Abstract
AIM: To describe the establishment of a Danish inflammatory bowel diseases (IBD) twin cohort with focus on concordance of treatment and inflammatory markers.
METHODS: We identified MZ twins, likely to be discordant or concordant for IBD, by merging information from the Danish Twin Register and the National Patient Register. The twins were asked to provide biological samples, questionnaires, and data access to patient files and public registries. Biological samples were collected via a mobile laboratory, which allowed for immediate centrifugation, fractionation, and storage of samples. The mean time from collection of samples to storage in the -80 °C mobile freezer was less than one hour. The diagnoses where validated using the Copenhagen diagnostic criteria.
RESULTS: We identified 159 MZ IBD twin pairs, in a total of 62 (39%) pairs both twins agreed to participate. Of the supposed 62 IBD pairs, the IBD diagnosis could be confirmed in 54 pairs. The cohort included 10 concordant pairs, whereof some were discordant for either treatment or surgery. The 10 concordant pairs, where both pairs suffered from IBD, included eight CD/CD pairs, one UC/UC pair and one UC/IBDU pair. The discordant pairs comprised 31 UC, 5 IBDU (IBD unclassified), and 8 CD discordant pairs. In the co-twins not affected by IBD, calprotectin was above 100 μg/g in 2 participants, and above 50 μg/g in a further 5 participants.
CONCLUSION: The presented IBD twin cohorts are an excellent resource for bioinformatics studies with proper adjustment for disease-associated exposures including medication and inflammatory activity in the co-twins.
Keywords: Digestive system diseases, Inflammatory bowel diseases, Crohn’s disease, Ulcerative colitis, Epidemiologic studies, Twins, Biobank
Core tip: Using co-twin study designs to segregate genetic and environmental factors in inflammatory bowel diseases (IBD) holds promise for future discovery, considering subclinical disease in the co-twins. However, as MZ IBD discordant twins are rarely seen this often-mean insufficient power for planned analyses. Hence, collaboration between IBD twin resources is crucial.
INTRODUCTION
The inflammatory bowel diseases (IBD), Crohn’s disease (CD) and ulcerative colitis (UC), affect a large number of Europeans[1,2]. Despite the introduction of new treatments, CD and UC remain chronic conditions with severe disease morbidity, often complicated by surgery and frequent admissions to hospital[1,3].
Although studies of the genome have found 200 loci associated with IBD, the variation in the IBD phenotype explained by these finding is still below 25%-30%[4,5] suggesting a role of environmental factors in IBD pathogenesis. Several studies indicate environmental impact on IBD pathogenesis including; exposure to pathogens[6], disease associated dysbiosis[7], metabolic disequilibrium[8], or epigenetic modifications[9]. More comprehensive studies, addressing these and other potential causes of IBD, could provide invaluable new insight into the pathogenesis of IBD[10], though studies using unrelated individuals would require large populations to overcome genetic variation between unrelated subjects[11].
Monozygotic (MZ) twins share common genotypes and epigenetic profiles at conception[12]. While some epigenetic differences arise during the lifetime of MZ twins[13], the inter-individual variation in relation to e.g., the epigenome and the gut microbiome remain lower between twin pairs than between unrelated persons[14]. Consequently, comprehensive studies of the exposome using IBD discordant MZ twin study designs could prove a powerful tool to assess the combined effects of environmental and endogenous factors, and identify targets for treatment and prevention[15].
A major challenge in such discordant twin pair studies is that quiescent or subclinical disease may blur the boundaries between cases and their co-twin controls[16,17]. Another major challenge is that IBD discordant twin pairs are also treatment discordant, hence observed differences might derive from differential medication rather than disease discordance. Given enough power, studies using concordant twin pairs in addition to discordant twin pairs could allow researchers to adjust for disease-associated exposures such as medication, as both twins have IBD but may be discordant for some of the applied treatments. Further, calprotectin correlates with intestinal inflammation[18-20], and could reflect quiescent or subclinical disease in the unaffected co-twins.
We describe the establishment of a Danish IBD twin cohort including sampling of biological material, and illustrate the importance of treatment discordance and measurement of inflammatory markers for future bioinformatics studies using IBD affected twins.
MATERIALS AND METHODS
We identified MZ twins, likely to be discordant or concordant for IBD, by merging information from the Danish Twin Register and the National Patient Register[21,22].
Danish twin register
The Danish Twin Register enabled identification of MZ twins living in Denmark at the time of inclusion, with assessment of zygosity correct in 96% of cases[23]. The Register contains 72% of all twin pairs born between 1931-1968, with complete ascertainment of all live born twins since 1968[21].
National patient register
The National Patient Register is a nationwide register of all hospital discharge diagnoses, including surgical and other procedures recorded in Danish hospitals since 1977[22]. The Register provides outpatient data from 1994 and surgical procedures since 1996. Diagnoses of UC and CD were identified using the international classification of diseases (ICD) 8th and 10th revision codes for CD (563.00-563.09 and K50) and UC (563.19, 569.04 and K51).
The diagnosis of IBD has previously been found to be accurate in over 90% of IBD cases in the national patient register, using a pathology register as reference[24].
Cohort recruitment
Merging the Danish Twin Register and the National Patient Register, identified 159 MZ twin pairs in which at least one twin had a diagnosis of either CD or UC according to the National Patient Register as of May 1st 2013. Of these, 113 index twins (the first twin to contract IBD according to the register) responded to the invitational letter of whom 42 twins declined to participate. Of the 71 positive index twin responders, nine co-twins did not wish to participate, leaving 62 pairs for inclusion, Figure 1.
Figure 1.
