Abstract
AIM: To investigate whether an endoscopy-based management could prevent the long-term risk of postoperative recurrence.
METHODS: From the pathology department database, we retrospectively retrieved the data of all the patients operated on for Crohn’s disease (CD) in our center (1986-2015). Endoscopy-based management was defined as systematic postoperative colonoscopy (median time after surgery = 9.5 mo) in patients with no clinical postoperative recurrence at the time of endoscopy.
RESULTS: From 205 patients who underwent surgery, 161 patients (follow-up > 6 mo) were included. Endoscopic postoperative recurrence occurred in 67.6%, 79.7%, and 95.5% of the patients, respectively 5, 10 and 20 years after surgery. The rate of clinical postoperative recurrence was 61.4%, 75.9%, and 92.5% at 5, 10 and 20 years, respectively. The rate of surgical postoperative recurrence was 19.0%, 38.9% and 64.7%, respectively, 5, 10 and 20 years after surgery. In multivariate analysis, previous intestinal resection, prior exposure to anti-TNF therapy before surgery, and fistulizing phenotype (B3) were postoperative risk factors. Previous perianal abscess/fistula (other perianal lesions excluded), were predictive of only symptomatic recurrence. In multivariate analysis, an endoscopy-based management (n = 49/161) prevented clinical (HR = 0.4, 95%CI: 0.25-0.66, P < 0.001) and surgical postoperative recurrence (HR = 0.30, 95%CI: 0.13-0.70, P = 0.006).
CONCLUSION: Endoscopy-based management should be recommended in all CD patients within the first year after surgery as it highly decreases the long-term risk of clinical recurrence and reoperation.
Keywords: Crohn’s disease, Postoperative recurrence, Endoscopy, Prevalence, Risk factors
Core tip: Although often recommended, the impact of an endoscopy-based management following surgery remains poorly investigated in Crohn’s patients. We aimed to investigate whether an endoscopy-based management could prevent the long-term risk of postoperative recurrence in Crohn’s disease (CD). We retrospectively retrieved the data of 161 patients operated on for CD in our center. We showed for the first time, that an endoscopy-based management decreased the long-term risk of clinical and surgical postoperative recurrence in CD and the risk of reoperation.
INTRODUCTION
Crohn’s disease (CD) is a chronic inflammatory bowel disease (IBD) of unknown etiology and can lead to digestive damage[1,2]. In the era of biologics, surgery still remains required in half of the patients ten years after diagnosis, especially in complicated diseases i.e. stenosis, abscess or fistula[3]. Surgical resection is unfortunately not curative in CD, and postoperative recurrence (POR) remains a crucial issue in these patients. The risk of reoperation is very heterogeneous in the medical literature due to different studies periods and designs, but ranges from 12% to 57% 10 years after surgery[4-7]. While clinical POR occurred in approximately half of the patients 10 years after surgery[8], three quarters (48%-93%) of patients experienced endoscopic POR within one year after surgery in referral centers[8-20].
More than 25 years ago, Rutgeerts et al[12] underlined that postoperative history of CD is very heterogeneous and highlighted the need to identify predictive factors of recurrence to stratify CD patients in order to optimize the therapeutic management in the immediate postoperative period. Several factors have been proposed as POR predictors (smoking, perianal lesions, previous intestinal resection, fistulizing phenotype and resection length > 50 cm), but their impact remains still debated[8,21].
Performing an endoscopy within the first year after surgery is often recommended in clinical practice[21,22]. However, the level of evidence suggesting the efficacy of such strategy remains low. Two retrospective studies reported no impact of an endoscopy-based management (EBM)[23,24]. A French group suggested, in a retrospective cohort, that an EBM was associated with a decrease risk of clinical POR at 5 years[25]. Recently, the landmark POCER trial showed that an early EBM decreased the risk of endoscopic POR at 18 mo post-surgery[26]. However the long-term impact of EBM on the risks of clinical and surgical POR remains unknown.
In the present study, we aimed to investigate whether an EBM could prevent the long-term risk of POR in CD. In addition, we aimed to report the prevalence and the risk factors of endoscopic, clinical and surgical POR, in our cohort, between 1986 and 2015.
MATERIALS AND METHODS
Ethical considerations
The study was performed in accordance with the Declaration of Helsinki, Good Clinical Practice and applicable regulatory requirements. The study was approved by local Ethics Committee (IRB number 00008526 - 2014/CE86).
Patients
We performed a retrospective study of a single-center cohort in which standardized evaluation was completed by experienced clinicians in all patients. From the electronic database of the Pathology Department of the University Hospital Estaing of Clermont-Ferrand, France, we identified 205 patients who underwent an intestinal resection for CD, between 1986 and 2015, at the Institution. Only CD patients with a follow-up of at least 6 mo were considered for the study. Clinical, biological, pathological and endoscopic data were retrospectively collected from medical records (Table 1). As we aimed to be close to the real-life practice, we chose to include all the types of intestinal resection including patients with a definitive ostomy. For the patients with a temporary ostomy, the time point zero was defined as the time of the intestinal resection since we aimed to investigate all the potential factors influencing the time to recur (including type of resection and the presence of temporary or definitive ostomy). Surgical recurrence was defined as reoperation for CD. Clinical POR was defined, according to De Cruz et al[23], as recurrence of symptoms leading to hospitalization or therapeutic modifications after exclusion of other causes of recurrent symptoms such as bile-salt diarrhea, bacterial overgrowth and adhesion-related obstruction. Endoscopic POR was defined as Rutgeerts’ score ≥ i2[12]. Regarding the endoscopies performed before the widespread of Rutgeerts’ score use or with no score specified on the colonoscopy report, the score was evaluated retrospectively based on the content of the colonoscopy report. Patients underwent colonoscopies at their physician’s discretion to assess potential subclinical disease. Patients were classified in endoscopy-based management (EBM) group if they underwent a systematic colonoscopy with no clinical POR at the time of endoscopy. All the patients included in the EBM group had a “step-up” therapeutic strategy in case of endoscopic POR[22]. The impact of the postoperative treatments was investigated in considering three groups: therapies to prevent endoscopic POR (treatment received during the period ranging from intestinal resection to endoscopic POR), therapies to prevent clinical POR (treatment received during the period ranging from endoscopic POR to clinical POR), and therapies to prevent surgical POR (treatment received during the period ranging from clinical POR to re-operation).
