73 years), two were female, all were right-handed by the Edinburgh Handedness Inventory (Oldfield, 1971), and mean time spent in full-time education was 11.6±1.5 years (range 0–16)
Table 1.
Patient data
| Patient | Age (years) |
Sex | Time after stroke (months) |
Left hemisphere lesion site and time post-stroke onset when MRI scan was obtained. |
Educational period (years) |
Western aphasia battery | Initial intervention |
||
|---|---|---|---|---|---|---|---|---|---|
| Aphasia quotient (%) |
Language quotient (%) |
Type of aphasia |
|||||||
| 1 | 60 | F | 87.3 | Fronto-parieto-temporal infarction, 4 days |
12.0 | 21.0 | 17.0 | Global | ctDCS |
| 2 | 56 | M | 44.3 | Fronto-parieto-temporal infarction, 18 days |
14.0 | 14.0 | 9.0 | Global | Sham |
| 3 | 46 | M | 8.0 | Fronto- temporal infarction, 5 days | 16.0 | 11.0 | 21.0 | Global | ctDCS |
| 4 | 60 | M | 180.6 | Frontal infarction, 13 years | 9.0 | 72.0 | 69.0 | Broca’s | Sham |
| 5 | 73 | F | 168.0 | Subcortical lesion, capsular-putaminal with superior lesion extension into PVWM, 13 years |
0.0 | 79.0 | 76.0 | Anomic | Sham |
| 6 | 65 | M | 6.0 | Fronto- temporal infarction, 48 days | 16.0 | 71.0 | 72.0 | Anomic | ctDCS |
| 7 | 73 | M | 6.0 | Temporo-parietal infarction, 1 days | 12.0 | 39.2 | -* | Broca’s | ctDCS |
| 8 | 66 | M | 7.1 | Subcortical lesion, capsular-putaminal with superior lesion extension into PVWM, 4 days |
16.0 | 24.0 | 31.0 | Broca’s | ctDCS |
| 9 | 53 | M | 10.4 | Fronto-temporal infarction, 6 days | 12.0 | 43.0 | 62.0 | Broca’s | Sham |
| 10 | 67 | M | 6.0 | Two lesions: subcortical lesion, capsular-putaminal with very small superior lesion extension into PVWM; and occipital lesion, 42 days |
9.0 | 21.0 | 32.0 | Transcortical motor |
Sham |
| Mean±SE | 61.9 ±2.7 | 52.4 ± 21.9 | 11.6 ± 1.5 | 39.5 ± 8.2 | 43.2 ± 8.3 | ||||
F = female; M= male; PVWM = periventricular white matter;
un-checkable writing ability.
Patients were tested at a mean 52.4 ± 21.9 months (range 6.0 – 180.6 months) after stroke. Types of aphasia were categorized using the Kertesz’ classification (Kertesz, 1979), and aphasia severity was determined using aphasia (Kertesz & Poole, 1974); AQ, range 0 ~ 100, normal range > 95) and language (Shewan, 1986) quotients (LQ, range 0 ~ 100, normal range > 95) according to the validated Korean version of the Western aphasia battery (WAB; Kim & Na, 2001).
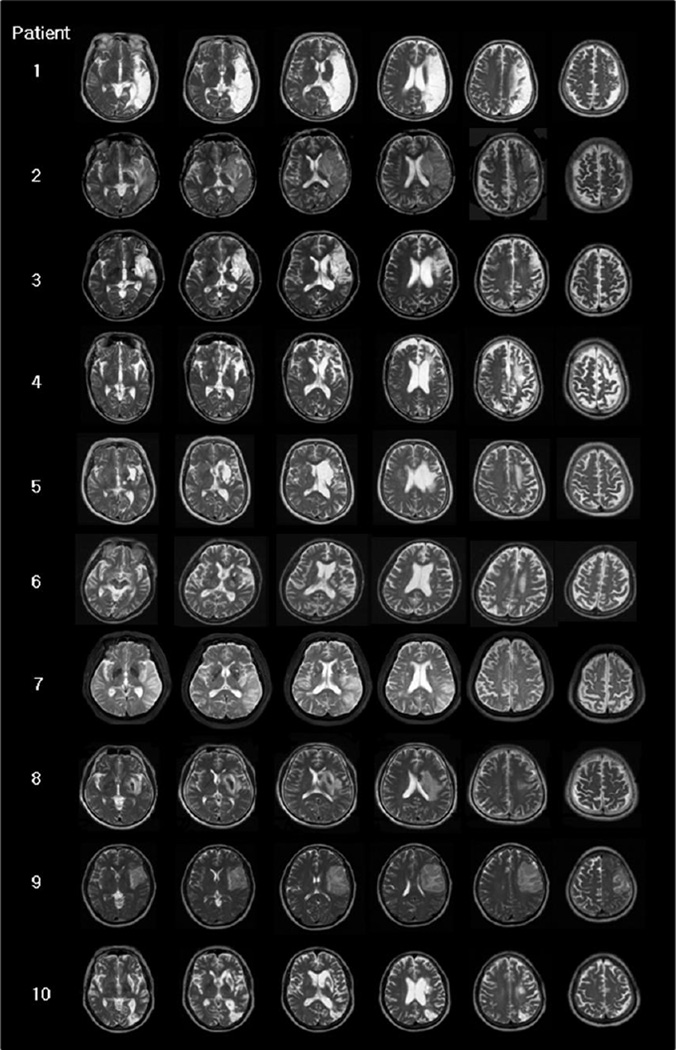
Lesion sites and patient aphasia profiles are presented in Table 1, and their T2-MRI scans in Fig. 1. For most cases (8/10), only acute or subacute, T2-weighted MRI scans were available (1 – 48 days poststroke onset). For two cases (case 4, and case 5), the MRIs were acquired at 13 years poststroke. Thus, the acute and subacute T2-weighted MRI scans may not have shown the full extent of the lesion that was present in the chronic state when the cases were treated (6 months to 13 years, poststroke).