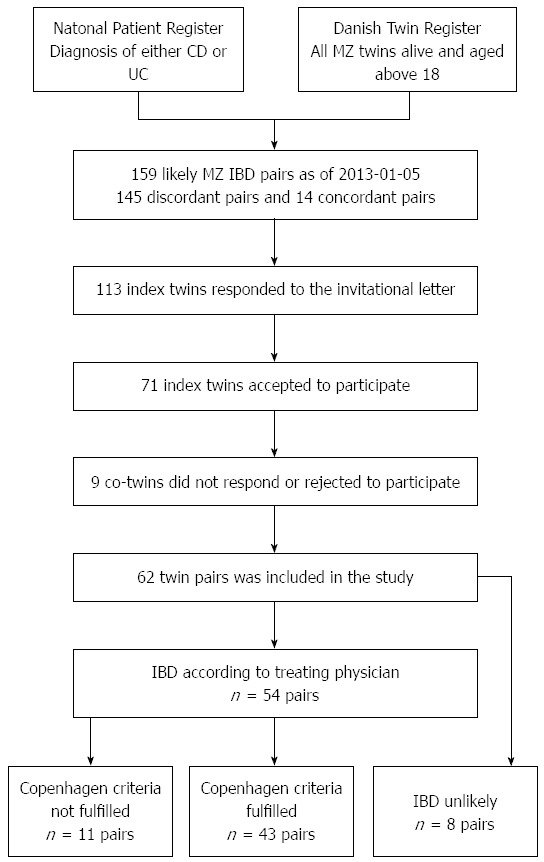
Collection of twin pairs. IBD: Inflammatory bowel diseases; CD: Crohn’s disease; UC: Ulcerative colitis.
Data collection
The participants filled out a questionnaire including age, sex, smoking status, medication, dietary patterns including a food frequency questionnaire, a 48-h dietary recall, time of last meal or exercise, travel history, and pregnancies and disease activity at time of sampling, either Harvey Bradshaw Index (CD) (33) or Simple Clinical Colitis Index (UC) (34).
Data collected from the patient record included disease staging using the Montreal classification (32), any IBD complications, extra intestinal manifestations, and gastrointestinal operations as well as prior IBD medication.
The register diagnosis of CD, UC or IBDU, was validated by hospital records and pathology descriptions using the Copenhagen criteria[25]: Copenhagen Diagnostic Criteria for CD (at least two of the criteria present)[26,27]: (1) History of abdominal pain, weight loss and/or diarrhoea for more than three months; (2) Characteristic endoscopic findings of ulceration (aphthous lesions, snail track ulceration) or cobble stoning or radiological features of stricture or cobble stoning; (3) Histopathology consistent with Crohn’s disease (epitheloid granuloma of Langerhans type or transmural discontinuous focal or patchy inflammation); and (4) Fistula and/or abscess in relation to affected bowel segments.
Copenhagen diagnostic criteria for UC (all three of the criteria present)[26,28]: (1) History of diarrhoea and/or rectal bleeding and pus for more than one week or repeated episodes; (2) characteristic endoscopic findings of continuous ulceration, vulnerability or granulated mucosa; and (3) histopathology consistent with ulcerative colitis (neutrophils within epithelial structures, cryptitis, crypt distortion, crypt abscesses).
Inter-observer variation has previously been found with regards to the Montreal classification[29]. To avoid potential inter-observer variation one researcher validated the diagnoses and assessed the Montreal classification (FTM). Furthermore, to improve validity of diagnoses and phenotypes, complicated cases were reviewed by a gastroenterological specialist and senior physician (VAN). In daily clinical practice, the diagnosis may remain difficult; therefore, we included cases, which were perceived to have IBD by the treating physician although not fulfilling the Copenhagen criteria as IBD cases according to available information from the files. Cases where the diagnosis of IBD was unlikely were designated “Gastrointestinal (GI) symptoms not IBD” for future reference.
Biological samples
Due to the geographical challenges in sampling a nationwide cohort, a mobile lab was set up using a camper previously fitted for a similar purpose. The camper was equipped with a small lab bench, heating, refrigeration, -20 °C freezer, a mobile -80 °C freezer, as well as a swinging bucket centrifuge.
The mobile lab setup allowed researchers to visit the twins in their home or another private location. The samples were collected adhering to the Sample PRE-analytical Code (SPREC) and Biospecimen Reporting for Improved Study Quality (BRISQ) guidelines, logging primary container, pre- and post-centrifugation conditions, centrifugation parameters and storage conditions, see supplementary materials Table 1[30,31]. Faecal specimens were sampled by participants up until 48 h before the visit and stored in their own freezer at -20 °C[32]. Samples were then transferred to a -80 °C freezer at the visit, under which conditions faecal samples have been found to be stable in composition[33]. Oral samples were collected with a cytobrush (Cytotak™ Transwab® Labelled Tube MW148) from the dorsum of the tongue, suspended in a buffer medium, and immediately frozen at -80 °C. Paraffin was used to collect sputum samples that were either suspended in RNA later or frozen directly at -80 °C. One researcher conducted the collection of all samples. All samples were analysed using standard methods centrally to avoid sampling variation between different centers.
Table 1.