Table 1.
Baseline characteristics of the 161 included Crohn’s disease patients at the time of surgery n (%)
| Mean age at the time of surgery (yr) | 36.4 ± 13.4 | Adalimumab | 22 (13.7) |
| Mean age at diagnosis (yr) | 28.7 ± 13.1 | Anti-TNF naive at the time of surgery | 37 (23.0) |
| Median disease duration (yr) (IQR) | 5.8 (2.0–11.7) | Type of surgery | |
| Female gender | 93 (57.8) | Ileocecal resection | 76 (47.2) |
| Mean weight (kg) | 60.2 ± 14.8 | Ileal resection | 21 (13.1) |
| Mean body mass index (kg/m²) | 21.5 ± 4.9 | Ileo-colectomy | 14 (8.7) |
| Active smoker | 53 (32.9) | Partial colectomy | 31 (19.2) |
| Familial history of IBD | 20 (12.4) | Subtotal colectomy | 8 (5.0) |
| Previous appendectomy | 67 (41.6) | Total colectomy | 9 (5.6) |
| Previous intestinal resection | 50 (31.1) | Abdomino-perianal amputation | 2 (1.2) |
| Montreal classification | Site of anastomosis | ||
| Age at diagnosis | Ileo-colic | 91 (66.4) | |
| A1 | 15 (9.3) | Ileo-rectal | 9 (6.6) |
| A2 | 116 (72.1) | Ileo-ileal | 21 (15.3) |
| A3 | 28 (17.4) | Colo-colonic | 31 (22.6) |
| Crohn’s disease location | Colo-rectal | 7 (5.1) | |
| L1 | 64 (39.8) | Stomia | |
| L2 | 21 (13.0) | None | 113 (70.2) |
| L3 | 75 (46.6) | Transitory | 39 (24.2) |
| L4 | 18 (11.2) | Definitive | 9 (5.6) |
| Crohn’s disease behaviour | Surgical technic of anastomosis | ||
| B1 | 12 (7.4) | Stapled | 46 (43.8) |
| B2 | 75 (46.6) | Handsewn | 59 (56.2) |
| B3 | 74 (46.0) | Type of anastomosis | |
| Perianal lesions | 69 (42.8) | Side-to-end | 18 (18.0) |
| Anal ulceration, fissure | 15 (9.3) | Side-to-side | 54 (54.0) |
| Fistula/abscess | 54 (33.5) | End-to-end | 28 (28.0) |
| Medication at the time of surgery | Mean length of ileal resection (cm) | 18.1 ± 17.1 | |
| 5-ASA | 24 (14.9) | Mean length of colonic resection (cm) | 14.3 ± 17.7 |
| Steroids | 38 (23.6) | Mean length of digestive resection (cm) | 31.6 ± 18.8 |
| Budesonide | 9 (5.6) | Perioperative complications | 25 (16.8) |
| Thiopurines | 36 (22.4) | Free margin resection | 21 (17.1) |
| Methotrexate | 5 (3.1) | Granuloma | 47 (40.5) |
| Infliximab | 15 (9.3) | Median CRP level, mg/L (IQR) | 17.0 (3.8-61.0) |
IQR: Interquartile; CRP: C-reactive protein; TNF: Tumor necrosis factor; IBD: Inflammatory bowel disease.
Data management and statistical analysis
Study data were collected and managed using REDCap electronic data capture tools hosted at Clermont-Ferrand University Hospital[27]. REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for importing data from external sources.
Statistical analysis was performed using Stata 13 software (StataCorp LP, College Station, TX, United States). The tests were two-sided, with a type I error set at a = 0.05. Subject’s characteristics were presented as mean ± SD or median (interquartile range) for continuous data (assumption of normality assessed using the Shapiro-Wilk test) and as the number of patients and associated percentages for categorical parameters. Comparisons between the independent groups were performed using the χ2 or Fisher’s exact tests for categorical variables, and using Student t-test or Mann-Whitney test for quantitative parameters (normality, assumption of homoscedasticity studied using Fisher-Snedecor test). Concerning the censored data, estimates were constructed using the Kaplan-Meier method. The log-rank test was used in a univariate analysis to test the prognostic value of patient characteristics for the occurrence of an event. Cox proportional hazards regression was used to investigate prognostic factors in a multivariate situation by backward and forward stepwise analysis of the factors considered significant in univariate analysis (entered into the model if P < 0.10) and according to clinically relevant parameters. The proportional hazard hypotheses were verified using Schoenfeld’s test and plotting residuals. The interactions between possible predictive factors were also tested. Results were expressed as HRs and 95%CI.
RESULTS
Baseline characteristics of the patients
Overall, 161 CD patients were included in the study. The characteristics of these patients at the time of surgery are given in Table 1.
Prevalence of surgical, clinical and endoscopic POR
We observed a prevalence of endoscopic POR of 31.7%, 67.6%, 79.7%, 91.1% and 95.5%, respectively 1, 5, 10, 15 and 20 years after surgery (Figure 1). In our cohort, 21.5%, 61.4%, 75.9%, 88.7% and 92.5% of the patients experienced clinical POR at 1, 5, 10, 15 and 20 years, respectively (Figure 1). The rate of surgical POR was 1.3%, 19.0%, 38.9%, 57.7% and 64.7%, respectively 1, 5, 10, 15 and 20 years after surgery (Figure 1).
Figure 1.
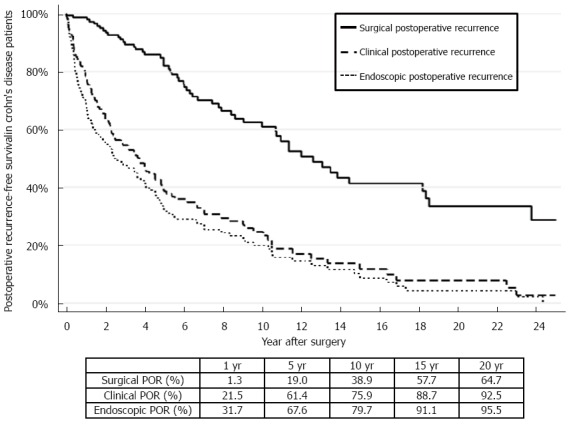
Kaplan Meir curves representing the prevalence of surgical, clinical and endoscopic postoperative recurrence in Crohn’s disease patients undergoing intestinal resection in the Clermont-Ferrand inflammatory bowel disease unit (1986-2015).
