Abstract
Peripheral artery disease (PAD) produces significant disability attributable to lower extremity ischemia. Limited treatment modalities exist to ameliorate clinical symptoms in patients with PAD. Growing evidence links microRNAs to key processes that govern disease expression in PAD including angiogenesis, endothelial function, inflammation, vascular regeneration, vascular smooth muscle cell function, restenosis, and mitochondrial function. MicroRNAs have been identified in circulation and may serve as novel biomarkers in PAD. This article reviews the potential contribution of microRNA to key pathways of disease development in PAD that may lead to microRNA-based diagnostic and therapeutic approaches.
Keywords: biomarker, peripheral arterial disease, epigenetics
There is a rising worldwide prevalence of disability attributable to peripheral artery disease (PAD).[1,2] Lower extremity ischemia in PAD causes suffering and functional impairment.[3] Cardiovascular complications of systemic atherosclerosis are markedly increased in PAD and persist despite available treatments.[4,5] In addition, the majority of patients with PAD remain undiagnosed and undertreated.[6] New methods for detecting PAD along with an improved understanding of the mechanisms driving vascular injury are critical to develop innovative strategies for prevention and management.
Emerging evidence identifies microRNAs (miRNAs) as novel regulators of vascular biology.[7] miRNAs are small, noncoding RNAs that interact with gene transcripts to repress expression.[8] Experimental work links miRNAs to key processes relevant to PAD including inflammation, angiogenesis, endothelial function, smooth muscle cell biology and restenosis (see Figure). Interestingly, miRNAs are present in circulating blood in humans and have potential as PAD disease biomarkers.[9] Endothelial-specific miRNAs may have specific relevance in atherosclerotic disease. Modulation of miRNA levels represents a novel treatment approach for limb ischemia. The current review focuses on miRNA in the mechanisms of disease development in PAD that may provide opportunities for miRNA-based therapies.
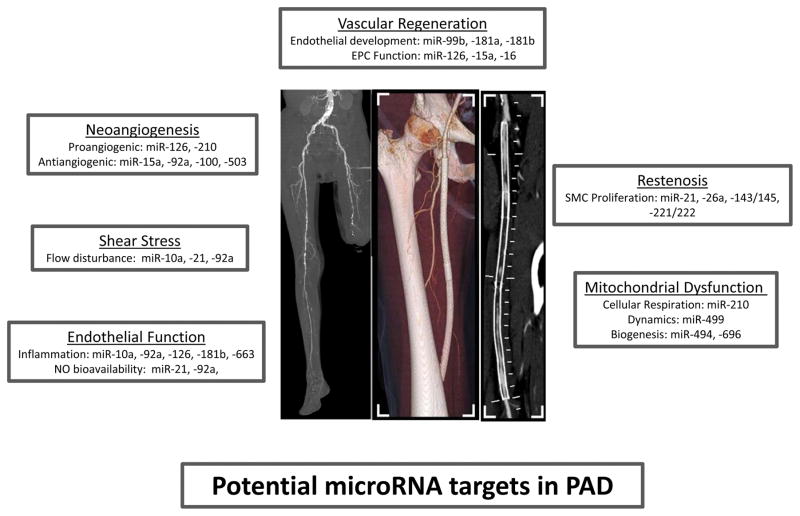
Figure 1.
Potential Contributions of MicroRNAs to PAD. MicroRNAs (miR) have been identified that determine key processes relevant to disease manifestation in PAD including neoangiogenesis, endothelial shear stress response, endothelial function, restenosis, vascular regenerative capacity, and mitochondrial function.
Determinants of Clinical Status in PAD
Atherosclerotic PAD involves the development of obstructive lesions in the arteries of the lower extremities. Patients with PAD have even higher cardiovascular event rates than patients with established coronary artery disease (CAD) that persist with aggressive risk factor control.[4] Epidemiologic evidence indicates that the relative impact of traditional risk factors differs between PAD and CAD.[10,11] In addition, prior studies report a stronger association of selected inflammatory markers with PAD as compared to CAD.[12,13] These findings substantiate the premise that the pathophysiology of PAD has distinctions from CAD. Thus, the miRNA signature and treatment approach may be different in PAD.
The determinants of clinical status and prognosis in PAD are complex. Classically, lower extremity symptoms have been attributed to fixed obstruction to flow. However, the severity of arterial obstruction is an incomplete predictor of clinical symptoms.[14–16] Vascular dysfunction may accelerate the clinical expression and progression of PAD.[17,18] Experimental studies suggest that endothelial-expressed miRNA have particular importance in vascular processes relevant to PAD.
MicroRNA in Vascular Function
miRNAs are small RNAs that regulate gene expression and direct vascular biology. Initially transcribed as long primary miRNAs, sequential processing by the enzymes Drosha and Dicer produces mature ≈22 nucleotide miRNAs. miRNA binding to the 3′ untranslated region of messenger RNA (mRNA) alters protein expression through translational repression or mRNA transcript degradation.[19] Individual miRNA may associate with functionally-related transcripts thereby governing complex processes in a coordinated fashion. There is considerable interest in miRNAs as therapeutic targets in vascular diseases as a single miRNA has the potential to influence entire gene networks.[8]
The broad role of miRNAs in vascular biology was established by studies examining genetic disruption of the processing enzyme, Dicer. Genetic disruption of Dicer impairs blood vessel formation leading to embryonic lethality in mice.[20] In cultured endothelial cells, silencing Dicer had marked effects on gene expression and functional properties. [21–23] In a mouse model, endothelial-targeted Dicer deletion reduced growth factor-mediated angiogenic responses confirming the importance of miRNAs to endothelial control of vascular growth.[24] Specific miRNAs demonstrate higher expression in endothelial cells.[25] Collectively, these findings indicate that miRNAs influence endothelial functions important to PAD.
MicroRNA in the Angiogenic Response to Limb Ischemia
An inadequate angiogenic response to lower extremity ischemia contributes to symptom manifestation in PAD patients. Growing evidence from animal models of limb ischemia confirms the physiological role of miRNAs in angiogenesis. Conditional inactivation of Dicer in the endothelium impairs capillary growth and blood flow recovery after femoral artery disruption.[24] Dicer knockdown reduced expression of endothelial specific miRNAs and altered expression of proteins in the vascular endothelial growth factor (VEGF) signaling pathways including VEGF receptor 2 and Tie-1.[21] Further, vascular endothelial growth factor regulates expression levels of angiogenesis-related miRNAs in endothelial cells.[24]
More recent work has identified many specific miRNAs that modify the vascular growth response to limb ischemia (see Table 1). The endothelial expressed miRNA, miR-92a, is induced by ischemia and blocks blood vessel growth.[26] Inhibition of miR-92a enhanced neovascularization, reduced tissue necrosis and increased blood flow in a mouse model of limb ischemia.[26] Several proteins that facilitate angiogenesis including the integrin subunit alpha 5 and endothelial nitric oxide synthase are potential miR-92a targets. Similarly, miR-100 is suppressed by hindlimb ischemia and represses angiogenesis through mammalian target of rapamycin (mTOR).[27] Endothelial-selective overexpression of the anti-angiogenic miR-15a diminished leg perfusion recovery and capillary density in response to limb ischemia.[28]
Table 1.