Fig. 1. Patient's T2-weighted MRI scans.
Most cases (8/10) have T2-weighted MRI scans acquired in the acute or subacute stage poststroke (1 to 48 days); two cases have MRIs acquired in the very chronic stage, 13 years poststroke (cases 4 and 5). Note, since all cases were treated with tDCS in the chronic stage poststroke (at least 6 months poststroke), the borders of the lesion on these acute and subacute MRI scans might have changed somewhat, by the time these patients were actually treated.
Patients with multiple brain lesions, unstable medical or neurologic conditions, a metallic foreign body within the brain, a pacemaker or artificial cochlear implant, and those with severe depression, a history of seizure, or unable to perform the protocol-related behavioral task were excluded.
This study protocol was approved by our local institutional review board, and all patients or their legal delegates provided signed, written, informed consent. The study was conducted in accordance with the regulatory standards of Good Clinical Practice and the Declaration of Helsinki ("World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects," 2000). The experiment was performed at our institution.
Experimental Design
All patients were initially familiarized with the experimental setting, and briefly practiced the behavioral task using pictures not used subsequently for testing. Each patient then underwent 5-day interventions of ctDCS+training or sham+training in a crossover manner separated by at least one rest week (maximum 10 days). Intervention orders were balanced across patients.
Word-retrieval training consisted of 30 minutes per day of training applied according to customary rehabilitation word-retrieval training protocols, and this training was designed to allow patients to advance to more difficult levels according to patient ability and a speech-language pathologist's decision (Nickels, 2002). Training consisted of modules, such as, answering yes/no to questions, cued naming about target pictures, and word/picture matching tasks based on pictures sorted by category; the focus was placed on nouns available from commercial picture books. Pictures used subsequently for testing were not used during training sessions. Word-retrieval training was performed while patients were being treated with tDCS or sham stimulation.
During training sessions, patient first received 10 minutes of word-retrieval training, and then 20 minutes of word-retrieval training+ctDCS or 20 minutes of word-retrieval training+sham stimulation. Word-retrieval training was provided by a speech language pathologist who was unaware of the type of stimulation administered (ctDCS or sham).
ctDCS (2 mA for 20 minutes) or sham (2 mA for 1 minute) stimulation were delivered through two 25 cm2 (5 cm × 5 cm) saline-soaked surface sponge electrodes (Phoresor® ΙΙ PM850; IOMED® Inc., Salt Lake City, Utah), using a previously described method (Gandiga, et al., 2006; Hummel, et al., 2005). For ctDCS, the current was slowly increased to 1 mA at the onset of stimulation and applied for 20 min, whereas for sham stimulation, tDCS was applied for 1min, after which the current was slowly tapered to 0. This sham procedure does not elicit patient’s perceptions and was performed out of the patients’ view. The configuration chosen was based on the findings of a previous report (Gandiga, et al., 2006). Briefly, the cathode position was centered over F8 according to the international 10–20 EEG system, which corresponds well with the right Broca’s homologue area (Friederici, et al., 1998; Herwig, et al., 2003; Okamoto, et al., 2004), and the anode was placed over the left supraorbital area (Nitsche & Paulus, 2000).
tDCS was administered by a separate investigator who did not participate in outcome measurements, training, or data analysis. The patients, the investigator who determined outcomes, and the speech language pathologist who provided standardized word-retrieval training were all unaware of the intervention type.
Outcome Measurements
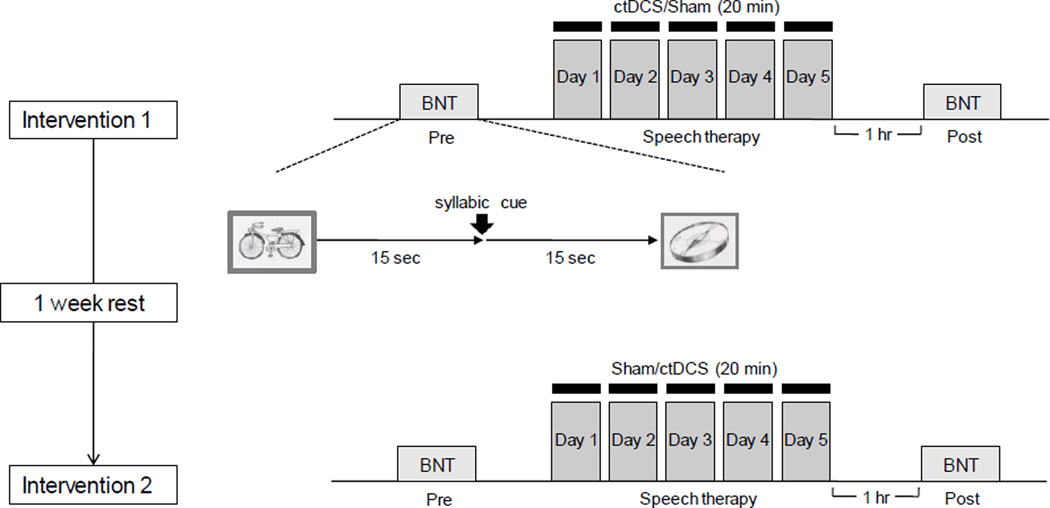
A standardized, validated Korean version of the BNT (Kim & Na, 1997), composed of 60 culturally customized pictures, was administered immediately before the first daily session and 1 hour after the end of the fifth daily session of ctDCS+training or sham+training (intervention 1 or intervention 2). See Figure 2.
Fig. 2. Experimental design of the behavioral study.
Each patient underwent two counterbalanced interventions of stimulation (ctDCS and sham) separated by a 1-week rest period. Each intervention consisted of five consecutive days of stimulation coupled with customary rehabilitative word-retrieval training (see Methods). A standardized, validated Korean version of the BNT (Kim & Na, 1997), composed of 60 culturally customized pictures was administered before and after the series of stimulation sessions as an outcome measure.