Risk factors of endoscopic POR
Among the 161 CD patients included in this study, 102 patients underwent a colonoscopy during their follow-up. The median interval for endoscopic POR was 2.0 years (0.6-3.6). While 54 patients (33.5%) received 5-ASA in prevention of endoscopic POR, 40 patients (24.8%), 7 patients (4.3%) and 41 patients (25.5%) were treated with thiopurines, methotrexate and anti-TNF, respectively. The postoperative endoscopic evaluation highlighted the following distribution: 19 patients (18.6%) classified as i0 according to the Rutgeerts’ score[12], 19 patients (18.6%) as i1, 17 patients (16.7%) as i2, 12 patients (11.8%) as i3 and 35 patients (34.3%) as i4. In univariate analysis, prior intestinal resection, prior exposure to anti-TNF therapy before surgery seemed to be associated with shorter time until endoscopic POR (20.5 mo vs 43.5 mo, P = 0.06) and (8.0 mo vs 41.5 mo, P < 0.001), respectively (Table 2). Patients operated during the 1986-1999 period experienced earlier endoscopic POR than those operated during the 2000-2015 period (P = 0.004). In multivariate analysis, prior exposure to anti-TNF therapy before surgery (HR = 2.55, 95%CI: 1.37-4.73) and undergoing surgery during the 1986-1999 period (HR = 1.61, 95%CI: 1.04-2.49) were predictive of endoscopic POR.
Table 2.
Univariate analysis of risk factors for endoscopic postoperative recurrence in Crohn’s disease
| Median time to endoscopic POR (mo) | HR [95%CI] | P value | |
| Age | 1.00 [0.99-1.00] | 0.2 | |
| Age | |||
| < 35 yr | 41.4 | Reference | |
| ≥ 35 yr | 24.0 | 1.26 [0.86-1.84] | 0.23 |
| Age at diagnosis | |||
| ≤ 16 yr | 38.1 | Reference | |
| 16-40 yr | 34.6 | 0.88 [0.47-1.67] | 0.71 |
| ≥ 40 yr | 17.6 | 1.41 [0.60-2.63] | 0.53 |
| Tobacco use | |||
| Non-smoker | 38.1 | Reference | |
| Active smoker | 27.9 | 1.28 [0.77-1.70] | 0.49 |
| Previous intestinal resection | |||
| No | 43.5 | Reference | |
| Yes | 20.5 | 1.22 [0.98-2.15] | 0.06 |
| Total resection length > 50 cm | |||
| No | 20.5 | Reference | |
| Yes | 30.2 | 0.98 [0.56-1.73] | 0.7 |
| Disease behavior (Montreal classification) | |||
| B1 | - | Reference | |
| B2 | 43.5 | 1.30 [0.46-3.75] | 0.62 |
| B3 | 22.6 | 1.34 [0.47-3.80] | 0.58 |
| Fistulizing Crohn’s disease (B3) | |||
| No | 29.3 | Reference | |
| Yes | 22.6 | 1.06 [0.73-1.53] | 0.75 |
| Perianal lesions | |||
| No | 34.6 | Reference | |
| Yes | 19.2 | 1.18 [0.82-1.71] | 0.37 |
| Type of perianal lesions | |||
| Non-fistulizing lesions | 33.4 | Reference | |
| Fistula, abscess | 20.5 | 1.10 [0.75-1.60] | 0.23 |
| Disease duration | 1.00 [0.99-1.01] | 0.77 | |
| Ileal resection > 50 cm | |||
| No | 25.9 | Reference | |
| Yes | 114.5 | 0.58 [0.21-1.60] | 0.29 |
| Prior exposure to anti-TNF therapy before surgery | |||
| No | 41.5 | Reference | |
| Yes | 8.0 | 3.91 [1.80-5.90] | < 0.001 |
| Thiopurines therapy in prevention of endoscopic postoperative recurrence | |||
| No | 43.5 | Reference | |
| Yes | 43.7 | 1.07 [0.69-1.65] | 0.75 |
| Anti-TNF therapy in prevention of endoscopic postoperative recurrence | |||
| No | 41.4 | Reference | |
| Yes | 20.5 | 1.28 [0.78-2.13] | 0.55 |
| Period of surgery | |||
| 1986-1999 | Reference | ||
| 2000-2015 | 1.00 [0.54-1.84] | 0.99 |
CRP: C-reactive protein; TNF: Tumor necrosis factor.
Risk factors of clinical POR
Among the 161 included patients, the median time to clinical POR was 2.5 years (0.7-4.9). While 54 patients (33.5%) were treated with 5-ASA in prevention of clinical POR, 34 patients (21.1%), 2 patients (1.2%) and 26 patients (16.1%) were treated with thiopurines, methotrexate and anti-TNF, respectively. In univariate analysis, we reported that previous intestinal resection (51.0 mo vs 26.6 mo, P = 0.02), previous perianal fistula or abscess (54.5 mo vs 20.5 mo, P = 0.049) and prior exposure to anti-TNF therapy before surgery (24.0 vs 48.0, P = 0.007) were risk factors regarding clinical POR (Table 3). Patients operated during the 1986-1999 period experienced also earlier endoscopic POR than those operated during the 2000-2015 period (P = 0.013). In contrast, age at the time of surgery, age at the time of diagnosis, disease duration, tobacco use, resection length, CD behavior according to Montreal classification and all the other studied factors were not associated to an increased risk to experience clinical POR, in our cohort (Table 3). In addition, neither the use of thiopurines nor the use anti-TNF was protective factor of clinical POR. In multivariate analysis, previous intestinal resection (HR = 1.62, 95%CI: 1.07-2.46, P = 0.02), previous perianal abscess or fistula (HR = 1.50, 95%CI: 1.01-2.24, P = 0.042) and prior exposure to anti-TNF therapy before surgery (HR = 1.91, 95%CI: 1.01-3.66, P = 0.049) were predictive of clinical POR.
