Abstract
AIM: To determine the efficacy of probiotic supplementation on intestinal transit time (ITT) in adults and to identify factors that influence these outcomes.
METHODS: We conducted a systematic review of randomized controlled trials of probiotic supplementation that measured ITT in adults. Study quality was assessed using the Jadad scale. A random effects meta-analysis was performed with standardized mean difference (SMD) of ITT between probiotic and control groups as the primary outcome. Meta-regression and subgroup analyses examined the impact of moderator variables on SMD of ITT.
RESULTS: A total of 15 clinical trials with 17 treatment effects representing 675 subjects were included in this analysis. Probiotic supplementation was moderately efficacious in decreasing ITT compared to control, with an SMD of 0.38 (95%CI: 0.23-0.53, P < 0.001). Subgroup analyses demonstrated statistically greater reductions in ITT with probiotics in subjects with vs without constipation (SMD: 0.57 vs 0.22, P < 0.01) and in studies with high vs low study quality (SMD: 0.45 vs 0.00, P = 0.01). Constipation (R2 = 38%, P < 0.01), higher study quality (R2 = 31%, P = 0.01), older age (R2 = 27%, P = 0.02), higher percentage of female subjects (R2 = 26%, P = 0.02), and fewer probiotic strains (R2 = 20%, P < 0.05) were predictive of decreased ITT with probiotics in meta-regression. Medium to large treatment effects were identified with B. lactis HN019 (SMD: 0.67, P < 0.001) and B. lactis DN-173 010 (SMD: 0.54, P < 0.01) while other probiotic strains yielded negligible reductions in ITT relative to control.
CONCLUSION: Probiotic supplementation is moderately efficacious for reducing ITT in adults. Probiotics were most efficacious in constipated subjects, when evaluated in high-quality studies, and with certain probiotic strains.
Keywords: Constipation, Gastrointestinal, Intestinal transit time, Meta-analysis, Probiotics
Core tip: We performed a contemporary systematic review and meta-analysis of randomized controlled trials to determine the effects of short-term probiotic supplementation on transit time in adults. Probiotic supplementation is moderately efficacious for reducing intestinal transit time in adults. Probiotics were most efficacious in constipated subjects, when evaluated in high-quality studies, and with certain probiotic strains.
INTRODUCTION
The human colonic microbiota is a complex ecosystem involved in maintenance of health and physiological functions of the host. Disturbances within the microbiota may result in gastrointestinal disorders such as constipation, irritable bowel syndrome, or periodic bouts of irregularity. Functional gastrointestinal disorders are a highly prevalent group of persistent and recurring conditions with a prevalence of 69% in the general population[1]. Slow intestinal transit is a common manifestation of functional gastrointestinal disorders affecting the bowel[2] and may also occasionally affect otherwise healthy individuals. Although the benefits of reducing intestinal transit time (ITT) in patients with constipation are obvious, reductions in ITT are also considered a beneficial physiological effect in the non-diseased general population[3]. Over-the-counter and prescription medications intended to normalize intestinal transit are widely utilized although no known treatment is considered efficacious, safe, and cost effective[4]. Probiotics are live micro-organisms that confer a health benefit on the host when administered in adequate dosages[5] and have been extensively studied for enhancement of gastrointestinal health[6,7]. Previously, we performed the first systematic review and meta-analysis on the efficacy of probiotic supplementation on ITT in adults[8]. The purpose of this study was to update these findings with data from randomized controlled trials (RCTs) published over the 3-year period since our last review.
MATERIALS AND METHODS
Literature search
This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)[9]. We searched MEDLINE and EMBASE for RCTs of probiotic supplementation that reported ITT in adults by using a combination of relevant keywords. The details of the MEDLINE search strategy are listed in Table 1. The syntax for EMBASE was similar, but adapted as necessary. Additionally, manual searches were conducted using the Directory of Open Access Journals, Google Scholar, and the reference lists of included papers and other relevant meta-analyses. No date restrictions were applied to the searches. The final search was conducted in October 2015.
Table 1.
MEDLINE search strategy
| Therapeutic search terms |
| Probiotic |
| Synbiotic |
| Lactobacill |
| Bifidobacteri |
| Yogurt (yoghurt) |
| Fermented milk |
| Main outcome search terms |
| Gastrointestinal |
| Transit |
| Gut |
| Motility |
| Colonic |
| Constipation |
| Irritable bowel |
| Combination terms |
| or/1-6 |
| or/7-13 |
| and/14-15 |
Study selection
Two researchers independently selected studies for inclusion in the review. Disagreements were resolved by consensus. Titles and abstracts were initially screened to exclude manuscripts published in non-English journals. Next, review articles, commentaries, letters, and case reports were excluded. Lastly, we excluded studies of subjects where ITT reduction was undesirable or uninterpretable (e.g., diarrhea or mixed IBS subtypes). Full-text of the remaining manuscripts was then retrieved and reviewed. Publications that failed to report ITT or that described non-randomized, non-controlled, or otherwise irrelevant studies were also excluded.
Data extraction
Data were extracted from eligible peer-reviewed articles by one author and then verified by a second author. Data extraction discrepancies between the two researchers were resolved by consensus. The following variables were recorded in a pre-designed database: general manuscript information (author, institution name and location, journal, year, volume, page numbers), study design characteristics (study quality, study design, sample size, method of ITT assessment, probiotic strain, daily dosage, product delivery method, and treatment duration), subject characteristics (age, gender, body mass index, and condition), and ITT summary statistics necessary for meta-analysis.