A single rater, unaware of stimulation type, administered the BNT using Superlab pro version 4.0 (Cedrus Corporation, San Pedro, CA). BNT pictures were randomly presented on a screen 75 cm from patients' eyes. Patients were instructed to name the picture shown as quickly and as accurately as possible. (Note, this randomized presentation of BNT pictures differs from the standard method of presentation of BNT pictures designed by Kaplan, et al. (Kaplan, et al., 2001), which involves presenting pictures in high to low frequency of occurrence order.
When a patient could not name the object on a picture correctly within 15 seconds, a syllabic cue was given, and the patient was allowed a further 15 seconds to name the object. Reaction times were taken from initial picture presentation. The number of correct responses to 60 pictures and reaction times were subjected to statistical analyses (Fig. 2).
Statistical analysis
Repeated measures ANOVA (ANOVARM) with the factor “INTERVENTION” (ctDCS+training versus Sham+training) as the within-subject factor, and “TIME” (pre- versus post-stimulation), as the repeated measure, were used to compare the effects of INTERVENTION and TIME on picture naming functions (accuracy and speed).
Post hoc analyses were performed, corrected for multiple comparisons using the Bonferroni test. The paired t-test was also used to evaluate the effect of INTERVENTION on picture naming.
The analysis was performed using SAS statistical software (version 9.1, Cary, N.C). All data are presented as means ± standard errors (SE).
Results
Each patient's individual scores and Pre- and Post-testing score changes according to the validated Korean version of the BNT (Kim & Na, 1997) are presented in Table 2.
Table 2.
Individual patient's pre- and post-stimulation Boston Naming Test scores and score changes
| Patient | Type of aphasia |
Time after stroke (months) |
Initial intervention |
ctDCS | Sham | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of correct responses |
Reaction time (msec) | Number of correct responses |
Reaction time (msec) | ||||||||||||
| Pre | Post | Δ | Pre | Post | Δ | Pre | Post | Δ | Pre | Post | Δ | ||||
| 1 | Global | 87.3 | ctDCS | 8.0 | 7.0 | −1.0 | 10337.5 | 11249.3 | 911.8 | 10.0 | 13.0 | 3.0 | 8538.1 | 13317.8 | 4779.7 |
| 2 | Global | 44.3 | Sham | 11.0 | 12.0 | 1.0 | 17349.5 | 13800.3 | −3549.2 | 11.0 | 12.0 | 1.0 | 17298.4 | 15876.7 | −1421.7 |
| 3 | Global | 8 | ctDCS | 4.0 | 17.0 | 13.0 | 16758.3 | 13562.4 | −3195.9 | 13.0 | 20.0 | 7.0 | 9955.4 | 11305.8 | 1350.4 |
| 4 | Broca’s | 180.6 | Sham | 53.0 | 57.0 | 4.0 | 6134.2 | 6617.3 | 483.1 | 49.0 | 52.0 | 3.0 | 9327.0 | 9059.1 | −267.9 |
| 5 | Anomic | 168 | Sham | 44.0 | 46.0 | 2.0 | 7672.7 | 7223.8 | −448.9 | 48.0 | 47.0 | −1.0 | 9146.8 | 7859.1 | −1287.6 |
| 6 | Anomic | 6 | ctDCS | 53.0 | 56.0 | 3.0 | 6768.3 | 5828.0 | −940.3 | 51.0 | 52.0 | 1.0 | 5567.8 | 4945.4 | −622.3 |
| 7 | Broca’s | 6 | ctDCS | 2.0 | 7.0 | 5.0 | 15988.0 | 13204.9 | −2783.1 | 4.0 | 4.0 | 0.0 | 20931.3 | 16026.0 | −4905.3 |
| 8 | Broca’s | 7.1 | ctDCS | 19.0 | 18.0 | −1.0 | 19283.1 | 17671.1 | −1612.0 | 17.0 | 13.0 | −4.0 | 20383.9 | 21321.7 | 937.8 |
| 9 | Broca’s | 10.4 | Sham | 54.0 | 60.0 | 6.0 | 8842.2 | 8856.5 | 14.3 | 50.0 | 54.0 | 4.0 | 11431.6 | 10104.4 | −1327.2 |
| 10 | Transcortical motor | 6 | Sham | 36.0 | 39.0 | 3.0 | 12443.5 | 14061.8 | 1618.3 | 30.0 | 32.0 | 2.0 | 14908.9 | 14846.0 | −62.9 |
Δ = post-pre
After initial familiarization, the baseline number of correct responses (28.4± 6.9 versus 28.3 ± 6.1; P=0.945) and reaction times (12157.7 ± 1539.8 msec versus 12748.9 ± 1680.4 msec; P=0.585) of were comparable prior to the ctDCS and Sham interventions.
ANOVARM revealed a significant effect for TIMEPre,Post [F(1, 9) = 6.02; P < 0.05] and a trend toward a significant interaction INTERVENTIONctDCS,Sham × TIMEPre,Post[F(1, 9) = 4.72; P = 0.058] without INTERVENTIONctDCS,Sham[F(1, 9) = 0.64; P =0.446] effect on the number of correct responses.