Clinical characteristics n (%)
| Pair type |
Discordant twin pairs |
Concordant twin pairs |
|||||
| Status | Co-twin | CD | IBDU | UC | non-IBD GI symptoms | IBD co-twin | IBD index twin |
| n | 52 | 8 | 5 | 31 | 8 | 10 | 10 |
| Males/Females | 23/29 | 2/6 | 1/4 | 16/15 | 4/4 | 4/6 | 4/6 |
| Age (yr) | 50(26-78) | 47 (26-67) | 57 (34-77) | 49 (32-70) | 55 (27-78) | 49 (28-68) | 49 (28-68) |
| Age at onset | 32 (21-47) | 43 (23-73) | 35 (20-59) | 38 (23-62) | 21 (14-29) | 23 (11-34) | |
| Age at diagnosis | 34 (25-46) | 41 (29-72) | 34 (17-66) | 48 (18-72) | 24 (11-37) | 31 (21-47) | |
| CPH criteria fulfilled | 6 (75) | 4 (80) | 24 (77) | 0 (0) | 8 (80) | 9 (90) | |
| Disease location | |||||||
| L1 ileal | 2 (25) | 2 (40) | 3 (30) | 2 (20) | |||
| L2 colonic | 3 (38) | 0 (0) | 1 (10) | 1 (10) | |||
| L3 ileocolonic | 0 (0) | 0 (0) | 4 (40) | 4 (40) | |||
| L4 isolated upper disease | 0 (0) | 0 (0) | 0 (0) | 1 (10) | |||
| B1 non stricturing non penetrating | 4 (50) | 2 (40) | 3 (30) | 1 (10) | |||
| B2 stricturing | 2 (25) | 0 (0) | 4 (40) | 5 (50) | |||
| B3 penetrating | 0 (0) | 0 (0) | 3 (30) | 2 (20) | |||
| P perianal disease | 1 (13) | 0 (0) | 2 (20) | 0 (0) | |||
| Proctitis | 1 (20) | 6 (19) | |||||
| Left sided | 0 (0) | 9 (29) | |||||
| Extensive | 1 (20) | 10 (32) | |||||
n denotes the number of participants with the phenotype described in status. IBD: Inflammatory bowel diseases; CD: Crohn’s disease; UC: Ulcerative colitis.
The mean time from collection of samples to storage in the -80 °C mobile freezer was less than one hour, except for blood samples, which were 60 min and 15 s please see Supplementary materials Table 2. Records where kept to ensure identification of any deviations from protocol in future analysis.
Table 2.
Complications, medication and smoking n (%)
| Pair type |
Discordant twin pairs |
Concordant twin pairs |
|||||
| Status | Co-twin | CD | IBDU | UC | non-IBD GI symptoms | IBD co-twin | IBD index twin |
| n | 52 | 8 | 5 | 31 | 8 | 10 | 10 |
| Complications | |||||||
| GI complications1 | 3 (6) | 2 (25) | 1 (20) | 2 (6) | 0 (0) | 4 (40) | 5 (50) |
| Extra intestinal manifestations2 | 5 (10) | 3 (38) | 2 (40) | 12 (39) | 2 (25) | 2 (20) | 6 (60) |
| Ever surgery | 0 (0) | 2 (25) | 1 (20) | 12 (39) | 2 (25) | 7 (70) | 5 (50) |
| Colectomy | 0 (0) | 1 (13) | 1 (20) | 7 (23) | 0 (0) | 0 (0) | 0 (0) |
| Medication | |||||||
| Ever TNF-inhibitor | 0 (0) | 2 (25) | 1 (20) | 4 (13) | 0 (0) | 1 (10) | 1 (10) |
| Ever glucocorticoids | 0 (0) | 3 (38) | 4 (80) | 17 (55) | 2 (25) | 6 (60) | 6 (60) |
| Ever other immunosupressor3 | 0 (0) | 4 (50) | 2 (40) | 6 (19) | 0 (0) | 2 (20) | 5 (50) |
| Ever 5-ASA | 1 (2) | 5 (63) | 4 (80) | 22 (71) | 1 (13) | 5 (50) | 6 (60) |
| Smoking | |||||||
| Never smoker | 28 (54) | 6 (75) | 2 (40) | 21 (68) | 5 (63) | 3 (30) | 2 (20) |
| Current smoker | 10 (19) | 1 (13) | 1 (20) | 2 (6) | 2 (25) | 3 (30) | 7 (70) |
| Former smoker | 13 (25) | 1 (13) | 2 (40) | 7 (23) | 1 (13) | 4 (40) | 1 (10) |
Fistula, adherences, strictures, toxic megacolon, abscess, perforation, colorectal cancer;
Hepatitis, primary sclerosing cholangitis, autoimmune pancreatitis, uveitis, erythema nodosum, pyoderma gangrenosum, arthritis, aphthous ulcers;
Methotrexate, azathioprine, and cyclosporine;. n denotes the number of participants with the phenotype described in status. IBD: Inflammatory bowel diseases; CD: Crohn’s disease; UC: Ulcerative colitis.
Statistical analysis
The study included only basic descriptive statistics using R version 3.2.0. In order to ensure confidentiality, no grouping of the twins below five pairs was presented. The statistical methods of this study were reviewed by statistician Mikael Andersson from department of epidemiology at Statens Serum Institut.
RESULTS
Study cohort
Out of 62 MZ twin pairs, after scrutinizing patient records, register data, and questionnaires, we found the index case of eight pairs unlikely to have IBD. The 8 cases were afflicted by the following diagnoses: lymphocytic colitis, irritable bowel syndrome, Clostridium Difficile infection, ischemic bowel changes and abscesses without pathologic CD features and grouped as GI symptoms not IBD. At least one twin suffered from IBD in all remaining 54 pairs according to patient records before verification of diagnostic criteria. Forty-four were discordant for IBD, of whom 24 out of 31 UC pairs, four out of five IBDU pairs, and six out of eight CD pairs fulfilled the Copenhagen diagnostic criteria. Of the 10 concordant pairs, there were eight CD/CD pairs, one UC/UC pair, and one UC/IBDU pair, where all but one CD index twin fulfilled the Copenhagen criteria. Both verified and suspected cases were included in the cohort, to reflect clinical practice.
Age at diagnosis
The mean age at diagnosis was lower in the CD concordant than CD discordant pairs (24.75 years vs 31.75 years). The timespan between the diagnosis of an index twin and the IBD co-twin was 6 years on average, ranging from 94 d to 14 years. The mean disease duration at sampling was 15 years on average, ranging from 295 d to 37 years.