MicroRNAs Relevant to Peripheral Artery Disease
| MicroRNA | Putative Target | Putative Mechanism |
|---|---|---|
| miR-92a | eNOS, integrin-α5 KLF2/4 |
Anti-angiogenic, Inhibit NO Pro-inflammatory |
| miR-100 | mTOR | Anti-angiogenic |
| miR-503 | Cell cycle regulators | Anti-angiogenic |
| miR-126 | SPRED1/PIK3R2/VEGF VCAM1 |
Pro-angiogenic, EPC function Anti-inflammatory |
| miR-10a | NFκB | Anti-inflammatory |
| miR-663 | KLF4, ATF6 | Pro-inflammatory |
| miR-21 | PPARα PTEN PCD4/JNK PTEN/BC12 |
Pro-inflammatory Increase NO Restenosis SMC Proliferation |
| miR-181b | Importin-α3 | Anti-inflammatory |
| miR-15a/16 | VEGF | Impair EPC function |
| miR-143/145 | KLF4/5, ACE, PDGFR | SMC Proliferation |
| miR-221/222 | p27, p57 | SMC Proliferation |
| miR-210 | Ephrin A3/VEGF | Pro-angiogenic |
| miR-499 | DRP-1 | Mitochondrial dynamics |
| miR-494 | MTF A | Mitochondrial biogenesis |
| miR-699 | PGC1α | Mitochondrial biogenesis |
Patients with diabetes have worse outcomes in PAD with a higher risk of limb loss reflective in part of limited angiogenic response.[29] A recent study provided evidence that microRNA are a novel mechanism for dysfunctional angiogenesis in diabetes.[30] MicroRNA analysis of endothelial cells exposed to high glucose demonstrated elevated miRNA-503. Overexpression of miRNA attenuated endothelial proliferation, migration and tube formation consistent with reduced angiogenic potential. In diabetic mice, delivery of a mir-503 inhibitory decoy restored the blood flow recovery response to hindlimb ischemia. The significance to clinical PAD was corroborated by the observation of higher miRNA-503 levels in the skeletal muscle of diabetic patients with critical limb ischemia, compared to controls. It should be noted that the sample size in the clinical portion of the study was small that limited the ability to control for potential confounders including diabetes and smoking. Taken together, these findings suggest that antagonism of miRNA-503 has therapeutic potential to improve limb ischemia in patients with diabetes.
Individual miRNAs may also facilitate angiogenesis. In limb ischemia models, injection of an antagomir that silenced the endothelial miR-126 reduced capillary growth though overall blood flow recovery was similar suggesting that compensatory mechanisms may exist to limit the effect of loss of a single miRNA.[31] The pro-angiogenic properties of miR-126 are mediated by targeting SPRED1 and PIK3R2, endogenous inhibitors of VEGF signaling.[32,33]
MicroRNA in the Regulation of Endothelial Function
In addition to participating in the control of angiogenesis, vascular miRNAs influence aspects of endothelial function critical to PAD. Recent investigations have characterized miRNAs that alter endothelial phenotype to affect atherogenic potential. Interestingly, global reduction of miRNAs through Dicer silencing increased eNOS expression and nitric oxide bioavailability in cultured endothelial cells.[21] Thus, miRNAs serve as endogenous suppressors of eNOS levels suggesting that targeting specific miRNAs may enhance nitric oxide production. Subsequent work showed that miR-92a antagonism improved endothelial nitric oxide signaling, improved nitric oxide bioavailability, and increased flow-mediated dilation in cell culture and animal models.[26] [34] Further studies are needed to evaluate the relation of miRNA levels and nitric-oxide mediated endothelial function in patients with PAD.
Developing evidence links miRNAs to the endothelial response to flow. Local shear stress patterns modify endothelial biology in part through altered gene expression levels.[35] Laminar flow maintains endothelial health, whereas disturbed flow produces an adverse, pro-inflammatory endothelial state.[36] Multiple studies have demonstrated changes in miRNA expression with endothelial cell exposure to varying shear stress patterns.[37] Arterial regions exposed to pro-atherogenic flow display distinct miRNA expression profiles.[38,39]
Flow-sensitive miRNAs have also been studied in hindlimb ischemia reinforcing a potential connection to PAD. In endothelial cells, flow patterns induce differential expression of miR-92a with increased levels with oscillatory compared to pulsatile shear.[34] Complementary findings from porcine aorta showed higher miR-92a levels in regions exposed to flow turbulence.[39] Conversion from atheroprotective to atheroprone gene expression by oscillatory shear was mediated by a post-transcriptional reduction in kruppel-like factor (KLF) 2 and 4 levels through miR-92a.[34,39] miR-92a inhibition also prevented tumor necrosis factor alpha-mediated endothelial inflammatory activation by augmenting KLF4 levels.[39] A study in zebrafish embryos suggested flow-induced modulation of miR-126; however, miR-126 was not upregulated by laminar flow in cultured human endothelial cells.[40,41] miR-126 does appear to moderate endothelial inflammation by reducing vascular cell adhesion molecule-1 expression and decreasing leukocyte adherence.[42]
Additional miRNAs have been characterized that impact flow-dependent vascular inflammation. The amount of miR-10a is diminished in abnormal flow areas. In endothelial cells, mir-10a has anti-inflammatory properties by reducing expression of nuclear factor kappa B activators.[38] Conversely, oscillatory shear stress stimulates pro-inflammatory miR-663 and upregulates inflammatory gene expression and enhances leukocyte adhesion to endothelial cells.[43] Separate reports showed higher miR-21 levels with both oscillatory shear and laminar shear.[44,45] miR-21 exerts diverse endothelial effects by decreasing peroxisome proliferators-activated receptor-α expression and promoting adhesion molecule expression. However, beneficial effects have also been shown with miR-21 overexpression with reduction of PTEN, an inhibitor of eNOS activation, and increased nitric oxide activity.[45] Overexpression of miR-181b blunted endothelial inflammatory activation by suppressing importin-α3, a facilitator of NFκB activation.[46] Additional work will be required to determine whether the vascular inflammation present in PAD can be mitigated by microRNA-based therapies.[47]
MicroRNA and Vascular Reparative Function
Considerable enthusiasm exists for the prospects of employing cell-based interventions to facilitate vascular growth in PAD. The isolation of bone-marrow derived progenitor cells that promote neovascularization has prompted the development of investigational therapeutic strategies for advanced vascular disease. [48] Results from early clinical studies in patients with PAD have revealed modest benefits; however, multiple impediments persist that limit clinical efficacy. There is accumulating support for the role of miRNAs in optimizing stem cell function.[49] Investigations using Dicer reduction established that miRNAs are an essential component of stem cell maintenance and differentiation.[50,51] A recent study in cardiomyocytes detected a set of miRNAs that stimulated cell proliferation and cardiac regeneration following myocardial infarction.[52] These findings provide support for the concept that miRNA administration may bolster functional recovery produced by stem cells.