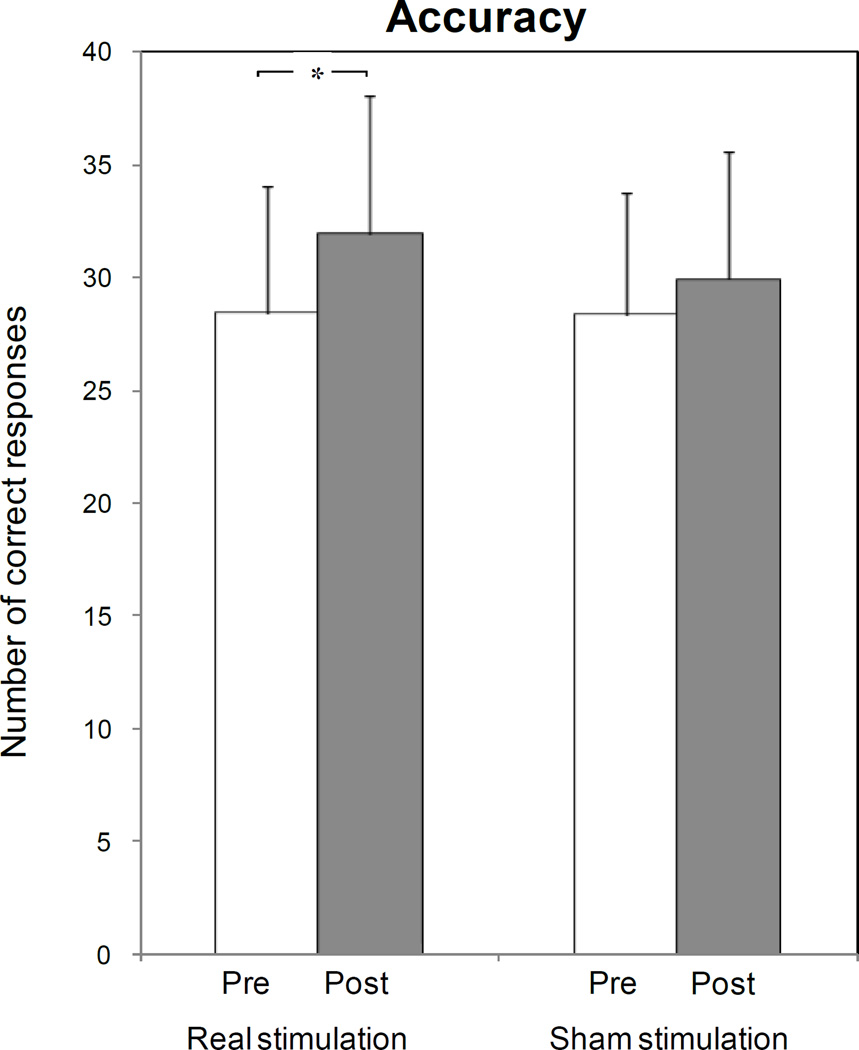
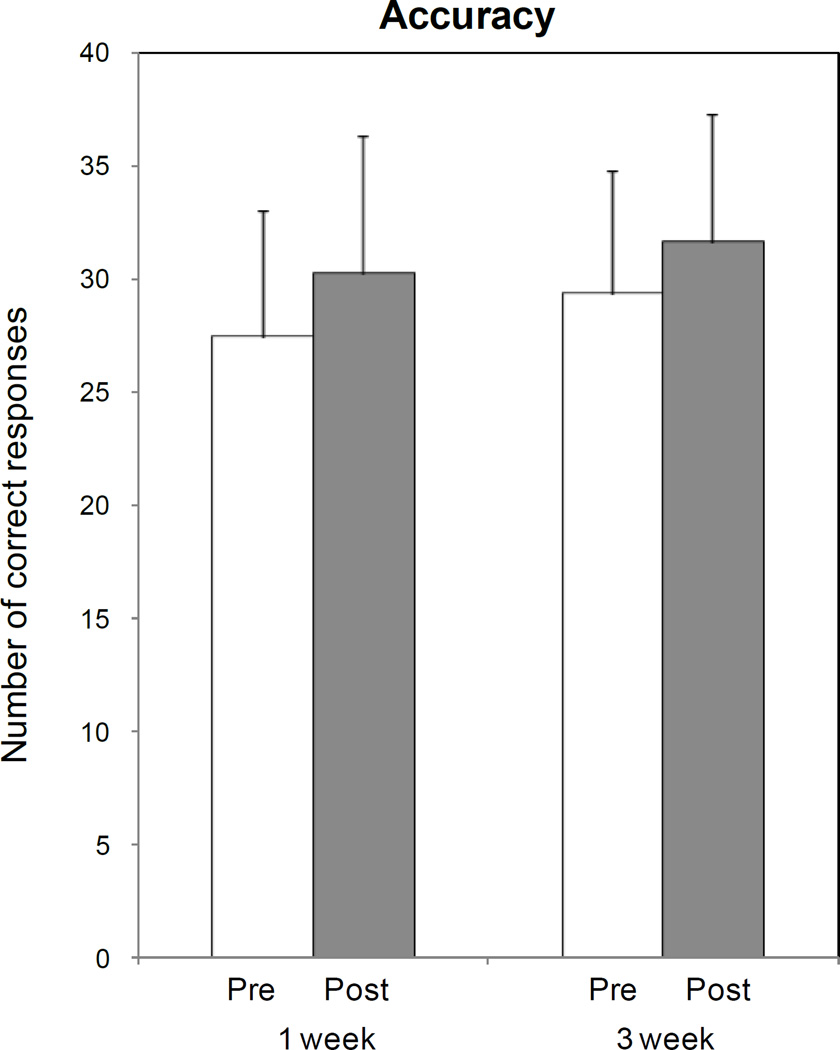
Paired t testing showed that ctDCS enhanced training effects on response accuracy relative to baseline (number of correct responses increased from 28.4 ± 6.9 at baseline to 31.9 ± 6.9 post-stimulation; P=0.023; Fig. 3-a, asterisk), but that Sham stimulation had only a minimal effect on response accuracy (from 28.3 ± 6.1 at baseline to 29.9 ± 6.2 post-stimulation; P=0.125; fig. 3-a).