Table 3.
Univariate analysis of risk factors for clinical postoperative recurrence in Crohn’s disease
| Median time to clinical POR (mo) | HR [95%CI] | P value | |
| Age | 1.00 [0.99-1.01] | 0.4 | |
| Age | |||
| < 35 yr | 45.2 | Reference | |
| ≥ 35 yr | 30.2 | 1.25 [0.84-1.85] | 0.27 |
| Age at diagnosis | |||
| ≤ 16 yr | 38.1 | Reference | |
| 16-40 yr | 48.0 | 0.81 [0.43-1.54] | 0.52 |
| ≥ 40 yr | 30.2 | 1.03 [0.48-2.23] | 0.93 |
| Tobacco use | |||
| Non-smoker | 45.2 | Reference | |
| Active smoker | 43.7 | 1.00 [0.66-1.53] | 0.98 |
| Previous intestinal resection | |||
| No | 51.0 | Reference | |
| Yes | 26.6 | 1.62 [1.07-2.44] | 0.02 |
| Total resection length > 50 cm | |||
| No | 38.1 | Reference | |
| Yes | 33.4 | 1.20 [0.66-2.16] | 0.55 |
| Disease behavior (Montreal classification) | |||
| B1 | 84.5 | Reference | |
| B2 | 54.5 | 1.39 [0.48-4.00] | 0.53 |
| B3 | 28.9 | 1.61 [0.56-4.56] | 0.37 |
| Fistulizing Crohn’s disease (B3) | |||
| No | 58.2 | Reference | |
| Yes | 28.9 | 1.21 [0.81-1.78] | 0.34 |
| Perianal lesions | |||
| No | 54.5 | Reference | |
| Yes | 26.9 | 1.26 [0.85-1.86] | 0.24 |
| Type of perianal lesions | |||
| Non-fistulizing lesions | 54.5 | Reference | |
| Fistula, abscess | 20.5 | 1.46 [1.01-2.16] | 0.05 |
| Disease duration | 1.00 [0.99-1.01] | 0.31 | |
| Ileal resection > 50 cm | |||
| No | 33.4 | Reference | |
| Yes | 59.5 | 0.72 [0.26-2.02] | 0.54 |
| Prior exposure to anti-TNF therapy before surgery | |||
| No | 48.0 | Reference | |
| Yes | 24.0 | 2.64 [1.24-4.33] | 0.007 |
| Thiopurines therapy in prevention of clinical postoperative recurrence | |||
| No | 44.8 | Reference | |
| Yes | 43.7 | 1.14 [0.74-1.76] | 0.53 |
| Anti-TNF therapy in prevention of clinical postoperative recurrence | |||
| No | 48.6 | Reference | |
| Yes | 29.3 | 1.39 [0.88-2.20] | 0.16 |
| Period of surgery | |||
| 1986-1999 | Reference | ||
| 2000-2015 | 1.71 [1.12-2.63] | 0.013 |
CRP: C-reactive protein; TNF: Tumor necrosis factor.
Risk factors of surgical POR
In our cohort (n = 161), the median time to surgical POR was 5.2 years (2.0-10.3). The medications used between the time of surgery and surgical POR were 5-ASA in 62 patients (38.5%), steroids in 78 patients (48.4%), thiopurines in 59 patients (36.6%) and anti-TNF in 69 patients (42.8%). In univariate analysis, previous intestinal resection (108.6 mo vs 173.4 mo, P = 0.04), fistulizing CD (B3 according to Montreal classification) (128.8 mo vs 162.1 mo, P = 0.03), prior exposure to anti-TNF therapy before surgery (P = 0.07) and anti-TNF therapy after surgery (136.2 mo vs 156.9 mo, P = 0.02) were associated with shorter time until reoperation (Table 4). The other potential risk factors investigated in the study were listed in Tables 1 and 2. In multivariate analysis, fistulizing CD (B3 according to Montreal classification) (HR = 1.78, 95%CI: 1.04-3.04, P = 0.003) and previous intestinal resection (HR = 1.7, 95%CI: 1.00-2.72, P = 0.05) were predictive of surgical POR.
Table 4.
Univariate analysis of risk factors for surgical postoperative recurrence in Crohn’s disease
| Median time to surgical POR (mo) | HR [95%CI] | P value | |
| Age | 1.00 [0.99-1.01] | 0.29 | |
| Age | |||
| < 35 yr | 218.3 | Reference | |
| ≥ 35 yr | 131.6 | 1.32 [0.77-2.26] | 0.30 |
| Age at diagnosis | |||
| ≤ 16 yr | Reference | ||
| 16-40 yr | 144.0 | 1.37 [0.42-4.42] | 0.60 |
| ≥ 40 yr | 108.6 | 1.73 [0.45-6.54] | 0.42 |
| Tobacco use | |||
| Non-smoker | 136.2 | Reference | |
| Active smoker | 173.4 | 0.84 [0.46-1.52] | 0.56 |
| Previous intestinal resection | |||
| No | 173.4 | Reference | |
| Yes | 108.6 | 1.74 [1.01-3.00] | 0.04 |
| Total resection length > 50 cm | |||
| No | 136.2 | Reference | |
| Yes | 120.0 | 1.50 [0.67-3.34] | 0.32 |
| Disease behavior (Montreal classification) | |||
| B1 | Reference | ||
| B2 | 162.1 | 3.93 [0.52-29.33] | 0.18 |
| B3 | 128.8 | 5.71 [0.77-42.23] | 0.09 |
| Fistulizing Crohn’s disease (B3) | |||
| No | 165.4 | Reference | |
| Yes | 128.8 | 1.78 [1.04-3.05] | 0.03 |
| Perianal lesions | |||
| No | 136.2 | Reference | |
| Yes | 151.1 | 0.99 [0.58-1.69] | 0.97 |
| Type of perianal lesions | |||
| Non-fistulizing lesions | 156.9 | Reference | |
| Fistula, abscess | 144.0 | 1.14 [0.66-1.97] | 0.63 |
| Disease duration | 1.00 [0.99-1.01] | 0.67 | |
| Ileal resection > 50 cm | |||
| No | 136.2 | Reference | |
| Yes | 120.0 | 1.23 [0.29-5.16] | 0.78 |
| Prior exposure to anti-TNF therapy before surgery | |||
| No | 151.1 | Reference | |
| Yes | . | 1.62 [0.92-7.08] | 0.07 |
| Thiopurines therapy in prevention of surgical postoperative recurrence | |||
| No | 144.0 | Reference | |
| Yes | 151.1 | 0.91 [0.50-1.65] | 0.75 |
| Anti-TNF therapy in prevention of surgical postoperative recurrence | |||
| No | 156.9 | Reference | |
| Yes | 136.2 | 2.09 [1.14-3.81] | 0.02 |
| Period of surgery | |||
| 1986-1999 | Reference | ||
| 2000-2015 | 1.85 [1.22-2.80] | 0.004 |
CRP: C-reactive protein; TNF: Tumor necrosis factor.