miRNAs have been detected that contribute to pluripotency, vascular differentiation, and adult progenitor cell function. [49,53,54] The transformation of embryonic stem cells to endothelial cells is accompanied by changes in miRNA levels relevant to angiogenesis including higher (miR-126, miR-210, let-7, mir-130a, miR-133, and miR-196) and lower (miR-20a, miR-20b, miR-221, and miR-222) expression.[55,56] In an animal model, genetic augmentation of embryonic stem cells to increase miRNAs critical to endothelial cell differentiation (miR-99b, miR-181a, miR-181b) improved blood flow recovery from hindlimb ischemia.[57]
Circulating pro-angiogenic endothelial progenitor cells have the capacity to foster vascular repair.[58] It has been proposed that in disease states, progenitor cell scarcity and dysfunction impair vascular regeneration.[59] In patients with coronary artery disease, endothelial progenitor cells showed differential expression of miRNAs related to angiogenesis, a phenotype that was reversed with statin therapy.[60] In patients with diabetes, endothelial progenitor cells displayed lower miR-126 expression that was associated with impaired angiogenic function.[61] A recent study demonstrated higher expression of miRNA-15a and 16 in endothelial progenitor cells from patients with critical limb ischemia.[62] These miRNAs reduced VEGF expression and alteration of endothelial progenitor cells to reduce the levels of miRNA-15a and 16 improved hindlimb blood flow recovery in a murine model. Together, the current evidence suggests that miRNA manipulation may be an approach to enhance cell-based therapies for PAD.
MicroRNA in the Development of Restenosis
In the past decade, the use of endovascular therapies to treat advance PAD has risen dramatically.[63] However, the efficacy of lower extremity revascularization is limited by the high rate of restenosis.[64] In vitro studies have identified miRNAs which regulate vascular SMC de-differentiation and processes relevant to myointimal hyperplasia.[65] Studies in animal models also demonstrate miRNA-dependent regulation of neointimal lesion formation.[66] Following carotid balloon injury in rats, expression profiling detects deviation in the vascular wall levels of multiple microRNAs.[67]
Endothelial damage with angioplasty depresses expression of miR-143 and 145, key supervisors of vascular smooth muscle cell phenotype.[67–69] Overexpression of miR-145 inhibits lesion formation and vascular smooth muscle proliferation.[70–72] Genetic models with miR-143/145 knockout mice have yielded complex information about neointimal growth. One study showed that young mice lacking miR-143/145 spontaneously develop femoral artery proliferative lesions.[69] Another report demonstrated limited neointimal growth to carotid artery injury in miR-143/145 depleted mice.[70] It may be that the proper regulation of miR-143/145 levels is required to stabilize downstream gene expression and prevent an aberrant injury response. Downstream targets of miR-143 and 145 that shift vascular smooth cell phenotype include KLF4, KLF5, platelet derived growth factor receptor, and angiotensin-converting enzyme.[68,69,71,73,74] Smooth muscle calcification is induced by bone morphogenetic protein-2 (BMP-2) downregulation of miR-30b and 30c, which increases Runx2 expression.[75]
Arterial injury also elevates levels of selected vascular smooth cell microRNAs. Angioplasty-induced vessel damage resulted in an increase in miR-21 levels in rats.[67] Depletion of miR-21 constrained the development of restenotic lesions along with lower vascular smooth muscle cell proliferation in both carotid and iliac artery models.[67,76] Fibroblast proliferation is also activated by miR-21 through programmed cell death 4/JNK pathway.[76] In vascular smooth muscle cells, the proliferative actions of miR-21 are mediated through PTEN and Bcl-2.[67,77] In patients with thromboangiitis obliterans, a non-atherosclerotic peripheral vascular disease, arterial miR-21 levels were increased and shown to modulate smooth muscle proliferation by targeting tropomyosin 1.[78] The number of samples in the clinical study was relatively small precluding any adjustment for potential confounders; thus a larger study will be necessary to replicate these findings. In a comparable fashion, angioplasty injury produces greater miR-221 and 222 expression.[79] Reduction of miR-221 and 222 interfered with vascular smooth muscle proliferation and neointimal hyperplasia through suppression of p27(Kip1) and p57 (Kip2). It is possible that derangements of miRNA expression translate to restenosis in PAD patients and could be manipulated by therapeutic inteventions.
MicroRNA and Mitochondrial Function
There is growing appreciation that peripheral artery disease produces skeletal muscle abnormalities characterized by mitochondrial dysfunction.[80,81] Abnormal muscle energetics combined with ischemia and endothelial dysfunction may amplify functional limitations in PAD.[82] Increasing experimental data associate miRNA with mitochondrial biology in muscle and the vasculature.[83] In skeletal muscle, limb ischemia induces differences in a set of miRNAs in mice.[84] Recent work shows detectable miRNA in the mitochondria that regulate energetics.[85,86]
Ischemia drives changes in miRNA expression relevant to mitochondrial function.[87] Multiple lines of evidence indicate that miR-210 is an integral regulator of the response to hypoxia.[88] miR-210 expression is stimulated in hypoxic conditions, in part, through HIF1α and suppresses mitochondrial respiration and reactive oxygen species generation.[89,90] MiR-210 expression, in the setting of hypoxia, augments the angiogenic response to VEGF by suppressing Ephrin-A3 expression.[91] Additional studies show that ischemia generates a transition to greater mitochondrial fragmentation and apoptosis. Ischemic conditions decrease miR-499 that promotes DRP-1 expression and increased mitochondrial fission.[92] In patients with diabetes, aberrant mitochondrial dynamics relates to endothelial dysfunction.[93]
Restoration of mitochondrial function is a proposed pathway underlying the dramatic benefits of exercise therapy in PAD patients.[3,94]In animal models, specific miRNA have been isolated that couple physical activity with mitochondrial biogenesis. Exercise intervention reduced the expression of miR-494, a microRNA that is stimulated by limb ischemia.[84,95] Through an interaction with mitochondrial transcription factor A, miR-494 modulates mitochondrial content.[95] In a similar fashion, skeletal muscle levels of miR-696 decreased with exercise training and increased with inactivity. [96] The amount of miR-696 associated with proliferator-activated receptor-gamma coactivator-1alpha (PGC-1α) expression and with mitochondrial number.[96] Notably, PGC-1α, a controller of mitochondrial biogenesis, also determines blood flow recovery from limb ischemia and exercise-induced angiogenesis.[97,98] Further studies are warranted to establish whether miRNAs contribute to mitochondrial dysfunction in patients with PAD.