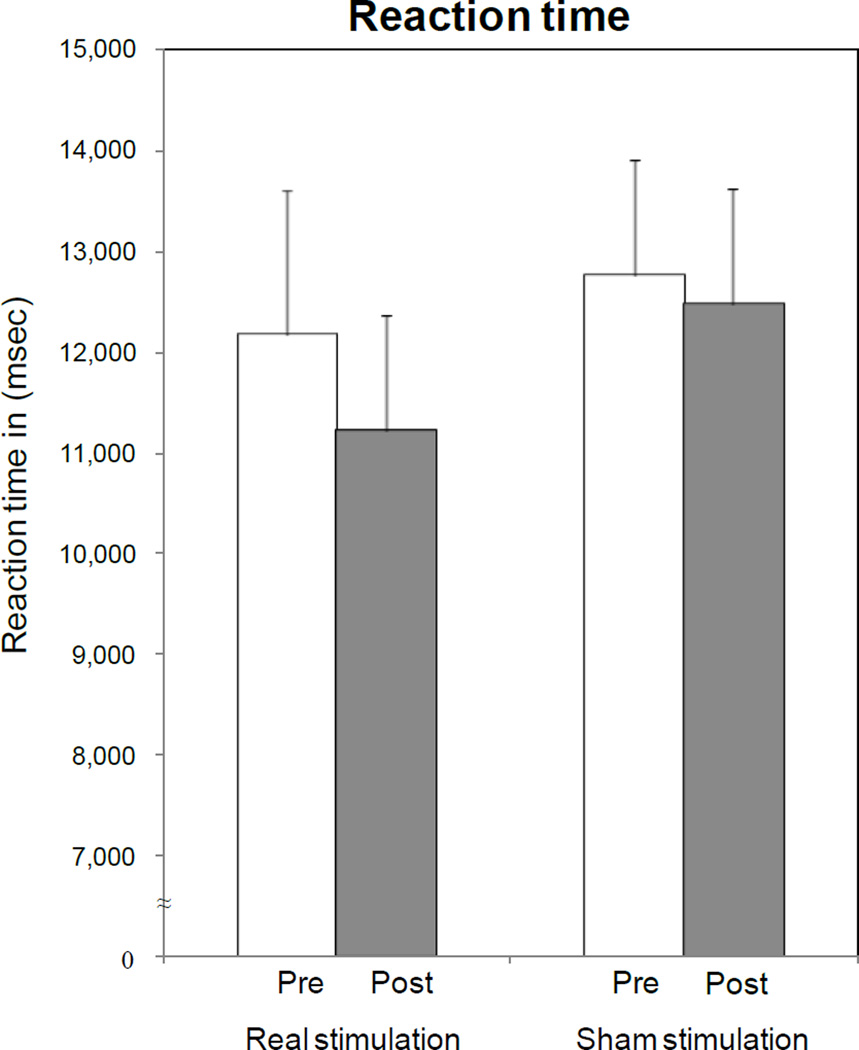
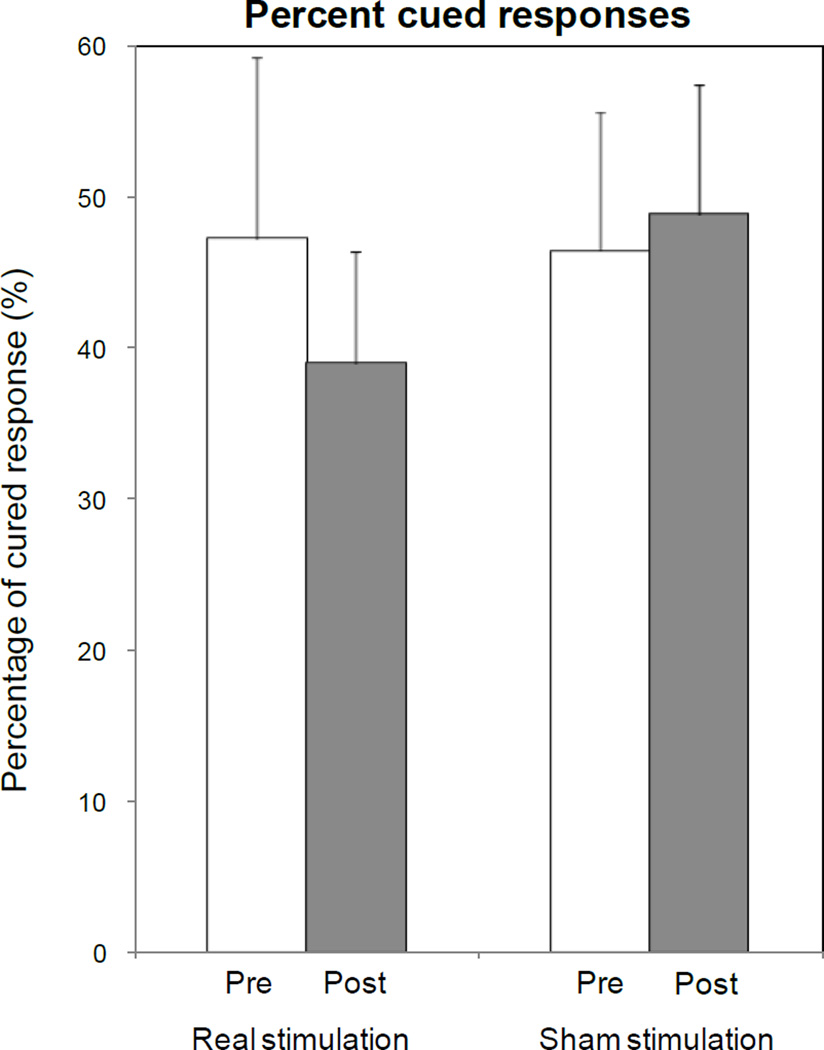
Fig. 3. Effects of ctDCS and Sham on picture naming task performance: accuracy (a), reaction time (b) and percentage of cued responses (c).
ctDCS + training improved accuracy relative to baseline (asterisk), with a pre-post difference larger than that induced by Sham tDCS (P=0.023 by paired t test). No significant differences were observed in terms of reaction times and percentages of cued response, despite a non-significant shortening of reaction time and reduction in the percentage of cued responses for ctDCS. Note that a general carry-over of response accuracy from week 1 to week 3 was observed, irrespective of the initial intervention type (d). Bars represent standard errors.
In terms of reaction times, ANOVARM showed no effects for INTERVENTIONctDCS,Sham[F(1, 9) = 0.20; P = 0.303], TIMEPre,Post[F(1, 9) = 1.15; P = 0.247], or INTERVENTIONctDCS,Sham × TIMEPre,Post [F(1, 9) = 0.76; P = 0.406] (Fig. 3-b).