Clinical characteristics, complications, medication and smoking
Table 1 shows clinical characteristics of the discordant twin pairs. Nine extra intestinal manifestations were present among co-twins, most often arthropathy. Table 2 shows complications, medication and smoking. Though numbers are small, 25% of CD index twins and 63% of CD co-twins received surgery after their IBD diagnosis. Conversely, 50% received azathioprine among the CD index twins vs 13% among the CD co-twins.
Assessment of inflammatory activity in concordant and discordant twin pairs
Figure 2A shows inflammatory activity in discordant co-twin pairs as measured by calprotectin, at the time of sample collection. There was evidence of gut inflammation in the apparently non-affected co-twin, with faecal calprotectin > 100 μg/g in two individuals and > 50 μg/g in a further five (Figure 2A). In two of the index twins whose IBD diagnosis could not be verified, faecal calprotectin was > 100 μg/g. Values of patient reported disease scores were also slightly increased though slightly less pronounced (Figure 2B and C).
Figure 2.
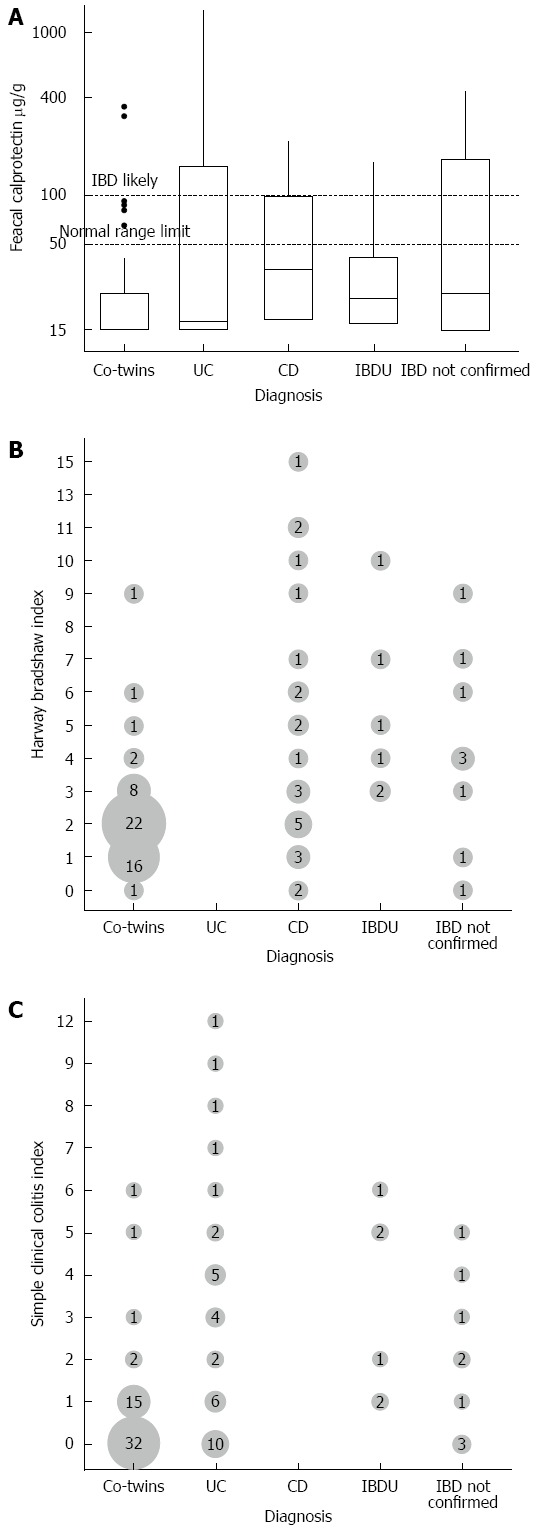
Figure shows fecal calprotectin measures stratified (A), harwey bradshaw index stratified by phenotype (B) and simple clinical colitis index stratified (C) by phenotype. IBD: Inflammatory bowel diseases; CD: Crohn’s disease; UC: Ulcerative colitis.
DISCUSSION
We have established a nationwide cohort of 62 affected or suspected IBD monozygotic twin pairs, which allow assessment of a range of disease- and treatment- associated and phenotypical traits amongst both discordant and concordant MZ IBD twins. Validation of the CD, UC, and IBDU diagnoses resulted in 8 pairs where the diagnosis was unlikely, and 11 pairs where the diagnosis was likely, but the clinical information was too sparse to validate this. Therefore, 43 twin pairs fulfilled the Copenhagen diagnostic criteria. The cohort included 10 concordant pairs, and several of these IBD concordant pairs were discordant for either treatment or surgery. The 44 IBD discordant pairs comprised 31 UC pairs, five IBDU pairs, and eight CD pairs. Inflammatory activity was above the normal range in 7 of the co-twins not affected by IBD, with calprotectin above 100 μg/g in two co-twin pairs and above 50 μg/g in a further five pairs.
The strength of the presented twin cohort lies in the wide range of data collected, from questionnaire data, patient file and public register data, to multiple biological samples. Our mobile laboratory enabled uniform collection of biological material with few deviations from existing guidelines regarding sample collection, storage, and handling. The average time from sampling to storage at -80 °C was 1 h or less for all samples. Our uniform sample collection method using a mobile laboratory reduced aberrant data handling normally affecting nationwide multicentre studies, and allowed for a single-site analysis of disease activity data. A drawback of this approach is that more advanced laboratory handling, like cell separation and preserving viable cells, was not performed. Instead, the study used CPT tubes, a commercial cell preservative, and gradual freezing of cells using a “mister frosty”, which has previously been shown to preserve viable cells and cell integrity[34].