Quality assessment
The Jadad scale was used to assess RCT study quality[10]. Studies were scored according to the presence of three key methodological features: randomization, blinding and subject accountability. Randomization was scored from 0 to 2, blinding was scored from 0 to 2, and subject accountability was scored 0 or 1. RCTs with a score of 3 to 5 were classified as high quality; studies with a score of 0 to 2 were classified as low quality.
Statistical analysis
A random effects meta-analysis model was selected a priori based on the assumption that treatment effects were heterogeneous given the differences in probiotic strain, study design characteristics, and subject characteristics among studies. The standardized mean difference (SMD) and 95% confidence interval (CI) were the statistics of interest to describe treatment effects since different measures of ITT (e.g., whole gut, colonic, oro-cecal, etc.) were utilized in the included studies. The SMD is calculated as the mean difference in ITT between probiotic and control groups divided by the pooled standard deviation in ITT. SMD values of 0.2, 0.5, and 0.8 are defined as small, medium, and large, respectively[11]. Positive SMDs imply that probiotics were more effective in reducing ITT vs control while negative SMDs imply a greater treatment effect with control vs probiotics. A forest plot was used to illustrate the individual study findings and the random effects meta-analysis results. Heterogeneity of effects across studies was estimated with the I2 statistic where values of ≤ 25%, 50%, and ≥ 75% represent low, moderate, and high inconsistency, respectively[12]. In addition, a one study removed meta-analysis was performed to assess the influence of individual studies on the meta-analysis findings. Publication bias was visually assessed with a funnel plot and quantitatively assessed using Egger’s test[13]. Meta-regression and subgroup analyses were performed to explore sources of heterogeneity. All analyses were performed using Comprehensive Meta-analysis (version 2.2, Biostat, Englewood NJ). The statistical methods of this study were reviewed by Clinton Hagen, MS (Mayo Clinic, Rochester, MN).
RESULTS
Study selection
Our initial database search retrieved 618 titles and abstracts; hand searching relevant bibliographies identified 3 additional records. After screening records for inclusion criteria, 101 full text articles were reviewed for eligibility. Ultimately, 15 RCTs with 17 treatment effects representing 675 unique subjects were included in the final analysis[14-28]. A flow chart of study identification and selection is shown in Figure 1.
Figure 1.
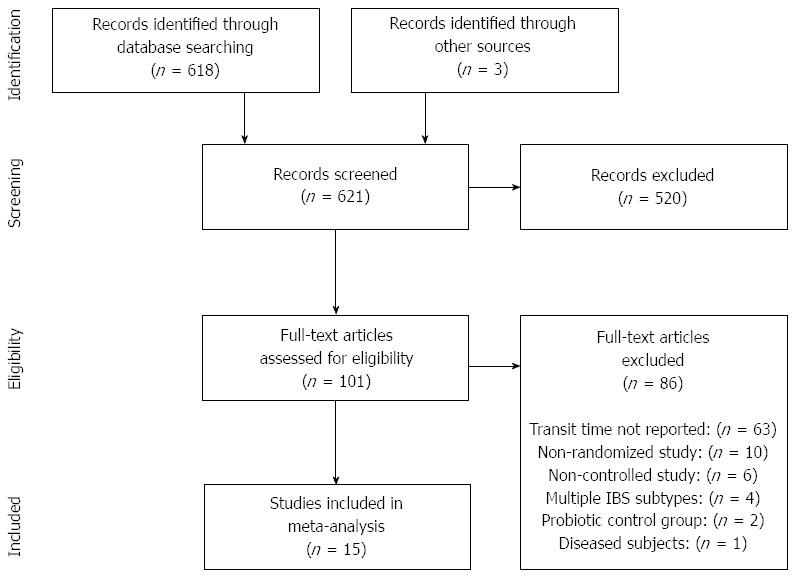
PRISMA flow diagram.
Study characteristics
Sample sizes ranged from 10 to 36 per treatment arm for parallel groups designs (9 studies) and from 12 to 83 for cross-over designs (6 studies). Thirteen RCTs contributed one treatment effect each and two RCTs contributed two effects each; the study of Rosenfeldt and colleagues[21] assessed two different probiotic formulations and the study of Waller and colleagues[23] assessed two different dosages of the same probiotic strain. Daily probiotic dosages varied considerably among studies, ranging from 5 × 108 to 9.8 × 1010 colony forming units (CFU) per day (median 1.6 × 1010 CFU per day). Probiotic treatment periods ranged from 10 to 28 d (median 18 d). Intestinal transit time was measured using radiopaque markers in 13 studies and with carmine red dye in 2 studies. The most commonly tested product format was yogurt or other forms of fermented milk. Six (40%) studies included other components in the active product known to influence ITT such as lactulose, psyllium, inulin, polydextrose, maltodextrose, and oligofructose (Table 2).
Table 2.