Regarding percentages of cued responses, ANOVARM also revealed no effects for INTERVENTIONctDCS,Sham [F(1, 9) = 1.019; P =0.339], TIMEPre,Post [F(1, 9) = 0.846; P =0.382] or INTERVENTIONctDCS,Sham× TIMEPre,Post[F(1, 9) = 2.269; P =0.166] (Fig. 3-c).
Improvements in correct responses (Fig. 3-a) were not found to be correlated with age, time after stroke, number of years in full-time education, or severity of aphasia as determined by AQ and pre-ctDCS BNT scores (P > 0.05 by Pearson’s correlation analysis).
Regarding the effect of order of stimulation (ctDCS versus sham first), ANOVARM incorporating ORDER as a between subject factor showed no significant effect of ORDERctDCS first,Sham first[F(1,8)=3.57; P=0.095], TIMEPre,Post[F(1,8)=3.10; P=0.116], or ORDERctDCS first,Sham first × TIMEPre,Post [F(1,8)=9.80; P=0.335] on numbers of correct responses, indicating that improvements in number of correct responses after ctDCS sessions were not affected by order of stimulation.
However, ANOVARM incorporating WEEK as the within subject factor irrespective of initial intervention type, showed a significant WEEK1week,3week[F(1,9)=6.02; P=0.037] effect in the absence of TIMEPre,Post[F(1,9)=1.75; P=0.219] or WEEK1week,3week × TIMEPre,Post [F(1,9)=0.22; P=0.651], indicating a general carry-over effect of response accuracy from week 1 to week 3 (Fig. 3-d).
Discussion
The main findings of this double blind, sham controlled, crossover study are that ctDCS applied to the right healthy Broca's homologue area at the same time as word-retrieval training improved picture naming accuracy in post-stroke aphasia patients as compared with sham stimulation.
The choice of the standardized, validated Korean version of the BNT task (Kim & Na, 1997) as an endpoint measure was based on its published sensitivity for measuring speech-language function (Petrovich Brennan, et al., 2007), a high test–retest reliability, and minimal practice effects (Flanagan & Jackson, 1997).
Previous reports have documented increased activation in right frontal areas during the performance of various language tasks in non-fluent aphasia (Belin, et al., 1996; Naeser, et al., 2004; Rosen, et al., 2000), and it has been proposed that this increased activation might be the consequence of a loss of active interhemispheric inhibition from homologous regions in the lesioned hemisphere (Blank, et al., 2003; Rosen, et al., 2000). In Wernicke's aphasia, when increased activation was observed in the right temporal area, it was believed to represent a compensatory neural response, which could conceivably contribute to functional recovery (Weiller, et al., 1995). Furthermore, in non-fluent aphasia cases, right frontal compensatory activation was observed following treatment with intention aphasia therapy (Crosson, et al., 2009).
However, more work is needed to understand the functional role of this activation, which could potentially differ across individuals depending on factors, such as, time after onset, lesion site, and magnitude of impairment.
The former scenario assumes an interhemispheric imbalance, postulated initially by Kinsbourne, which could theoretically be present in stroke patients (F. C. Hummel & Cohen, 2006; Kinsbourne, 1970; Rosen, et al., 2000). Observations of persistent over-activation of right frontal areas in functional imagining studies in non-fluent aphasia patients supports this interhemispheric imbalance theory (Belin, et al., 1996; Martin, et al., 2009; Naeser, et al., 2004; Rosen, et al., 2000).
The phenomenon by which a lesion (real or "virtual" as in this study with ctDCS) to a healthy part of the brain results in improvement in function mediated by another part of the brain, has been described as paradoxical functional facilitation (Kapur, 1996). This concept has been well documented in the motor (Fregni, et al., 2005; Fregni, et al., 2006; Mansur, et al., 2005; Takeuchi, et al., 2005) and various cognitive (Brighina, et al., 2003; Oliveri, et al., 2001; Shindo, et al., 2006) domains after stroke using a “virtual” lesion approach.
Based on these reports we hypothesized that the downregulation of activity in the right healthy Broca's homologue area could paradoxically facilitate language functions after left hemispheric stroke (Kapur, 1996), perhaps by reducing abnormally increased interhemispheric transcallosal inhibition from the contralesional to the ipsilesional cortex (Fregni, et al., 2006; Mansur, et al., 2005; Naeser, et al., 2005; Oliveri, et al., 2001; Takeuchi, et al., 2005). A previous open-protocol study also pointed in this direction (Naeser, et al., 2005). However, the interhemispheric transcallosal inhibition model in aphasia is still not clear, and needs further elucidation.
In the present double-blind, sham controlled, crossover study, we found that ctDCS over the right healthy Broca's homologue area led to significant improvements in the accuracy of naming in aphasia. However, although our results showed significant improvements in accuracy, they only showed a non-significant trend for improvements in speed. These findings are encouraging as they indicate that gains in accuracy induced by the combination of ctDCS and training were not accomplished at the cost of a slower speed. Therefore, such improvements did not rely on a simple movement along an unchanged speed/accuracy trade off curve. For a discussion of this issue in the motor domain refer to (Reis, et al., 2009).
When we examined the data for patients who received sham stimulation first (patients 2, 4, 5, 9 and 10), three of the 5 achieved their highest naming scores (patients 4, 9 and 10, who achieved final BNT scores of 57, 60, and 39, respectively) only after second real stimulation. This compelling finding suggests that tDCS combined with simultaneous word-retrieval training is a worthwhile approach.
When considering the individual patients, two patients (patients 3 and 7) made the greatest change in naming accuracy with tDCS. These patients were the most severe in their baseline naming accuracy scores (2 and 4), and amongst the earliest post-stroke (6 and 8 months poststroke), and they both received the tDCS first.