Circulating MicroRNA in PAD
Recent investigations demonstrate detectable levels of circulating miRNAs that serve as novel biomarkers in cardiovascular disease.[9,99,100] Prior studies show elevation of cardiac-specific miRNAs following acute myocardial infarction.[101–103] In prospective studies, miRNA expression profiles in plasma predicted cardiovascular outcomes in patients with atherosclerotic disease.[104,105] Levels of multiple miRNAs known to be expressed in cultured endothelial cells were lower in CAD patients.[106] Importantly, changes in circulating miRNA expression differ in animal models of myocardial infarction as compared to limb ischemia.[103] Potential advantages of miRNAs as biomarkers include: the ability to use amplification techniques to detect low level expression; long-term stability in blood; and, most interestingly, the possibility of evaluating expression networks across multiple miRNAs to examine coordinated responses to disease.[107]
In patients with PAD, individual miRNAs have been shown to be differentially expressed in circulation (see Table 2). Many of the miRNAs that have been studied in PAD were selected based on the animal studies discussed above that demonstrate relevance to limb ischemia. In diabetic patients, there was an association of lower circulating miR-126 with lower ankle-brachial index.[108] In patients with critical limb ischemia, levels of the anti-angiogenic miRs 15a and 16 were higher in serum and predicted the occurrence of amputation amongst diabetic individuals.[62] Similarly, plasma levels of miR-503 were higher in diabetic patients with critical limb ischemia.[30] In patients with thromboangiitis obliterans, circulating levels of miR-130, miR-27b, and miR-210 were increased.[109] Future studies are needed to perform a full profile of circulating miRNAs in a large cohort of PAD patients and controls.
Table 2.
Circulating MicroRNAs as Biomarkers in Peripheral Artery Disease
| MicroRNA | Clinical Findings |
|---|---|
| miR-503 | Higher in patients with diabetes and critical limb ischemia |
| miR-126 | Lower levels correlate with lower ankle-brachial index in patients with diabetes |
| miR-15a/16 | Lower in patients with critical limb ischemia |
| miR-130, -27b,-210 | Higher in patients with thromboangiitis obliterans |
The source and biological function of circulating miRNA remain a subject of active investigation. Developing evidence suggests cells actively release miRNA into microvesicles, which protects them from degradation by endogenous RNAses.[32,110] miRNAs specifically expressed in endothelial cells are enriched in plasma suggesting endothelial injury as a source.[106] In addition, miRNAs can be delivered to target cells.[111,112] Thus, there is the potential that the endothelium absorbs circulating miRNAs thereby regulating cell phenotype.[113,114] Transfer of microRNA from endothelial progenitor cell-derived circulating microvesicles improved perfusion recovery after hindlimb ischemia.[115] Platelets may also be an important determinant of circulating miRNA levels.[116] The diagnostic and prognostic implications of miRNA profiles require further characterization in patients with PAD.
MicroRNA therapies in PAD
Treatments based on miRNA targets are an area of active investigation.[8] In principle, both stimulation of beneficial miRNA pathways and inhibition of adverse miRNAs could be employed to confer clinical benefit in PAD.[117,118] Administration of mimics of pro-angiogenic miRNA or delivery with trophic expression vectors could promote neovascularization in PAD. The technology for opposing microRNA action systemically is further developed. Antimir development involves the creation of complementary oligonucleotides that reduce the levels of specific miRNAs. Multiple strategies permit adequate delivery and repression of miRNAs by antimirs including modification to increase binding capacity, avoid breakdown by nucleases, and enhance cell uptake, though novel tissue targeting modalities are sought. As described above, several strategies have been used successfully to promote vascular growth in animal models of hindlimb ischemia by antagonizing miRNAs known to be upregulated in ischemic tissue. Advantages of antimir therapies include the coordinated regulation of multiple gene targets by single miRNAs and the potential specificity of miRNA dysregulation to ischemic tissues. However, the fact that individual miRNAs have multiple targets also raises concern for off-target effects of antimirs. Additional approaches may be required to restrict the influence of antimirs to specific tissues or regions.
Conclusions
In summary, there is extensive support for intersections of miRNAs and vascular functions that determine clinical disease status in PAD. Individual miRNAs have been characterized that influence the lower extremity response to ischemia in animal models. Clinical studies in patients with PAD confirm alterations in vascular miRNAs in ischemic regions and in circulation. The translation of miRNA biology to the clinical arena for detection and management of clinical PAD holds significant promise.
Acknowledgments
We thank Dr. Dominik Fleischmann, Professor of Radiology and Chief of Cardiovascular Imaging at Stanford University, for providing the radiologic images used in the Figure.
Funding Sources
Dr. Hamburg is supported by the Boston University Leadership Program in Vascular Medicine (K12 HL083781) and by NIH HL102299 and NIH HL109790.
Dr. Leeper is supported by NIH K12HL087746 and NIH K08 HL103605.