Study characteristics
| Study | Country | Study design | n(active: control) | Transit timeoutcome, method | Probiotic strain | Daily dosage(109 CFU) | Delivery method | Treatmentduration(d) |
| Agrawal et al[14], 2009 | United Kingdom | Parallel groups | 17:17 | CTT, radiopaque markers | B. lactis DN-173 010 | 25 | Active: Yogurt + probiotic | 28 |
| Control: Nonfermented milk-based product | ||||||||
| Bartram et al[15], 1994 | Germany | Cross-over | 12 | OATT, radiopaque markers | B. longum | > 0.5 | Active: Yogurt with 2.5 g lactulose + probiotic | 21 |
| Control: Yogurt | ||||||||
| Bazzocchi et al[25], 2014 | Italy | Parallel groups | 19:12 | TITT, radiopaque markers | L. plantarum, L. acidophilus, L. rhamnosus, B. longum, B. breve | - | Active: Sachet with psyllium+probiotic | 56 |
| Control: Sachet with 2.8 g maltodextrin | ||||||||
| Bouvier et al[16], 2001 | France | Parallel groups | 36:36 | CTT, radiopaque markers | B. lactis DN-173 010 | 97.5 | Active: Probiotic fermented milk | 11 |
| Control: Heat-treated probiotic fermented milk | ||||||||
| Holma et al[17], 2010 | Finland | Parallel groups | 12:10 | TITT, radiopaque markers | L. rhamnosus GG | 20 | Active: Buttermilk + probiotic and white wheat bread | 21 |
| Control: White wheat bread | ||||||||
| Hongisto et al[18], 2006 | Finland | Parallel groups | 16:14 | TITT, radiopaque markers | L. rhamnosus GG | 15 | Active: Yogurt + probiotic and low fiber toast | 21 |
| Control: Low fiber toast | ||||||||
| Krammer et al[24], 2011 | Germany | Parallel groups | 12:12 | CTT, radiopaque markers | L. casei Shirota | 6.5 | Active: Probiotic fermented milk drink | 28 |
| Control: Nonfermented milk drink | ||||||||
| Magro et al[26], 2014 | Brazil | Parallel groups | 26:21 | CTT, radiopaque markers | L. acidophilus NCFM, B. lactis HN019 | 2 | Active: Yogurt + polydextrose + probiotic Control: Yogurt | 14 |
| Malpeli et al[19], 2012 | Argentina | Cross-over | 83 | OCTT, carmine red dye | B. lactis BB12 | 2-20 | Active: Yogurt with 0.625 g inulin and oligofructose + probiotic | 15 |
| L. casei CRL 431 | 2-12 | Control: Yogurt | ||||||
| Marteau et al[20], 2002 | France | Cross-over | 32 | CTT, radiopaque markers | B. lactis DN-173 010 | 18.75 | Active: Yogurt + probiotic | 10 |
| Control: Yogurt | ||||||||
| Merenstein et al[27], 2014 | United States | Crossover | 68 | CTT, radiopaque markers | B. animalis ssp. lactis Bf-6 | 20-56 | Active: Yogurt + probiotic | 14 |
| Control: Yogurt | ||||||||
| Rosenfeldt et al[21], 2003a | Denmark | Cross-over | 13 | GTT, radiopaque markers | L. rhamnosus 19070-2 | 20 | Active: Freeze-dried powder + probiotic | 18 |
| L. reuteri DSM 12246 | 20 | Control: Skimmed milk powder w/dextrose | ||||||
| Rosenfeldt et al[21], 2003b | Denmark | Cross-over | 13 | GTT, radiopaque markers | L. casei subsp. alactus CHCC 3137 | 20 | Active: Freeze-dried powder + probiotic | 18 |
| L. delbrueckii subsp. lactis CHCC 2329 | 20 | Control: Skimmed milk powder w/dextrose | ||||||
| L. rhamnosus GG | 20 | |||||||
| Sairanen et al[22], 2007 | Finland | Parallel groups | 22:20 | CTT, radiopaque markers | B. longum BB536, B. lactis 420 | 2.4-181 | Active: Probiotic fermented milk | 21 |
| L. acidophilus 145 | 0.48 | Control: Fermented milk | ||||||
| Tulk et al[28], 2013 | Canada | Crossover | 65 | GTT, carmine red/carbon black capsules | B. lactis Bb12, L. acidophilus La5, L. casei CRL431 | 2 | Active: Yogurt + probiotic + inulin | 15 |
| Control: Yogurt | ||||||||
| Waller et al[23], 2011a | United States | Parallel groups | 33:34 | WGTT; radiopaque markers | B. lactis HN019 | 1.8 | Active: Capsule, maltodextrin, probiotic | 14 |
| Control: Capsule, maltodextrin | ||||||||
| Waller et al[23], 2011b | United States | Parallel groups | 33:34 | WGTT; radiopaque markers | B. lactis HN019 | 17.2 | Active: Capsule, maltodextrin, probiotic | 14 |
| Control: Capsule, maltodextrin |
Represents the reported range of total Bifidobacterium. CFU: Colony-forming units; CTT: Colonic transit time; GTT: Gastrointestinal transit time; OATT: Oro-anal transit time; OCTT: Oro-cecal TT; TITT: Total intestinal transit time; WGTT: Whole gut transit time.
Subject characteristics
Nine treatment effects were calculated for subjects with constipation or IBS-C while 8 effects were based on healthy subjects. Subjects were predominantly female, mean age ranged from 23 to 50 years, and mean body mass index ranged from 19 to 32 kg/m2 (Table 3).
Table 3.