This study represents a step forward because it characterizes performance improvements in a heterogeneous group of aphasia patients treated by ctDCS administration to the intact hemisphere in a double blind, crossover manner. On the other hand, several study shortcomings should be considered. First, the anode in our study was placed on the left supraorbital area, and therefore, we cannot rule out the possibility of a partial anodal contribution to performance improvements. Second, although baseline accuracies were comparable before ctDCS and Sham sessions, and there was no ORDER effect (ctDCS first or Sham first), patients showed a carry-over effect from the first to the second session, irrespective of the type of initial intervention and despite the 1 week interval between sessions. It is noteworthy that Naeser et al. observed a significant improvement in naming at 2 months after 10 rTMS treatment sessions to inhibit the right pars triangularis portion of the right Broca's homologue (Naeser, et al., 2005). To advance our understanding of the effects of tDCS on naming, we suggest a parallel group design study be undertaken. Third, our experimental design did not include a session in which patients received only ctDCS but not word-retrieval training, and as a consequence we cannot rule out the possibility that ctDCS alone contributed to the performance improvements reported here. However, this possibility seems less than likely since the after-effect of tDCS lasts less than an hour (the time post stimulation at which our endpoint measure was purposefully obtained) (Purpura & McMurtry, 1965; Wassermann & Grafman, 2005) and since in other modalities stimulation requires Hebbian pairing with training to facilitate learning processes (Butefisch, et al., 2004; Reis, et al., 2009). Fourth, variable types of aphasia were included in this study, and therefore, we suggest that more cases with the same category of aphasia should be recruited in future studies. We would also suggest that more focus be placed on selecting patient populations to determine whether treatment response depends on lesion site, extension, acuteness, age, or aphasia severity. Similarly, it will be important to determine in the future, the best parameters, frequency and duration of tDCS stimulation to optimize beneficial effects on naming, and the long-term effects of treatment
Conclusion
This double-blind, sham-controlled, crossover study of patients with aphasia supports the view that cathodal tDCS over the right healthy Broca's homologue area combined with simultaneously delivered conventional word-retrieval training can contribute to functional recovery from aphasia after stroke.
Acknowledgments
This research was supported by grants from the Seoul National University College of Medicine (Grant No. 800-20060236) and the Seoul National University Bundang Hospital (Grant No. 03-2008-004).
References
- Belin P, Van Eeckhout P, Zilbovicius M, Remy P, Francois C, Guillaume S, et al. Recovery from nonfluent aphasia after melodic intonation therapy: a PET study. Neurology. 1996;47(6):1504–1511. doi: 10.1212/wnl.47.6.1504. [DOI] [PubMed] [Google Scholar]
- Blank SC, Bird H, Turkheimer F, Wise RJ. Speech production after stroke: the role of the right pars opercularis. Ann Neurol. 2003;54(3):310–320. doi: 10.1002/ana.10656. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci. 2007;25(2):123–129. [PubMed] [Google Scholar]
- Brighina F, Bisiach E, Oliveri M, Piazza A, La Bua V, Daniele O, et al. 1 Hz repetitive transcranial magnetic stimulation of the unaffected hemisphere ameliorates contralesional visuospatial neglect in humans. Neurosci Lett. 2003;336(2):131–133. doi: 10.1016/s0304-3940(02)01283-1. [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Khurana V, Kopylev L, Cohen LG. Enhancing encoding of a motor memory in the primary motor cortex by cortical stimulation. J Neurophysiol. 2004;91(5):2110–2116. doi: 10.1152/jn.01038.2003. [DOI] [PubMed] [Google Scholar]
- Crosson B, Moore AB, McGregor KM, Chang YL, Benjamin M, Gopinath K, et al. Regional changes in word-production laterality after a naming treatment designed to produce a rightward shift in frontal activity. Brain Lang. 2009;111(2):73–85. doi: 10.1016/j.bandl.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan JL, Jackson ST. Test-retest reliability of three aphasia tests: performance of non-brain-damaged older adults. J Commun Disord. 1997;30(1):33–42. doi: 10.1016/s0021-9924(96)00039-1. quiz 42-33. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Mansur CG, Wagner T, Ferreira MJ, Lima MC, et al. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport. 2005;16(14):1551–1555. doi: 10.1097/01.wnr.0000177010.44602.5e. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Valle AC, Rocha RR, Duarte J, Ferreira MJ, et al. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke. 2006;37(8):2115–2122. doi: 10.1161/01.STR.0000231390.58967.6b. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Hahne A, von Cramon DY. First-pass versus second-pass parsing processes in a Wernicke's and a Broca's aphasic: electrophysiological evidence for a double dissociation. Brain Lang. 1998;62(3):311–341. doi: 10.1006/brln.1997.1906. [DOI] [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117(4):845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Herwig U, Satrapi P, Schonfeldt-Lecuona C. Using the international 10–20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr. 2003;16(2):95–99. doi: 10.1023/b:brat.0000006333.93597.9d. [DOI] [PubMed] [Google Scholar]
- Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128(Pt 3):490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol. 2006;5(8):708–712. doi: 10.1016/S1474-4422(06)70525-7. [DOI] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S. The Boston naming test. 2. Philadelphia, PA: Lippincott, Williams and Wilkins; 2001. [Google Scholar]