Footnotes
Conflict of Interest Disclosures:
None
Reference List
- 1.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013 Dec 15;380(9859):2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, et al. Heart Disease and Stroke Statistics--2013 Update: A Report From the American Heart Association. Circulation. 2013 Jan 1;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamburg NM, Balady GJ. Exercise rehabilitation in peripheral artery disease: functional impact and mechanisms of benefits. Circulation. 2011 Jan 4;123(1):87–97. doi: 10.1161/CIRCULATIONAHA.109.881888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steg PG, Bhatt DL, Wilson PW, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007 Mar 21;297(11):1197–206. doi: 10.1001/jama.297.11.1197. [DOI] [PubMed] [Google Scholar]
- 5.Cacoub PP, Zeymer U, Limbourg T, et al. Effects of adherence to guidelines for the control of major cardiovascular risk factors on outcomes in the REduction of Atherothrombosis for Continued Health (REACH) Registry Europe. Heart. 2011 Apr;97(8):660–7. doi: 10.1136/hrt.2010.213710. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001 Sep 19;286(11):1317–24. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 7.Quiat D, Olson EN. MicroRNAs in cardiovascular disease: from pathogenesis to prevention and treatment. J Clin Invest. 2013 Jan 2;123(1):11–8. doi: 10.1172/JCI62876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Rooij E, Olson EN. MicroRNA therapeutics for cardiovascular disease: opportunities and obstacles. Nat Rev Drug Discov. 2012 Nov;11(11):860–72. doi: 10.1038/nrd3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimmeler S, Zeiher AM. Circulating microRNAs: novel biomarkers for cardiovascular diseases? Eur Heart J. 2010 Jul 6; doi: 10.1093/eurheartj/ehq221. [DOI] [PubMed] [Google Scholar]
- 10.Murabito JM, Evans JC, Nieto K, et al. Prevalence and clinical correlates of peripheral arterial disease in the Framingham Offspring Study. Am Heart J. 2002 Jun;143(6):961–5. doi: 10.1067/mhj.2002.122871. [DOI] [PubMed] [Google Scholar]
- 11.Murabito JM, D’Agostino RB, Silbershatz H, Wilson WF. Intermittent claudication. A risk profile from The Framingham Heart Study. Circulation. 1997 Jul 1;96(1):44–9. doi: 10.1161/01.cir.96.1.44. [DOI] [PubMed] [Google Scholar]
- 12.Pradhan AD, Shrivastava S, Cook NR, et al. Symptomatic peripheral arterial disease in women: nontraditional biomarkers of elevated risk. Circulation. 2008 Feb 12;117(6):823–31. doi: 10.1161/CIRCULATIONAHA.107.719369. [DOI] [PubMed] [Google Scholar]
- 13.Wilson AM, Kimura E, Harada RK, et al. Beta2-microglobulin as a biomarker in peripheral arterial disease: proteomic profiling and clinical studies. Circulation. 2007 Sep 18;116(12):1396–403. doi: 10.1161/CIRCULATIONAHA.106.683722. [DOI] [PubMed] [Google Scholar]
- 14.McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001 Oct 3;286(13):1599–606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 15.Szuba A, Oka RK, Harada R, Cooke JP. Limb hemodynamics are not predictive of functional capacity in patients with PAD. Vasc Med. 2006 Nov;11(3):155–63. doi: 10.1177/1358863x06074828. [DOI] [PubMed] [Google Scholar]
- 16.Anderson JD, Epstein FH, Meyer CH, et al. Multifactorial determinants of functional capacity in peripheral arterial disease: uncoupling of calf muscle perfusion and metabolism. J Am Coll Cardiol. 2009 Aug 11;54(7):628–35. doi: 10.1016/j.jacc.2009.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vita JA, Hamburg NM. Does endothelial dysfunction contribute to the clinical status of patients with peripheral arterial disease? Can J Cardiol. 2010 Mar;26( Suppl A):45A–50A. doi: 10.1016/s0828-282x(10)71062-x. [DOI] [PubMed] [Google Scholar]
- 18.Gokce N, Keaney JF, Jr, Hunter LM, et al. Predictive value of non-invasively-determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;(41):1769–75. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 19.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009 Jan 23;136(2):215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang WJ, Yang DD, Na S, et al. Dicer is required for embryonic angiogenesis during mouse development. J Biol Chem. 2005 Mar 11;280(10):9330–5. doi: 10.1074/jbc.M413394200. [DOI] [PubMed] [Google Scholar]
- 21.Suarez Y, Fernandez-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007 Apr 27;100(8):1164–73. doi: 10.1161/01.RES.0000265065.26744.17. [DOI] [PubMed] [Google Scholar]
- 22.Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res. 2007 Jul 6;101(1):59–68. doi: 10.1161/CIRCRESAHA.107.153916. [DOI] [PubMed] [Google Scholar]
- 23.Shilo S, Roy S, Khanna S, Sen CK. Evidence for the involvement of miRNA in redox regulated angiogenic response of human microvascular endothelial cells. Arterioscler Thromb Vasc Biol. 2008 Mar;28(3):471–7. doi: 10.1161/ATVBAHA.107.160655. [DOI] [PubMed] [Google Scholar]
- 24.Suarez Y, Fernandez-Hernando C, Yu J, et al. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc Natl Acad Sci U S A. 2008 Sep 16;105(37):14082–7. doi: 10.1073/pnas.0804597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poliseno L, Tuccoli A, Mariani L, et al. MicroRNAs modulate the angiogenic properties of HUVECs. Blood. 2006 Nov 1;108(9):3068–71. doi: 10.1182/blood-2006-01-012369. [DOI] [PubMed] [Google Scholar]
- 26.Bonauer A, Carmona G, Iwasaki M, et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009 Jun 26;324(5935):1710–3. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 27.Grundmann S, Hans FP, Kinniry S, et al. MicroRNA-100 regulates neovascularization by suppression of mammalian target of rapamycin in endothelial and vascular smooth muscle cells. Circulation. 2011 Mar 8;123(9):999–1009. doi: 10.1161/CIRCULATIONAHA.110.000323. [DOI] [PubMed] [Google Scholar]
- 28.Yin KJ, Olsen K, Hamblin M, et al. Vascular endothelial cell-specific microRNA-15a inhibits angiogenesis in hindlimb ischemia. J Biol Chem. 2012 Aug 3;287(32):27055–64. doi: 10.1074/jbc.M112.364414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leeper NJ, Cooke JP. MicroRNA and mechanisms of impaired angiogenesis in diabetes mellitus. Circulation. 2011 Jan 25;123(3):236–8. doi: 10.1161/CIRCULATIONAHA.110.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caporali A, Meloni M, Vollenkle C, et al. Deregulation of microRNA-503 contributes to diabetes mellitus-induced impairment of endothelial function and reparative angiogenesis after limb ischemia. Circulation. 2011 Jan 25;123(3):282–91. doi: 10.1161/CIRCULATIONAHA.110.952325. [DOI] [PubMed] [Google Scholar]