Impact of an endoscopic-based management on the risk of POR
Overall, 49 of the 161 patients were included in the endoscopic-based management group. The median interval between initial surgery and endoscopy was 9.5 mo (6.0-22.9) in this group, including 63.2% of the patients (31/49) having a colonoscopy within the first year. Endoscopic POR occurred in 18 patients (36.7%) in the EBM-group. All of them underwent step-up therapeutic strategy as described in Table 5. In univariate analysis, an EBM was associated with a delayed time to clinical (33.4 mo vs 84.5 mo) and surgical recurrence. In multivariate analysis, an EBM decreased the risk of clinical POR (HR = 0.4, 95%CI: 0.25-0.66, P < 0.001) (Figure 2A) and surgical POR (HR = 0.30, 95%CI: 0.13-0.70, P = 0.006) (Figure 2B).
Table 5.
Step-up strategies in patients experiencing endoscopic postoperative recurrence in the endoscopic management-based group
| Number of patient | Treatment before endoscopic evaluation | Rutgeerts’ score | Treatment after endoscopic evaluation |
| 1 | None | i2 | AZA |
| 2 | AZA | i3 | IFX |
| 3 | AZA | i4 | IFX |
| 4 | AZA | i2 | AZA |
| 5 | AZA | i2 | AZA (increased dose) |
| 6 | 5-ASA | i4 | IFX |
| 7 | ADA eow | i3 | ADA ew |
| 8 | None | i4 | ADA |
| 9 | 5-ASA | i4 | IFX + MTX |
| 10 | AZA | i4 | IFX |
| 11 | AZA | i2 | AZA (increased dose) |
| 12 | IFX + MTX | i3 | IFX (increased dose) + MTX |
| 13 | ADA eow | i4 | ADA ew |
| 14 | None | i2 | AZA |
| 15 | None | i3 | ADA |
| 16 | None | i2 | AZA |
| 17 | None | i2 | AZA |
| 18 | ADA eow | i3 | ADA ew |
AZA: Azathioprine; MTX: Methotrexate; IFX: Infliximab; ADA: Adalimumab; eow: Every other week; ew: Every week.
Figure 2.
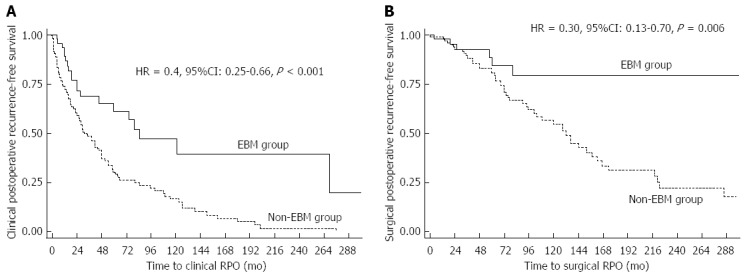
Long-term impact of endoscopic-based management on and clinical (A) and surgical (B) postoperative recurrence in Crohn’s disease.
DISCUSSION
Although performing a colonoscopy within the first year following surgery is commonly recommended in daily practice, the level of evidence suggesting that an EBM is an efficient strategy remains poorly investigated and is limited to short-term outcomes[23-26]. We reported here, the long-term impact of an EBM on the surgical and clinical POR risk that it has never been reported so far.
The prevalence of endoscopic POR in our cohort was perfectly in line with data from population-based cohort, which showed more than half of patients are experiencing endoscopic POR at 5 years, three quarters at 10 years and more than 90% at 15 years[3,28-30]. Our results also highlighted that more than three quarters (75.9%) of the patients experienced clinical POR within 10 years after surgery, that clinical symptoms occurred in almost all the CD patients followed in referral centers (92.5% at 20 years) and that almost two thirds (64.7%) of the CD patients were re-operated within 20 years of surgery. These data confirmed that surgery is not curative in CD in the large majority of the cases.
In our cohort, we confirmed that patients who underwent prior intestinal resection for CD, had higher risks of surgical, clinical and endoscopic POR, as previously showed in both population-based cohort[29] and referral centers[19]. In addition, we found that a fistulizing phenotype (B3 according to the Montreal classification) was associated with higher risk of endoscopic and surgical POR according to the results of a meta-analysis including 13 studies and 3044 patients (OR = 1.5, P = 0.002)[31] and several referral center-based studies[8]. Surprisingly, we did not show any influence of tobacco use on the risk of POR in our cohort. However, smoking is often considered as the strongest risk factor for postoperative recurrence, increasing by twofold the risk of clinical recurrence and multiplying by 2.5 the risk for surgical POR within 10 years, with a dose-response relationship[21,32,33]. It could be partly explained by the retrospective design of our study and the fact that studying smoking habits is very difficult due to a wide modification of the smoking status during this long-term follow-up, the hardness to evaluate accurately the consumption of cigarettes and the underestimation of the number of smokers. Perianal disease is often admitted as predictor for POR. However, it remains unclear whether perianal lesions directly impacted the postoperative course of luminal disease or was only associated with perianal disease relapse leading to therapeutic modifications. In our cohort, the overall perianal lesions including both fistulizing and non-fistulizing (ulceration, fissure) lesions did not show any impact on the rate of recurrence. In contrast, we observed that prior perianal fistula or abscess was associated with increased clinical POR rate, but it did not influence the risk of both endoscopic and surgical POR. Most of the previous data indicated that perianal lesions were associated with clinical POR[28,34,35], while neither the studies investigating the risk factors for surgical POR[8,36,37], nor those interested in risk factors for endoscopic POR[8] achieved to prove the role of perianal involvement in the postoperative course of CD. Our results seemed to confirm that perianal involvement did not influence the risk of luminal recurrence, but underlined the fact that patients with perianal involvement had an increased risk of perianal symptomatic recurrence. Accordingly, we suggest that these patients require aggressive treatment after surgery, preferably to prevent perianal relapse rather than luminal recurrence, but this point warrants to be validated in additional studies. Some authors suggested using anti-TNF therapy in prevention of endoscopic POR in patients with prior exposure to anti-TNF before surgery[22]. This statement is based on experts’ opinion rather than evidence-based medicine. However, in our cohort, we found that prior exposure to anti-TNF therapy before surgery is the most relevant risk factor for POR. It could mean that anti-TNF agents prescription associated to the most severe disease could predict an unfavorable postoperative course in CD. Stratifying the patients according to their risk factors of POR remains a key point in the management of the postoperative period in CD. However, the known risk factors do not allowed to accurately select the high-risk patients. Histologic factors, especially plexitis, could improve the selection of CD patients with ileocolic resection[38-41].