Subject characteristics
| Study | Mean age (yr) | Female gender (%) | Mean BMI (kg/m2) | Condition |
| Agrawal et al[14], 2009 | 40 | 100 | 25 | IBS-C |
| Bartram et al[15], 1994 | 23 | 58 | -2 | None |
| Bazzocchi et al[25], 2014 | 40 | 86 | 19 | Constipation |
| Bouvier et al[16], 2001 | 33 | 50 | 22 | None |
| Holma et al[17], 2010 | 44 | 921 | 24 | Constipation |
| Hongisto et al[18], 2006 | 43 | 100 | 24 | Constipation |
| Krammer et al[24], 2011 | 50 | 100 | -2 | Constipation |
| Magro et al[26], 2014 | 32 | 91 | 28 | Constipation |
| Malpeli et al[19], 2012 | 41 | 100 | -2 | Constipation |
| Marteau et al[20], 2002 | 27 | 100 | 21 | None |
| Merenstein et al[27], 2014 | 29 | 100 | 23 | None |
| Rosenfeldt et al[21], 2003a | 25 | 0 | -2 | None |
| Rosenfeldt et al[21], 2003b | 25 | 0 | -2 | None |
| Sairanen et al[22], 2007 | 39 | 64 | 25 | None |
| Tulk et al[28], 2013 | 29 | 60 | 24 | None |
| Waller et al[23], 2011a | 44 | 65 | 31 | Constipation |
| Waller et al[23], 2011b | 44 | 65 | 32 | Constipation |
Percentage estimated from larger study cohort;
Represents missing data. BMI: Body mass index; IBS-C: Irritable bowel syndrome, constipation predominant.
Study quality assessment
Overall, the quality of RCT reporting was medium with a median Jadad score of 3 (range: 1-5). Twelve of 17 treatment effects were based on high quality (Jadad score 3-5) trials. The method of randomization was inadequately described in most studies. Descriptions of blinding were adequate overall. Subject accountability in RCTs was sufficiently detailed in 11 of 17 cases (Table 4).
Table 4.
Assessment of study quality
| Study |
Jadad scale |
|||
| Randomization range: 0-2 | Double blinding range: 0-2 | Subject account range: 0-1 | Total score1 range: 0-5 | |
| Agrawal et al[14], 2009 | 1 | 2 | 1 | 4 |
| Bartram et al[15], 1994 | 1 | 2 | 0 | 3 |
| Bazzocchi et al[25], 2014 | 1 | 2 | 1 | 4 |
| Bouvier et al[16], 2001 | 1 | 2 | 0 | 3 |
| Holma et al[17], 2010 | 1 | 0 | 1 | 2 |
| Hongisto et al[18], 2006 | 1 | 0 | 0 | 1 |
| Krammer et al[24], 2011 | 1 | 1 | 1 | 3 |
| Magro et al[26], 2014 | 2 | 2 | 1 | 5 |
| Malpeli et al[19], 2012 | 0 | 2 | 1 | 3 |
| Marteau et al[20], 2002 | 1 | 2 | 1 | 4 |
| Merenstein et al[27], 2014 | 2 | 2 | 1 | 5 |
| Rosenfeldt et al[21], 2003a | 1 | 1 | 0 | 2 |
| Rosenfeldt et al[21], 2003b | 1 | 1 | 0 | 2 |
| Sairanen et al[22], 2007 | 1 | 1 | 0 | 2 |
| Tulk et al[28], 2013 | 1 | 1 | 1 | 3 |
| Waller et al[23], 2011a | 2 | 2 | 1 | 5 |
| Waller et al[23], 2011b | 2 | 2 | 1 | 5 |
Higher scores represent better study quality.
Main results
In relation to controls, probiotic supplementation statistically decreased ITT, with an SMD of 0.38 (95%CI: 0.23-0.53, P < 0.001) (Figure 2). Only 5 of 17 treatment effects statistically favored probiotic supplementation. There was low heterogeneity among studies (I2 = 20%, P = 0.22) with no evidence of publication bias (Egger’s regression test: P = 0.44) (Figure 3). A one study removed sensitivity analysis was performed to determine the influence of individual studies on main outcomes. Overall, no single study significantly influenced the observed SMD of ITT with probiotics vs control. SMDs ranged from 0.35 to 0.42 (all P < 0.001) following removal of each study one at a time from the meta-analysis (Figure 4).
Figure 2.
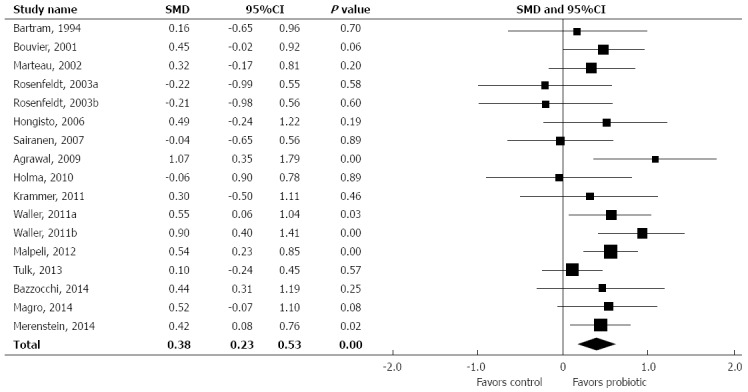
Forest plot of standardized mean difference in intestinal transit time across studies. Random effects model. I2 = 20%, P = 0.22. SMD: Standardized mean difference.
Figure 3.
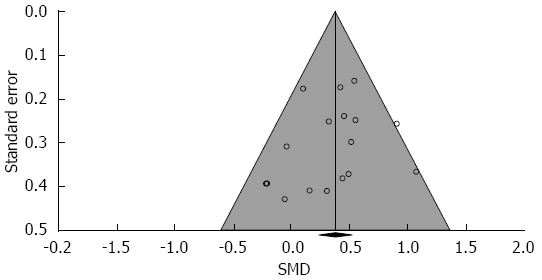
Funnel plot of standardized mean difference in intestinal transit time across studies. Eggar’s P value = 0.44 for publication bias. SMD: Standardized mean difference.