- 31.van SC, Seghers L, Bijkerk R, et al. Antagomir-mediated silencing of endothelial cell specific microRNA-126 impairs ischemia-induced angiogenesis. J Cell Mol Med. 2009 Aug;13(8A):1577–85. doi: 10.1111/j.1582-4934.2008.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zernecke A, Bidzhekov K, Noels H, et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009 Dec 8;2(100):ra81. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 33.Fish JE, Santoro MM, Morton SU, et al. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008 Aug;15(2):272–84. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu W, Xiao H, Laguna-Fernandez A, et al. Flow-Dependent Regulation of Kruppel-Like Factor 2 Is Mediated by MicroRNA-92a. Circulation. 2011 Aug 2;124(5):633–41. doi: 10.1161/CIRCULATIONAHA.110.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Cardena G, Comander J, Anderson KR, Blackman BR, Gimbrone MA., Jr Biomechanical activation of vascular endothelium as a determinant of its functional phenotype. Proc Natl Acad Sci U S A. 2001 Apr 10;98(8):4478–85. doi: 10.1073/pnas.071052598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011 Jan;91(1):327–87. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boon RA, Hergenreider E, Dimmeler S. Atheroprotective mechanisms of shear stress-regulated microRNAs. Thromb Haemost. 2012 Oct;108(4):616–20. doi: 10.1160/TH12-07-0491. [DOI] [PubMed] [Google Scholar]
- 38.Fang Y, Shi C, Manduchi E, Civelek M, Davies PF. MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc Natl Acad Sci U S A. 2010 Jul 27;107(30):13450–5. doi: 10.1073/pnas.1002120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fang Y, Davies PF. Site-specific microRNA-92a regulation of Kruppel-like factors 4 and 2 in atherosusceptible endothelium. Arterioscler Thromb Vasc Biol. 2012 Apr;32(4):979–87. doi: 10.1161/ATVBAHA.111.244053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicoli S, Standley C, Walker P, et al. MicroRNA-mediated integration of haemodynamics and Vegf signalling during angiogenesis. Nature. 2010 Apr 22;464(7292):1196–200. doi: 10.1038/nature08889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hergenreider E, Heydt S, Treguer K, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012 Mar;14(3):249–56. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 42.Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci U S A. 2008 Feb 5;105(5):1516–21. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ni CW, Qiu H, Jo H. MicroRNA-663 upregulated by oscillatory shear stress plays a role in inflammatory response of endothelial cells. Am J Physiol Heart Circ Physiol. 2011 May;300(5):H1762–H1769. doi: 10.1152/ajpheart.00829.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou J, Wang KC, Wu W, et al. MicroRNA-21 targets peroxisome proliferators-activated receptor-{alpha} in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc Natl Acad Sci U S A. 2011 Jun 21;108(25):10355–60. doi: 10.1073/pnas.1107052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weber M, Baker MB, Moore JP, Searles CD. MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. Biochem Biophys Res Commun. 2010 Mar 19;393(4):643–8. doi: 10.1016/j.bbrc.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun X, Icli B, Wara AK, et al. MicroRNA-181b regulates NF-kappaB-mediated vascular inflammation. J Clin Invest. 2012 Jun 1;122(6):1973–90. doi: 10.1172/JCI61495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McDermott MM, Ferrucci L, Liu K, et al. D-dimer and inflammatory markers as predictors of functional decline in men and women with and without peripheral arterial disease. J Am Geriatr Soc. 2005 Oct;53(10):1688–96. doi: 10.1111/j.1532-5415.2005.53510.x. [DOI] [PubMed] [Google Scholar]
- 48.Leeper NJ, Hunter AL, Cooke JP. Stem cell therapy for vascular regeneration: adult, embryonic, and induced pluripotent stem cells. Circulation. 2010 Aug 3;122(5):517–26. doi: 10.1161/CIRCULATIONAHA.109.881441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heinrich EM, Dimmeler S. MicroRNAs and stem cells: control of pluripotency, reprogramming, and lineage commitment. Circ Res. 2012 Mar 30;110(7):1014–22. doi: 10.1161/CIRCRESAHA.111.243394. [DOI] [PubMed] [Google Scholar]
- 50.Murchison EP, Partridge JF, Tam OH, Cheloufi S, Hannon GJ. Characterization of Dicer-deficient murine embryonic stem cells. Proc Natl Acad Sci U S A. 2005 Aug 23;102(34):12135–40. doi: 10.1073/pnas.0505479102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanellopoulou C, Muljo SA, Kung AL, et al. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 2005 Feb 15;19(4):489–501. doi: 10.1101/gad.1248505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eulalio A, Mano M, Dal FM, et al. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012 Dec 20;492(7429):376–81. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- 53.Ivey KN, Srivastava D. MicroRNAs as regulators of differentiation and cell fate decisions. Cell Stem Cell. 2010 Jul 2;7(1):36–41. doi: 10.1016/j.stem.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 54.Martinez NJ, Gregory RI. MicroRNA gene regulatory pathways in the establishment and maintenance of ESC identity. Cell Stem Cell. 2010 Jul 2;7(1):31–5. doi: 10.1016/j.stem.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kane NM, Meloni M, Spencer HL, et al. Derivation of endothelial cells from human embryonic stem cells by directed differentiation: analysis of microRNA and angiogenesis in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2010 Jul;30(7):1389–97. doi: 10.1161/ATVBAHA.110.204800. [DOI] [PubMed] [Google Scholar]
- 56.Ivey KN, Muth A, Arnold J, et al. MicroRNA regulation of cell lineages in mouse and human embryonic stem cells. Cell Stem Cell. 2008 Mar 6;2(3):219–29. doi: 10.1016/j.stem.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kane NM, Howard L, Descamps B, et al. Role of microRNAs 99b, 181a, and 181b in the differentiation of human embryonic stem cells to vascular endothelial cells. Stem Cells. 2012 Apr;30(4):643–54. doi: 10.1002/stem.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012 Feb 17;110(4):624–37. doi: 10.1161/CIRCRESAHA.111.243386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heeschen C, Lehmann R, Honold J, et al. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004 Apr 6;109(13):1615–22. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Q, Kandic I, Kutryk MJ. Dysregulation of angiogenesis-related microRNAs in endothelial progenitor cells from patients with coronary artery disease. Biochem Biophys Res Commun. 2010 Dec 30; doi: 10.1016/j.bbrc.2010.12.119. [DOI] [PubMed] [Google Scholar]