Although early colonoscopy after surgery is recommended in ECCO guidelines[21], low evidence supports this recommendation to date. Two retrospective studies evaluating the impact of postoperative EBM with tailored treatment according to the endoscopic findings did not report any benefit of this strategy on both clinical and surgical POR[23,24]. Bordeianou et al[24] reported no significant difference in time to clinical POR among the three following groups (n = 199 patients): immediate postoperative treatment, tailored treatment after endoscopy and no treatment. Similarly, De Cruz et al[23] reported no clinical benefit from an EBM in 136 CD patients. The authors explained their negative results in noting that the response to the endoscopic findings was not standardized and immunosuppressive therapy was uncommon during their study period. More recently, among 132 operated on for CD from the Saint-Louis Hospital, Paris, France, the authors reported a decreased clinical POR rate 5 years after surgery, in the patients with EBM, compared to the non-EBM group (26% vs 52%)[25]. Recently, the landmark POCER trial, a prospective, well-designed study, compared the impact of a tailored management according to clinical risk of recurrence, with early colonoscopy and treatment step-up on recurrence[26]. The results showed that an early EBM, performed 6 mo after surgery, decreased the rate of endoscopic POR at 18 mo[26]. However the long-term impact of EBM on the risk of POR (especially surgical) remained unknown. Our results indicated for the first time that an EBM influenced the risk of reoperation for CD, leading to a delayed time before surgical POR. In addition, we confirmed that the EBM group experienced less clinical POR over time than the non-EBM group, in a long period of follow-up. As our cohort overlapped a very long period with different available medications overtime, we did not show any impact of the postoperative treatment, especially biologics, in this population.
The main limits of this study were the retrospective and monocentric design. In addition, the time of endoscopy (median = 9.5 mo after surgery) and the step-up strategy were not standardized for all the patients. However, we observed for the first time the positive impact of an EBM on the risk of reoperation in CD, in a cohort monitored during almost 30 years (1986-2015) and based on a Pathology Department electronic database (consecutive patients).
In conclusion, POR is very frequent in CD and remains a critical issue in the management of the postoperative period. The identification of predictors to select the high-risk patients warranting top-down strategy in the postoperative period is crucial. An endoscopic-based management within the first year after surgery decreases the risk of symptoms recurrence and reoperation and then have to be recommended in daily practice.
ACKNOWLEDGMENTS
Thank you to the company “MG translate” for reviewing the manuscript.
COMMENTS
Background
Surgical resection is unfortunately not curative in Crohn’s disease (CD), and postoperative recurrence (POR) remains a crucial issue in these patients. Performing an endoscopy within the first year after surgery is often recommended in clinical practice. However, the level of evidence suggesting the efficacy of such strategy remains low. Two retrospective studies reported no impact of an endoscopy-based management (EBM). A French group suggested, in a retrospective cohort, that an EBM was associated with a decrease risk of clinical POR at 5 years. Recently, the landmark POCER trial showed that an early EBM decreased the risk of endoscopic POR at 18 months post-surgery. However the long-term impact of EBM on the risks of clinical and surgical POR remains unknown.
Research frontiers
The level of evidence suggesting the efficacy of an endoscopy-based strategy in CD remains low especially in the long-term. In the present study, the authors aimed to investigate whether an endoscopy-based strategy could prevent the long-term risk of POR in CD.
Innovations and breakthroughs
This paper showed for the first time, that an endoscopic-based management within the first year after surgery decreases the long-term risk of symptoms recurrence and reoperation.
Applications
Endoscopy-based management should be recommended in all CD patients within the first year after surgery in daily practice as it highly decreases the long-term risk of clinical recurrence and reoperation.
Terminology
An endoscopy-based strategy in CD means treatment intensification in case of endoscopic recurrence to prevent symptoms reappearance.
Peer-review
This article deals with an important aspect of CD- post operative recurrence. The article is well written in general.
Footnotes
Institutional review board statement: The study was approved by local Ethics Committee (IRB number 00008526 - 2014/CE86).
Informed consent statement: The study was performed in accordance with the Declaration of Helsinki, Good Clinical Practice and applicable regulatory requirements.
Conflict-of-interest statement: All the authors have no conflict of interest related to the manuscript.