Figure 4.
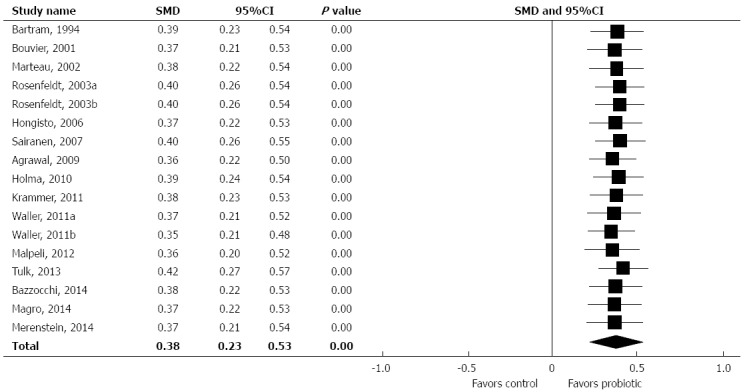
One study removed forest plot of standardized mean difference in intestinal transit time across studies. SMD: Standardized mean difference.
Additional analyses
Subgroup analyses (SA) (Table 5) and meta-regression (MR) (Table 6) were performed to determine the influence of study- and subject-related characteristics on ITT. Probiotic supplementation reduced ITT in comparison to controls in several of the analyzed subgroups. Greater reductions in ITT were observed with probiotics in subjects with vs without constipation (SA and MR, P < 0.01) and in high-quality (Jadad score ≥ 3) vs low-quality (Jadad score < 3) studies (SA and MR, P = 0.01). There were trends for greater probiotic efficacy with older age (SA, P = 0.08, MR, P = 0.02), in recently published studies (SA, P = 0.08), with parallel groups study designs (SA, P = 0.08), higher percentage of female subjects (SA, P = 0.08, MR, P = 0.02), single-strain probiotics (SA, P = 0.09, MR, P < 0.05) and higher body mass index (SA, P = 0.16, MR, P = 0.08). Treatment duration, geographic location of study, inclusion of potentially confounding treatments, and daily probiotic dosage were not found to have a significant influence on probiotic efficacy in subgroup analysis and meta-regression. Analysis of outcomes by probiotic strain identified medium to large treatment effects with B. lactis HN019 (SMD: 0.67, P < 0.001) and B. lactis DN-173 010 (SMD: 0.54, P < 0.01) while treatment effects with other strains were small (SMD: 0.10-0.33) and not statistically significant (Table 7).
Table 5.
Subgroup analysis of study- and subject-related factors on intestinal transit time
| Study | SMD | 95%CI | P value | P value |
| (pre-post) | (between groups) | |||
| Subject condition | ||||
| Constipation/IBS-C (n = 9) | 0.57 | 0.39 to 0.75 | < 0.001 | < 0.01 |
| Healthy (n = 8) | 0.22 | 0.05 to 0.39 | 0.01 | |
| Study quality | ||||
| Jadad score ≥ 3 (n = 12) | 0.45 | 0.31 to 0.59 | < 0.001 | 0.01 |
| Jadad score < 3 (n = 5) | 0.00 | -0.33 to 0.33 | > 0.99 | |
| Age1 | ||||
| ≥ 39 yr (n = 9) | 0.51 | 0.29 to 0.73 | < 0.001 | 0.08 |
| < 39 yr (n = 8) | 0.27 | 0.09 to 0.44 | < 0.01 | |
| Publication year | ||||
| After 2008 (n = 10) | 0.47 | 0.29 to 0.65 | < 0.001 | 0.08 |
| Before 2008 (n = 7) | 0.20 | -0.03 to 0.44 | 0.09 | |
| Number of probiotic strains | ||||
| Single strain (n = 10) | 0.49 | 0.32 to 0.66 | < 0.001 | 0.09 |
| Multiple strains (n = 7) | 0.23 | -0.01 to 0.47 | 0.06 | |
| Study design | ||||
| Parallel groups (n = 11) | 0.48 | 0.31 to 0.65 | < 0.001 | 0.09 |
| Cross-over (n = 6) | 0.26 | -0.02 to 0.46 | 0.07 | |
| Body mass index12 | ||||
| ≥ 25 kg/m2 (n = 5) | 0.59 | 0.24 to 0.94 | < 0.001 | 0.16 |
| < 25 kg/m2 (n = 7) | 0.31 | 0.13 to 0.49 | < 0.001 | |
| Treatment duration1 | ||||
| < 18 d (n = 8) | 0.45 | 0.29 to 0.60 | < 0.001 | 0.17 |
| ≥ 18 d (n = 9) | 0.22 | -0.06 to 0.50 | 0.12 | |
| Geographic location | ||||
| Americas (n = 6) | 0.47 | 0.26 to 0.67 | < 0.001 | 0.20 |
| Europe (n = 11) | 0.28 | 0.07 to 0.49 | < 0.01 | |
| Female gender proportion1 | ||||
| ≥ 86% (n = 9) | 0.47 | 0.30 to 0.64 | < 0.01 | 0.22 |
| < 86% (n = 8) | 0.27 | 0.00 to 0.54 | < 0.05 | |
| Confounding treatments3 | ||||
| Yes (n = 7) | 0.46 | 0.24 to 0.67 | < 0.001 | 0.32 |
| No (n = 10) | 0.30 | 0.10 to 0.51 | < 0.01 | |
| Daily probiotic dosage1 | ||||
| ≥ 1.610 CFU (n = 8) | 0.40 | 0.12 to 0.67 | < 0.01 | 0.74 |
| < 1.610 CFU (n = 7) | 0.34 | 0.16 to 0.52 | < 0.001 |
Categorized by median value;
Body mass index not reported for 5 treatment effects;
Includes studies where treatment included probiotics plus fiber or non-digestible sugar. Variables sorted from lowest to highest between-groups P value; n represents the number of treatment effects. IBS-C: Irritable bowel syndrome, constipation predominant; SMD: Standardized mean difference.