While the collection of biological material in this study is more uniform than previous twin studies[14,35-40], we were unable to perform invasive manoeuvres such as endoscopy with our mobile setup. Though we expect to achieve access to some biopsy material taken from routine endoscopies, a large proportion of the healthy twins had not recently undergone endoscopy, thus limiting opportunities for comparison. Our assessment of clinical characteristics and IBD medication use aggregated data from patient files and questionnaire data. Consequently, treatment not documented by hospital-based physicians or recalled by patients may remain unaccounted for, but this potential bias should be similar between concordant and discordant pairs.
Our inclusion rate was lower than expected at 62 pairs out of the contacted 159, perhaps due to the extent of collected samples, and the need for including both twins. Indeed, some selection bias favouring the inclusion of concordant pairs over discordant pairs could not be ruled out, based on the proportion of concordant pairs invited to the proportion of concordant pairs accepting to participate.
The IBD twins were identified using nationwide registers, reducing bias often bestowed upon twin studies relying on advertising for recruitment. Given sufficient power, concordant pairs may play a crucial role in discerning the effects of disease-associated traits, such as medical therapy, from the effects of IBD, e.g., on the methylome or metagenome. In addition, though we did not have the power to test this formally, the mean age at diagnosis seemed lower in the CD concordant pairs at 25 years vs 32 years among CD discordant pairs. Results from previous twin studies are conflicting on this point[41-43]. If indeed such a difference exists, one possible explanation might be that concordant pairs carry a larger genetic liability to disease, with a lower threshold for disease throughout life, increasing the risk of both twins contracting IBD, resulting in twin concordance. A previous Swedish twin study found the total allele frequency of Nod/Card mutations to be 4.4 times higher among concordant twin pairs compared to discordant twin pairs contributing to, but not explaining concordance[41].
Phenotypical characteristics differentiate CD and UC from each other and from other conditions such as IBS, microscopic colitis and infections. A combination of clinical evaluation, endoscopic, histological, radiological, and/or bio-chemical investigations provides the diagnostic foundation for CD and UC[44,45]. The correct classification of twin discordance is paramount. Of note, classification is not only dependent on the correct diagnosis but also on time interval following the diagnosis of the index twin, with the risk of contracting IBD declining with time for the co-twin. Although the maximum time-span between concordant pairs in this cohort was below the mean disease duration of 15 years in the present cohort, final verification of twin discordance can only be assessed at the end of the lifespan of both twins. Methods do however exist that take time to event into account[46]. While future disease may not be a problem in a discordant twin study design, this is only true if the exposures causing this disease are not already present. Quiescent and subclinical disease may complicate the distinction between cases and their co-twin controls. Indeed, a newly published study indicates the presence of latent or emerging disease in family members of affected IBD cases[16]. In addition, family members of affected IBD cases have increased calprotectin levels as compared to the background population[17]. Though a normal calprotectin level does not exclude IBD, due to the dynamic nature of this condition, and a low level in well treated IBD patients, calprotectin levels correlate with intestinal inflammation[18-20], and could thus reflect an increased liability to IBD in familial members of IBD-affected cases[47,48]. One[48] twin study published in the past 10 years has reported on increased levels of intestinal inflammatory activity biomarkers such as calprotectin among the unaffected co-twins, while the majority of previous twin studies have not[14,35-40,49]. We found two co-twins with no history of IBD with calprotectin values exceeding 100 μg/g, and a further 5 with values above the normal range. This may be important, as increased calprotectin may reflect that many of the exposures leading to disease may already be present in a co-twin, if subclinical disease is not already present. As a result, inter-individual differences with impact on disease pathogenesis within pairs may be harder to assess, suggesting that calprotectin levels should be considered in analysis.
Disease discordant IBD twins remain rare and precious to research[14,35-40,49]. Though providing a powerful model for research, this will often mean insufficient power for planned analyses. Hence, collaboration between twin resources is crucial. Collaboration with the Nixon Twin and Multiplex (TAM) United Kingdom IBD cohort, analysing epigenetic data within similar biological material, has already been established[50]. Thus, both the Danish and the British IBD twin cohorts will include a range of clinical, epidemiological and biological data enabling researchers to study a cross section of the IBD exposome (Figure 3).
Figure 3.
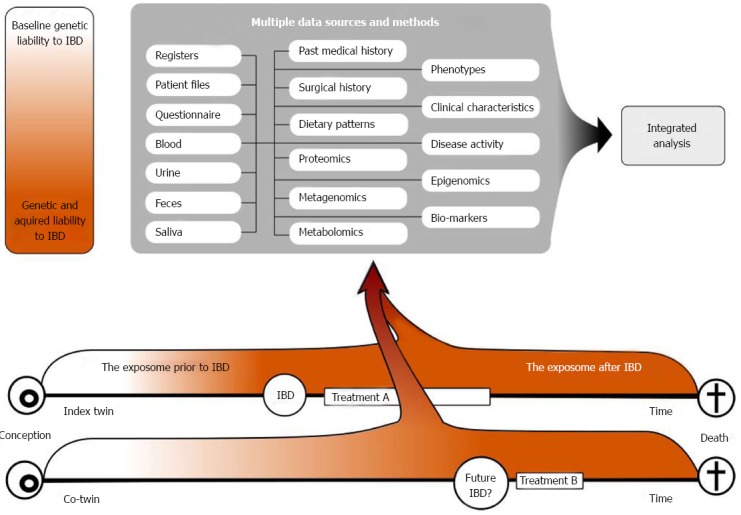
Figure shows how the collected twin data may be used in different downstream analyses. The figure illustrates the initial genetic concordance in liability, the progressive discordance for disease liability due to heterogeneous exposures, and the possible future concordance for IBD, but not necessarily for treatment. IBD: Inflammatory bowel diseases.