- Kapur N. Paradoxical functional facilitation in brain-behaviour research. A critical review. Brain. 1996;119(Pt 5):1775–1790. doi: 10.1093/brain/119.5.1775. [DOI] [PubMed] [Google Scholar]
- Kertesz A. Aphasia and associated disorders: taxonomy, localization, and recovery. New York: Grune & Stratton; 1979. [Google Scholar]
- Kertesz A, Poole E. The aphasia quotient: the taxonomic approach to measurement of aphasic disability. Can J Neurol Sci. 1974;1(1):7–16. [PubMed] [Google Scholar]
- Kim H, Na DL. Paradise Korean version - the Western Aphasia Battery. Seoul: Paradise Welfare Foundation; 2001. [Google Scholar]
- Kim H, Na DL. Korean Verion - Boston Naming Test. Seoul: Hakji-sa; 1997. [Google Scholar]
- Kinsbourne M. A model for the mechanism of unilateral neglect of space. Trans Am Neurol Assoc. 1970;95:143–146. [PubMed] [Google Scholar]
- Mansur CG, Fregni F, Boggio PS, Riberto M, Gallucci-Neto J, Santos CM, et al. A sham stimulation-controlled trial of rTMS of the unaffected hemisphere in stroke patients. Neurology. 2005;64(10):1802–1804. doi: 10.1212/01.WNL.0000161839.38079.92. [DOI] [PubMed] [Google Scholar]
- Martin PI, Naeser MA, Ho M, Doron KW, Kurland J, Kaplan J, et al. Overt naming fMRI pre- and post-TMS: Two nonfluent aphasia patients, with and without improved naming post-TMS. Brain Lang. 2009;111(1):20–35. doi: 10.1016/j.bandl.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M, Elbert T, Djundja D, Taub E, Rockstroh B. Extending the Constraint-Induced Movement Therapy (CIMT) approach to cognitive functions: Constraint-Induced Aphasia Therapy (CIAT) of chronic aphasia. NeuroRehabilitation. 2007;22(4):311–318. [PubMed] [Google Scholar]
- Monti A, Cogiamanian F, Marceglia S, Ferrucci R, Mameli F, Mrakic-Sposta S, et al. Improved naming after transcranial direct current stimulation in aphasia. J Neurol Neurosurg Psychiatry. 2008;79(4):451–453. doi: 10.1136/jnnp.2007.135277. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Baker EH, Hodge SM, Sczerzenie SE, Nicholas M, et al. Overt propositional speech in chronic nonfluent aphasia studied with the dynamic susceptibility contrast fMRI method. Neuroimage. 2004;22(1):29–41. doi: 10.1016/j.neuroimage.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Kobayashi M, et al. Improved picture naming in chronic aphasia after TMS to part of right Broca's area: an open-protocol study. Brain Lang. 2005;93(1):95–105. doi: 10.1016/j.bandl.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Nickels N. Therapy for naming disorders: revisiting, revising and reviewing. Aphasiology. 2002;16:935–979. [Google Scholar]
- Nitsche MA, Liebetanz D, Antal A, Lang N, Tergau F, Paulus W. Modulation of cortical excitability by weak direct current stimulation--technical, safety and functional aspects. Suppl Clin Neurophysiol. 2003;56:255–276. doi: 10.1016/s1567-424x(09)70230-2. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Dan H, Sakamoto K, Takeo K, Shimizu K, Kohno S, et al. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. Neuroimage. 2004;21(1):99–111. doi: 10.1016/j.neuroimage.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Bisiach E, Brighina F, Piazza A, La Bua V, Buffa D, et al. rTMS of the unaffected hemisphere transiently reduces contralesional visuospatial hemineglect. Neurology. 2001;57(7):1338–1340. doi: 10.1212/wnl.57.7.1338. [DOI] [PubMed] [Google Scholar]
- Pedersen PM, Vinter K, Olsen TS. Aphasia after stroke: type, severity and prognosis. The Copenhagen aphasia study. Cerebrovasc Dis. 2004;17(1):35–43. doi: 10.1159/000073896. [DOI] [PubMed] [Google Scholar]
- Petrovich Brennan NM, Whalen S, de Morales Branco D, O'Shea JP, Norton IH, Golby AJ. Object naming is a more sensitive measure of speech localization than number counting: Converging evidence from direct cortical stimulation and fMRI. Neuroimage. 2007;37(Suppl 1):S100–S108. doi: 10.1016/j.neuroimage.2007.04.052. [DOI] [PubMed] [Google Scholar]
- Purpura DP, McMurtry JG. Intracellular Activities and Evoked Potential Changes During Polarization of Motor Cortex. J Neurophysiol. 1965;28:166–185. doi: 10.1152/jn.1965.28.1.166. [DOI] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A. 2009;106(5):1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Petersen SE, Linenweber MR, Snyder AZ, White DA, Chapman L, et al. Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology. 2000;55(12):1883–1894. doi: 10.1212/wnl.55.12.1883. [DOI] [PubMed] [Google Scholar]
- Shewan CM. The Language Quotient (LQ): a new measure for the Western Aphasia Battery. J Commun Disord. 1986;19(6):427–439. doi: 10.1016/0021-9924(86)90018-3. [DOI] [PubMed] [Google Scholar]
- Shindo K, Sugiyama K, Huabao L, Nishijima K, Kondo T, Izumi S. Long-term effect of low-frequency repetitive transcranial magnetic stimulation over the unaffected posterior parietal cortex in patients with unilateral spatial neglect. J Rehabil Med. 2006;38(1):65–67. doi: 10.1080/16501970500441807. [DOI] [PubMed] [Google Scholar]
- Takeuchi N, Chuma T, Matsuo Y, Watanabe I, Ikoma K. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke. 2005;36(12):2681–2686. doi: 10.1161/01.STR.0000189658.51972.34. [DOI] [PubMed] [Google Scholar]
- Wade DT, Hewer RL, David RM, Enderby PM. Aphasia after stroke: natural history and associated deficits. J Neurol Neurosurg Psychiatry. 1986;49(1):11–16. doi: 10.1136/jnnp.49.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Arch Neurol. 2004;61(12):1844–1848. doi: 10.1001/archneur.61.12.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM, Grafman J. Recharging cognition with DC brain polarization. Trends Cogn Sci. 2005;9(11):503–505. doi: 10.1016/j.tics.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Weiller C, Isensee C, Rijntjes M, Huber W, Muller S, Bier D, et al. Recovery from Wernicke's aphasia: a positron emission tomographic study. Ann Neurol. 1995;37(6):723–732. doi: 10.1002/ana.410370605. [DOI] [PubMed] [Google Scholar]
- World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Jama. 2000;284(23):3043–3045. [PubMed] [Google Scholar]