- 61.Meng S, Cao JT, Zhang B, et al. Downregulation of microRNA-126 in endothelial progenitor cells from diabetes patients, impairs their functional properties, via target gene Spred-1. J Mol Cell Cardiol. 2012 Jul;53(1):64–72. doi: 10.1016/j.yjmcc.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 62.Spinetti G, Fortunato O, Caporali A, et al. MicroRNA-15a and MicroRNA-16 Impair Human Circulating Pro-Angiogenic Cell (PAC) Functions and are Increased in the PACs and Serum of Patients with Critical Limb Ischemia. Circ Res. 2012 Dec 11; doi: 10.1161/CIRCRESAHA.111.300418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goodney PP, Beck AW, Nagle J, Welch HG, Zwolak RM. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vasc Surg. 2009 Jul;50(1):54–60. doi: 10.1016/j.jvs.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 64.Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007 Jan;45(Suppl S):S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 65.Leeper NJ, Raiesdana A, Kojima Y, et al. MicroRNA-26a is a novel regulator of vascular smooth muscle cell function. J Cell Physiol. 2011 Apr;226(4):1035–43. doi: 10.1002/jcp.22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zampetaki A, Mayr M. MicroRNAs in vascular and metabolic disease. Circ Res. 2012 Feb 3;110(3):508–22. doi: 10.1161/CIRCRESAHA.111.247445. [DOI] [PubMed] [Google Scholar]
- 67.Ji R, Cheng Y, Yue J, et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 2007 Jun 8;100(11):1579–88. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 68.Cordes KR, Sheehy NT, White MP, et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009 Aug 6;460(7256):705–10. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boettger T, Beetz N, Kostin S, et al. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest. 2009 Sep;119(9):2634–47. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xin M, Small EM, Sutherland LB, et al. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009 Sep 15;23(18):2166–78. doi: 10.1101/gad.1842409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng Y, Liu X, Yang J, et al. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res. 2009 Jul 17;105(2):158–66. doi: 10.1161/CIRCRESAHA.109.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Elia L, Quintavalle M, Zhang J, et al. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ. 2009 Dec;16(12):1590–8. doi: 10.1038/cdd.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Quintavalle M, Elia L, Condorelli G, Courtneidge SA. MicroRNA control of podosome formation in vascular smooth muscle cells in vivo and in vitro. J Cell Biol. 2010 Apr 5;189(1):13–22. doi: 10.1083/jcb.200912096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.vis-Dusenbery BN, Chan MC, Reno KE, et al. down-regulation of Kruppel-like factor-4 (KLF4) by microRNA-143/145 is critical for modulation of vascular smooth muscle cell phenotype by transforming growth factor-beta and bone morphogenetic protein 4. J Biol Chem. 2011 Aug 12;286(32):28097–110. doi: 10.1074/jbc.M111.236950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Balderman JA, Lee HY, Mahoney CE, et al. Bone Morphogenetic Protein-2 Decreases MicroRNA-30b and MicroRNA-30c to Promote Vascular Smooth Muscle Cell Calcification. J Am Heart Assoc. 2012 Dec;1(6):e003905. doi: 10.1161/JAHA.112.003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang F, Zhao XQ, Liu JN, et al. Antagonist of microRNA-21 improves balloon injury-induced rat iliac artery remodeling by regulating proliferation and apoptosis of adventitial fibroblasts and myofibroblasts. J Cell Biochem. 2012 Sep;113(9):2989–3001. doi: 10.1002/jcb.24176. [DOI] [PubMed] [Google Scholar]
- 77.Maegdefessel L, Azuma J, Toh R, et al. MicroRNA-21 blocks abdominal aortic aneurysm development and nicotine-augmented expansion. Sci Transl Med. 2012 Feb 22;4(122):122ra22. doi: 10.1126/scitranslmed.3003441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang M, Li W, Chang GQ, et al. MicroRNA-21 regulates vascular smooth muscle cell function via targeting tropomyosin 1 in arteriosclerosis obliterans of lower extremities. Arterioscler Thromb Vasc Biol. 2011 Sep;31(9):2044–53. doi: 10.1161/ATVBAHA.111.229559. [DOI] [PubMed] [Google Scholar]
- 79.Liu X, Cheng Y, Zhang S, et al. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2009 Feb 27;104(4):476–87. doi: 10.1161/CIRCRESAHA.108.185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pipinos II, Sharov VG, Shepard AD, et al. Abnormal mitochondrial respiration in skeletal muscle in patients with peripheral arterial disease. J Vasc Surg. 2003 Oct;38(4):827–32. doi: 10.1016/s0741-5214(03)00602-5. [DOI] [PubMed] [Google Scholar]
- 81.Pipinos II, Judge AR, Zhu Z, et al. Mitochondrial defects and oxidative damage in patients with peripheral arterial disease. Free Radic Biol Med. 2006 Jul 15;41(2):262–9. doi: 10.1016/j.freeradbiomed.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 82.Brass EP, Hiatt WR, Green S. Skeletal muscle metabolic changes in peripheral arterial disease contribute to exercise intolerance: a point-counterpoint discussion. Vasc Med. 2004 Nov;9(4):293–301. doi: 10.1191/1358863x04vm572ra. [DOI] [PubMed] [Google Scholar]
- 83.Li P, Jiao J, Gao G, Prabhakar BS. Control of mitochondrial activity by miRNAs. J Cell Biochem. 2012 Apr;113(4):1104–10. doi: 10.1002/jcb.24004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Greco S, De Simone M, Colussi C, et al. Common micro-RNA signature in skeletal muscle damage and regeneration induced by Duchenne muscular dystrophy and acute ischemia. FASEB J. 2009 Oct;23(10):3335–46. doi: 10.1096/fj.08-128579. [DOI] [PubMed] [Google Scholar]
- 85.Latronico MV, Condorelli G. The might of microRNA in mitochondria. Circ Res. 2012 Jun 8;110(12):1540–2. doi: 10.1161/CIRCRESAHA.112.271312. [DOI] [PubMed] [Google Scholar]