Table 6.
Meta-regression of study- and subject-related factors on intestinal transit time
| Variable | Unit of measure | Intercept | Point estimate | Explained variance (%) | P value |
| Constipation/IBS-C | 1 = Yes; 0 = No | 0.218 | 0.352 | 38 | < 0.01 |
| Jadad score | Per 1 unit | -0.117 | 0.141 | 31 | 0.01 |
| Age | Per 1 yr | -0.352 | 0.021 | 27 | 0.02 |
| Female gender proportion | Per 10% | -0.045 | 0.055 | 26 | 0.02 |
| Number of probiotic strains | Per 1 strain | 0.618 | -0.133 | 20 | < 0.05 |
| Body mass index1 | Per 1 kg/m2 | -0.526 | 0.037 | 22 | 0.08 |
| Treatment duration | Per 1 d | 0.392 | -0.004 | 0 | 0.96 |
| Daily probiotic dosage | Per 10 × 109 CFU | 0.385 | -0.001 | 0 | 0.98 |
Body mass index not reported for 5 treatment effects. Variables sorted from greatest to least explained variance.
Table 7.
Subgroup analysis of probiotic strains on intestinal transit time
| Probiotic strain | No. of treatment effects | SMD | 95%CI | P value |
| B. lactis HN019 | 3 | 0.67 | 0.37-0.97 | < 0.001 |
| B. lactis DN-173 010 | 3 | 0.54 | 0.16-0.92 | < 0.01 |
| L. casei CRL 431 | 2 | 0.33 | -0.10-0.75 | 0.14 |
| B. lactis BB12 | 2 | 0.33 | -0.10-0.75 | 0.14 |
| L. rhamnosus GG | 3 | 0.10 | -0.35-0.55 | 0.67 |
Probiotic strains sorted from highest to lowest standard mean difference. SMD: Standardized mean difference.
DISCUSSION
An ever-increasing body of evidence implicates the gastrointestinal microbiome in defining states of health and disease[29]. Probiotics may restore the composition of the gut microbiome and support beneficial functions to gut microbial communities, resulting in amelioration of gut inflammation and other disease phenotypes[30]. Consequently, probiotic supplementation is increasingly touted as an effective and accessible means of improving gut health, even in the general population of healthy adults. The current systematic review and meta-analysis demonstrates that short-term probiotic supplementation yielded moderate ITT reductions in adults. Additionally, the treatment effect of probiotics was greater in subjects with constipation, in high-quality studies, and with certain probiotic strains. In contrast to the moderate treatment effect observed in constipated subjects, probiotics only minimally influenced ITT in non-constipated adults. Given this finding, it appears that probiotic consumption will not lead to undesired short ITT or diarrhea. However, probiotic consumption for the sole purpose of reducing ITT is unjustified in healthy adults. Nevertheless, this finding does not diminish other beneficial effects that have been observed with probiotics in healthy adults[31,32].
In this meta-analysis, there was a trend for greater treatment effects with probiotics in parallel groups study designs compared to crossover studies (SMD: 0.48 vs 0.26, P = 0.09). Although there is no clear explanation for this finding, data from one included study deserves further discussion. The study of Merenstein et al[27] enrolled 68 healthy women using a crossover design, with a 6-wk washout between treatment periods. However, a significant carry-over effect was observed at the start of the second treatment period. For purposes of this meta-analysis, we treated this study as a parallel groups design using data from the first treatment period only[33]. Although the presence of a carry-over effect was not mentioned in the other crossover studies included in this analysis, the fact that washout periods ranged from 2 to 6 wk with significant carryover identified even after 6 wk in the Merenstein study raises the question of whether carry-over effects may have influenced outcomes of other crossover studies. Although crossover studies may initially appear attractive to researchers given the smaller sample size requirements compared to parallel groups designs, we propose that crossover designs are inappropriate in probiotic clinical trials unless the washout period for the probiotic has been previously established for the specific condition under study.
In comparison to our previous meta-analysis on this topic, the treatment effect of probiotics on ITT was largely unchanged (SMD: 0.40 vs 0.38). Importantly, with the addition of more studies, we were able to explore potential sources of heterogeneity among studies with greater precision. Novel subgroup findings included the observation of moderate probiotic treatment effects (SMD: 0.45) in high-quality studies, but no treatment effect (SMD: 0.0) in low-quality studies. Although the treatment effect sizes in parallel groups and crossover studies remained largely unchanged, study design is now a considerably stronger predictor of heterogeneity in ITT outcomes given the inclusion of additional studies. We also identified that single-strain probiotics were more efficacious than multiple strain probiotics. Although B. lactis HN019 and B. lactis DN-173 010 remained the most efficacious probiotic strains, we were able to analyze additional probiotic strains that yielded modest improvements in ITT relative to placebo.