The present cohort demonstrates the importance of assessing inflammatory biomarkers reflecting subclinical inflammatory activity among otherwise healthy co-twins in discordant twin studies. The present cohort will be part of international collaborations, thereby increasing the power to detect disease-associated factors, and allow sufficient concordant twins to be included in studies to adjust for treatment effects. Hypotheses that may be tested include whether epigenetic differences controlling IBD loci previously identified in GWAS studies exists within the twin pairs. Other approaches may include rodent models where rodent responses to biological material from discordant pairs may differ. Consequently, analysis of a range of data from cohorts of monozygotic IBD pairs using bioinformatic methods such as metagenomics, metabolomics, proteomic and epigenetics could provide new insight into the role of the exposome in IBD pathogenesis.
COMMENTS
Background
Although studies of the genome have found 200 loci associated with inflammatory bowel diseases (IBD), the variation in the IBD phenotype explained by these finding is still below 25%-30%, suggesting a role of environmental factors in IBD pathogenesis. Several studies indicate environmental impact on IBD pathogenesis, including exposure to pathogens, disease-associated dysbiosis, metabolic disequilibrium, or epigenetic modifications. Comprehensive studies of the exposome using IBD discordant MZ twin co-twin study designs could prove a powerful tool to assess the combined effects of environmental and endogenous factors, and identify targets for treatment and prevention.
Research frontiers
Historically twin studies have been used to calculate the heritability of complex traits and diseases. The co-twin control design constitutes an excellent model to investigate environmental factors associated with disease due to the genetic match between monozygotic twins. To date only a few studies have applied this method using bioinformatics methods in IBD. Most prominent is the work of Jonas Halvforsen and his group in Orebro Sweden that identified differential microbial stool patterns between IBD discordant twin pairs, underlining the potential of this methodology.
Innovations and breakthroughs
Co-twin control designs may result in complexity reduction, thus increasing power to identify microbial or epigenetic patterns associated with IBD and the interplay between these complex traits. Such studies necessitate cohorts as the one described in this study designed for downstream bioinformatics studies, and special emphasis was on pre-analytical sample handling.
Applications
The present cohort demonstrates the importance of assessing inflammatory biomarkers reflecting subclinical inflammatory activity among otherwise healthy co-twins in discordant twin studies. Using co-twin study designs to investigate environmental determinants of disease holds promise for future discovery. However, as MZ IBD discordant twins are rare this often means insufficient statistical power. Hence, collaboration between twin resources is crucial. Through international collaborations analysis of a range of data from cohorts of monozygotic IBD pairs using bioinformatic methods such as metagenomics, metabolomics, proteomics and epigenetics could provide new insight into the role of the exposome in IBD pathogenesis.
Terminology
Concordant twin pairs: twin pairs where both twins are affected by disease or trait. Discordant twin pairs: twin pairs where only one twin is affected by disease or trait. According to Wild (2005), the exposome encompasses all human environmental exposures from conception onwards.
Peer-review
As the authors realize, the real strength of this cohort is in the future translational studies, primarily as it relates to epigenetics. While they very briefly and superficially discuss these plans in the last paragraph of the conclusion, expanding on future plans for hypothesis-driven translational research would further strengthen the manuscript. Otherwise, this is a nice introduction to a novel cohort that hopes to generate fascinating future work.
Footnotes
Supported by Lundbeck foundation, Region of Southern Denmark, University of Southern Denmark, Hospital of Southern Jutland.
Institutional review board statement: The study was approved by the ethics committee of the region of southern Denmark (approval No: S20120176). Further, the study is included in the regional application to The Data Protection Agency (Institutional Southern Region of Denmark J.nr. 2008-58-0035). To ensure confidentiality direct paired comparisons between twin pairs are not shown.
Informed consent statement: Verbal as well as written informed consent was obtained from participants. This included consent to contact co-twins of the index twins, even if that included informing the co-twin of the diagnosis of the index twin.
Conflict-of-interest statement: Vibeke Andersen is an adviser for MSD/Merck, Jansen, and member of advisory board for MSD/Merck; Tine Jess has received funding for travel and speakers fee from AbbVie; Frederik Trier Moller, Lina Knudsen, Marcus Harbord, Jack Satsangi, Hannah Gordon, Lene Christiansen, Kaare Christensen: no conflicts of interest.
Data sharing statement: Technical appendix, code is available from the corresponding author at (frtm@ssi.dk). Additional data are available on request but may require further IRB approval/ approvals from the data protection agency, to be shared outside the research group.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: February 10, 2016
First decision: March 7, 2016
Article in press: May 4, 2016
P- Reviewer: Shapiro JM S- Editor: Qi Y L- Editor: A E- Editor: Ma S
References