- 86.Das S, Ferlito M, Kent OA, et al. Nuclear miRNA regulates the mitochondrial genome in the heart. Circ Res. 2012 Jun 8;110(12):1596–603. doi: 10.1161/CIRCRESAHA.112.267732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Voellenkle C, Rooij J, Guffanti A, et al. Deep-sequencing of endothelial cells exposed to hypoxia reveals the complexity of known and novel microRNAs. RNA. 2012 Mar;18(3):472–84. doi: 10.1261/rna.027615.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chan SY, Loscalzo J. MicroRNA-210: a unique and pleiotropic hypoxamir. Cell Cycle. 2010 Mar 15;9(6):1072–83. doi: 10.4161/cc.9.6.11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chan SY, Zhang YY, Hemann C, et al. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab. 2009 Oct;10(4):273–84. doi: 10.1016/j.cmet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mutharasan RK, Nagpal V, Ichikawa Y, Ardehali H. microRNA-210 is upregulated in hypoxic cardiomyocytes through Akt- and p53-dependent pathways and exerts cytoprotective effects. Am J Physiol Heart Circ Physiol. 2011 Oct;301(4):H1519–H1530. doi: 10.1152/ajpheart.01080.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fasanaro P, D’Alessandra Y, Di SV, et al. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem. 2008 Jun 6;283(23):15878–83. doi: 10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang JX, Jiao JQ, Li Q, et al. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med. 2011 Jan;17(1):71–8. doi: 10.1038/nm.2282. [DOI] [PubMed] [Google Scholar]
- 93.Shenouda SM, Widlansky ME, Chen K, et al. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation. 2011 Jul 26;124(4):444–53. doi: 10.1161/CIRCULATIONAHA.110.014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Murphy TP, Cutlip DE, Regensteiner JG, et al. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation. 2012 Jan 3;125(1):130–9. doi: 10.1161/CIRCULATIONAHA.111.075770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yamamoto H, Morino K, Nishio Y, et al. MicroRNA-494 regulates mitochondrial biogenesis in skeletal muscle through mitochondrial transcription factor A and Forkhead box j3. Am J Physiol Endocrinol Metab. 2012 Dec;303(12):E1419–E1427. doi: 10.1152/ajpendo.00097.2012. [DOI] [PubMed] [Google Scholar]
- 96.Aoi W, Naito Y, Mizushima K, et al. The microRNA miR-696 regulates PGC-1{alpha} in mouse skeletal muscle in response to physical activity. Am J Physiol Endocrinol Metab. 2010 Apr;298(4):E799–E806. doi: 10.1152/ajpendo.00448.2009. [DOI] [PubMed] [Google Scholar]
- 97.Arany Z, Foo SY, Ma Y, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008 Feb 21;451(7181):1008–12. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 98.Chinsomboon J, Ruas J, Gupta RK, et al. The transcriptional coactivator PGC-1alpha mediates exercise-induced angiogenesis in skeletal muscle. Proc Natl Acad Sci U S A. 2009 Dec 15;106(50):21401–6. doi: 10.1073/pnas.0909131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mayr M, Zampetaki A, Willeit P, Willeit J, Kiechl S. MicroRNAs Within the Continuum of Postgenomics Biomarker Discovery. Arterioscler Thromb Vasc Biol. 2013 Feb;33(2):206–14. doi: 10.1161/ATVBAHA.112.300141. [DOI] [PubMed] [Google Scholar]
- 100.Creemers EE, Tijsen AJ, Pinto YM. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res. 2012 Feb 3;110(3):483–95. doi: 10.1161/CIRCRESAHA.111.247452. [DOI] [PubMed] [Google Scholar]
- 101.Ai J, Zhang R, Li Y, et al. Circulating microRNA-1 as a potential novel biomarker for acute myocardial infarction. Biochem Biophys Res Commun. 2010 Jan 1;391(1):73–7. doi: 10.1016/j.bbrc.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 102.Wang GK, Zhu JQ, Zhang JT, et al. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur Heart J. 2010 Mar;31(6):659–66. doi: 10.1093/eurheartj/ehq013. [DOI] [PubMed] [Google Scholar]
- 103.D’Alessandra Y, Devanna P, Limana F, et al. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur Heart J. 2010 Jun 9; doi: 10.1093/eurheartj/ehq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Widera C, Gupta SK, Lorenzen JM, et al. Diagnostic and prognostic impact of six circulating microRNAs in acute coronary syndrome. J Mol Cell Cardiol. 2011 Nov;51(5):872–5. doi: 10.1016/j.yjmcc.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 105.Zampetaki A, Willeit P, Tilling L, et al. Prospective study on circulating MicroRNAs and risk of myocardial infarction. J Am Coll Cardiol. 2012 Jul 24;60(4):290–9. doi: 10.1016/j.jacc.2012.03.056. [DOI] [PubMed] [Google Scholar]
- 106.Fichtlscherer S, De RS, Fox H, et al. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010 Sep 3;107(5):677–84. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 107.Engelhardt S. Small RNA biomarkers come of age. J Am Coll Cardiol. 2012 Jul 24;60(4):300–3. doi: 10.1016/j.jacc.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 108.Zampetaki A, Kiechl S, Drozdov I, et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res. 2010 Sep 17;107(6):810–7. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- 109.Li T, Cao H, Zhuang J, et al. Identification of miR-130a, miR-27b and miR-210 as serum biomarkers for atherosclerosis obliterans. Clin Chim Acta. 2011 Jan 14;412(1–2):66–70. doi: 10.1016/j.cca.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Y, Liu D, Chen X, et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010 Jul 9;39(1):133–44. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 111.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011 Apr;13(4):423–33. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boon RA, Vickers KC. Intercellular Transport of MicroRNAs. Arterioscler Thromb Vasc Biol. 2013 Feb;33(2):186–92. doi: 10.1161/ATVBAHA.112.300139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gupta SK, Bang C, Thum T. Circulating microRNAs as biomarkers and potential paracrine mediators of cardiovascular disease. Circ Cardiovasc Genet. 2010 Oct 1;3(5):484–8. doi: 10.1161/CIRCGENETICS.110.958363. [DOI] [PubMed] [Google Scholar]
- 114.Mocharla P, Briand S, Giannotti G, et al. AngiomiR-126 expression and secretion from circulating CD34+ and CD14+ PBMCs: role for proangiogenic effects and alterations in type 2 diabetics. Blood. 2013 Jan 3;121(1):226–36. doi: 10.1182/blood-2012-01-407106. [DOI] [PubMed] [Google Scholar]
- 115.Ranghino A, Cantaluppi V, Grange C, et al. Endothelial progenitor cell-derived microvesicles improve neovascularization in a murine model of hindlimb ischemia. Int J Immunopathol Pharmacol. 2012 Jan;25(1):75–85. doi: 10.1177/039463201202500110. [DOI] [PubMed] [Google Scholar]
- 116.Willeit P, Zampetaki A, Dudek K, et al. Circulating MicroRNAs as Novel Biomarkers for Platelet Activation. Circ Res. 2013 Jan 2; doi: 10.1161/CIRCRESAHA.111.300539. [DOI] [PubMed] [Google Scholar]
- 117.Krutzfeldt J, Rajewsky N, Braich R, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005 Dec 1;438(7068):685–9. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 118.Fasanaro P, Greco S, Ivan M, Capogrossi MC, Martelli F. microRNA: emerging therapeutic targets in acute ischemic diseases. Pharmacol Ther. 2010 Jan;125(1):92–104. doi: 10.1016/j.pharmthera.2009.10.003. [DOI] [PubMed] [Google Scholar]