Data sharing statement: The original anonymous dataset is available on request from the corresponding author at a_buisson@hotmail.fr.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: February 26, 2016
First decision: March 31, 2016
Article in press: April 20, 2016
P- Reviewer: Desai DC, Osawa S S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
References
- 1.Pariente B, Cosnes J, Danese S, Sandborn WJ, Lewin M, Fletcher JG, Chowers Y, D’Haens G, Feagan BG, Hibi T, et al. Development of the Crohn’s disease digestive damage score, the Lémann score. Inflamm Bowel Dis. 2011;17:1415–1422. doi: 10.1002/ibd.21506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pariente B, Mary JY, Danese S, Chowers Y, De Cruz P, D’Haens G, Loftus EV, Louis E, Panés J, Schölmerich J, et al. Development of the Lémann index to assess digestive tract damage in patients with Crohn’s disease. Gastroenterology. 2015;148:52–63.e3. doi: 10.1053/j.gastro.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Peyrin-Biroulet L, Loftus EV, Colombel JF, Sandborn WJ. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol. 2010;105:289–297. doi: 10.1038/ajg.2009.579. [DOI] [PubMed] [Google Scholar]
- 4.Riss S, Schuster I, Papay P, Herbst F, Mittlböck M, Chitsabesan P, Stift A. Surgical recurrence after primary ileocolic resection for Crohn’s disease. Tech Coloproctol. 2014;18:365–371. doi: 10.1007/s10151-013-1061-4. [DOI] [PubMed] [Google Scholar]
- 5.Shivananda S, Hordijk ML, Pena AS, Mayberry JF. Crohn’s disease: risk of recurrence and reoperation in a defined population. Gut. 1989;30:990–995. doi: 10.1136/gut.30.7.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lock MR, Farmer RG, Fazio VW, Jagelman DG, Lavery IC, Weakley FL. Recurrence and reoperation for Crohn’s disease: the role of disease location in prognosis. N Engl J Med. 1981;304:1586–1588. doi: 10.1056/NEJM198106253042607. [DOI] [PubMed] [Google Scholar]
- 7.Borley NR, Mortensen NJ, Chaudry MA, Mohammed S, Warren BF, George BD, Clark T, Jewell DP, Kettlewell MG. Recurrence after abdominal surgery for Crohn’s disease: relationship to disease site and surgical procedure. Dis Colon Rectum. 2002;45:377–383. doi: 10.1007/s10350-004-6186-0. [DOI] [PubMed] [Google Scholar]
- 8.Buisson A, Chevaux JB, Allen PB, Bommelaer G, Peyrin-Biroulet L. Review article: the natural history of postoperative Crohn’s disease recurrence. Aliment Pharmacol Ther. 2012;35:625–633. doi: 10.1111/j.1365-2036.2012.05002.x. [DOI] [PubMed] [Google Scholar]
- 9.Greenstein AJ, Sachar DB, Pasternack BS, Janowitz HD. Reoperation and recurrence in Crohn’s colitis and ileocolitis Crude and cumulative rates. N Engl J Med. 1975;293:685–690. doi: 10.1056/NEJM197510022931403. [DOI] [PubMed] [Google Scholar]
- 10.Nygaard K, Fausa O. Crohn’s disease. Recurrence after surgical treatment. Scand J Gastroenterol. 1977;12:577–584. doi: 10.3109/00365527709181336. [DOI] [PubMed] [Google Scholar]
- 11.Tytgat GN, Mulder CJ, Brummelkamp WH. Endoscopic lesions in Crohn’s disease early after ileocecal resection. Endoscopy. 1988;20:260–262. doi: 10.1055/s-2007-1018188. [DOI] [PubMed] [Google Scholar]
- 12.Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology. 1990;99:956–963. doi: 10.1016/0016-5085(90)90613-6. [DOI] [PubMed] [Google Scholar]
- 13.Rutgeerts P, Geboes K, Vantrappen G, Kerremans R, Coenegrachts JL, Coremans G. Natural history of recurrent Crohn’s disease at the ileocolonic anastomosis after curative surgery. Gut. 1984;25:665–672. doi: 10.1136/gut.25.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gabbert HE, Ewe K, Singe CC, Junginger T, Gerharz CD, Köther K. [The early recurrence of Crohn’s disease after “curative” ileocecal resection. A prospective endoscopic and histological study] Dtsch Med Wochenschr. 1990;115:447–451. doi: 10.1055/s-2008-1065028. [DOI] [PubMed] [Google Scholar]
- 15.Olaison G, Smedh K, Sjödahl R. Natural course of Crohn’s disease after ileocolic resection: endoscopically visualised ileal ulcers preceding symptoms. Gut. 1992;33:331–335. doi: 10.1136/gut.33.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heimann TM, Greenstein AJ, Lewis B, Kaufman D, Heimann DM, Aufses AH. Prediction of early symptomatic recurrence after intestinal resection in Crohn’s disease. Ann Surg. 1993;218:294–298; discussion 298-299. doi: 10.1097/00000658-199309000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meresse B, Rutgeerts P, Malchow H, Dubucquoi S, Dessaint JP, Cohard M, Colombel JF, Desreumaux P. Low ileal interleukin 10 concentrations are predictive of endoscopic recurrence in patients with Crohn’s disease. Gut. 2002;50:25–28. doi: 10.1136/gut.50.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurer MA, Stamou KM, Wilson TR, Bradford IM, Leveson SH. Early symptomatic recurrence after intestinal resection in Crohn’s disease is unpredictable. Colorectal Dis. 2007;9:567–571. doi: 10.1111/j.1463-1318.2006.01202.x. [DOI] [PubMed] [Google Scholar]
- 19.Ng SC, Lied GA, Arebi N, Phillips RK, Kamm MA. Clinical and surgical recurrence of Crohn’s disease after ileocolonic resection in a specialist unit. Eur J Gastroenterol Hepatol. 2009;21:551–557. doi: 10.1097/MEG.0b013e328326a01e. [DOI] [PubMed] [Google Scholar]
- 20.Onali S, Petruzziello C, Calabrese E, Condino G, Zorzi F, Sica GS, Pallone F, Biancone L. Frequency, pattern, and risk factors of postoperative recurrence of Crohn’s disease after resection different from ileo-colonic. J Gastrointest Surg. 2009;13:246–252. doi: 10.1007/s11605-008-0726-1. [DOI] [PubMed] [Google Scholar]