- 1.Burisch J, Jess T, Martinato M, Lakatos PL. The burden of inflammatory bowel disease in Europe. J Crohns Colitis. 2013;7:322–337. doi: 10.1016/j.crohns.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42; quiz e30. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 4.Gordon H, Trier Moller F, Andersen V, Harbord M. Heritability in inflammatory bowel disease: from the first twin study to genome-wide association studies. Inflamm Bowel Dis. 2015;21:1428–1434. doi: 10.1097/MIB.0000000000000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jess T, Simonsen J, Nielsen NM, Jørgensen KT, Bager P, Ethelberg S, Frisch M. Enteric Salmonella or Campylobacter infections and the risk of inflammatory bowel disease. Gut. 2011;60:318–324. doi: 10.1136/gut.2010.223396. [DOI] [PubMed] [Google Scholar]
- 7.Gevers D, Kugathasan S, Denson LA, Vázquez-Baeza Y, Van Treuren W, Ren B, Schwager E, Knights D, Song SJ, Yassour M, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15:382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin HM, Helsby NA, Rowan DD, Ferguson LR. Using metabolomic analysis to understand inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:1021–1029. doi: 10.1002/ibd.21426. [DOI] [PubMed] [Google Scholar]
- 9.Nimmo ER, Prendergast JG, Aldhous MC, Kennedy NA, Henderson P, Drummond HE, Ramsahoye BH, Wilson DC, Semple CA, Satsangi J. Genome-wide methylation profiling in Crohn’s disease identifies altered epigenetic regulation of key host defense mechanisms including the Th17 pathway. Inflamm Bowel Dis. 2012;18:889–899. doi: 10.1002/ibd.21912. [DOI] [PubMed] [Google Scholar]
- 10.Huang H, Vangay P, McKinlay CE, Knights D. Multi-omics analysis of inflammatory bowel disease. Immunol Lett. 2014;162:62–68. doi: 10.1016/j.imlet.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, Handsaker RE, Kang HM, Marth GT, McVean GA. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bell JT, Spector TD. A twin approach to unraveling epigenetics. Trends Genet. 2011;27:116–125. doi: 10.1016/j.tig.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, Heine-Suñer D, Cigudosa JC, Urioste M, Benitez J, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci USA. 2005;102:10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willing BP, Dicksved J, Halfvarson J, Andersson AF, Lucio M, Zheng Z, Järnerot G, Tysk C, Jansson JK, Engstrand L. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology. 2010;139:1844–1854.e1. doi: 10.1053/j.gastro.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 15.van Dongen J, Slagboom PE, Draisma HH, Martin NG, Boomsma DI. The continuing value of twin studies in the omics era. Nat Rev Genet. 2012;13:640–653. doi: 10.1038/nrg3243. [DOI] [PubMed] [Google Scholar]
- 16.Biancone L, Calabrese E, Petruzziello C, Capanna A, Zorzi F, Onali S, Condino G, Lolli E, Ciccacci C, Borgiani P, et al. A family study of asymptomatic small bowel Crohn’s disease. Dig Liver Dis. 2014;46:276–278. doi: 10.1016/j.dld.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Thjodleifsson B, Sigthorsson G, Cariglia N, Reynisdottir I, Gudbjartsson DF, Kristjansson K, Meddings JB, Gudnason V, Wandall JH, Andersen LP, et al. Subclinical intestinal inflammation: an inherited abnormality in Crohn’s disease relatives? Gastroenterology. 2003;124:1728–1737. doi: 10.1016/s0016-5085(03)00383-4. [DOI] [PubMed] [Google Scholar]
- 18.van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ. 2010;341:c3369. doi: 10.1136/bmj.c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Limburg PJ, Ahlquist DA, Sandborn WJ, Mahoney DW, Devens ME, Harrington JJ, Zinsmeister AR. Fecal calprotectin levels predict colorectal inflammation among patients with chronic diarrhea referred for colonoscopy. Am J Gastroenterol. 2000;95:2831–2837. doi: 10.1111/j.1572-0241.2000.03194.x. [DOI] [PubMed] [Google Scholar]
- 20.Summerton CB, Longlands MG, Wiener K, Shreeve DR. Faecal calprotectin: a marker of inflammation throughout the intestinal tract. Eur J Gastroenterol Hepatol. 2002;14:841–845. doi: 10.1097/00042737-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Skytthe A, Kyvik KO, Holm NV, Christensen K. The Danish Twin Registry. Scand J Public Health. 2011;39:75–78. doi: 10.1177/1403494810387966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39:30–33. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 23.Christiansen L, Frederiksen H, Schousboe K, Skytthe A, von Wurmb-Schwark N, Christensen K, Kyvik K. Age- and sex-differences in the validity of questionnaire-based zygosity in twins. Twin Res. 2003;6:275–278. doi: 10.1375/136905203322296610. [DOI] [PubMed] [Google Scholar]
- 24.Fonager K, Sørensen HT, Rasmussen SN, Møller-Petersen J, Vyberg M. Assessment of the diagnoses of Crohn’s disease and ulcerative colitis in a Danish hospital information system. Scand J Gastroenterol. 1996;31:154–159. doi: 10.3109/00365529609031980. [DOI] [PubMed] [Google Scholar]
- 25.Burisch J, Cukovic-Cavka S, Kaimakliotis I, Shonová O, Andersen V, Dahlerup JF, Elkjaer M, Langholz E, Pedersen N, Salupere R, et al. Construction and validation of a web-based epidemiological database for inflammatory bowel diseases in Europe An EpiCom study. J Crohns Colitis. 2011;5:342–349. doi: 10.1016/j.crohns.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 26.Binder V, Both H, Hansen PK, Hendriksen C, Kreiner S, Torp-Pedersen K. Incidence and prevalence of ulcerative colitis and Crohn’s disease in the County of Copenhagen, 1962 to 1978. Gastroenterology. 1982;83:563–568. [PubMed] [Google Scholar]
- 27.Munkholm P. Crohn’s disease--occurrence, course and prognosis. An epidemiologic cohort-study. Dan Med Bull. 1997;44:287–302. [PubMed] [Google Scholar]
- 28.Langholz E. Ulcerative colitis. An epidemiological study based on a regional inception cohort, with special reference to disease course and prognosis. Dan Med Bull. 1999;46:400–415. [PubMed] [Google Scholar]