The strengths of this systematic review and meta-analysis are inclusion of only RCTs and a comprehensive assessment of the influence of moderator variables on ITT with probiotic supplementation. Our study also revealed several limitations in the design of ITT studies with probiotics. First, the treatment duration of included studies ranged from 10 to 56 d. Although the long-term safety of probiotics is well established[34], probiotic efficacy on ITT beyond 8 wk cannot be interpreted with the current analysis. Second, although the therapeutic benefit of probiotics appears to be strain-specific, the small number of studies performed with each strain prevented robust strain-specific comparisons. Finally, subject characteristics were relatively homogenous among studies with regard to age and gender. Therefore, the generalizability of these findings to the general population, particularly males and the elderly, is unknown. These findings give specific suggestions for future research in this field.
In conclusion, probiotic supplementation is moderately efficacious for reducing ITT in adults. Probiotics were most efficacious in constipated subjects, when evaluated in high-quality studies, and with certain probiotic strains.
COMMENTS
Background
Functional gastrointestinal disorders are common in the general population, with slow intestinal transit a common symptom. No therapy is highly efficacious, safe, and cost effective for treatment of slow-transit bowel disorders. Probiotics have been extensively studied for treatment of gastrointestinal disorders and may confer improvements in bowel regularity.
Research frontiers
Clinical trials of probiotic supplementation on intestinal transit time (ITT) yield discrepant results. The authors performed a contemporary systematic review and meta-analysis on the efficacy of probiotic supplementation on ITT in adults, with a secondary focus on exploring sources of heterogeneity through meta-regression and subgroup analyses.
Innovations and breakthroughs
Probiotics are most efficacious in constipated subjects, when evaluated in high-quality studies, and with certain probiotic strains.
Applications
Probiotic supplementation appears to confer clinically meaningful improvements in intestinal transit in subjects with constipation. Probiotic efficacy also significantly differs according to strain.
Terminology
Probiotics are live micro-organisms that confer a health benefit on the host when administered in adequate dosages. Intestinal transit time is an indicator of the time taken for a food bolus to travel through the gastrointestinal system. The standardized mean difference is a statistical measure of effect size for continuous outcomes, defined as the mean difference between groups divided by the pooled standard deviation.
Peer-review
Very nice manuscript.
Footnotes
Supported by Danisco Sweeteners.
Conflict-of-interest statement: Miller LE is a consultant to Bio-K Plus, DuPont, Fonterra, Natren, and UAS Laboratories. Ouwehand AC is an employee of DuPont Nutrition and Health. Zimmermann AC denies any conflict of interest.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: December 7, 2015
First decision: January 28, 2016
Article in press: March 2, 2016
P- Reviewer: Pehl C, Thompson JR S- Editor: Yu J L- Editor: A E- Editor: Wang CH
References
- 1.Drossman DA, Li Z, Andruzzi E, Temple RD, Talley NJ, Thompson WG, Whitehead WE, Janssens J, Funch-Jensen P, Corazziari E. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569–1580. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 2.Camilleri M. Review article: biomarkers and personalised therapy in functional lower gastrointestinal disorders. Aliment Pharmacol Ther. 2015;42:818–828. doi: 10.1111/apt.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Food Safety Authority. Guidance on the scientific requirements for health claims related to gut and immune function. EFSA J. 2011;9:1984. [Google Scholar]
- 4.Tack J, Müller-Lissner S. Treatment of chronic constipation: current pharmacologic approaches and future directions. Clin Gastroenterol Hepatol. 2009;7:502–508; quiz 496. doi: 10.1016/j.cgh.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 6.Malaguarnera G, Leggio F, Vacante M, Motta M, Giordano M, Bondi A, Basile F, Mastrojeni S, Mistretta A, Malaguarnera M, et al. Probiotics in the gastrointestinal diseases of the elderly. J Nutr Health Aging. 2012;16:402–410. doi: 10.1007/s12603-011-0357-1. [DOI] [PubMed] [Google Scholar]
- 7.Girardin M, Seidman EG. Indications for the use of probiotics in gastrointestinal diseases. Dig Dis. 2011;29:574–587. doi: 10.1159/000332980. [DOI] [PubMed] [Google Scholar]
- 8.Miller LE, Ouwehand AC. Probiotic supplementation decreases intestinal transit time: meta-analysis of randomized controlled trials. World J Gastroenterol. 2013;19:4718–4725. doi: 10.3748/wjg.v19.i29.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 10.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 11.Cohen J. Statistical power analysis for the behavioral sciences. Hillside, NJ: Lawrence Erlbaum Associates; 1987. [Google Scholar]
- 12.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agrawal A, Houghton LA, Morris J, Reilly B, Guyonnet D, Goupil Feuillerat N, Schlumberger A, Jakob S, Whorwell PJ. Clinical trial: the effects of a fermented milk product containing Bifidobacterium lactis DN-173 010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2009;29:104–114. doi: 10.1111/j.1365-2036.2008.03853.x. [DOI] [PubMed] [Google Scholar]