- 21.Van Assche G, Dignass A, Reinisch W, van der Woude CJ, Sturm A, De Vos M, Guslandi M, Oldenburg B, Dotan I, Marteau P, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Special situations. J Crohns Colitis. 2010;4:63–101. doi: 10.1016/j.crohns.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Buisson A, Chevaux JB, Bommelaer G, Peyrin-Biroulet L. Diagnosis, prevention and treatment of postoperative Crohn’s disease recurrence. Dig Liver Dis. 2012;44:453–460. doi: 10.1016/j.dld.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 23.De Cruz P, Bernardi MP, Kamm MA, Allen PB, Prideaux L, Williams J, Johnston MJ, Keck J, Brouwer R, Heriot A, et al. Postoperative recurrence of Crohn’s disease: impact of endoscopic monitoring and treatment step-up. Colorectal Dis. 2013;15:187–197. doi: 10.1111/j.1463-1318.2012.03168.x. [DOI] [PubMed] [Google Scholar]
- 24.Bordeianou L, Stein SL, Ho VP, Dursun A, Sands BE, Korzenik JR, Hodin RA. Immediate versus tailored prophylaxis to prevent symptomatic recurrences after surgery for ileocecal Crohn’s disease? Surgery. 2011;149:72–78. doi: 10.1016/j.surg.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Baudry C, Pariente B, Lourenço N, Simon M, Chirica M, Cattan P, Munoz-Bongrand N, Gornet JM, Allez M. Tailored treatment according to early post-surgery colonoscopy reduces clinical recurrence in Crohn’s disease: a retrospective study. Dig Liver Dis. 2014;46:887–892. doi: 10.1016/j.dld.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 26.De Cruz P, Kamm MA, Hamilton AL, Ritchie KJ, Krejany EO, Gorelik A, Liew D, Prideaux L, Lawrance IC, Andrews JM, et al. Crohn’s disease management after intestinal resection: a randomised trial. Lancet. 2015;385:1406–1417. doi: 10.1016/S0140-6736(14)61908-5. [DOI] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernell O, Lapidus A, Hellers G. Risk factors for surgery and recurrence in 907 patients with primary ileocaecal Crohn’s disease. Br J Surg. 2000;87:1697–1701. doi: 10.1046/j.1365-2168.2000.01589.x. [DOI] [PubMed] [Google Scholar]
- 29.Hellers G. Crohn’s disease in Stockholm county 1955-1974. A study of epidemiology, results of surgical treatment and long-term prognosis. Acta Chir Scand Suppl. 1979;490:1–84. [PubMed] [Google Scholar]
- 30.Agrez MV, Valente RM, Pierce W, Melton LJ, van Heerden JA, Beart RW. Surgical history of Crohn’s disease in a well-defined population. Mayo Clin Proc. 1982;57:747–752. [PubMed] [Google Scholar]
- 31.Simillis C, Yamamoto T, Reese GE, Umegae S, Matsumoto K, Darzi AW, Tekkis PP. A meta-analysis comparing incidence of recurrence and indication for reoperation after surgery for perforating versus nonperforating Crohn’s disease. Am J Gastroenterol. 2008;103:196–205. doi: 10.1111/j.1572-0241.2007.01548.x. [DOI] [PubMed] [Google Scholar]
- 32.Reese GE, Nanidis T, Borysiewicz C, Yamamoto T, Orchard T, Tekkis PP. The effect of smoking after surgery for Crohn’s disease: a meta-analysis of observational studies. Int J Colorectal Dis. 2008;23:1213–1221. doi: 10.1007/s00384-008-0542-9. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto T, Keighley MR. Smoking and disease recurrence after operation for Crohn’s disease. Br J Surg. 2000;87:398–404. doi: 10.1046/j.1365-2168.2000.01443.x. [DOI] [PubMed] [Google Scholar]
- 34.Parente F, Sampietro GM, Molteni M, Greco S, Anderloni A, Sposito C, Danelli PG, Taschieri AM, Gallus S, Bianchi Porro G. Behaviour of the bowel wall during the first year after surgery is a strong predictor of symptomatic recurrence of Crohn’s disease: a prospective study. Aliment Pharmacol Ther. 2004;20:959–968. doi: 10.1111/j.1365-2036.2004.02245.x. [DOI] [PubMed] [Google Scholar]
- 35.Yang RP, Gao X, Chen MH, Xiao YL, Chen BL, Hu PJ. [Risk factors for initial bowel resection and postoperative recurrence in patients with Crohn disease] Zhonghua Wei Chang Wai Ke Zazhi. 2011;14:176–180. [PubMed] [Google Scholar]
- 36.Lee SM, Han EC, Ryoo SB, Oh HK, Choe EK, Moon SH, Kim JS, Jung HC, Park KJ. Long-term Outcomes and Risk Factors for Reoperation After Surgical Treatment for Gastrointestinal Crohn Disease According to Anti-tumor Necrosis Factor-α Antibody Use: 35 Years of Experience at a Single Institute in Korea. Ann Coloproctol. 2015;31:144–152. doi: 10.3393/ac.2015.31.4.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khoshkish S, Arefi K, Charmehali M, Vahedi H, Malekzadeh R. Risk factors for postoperative recurrence of Crohn’s disease. Middle East J Dig Dis. 2012;4:199–205. [PMC free article] [PubMed] [Google Scholar]
- 38.Ferrante M, de Hertogh G, Hlavaty T, D’Haens G, Penninckx F, D’Hoore A, Vermeire S, Rutgeerts P, Geboes K, van Assche G. The value of myenteric plexitis to predict early postoperative Crohn’s disease recurrence. Gastroenterology. 2006;130:1595–1606. doi: 10.1053/j.gastro.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 39.Sokol H, Polin V, Lavergne-Slove A, Panis Y, Treton X, Dray X, Bouhnik Y, Valleur P, Marteau P. Plexitis as a predictive factor of early postoperative clinical recurrence in Crohn’s disease. Gut. 2009;58:1218–1225. doi: 10.1136/gut.2009.177782. [DOI] [PubMed] [Google Scholar]
- 40.Ng SC, Lied GA, Kamm MA, Sandhu F, Guenther T, Arebi N. Predictive value and clinical significance of myenteric plexitis in Crohn’s disease. Inflamm Bowel Dis. 2009;15:1499–1507. doi: 10.1002/ibd.20932. [DOI] [PubMed] [Google Scholar]
- 41.Bressenot A, Peyrin-Biroulet L. Histologic features predicting postoperative Crohn’s disease recurrence. Inflamm Bowel Dis. 2015;21:468–475. doi: 10.1097/MIB.0000000000000224. [DOI] [PubMed] [Google Scholar]