- 29.Krishnaprasad K, Andrews JM, Lawrance IC, Florin T, Gearry RB, Leong RW, Mahy G, Bampton P, Prosser R, Leach P, et al. Inter-observer agreement for Crohn’s disease sub-phenotypes using the Montreal Classification: How good are we? A multi-centre Australasian study. J Crohns Colitis. 2012;6:287–293. doi: 10.1016/j.crohns.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 30.Moore HM, Kelly A, Jewell SD, McShane LM, Clark DP, Greenspan R, Hainaut P, Hayes DF, Kim P, Mansfield E, et al. Biospecimen Reporting for Improved Study Quality. Biopreserv Biobank. 2011;9:57–70. doi: 10.1089/bio.2010.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Betsou F, Lehmann S, Ashton G, Barnes M, Benson EE, Coppola D, DeSouza Y, Eliason J, Glazer B, Guadagni F, et al. Standard preanalytical coding for biospecimens: defining the sample PREanalytical code. Cancer Epidemiol Biomarkers Prev. 2010;19:1004–1011. doi: 10.1158/1055-9965.EPI-09-1268. [DOI] [PubMed] [Google Scholar]
- 32.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu GD, Lewis JD, Hoffmann C, Chen YY, Knight R, Bittinger K, Hwang J, Chen J, Berkowsky R, Nessel L, et al. Sampling and pyrosequencing methods for characterizing bacterial communities in the human gut using 16S sequence tags. BMC Microbiol. 2010;10:206. doi: 10.1186/1471-2180-10-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clarke DM, Yadock DJ, Nicoud IB, Mathew AJ, Heimfeld S. Improved post-thaw recovery of peripheral blood stem/progenitor cells using a novel intracellular-like cryopreservation solution. Cytotherapy. 2009;11:472–479. doi: 10.1080/14653240902887242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petersen BS, Spehlmann ME, Raedler A, Stade B, Thomsen I, Rabionet R, Rosenstiel P, Schreiber S, Franke A. Whole genome and exome sequencing of monozygotic twins discordant for Crohn’s disease. BMC Genomics. 2014;15:564. doi: 10.1186/1471-2164-15-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spehlmann ME, Begun AZ, Saroglou E, Hinrichs F, Tiemann U, Raedler A, Schreiber S. Risk factors in German twins with inflammatory bowel disease: results of a questionnaire-based survey. J Crohns Colitis. 2012;6:29–42. doi: 10.1016/j.crohns.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 37.Lepage P, Häsler R, Spehlmann ME, Rehman A, Zvirbliene A, Begun A, Ott S, Kupcinskas L, Doré J, Raedler A, et al. Twin study indicates loss of interaction between microbiota and mucosa of patients with ulcerative colitis. Gastroenterology. 2011;141:227–236. doi: 10.1053/j.gastro.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 38.Halfvarson J. Genetics in twins with Crohn’s disease: less pronounced than previously believed? Inflamm Bowel Dis. 2011;17:6–12. doi: 10.1002/ibd.21295. [DOI] [PubMed] [Google Scholar]
- 39.Bengtson MB, Aamodt G, Vatn MH, Harris JR. Concordance for IBD among twins compared to ordinary siblings--a Norwegian population-based study. J Crohns Colitis. 2010;4:312–318. doi: 10.1016/j.crohns.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Willing B, Halfvarson J, Dicksved J, Rosenquist M, Järnerot G, Engstrand L, Tysk C, Jansson JK. Twin studies reveal specific imbalances in the mucosa-associated microbiota of patients with ileal Crohn’s disease. Inflamm Bowel Dis. 2009;15:653–660. doi: 10.1002/ibd.20783. [DOI] [PubMed] [Google Scholar]
- 41.Halfvarson J, Bresso F, D’Amato M, Järnerot G, Pettersson S, Tysk C. CARD15/NOD2 polymorphisms do not explain concordance of Crohn’s disease in Swedish monozygotic twins. Dig Liver Dis. 2005;37:768–772. doi: 10.1016/j.dld.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 42.Spehlmann ME, Begun AZ, Burghardt J, Lepage P, Raedler A, Schreiber S. Epidemiology of inflammatory bowel disease in a German twin cohort: results of a nationwide study. Inflamm Bowel Dis. 2008;14:968–976. doi: 10.1002/ibd.20380. [DOI] [PubMed] [Google Scholar]
- 43.Tysk C, Lindberg E, Järnerot G, Flodérus-Myrhed B. Ulcerative colitis and Crohn’s disease in an unselected population of monozygotic and dizygotic twins. A study of heritability and the influence of smoking. Gut. 1988;29:990–996. doi: 10.1136/gut.29.7.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dignass A, Eliakim R, Magro F, Maaser C, Chowers Y, Geboes K, Mantzaris G, Reinisch W, Colombel JF, Vermeire S, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012;6:965–990. doi: 10.1016/j.crohns.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 45.Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, Ochsenkühn T, Orchard T, Rogler G, Louis E, Kupcinskas L, Mantzaris G, Travis S, Stange E; European Crohn's and Colitis Organisation (ECCO) The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: Definitions and diagnosis. J Crohns Colitis. 2010;4:7–27. doi: 10.1016/j.crohns.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Scheike TH, Holst KK, Hjelmborg JB. Estimating twin concordance for bivariate competing risks twin data. Stat Med. 2014;33:1193–1204. doi: 10.1002/sim.6016. [DOI] [PubMed] [Google Scholar]
- 47.Moller FT, Andersen V, Wohlfahrt J, Jess T. Familial risk of inflammatory bowel disease: a population-based cohort study 1977-2011. Am J Gastroenterol. 2015;110:564–571. doi: 10.1038/ajg.2015.50. [DOI] [PubMed] [Google Scholar]
- 48.Zhulina Y, Hahn-Strömberg V, Shamikh A, Peterson CG, Gustavsson A, Nyhlin N, Wickbom A, Bohr J, Bodin L, Tysk C, et al. Subclinical inflammation with increased neutrophil activity in healthy twin siblings reflect environmental influence in the pathogenesis of inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1725–1731. doi: 10.1097/MIB.0b013e318281f2d3. [DOI] [PubMed] [Google Scholar]
- 49.Jess T, Riis L, Jespersgaard C, Hougs L, Andersen PS, Orholm MK, Binder V, Munkholm P. Disease concordance, zygosity, and NOD2/CARD15 status: follow-up of a population-based cohort of Danish twins with inflammatory bowel disease. Am J Gastroenterol. 2005;100:2486–2492. doi: 10.1111/j.1572-0241.2005.00224.x. [DOI] [PubMed] [Google Scholar]
- 50.IBD Nixon Twin and Multiplex Registry. Available from: http://www.ibdtam.org.uk/