- 15.Bartram HP, Scheppach W, Gerlach S, Ruckdeschel G, Kelber E, Kasper H. Does yogurt enriched with Bifidobacterium longum affect colonic microbiology and fecal metabolites in health subjects? Am J Clin Nutr. 1994;59:428–432. doi: 10.1093/ajcn/59.2.428. [DOI] [PubMed] [Google Scholar]
- 16.Bouvier M, Meance S, Bouley C, Berta J, Grimaud J. Effects of consumption of a milk fermented by the probiotic strain Bifidobacterium animalis DN-173 010 on colonic transit time in healthy humans. Biosci Microflora. 2001;20:43–48. [Google Scholar]
- 17.Holma R, Hongisto SM, Saxelin M, Korpela R. Constipation is relieved more by rye bread than wheat bread or laxatives without increased adverse gastrointestinal effects. J Nutr. 2010;140:534–541. doi: 10.3945/jn.109.118570. [DOI] [PubMed] [Google Scholar]
- 18.Hongisto SM, Paajanen L, Saxelin M, Korpela R. A combination of fibre-rich rye bread and yoghurt containing Lactobacillus GG improves bowel function in women with self-reported constipation. Eur J Clin Nutr. 2006;60:319–324. doi: 10.1038/sj.ejcn.1602317. [DOI] [PubMed] [Google Scholar]
- 19.Malpeli A, González S, Vicentin D, Apás A, González HF. Randomised, double-blind and placebo-controlled study of the effect of a synbiotic dairy product on orocecal transit time in healthy adult women. Nutr Hosp. 2012;27:1314–1319. doi: 10.3305/nh.2012.27.4.5770. [DOI] [PubMed] [Google Scholar]
- 20.Marteau P, Cuillerier E, Meance S, Gerhardt MF, Myara A, Bouvier M, Bouley C, Tondu F, Bommelaer G, Grimaud JC. Bifidobacterium animalis strain DN-173 010 shortens the colonic transit time in healthy women: a double-blind, randomized, controlled study. Aliment Pharmacol Ther. 2002;16:587–593. doi: 10.1046/j.1365-2036.2002.01188.x. [DOI] [PubMed] [Google Scholar]
- 21.Rosenfeldt V, Paerregaard A, Nexmann Larsen C, Moller PL, Tvede M, Sandstrom B, Jakobsen M, Michaelsen KF. Faecal recovery, mucosal adhesion, gastrointestinal effects and tolerance of mixed cultures of potential prebiotic lactobacilli. Microbial Ecology in Health and Disease. 2003;15:2–9. [Google Scholar]
- 22.Sairanen U, Piirainen L, Gråsten S, Tompuri T, Mättö J, Saarela M, Korpela R. The effect of probiotic fermented milk and inulin on the functions and microecology of the intestine. J Dairy Res. 2007;74:367–373. doi: 10.1017/S0022029907002713. [DOI] [PubMed] [Google Scholar]
- 23.Waller PA, Gopal PK, Leyer GJ, Ouwehand AC, Reifer C, Stewart ME, Miller LE. Dose-response effect of Bifidobacterium lactis HN019 on whole gut transit time and functional gastrointestinal symptoms in adults. Scand J Gastroenterol. 2011;46:1057–1064. doi: 10.3109/00365521.2011.584895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krammer HJ, Seggem HV, Schaumburg J, Neumer F. Effect of Lactobacillus casei Shirota on colonic transit time in patients with chronic constipation. Coloproctology. 2011;33:109–113. [Google Scholar]
- 25.Bazzocchi G, Giovannini T, Giussani C, Brigidi P, Turroni S. Effect of a new synbiotic supplement on symptoms, stool consistency, intestinal transit time and gut microbiota in patients with severe functional constipation: a pilot randomized double-blind, controlled trial. Tech Coloproctol. 2014;18:945–953. doi: 10.1007/s10151-014-1201-5. [DOI] [PubMed] [Google Scholar]
- 26.Magro DO, de Oliveira LM, Bernasconi I, Ruela Mde S, Credidio L, Barcelos IK, Leal RF, Ayrizono Mde L, Fagundes JJ, Teixeira Lde B, et al. Effect of yogurt containing polydextrose, Lactobacillus acidophilus NCFM and Bifidobacterium lactis HN019: a randomized, double-blind, controlled study in chronic constipation. Nutr J. 2014;13:75. doi: 10.1186/1475-2891-13-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merenstein DJ, D’Amico F, Palese C, Hahn A, Sparenborg J, Tan T, Scott H, Polzin K, Kolberg L, Roberts R. Short-term, daily intake of yogurt containing Bifidobacterium animalis ssp. lactis Bf-6 (LMG 24384) does not affect colonic transit time in women. Br J Nutr. 2014;111:279–286. doi: 10.1017/S0007114513002237. [DOI] [PubMed] [Google Scholar]
- 28.Tulk HM, Blonski DC, Murch LA, Duncan AM, Wright AJ. Daily consumption of a synbiotic yogurt decreases energy intake but does not improve gastrointestinal transit time: a double-blind, randomized, crossover study in healthy adults. Nutr J. 2013;12:87. doi: 10.1186/1475-2891-12-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guinane CM, Cotter PD. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Therap Adv Gastroenterol. 2013;6:295–308. doi: 10.1177/1756283X13482996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol. 2013;6:39–51. doi: 10.1177/1756283X12459294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sleator RD. Designer probiotics: Development and applications in gastrointestinal health. World J Gastrointest Pathophysiol. 2015;6:73–78. doi: 10.4291/wjgp.v6.i3.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pandey V, Berwal V, Solanki N, Malik NS. Probiotics: Healthy bugs and nourishing elements of diet. J Int Soc Prev Community Dent. 2015;5:81–87. doi: 10.4103/2231-0762.155726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freeman PR. The performance of the two-stage analysis of two-treatment, two-period crossover trials. Stat Med. 1989;8:1421–1432. doi: 10.1002/sim.4780081202. [DOI] [PubMed] [Google Scholar]
- 34.Didari T, Solki S, Mozaffari S, Nikfar S, Abdollahi M. A systematic review of the safety of probiotics. Expert Opin Drug Saf. 2014;13:227–239. doi: 10.1517/14740338.2014.872627. [DOI] [PubMed] [Google Scholar]


